Abstract
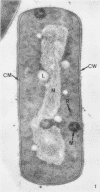
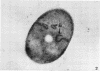
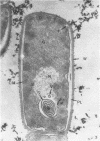
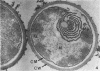
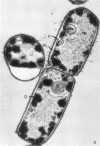
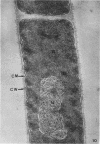
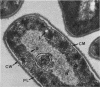
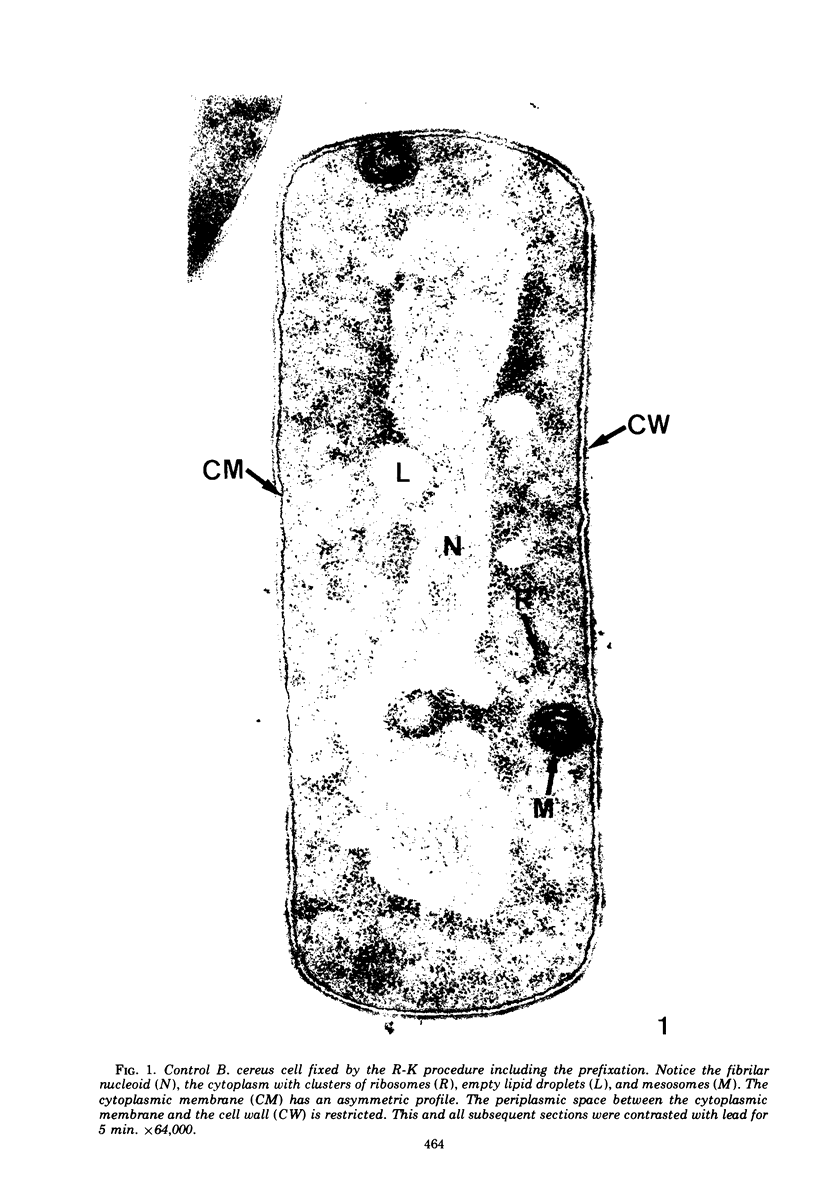
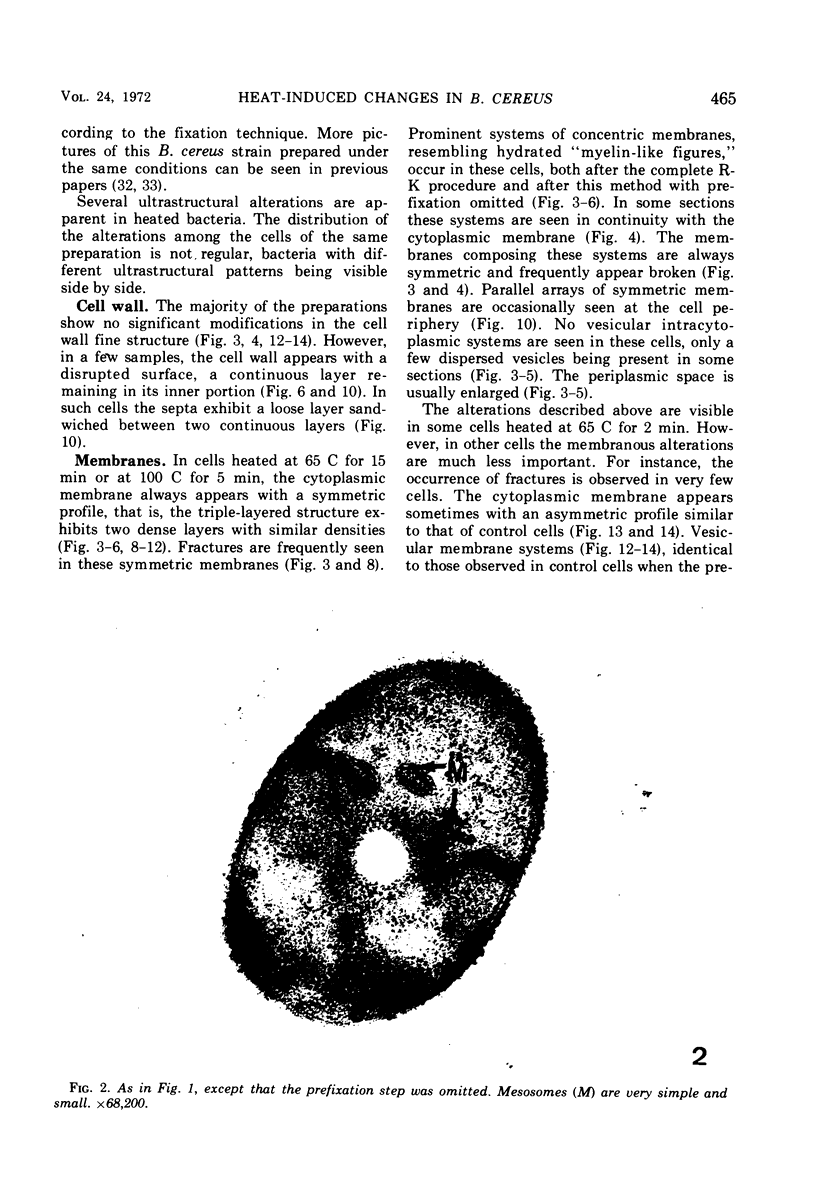
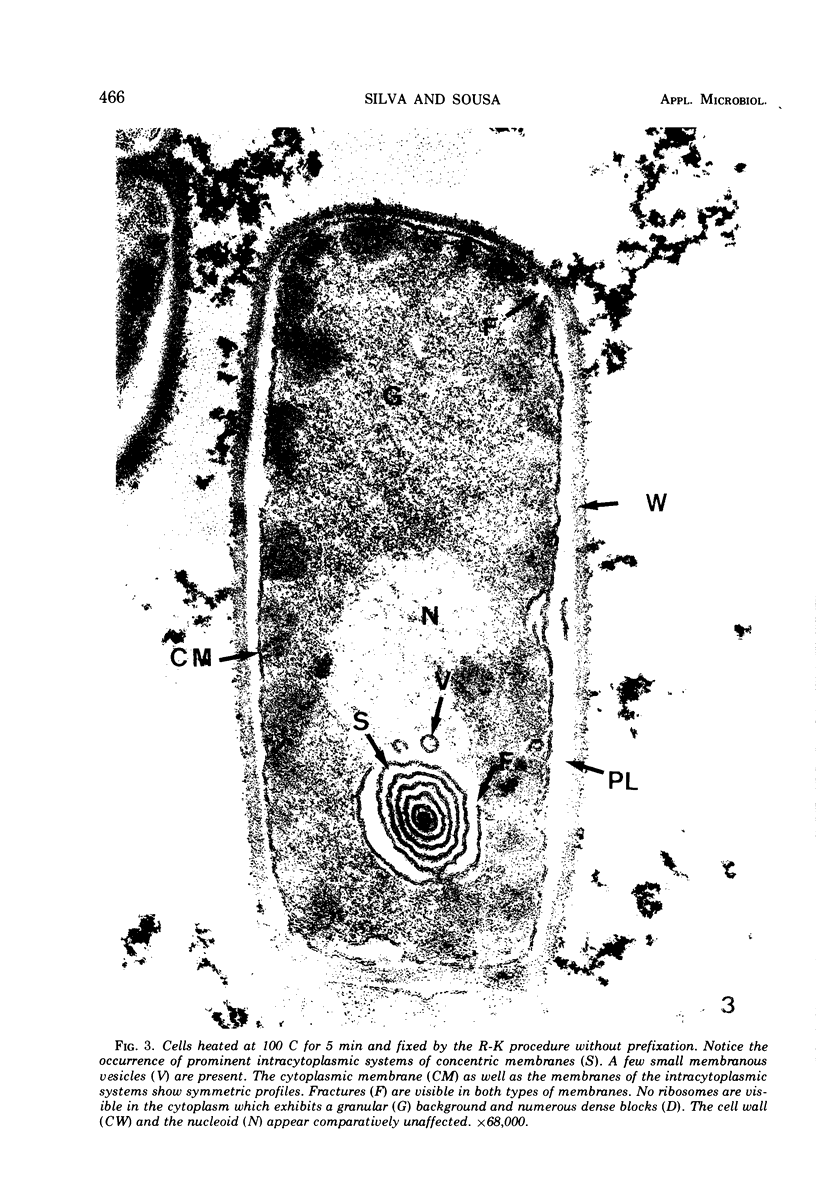
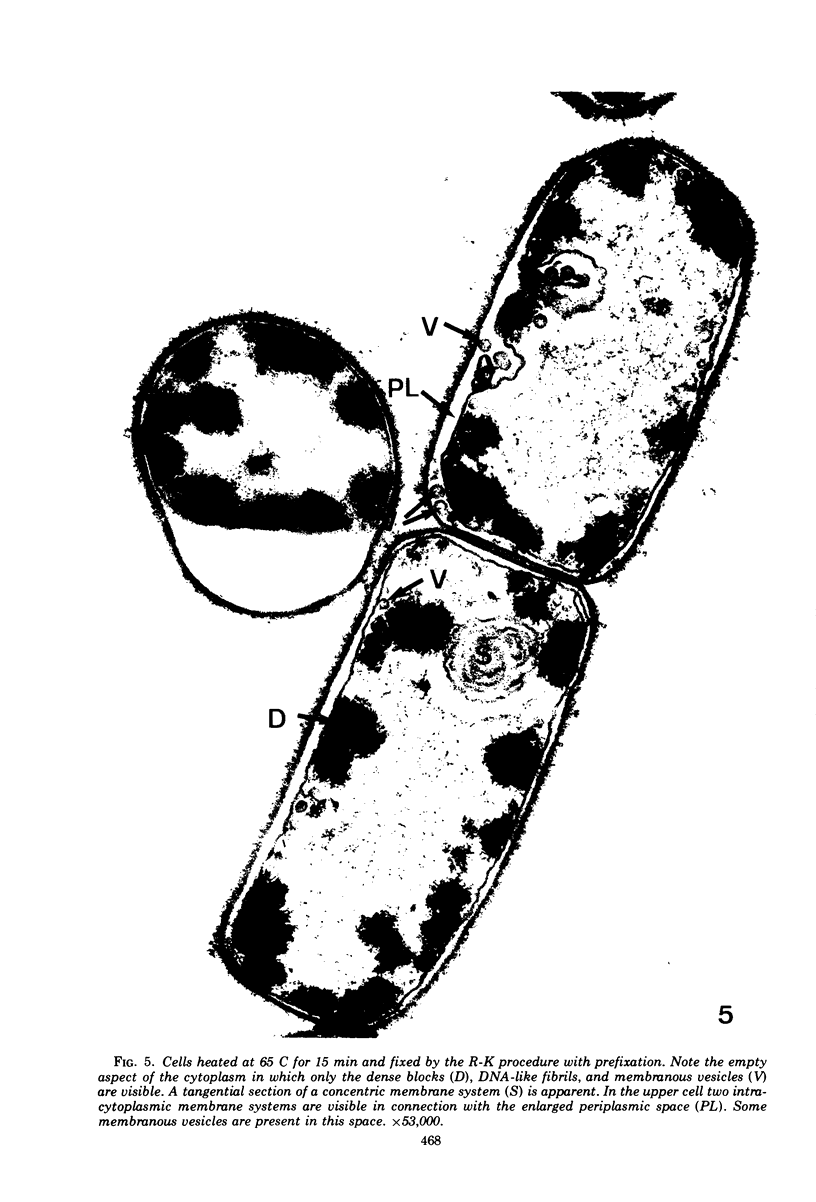
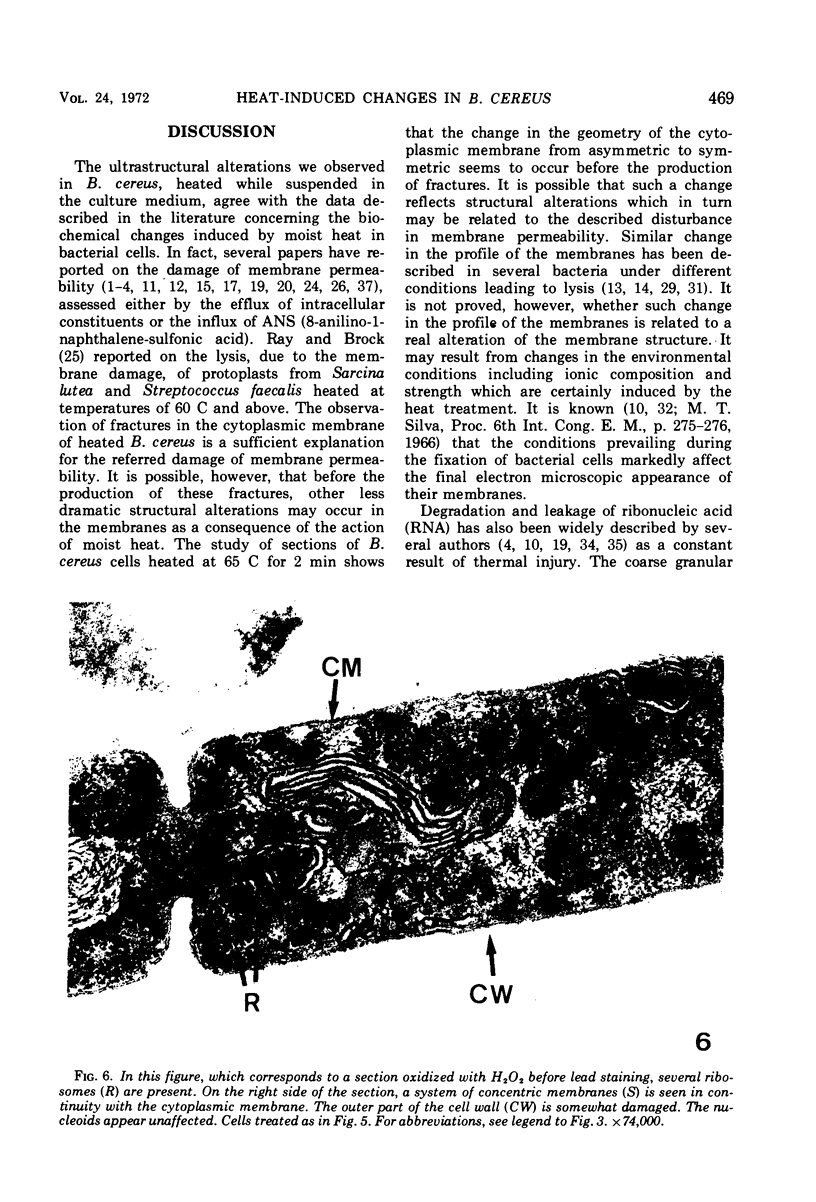
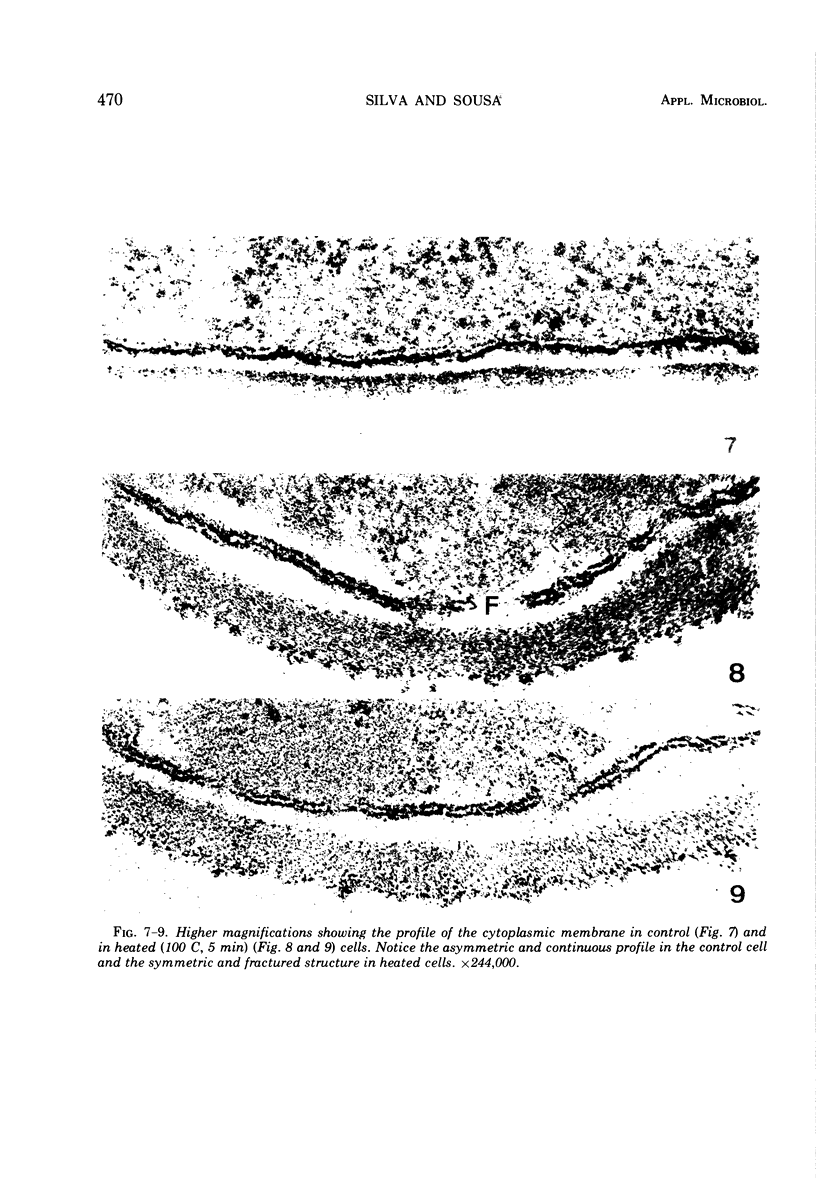
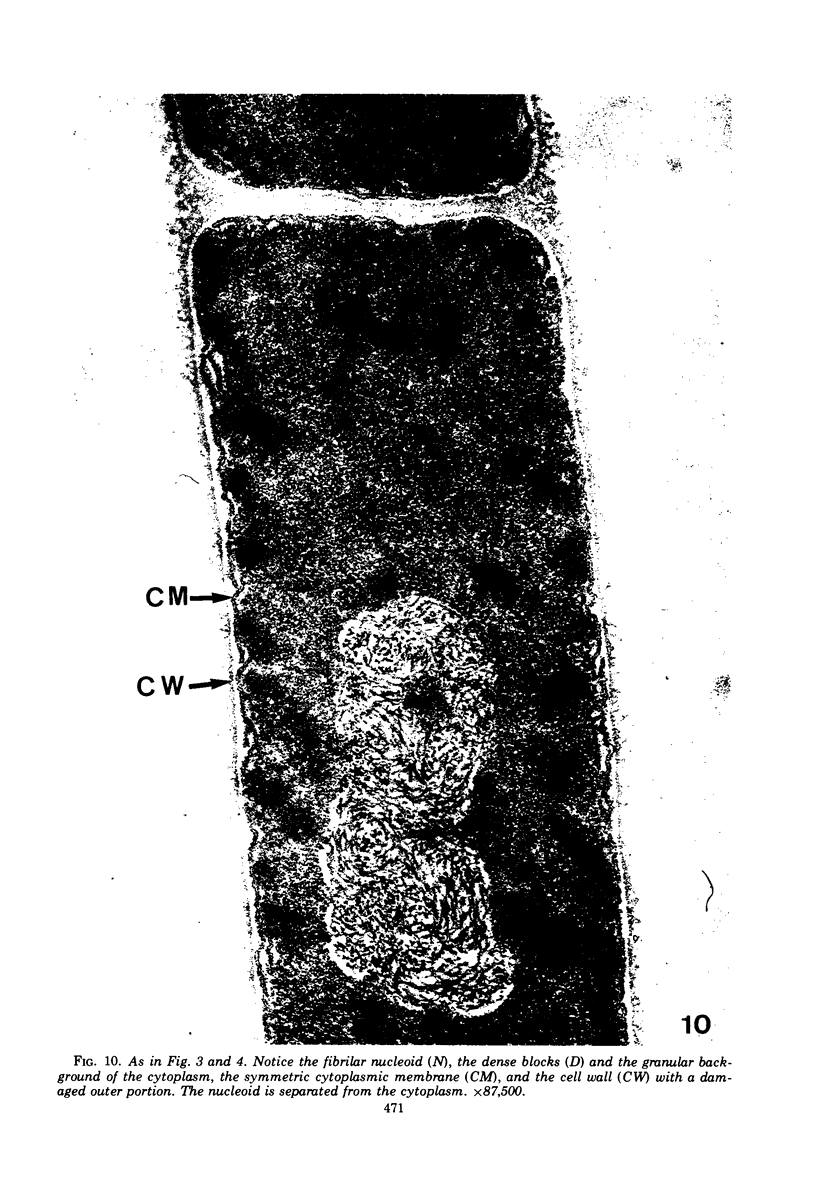
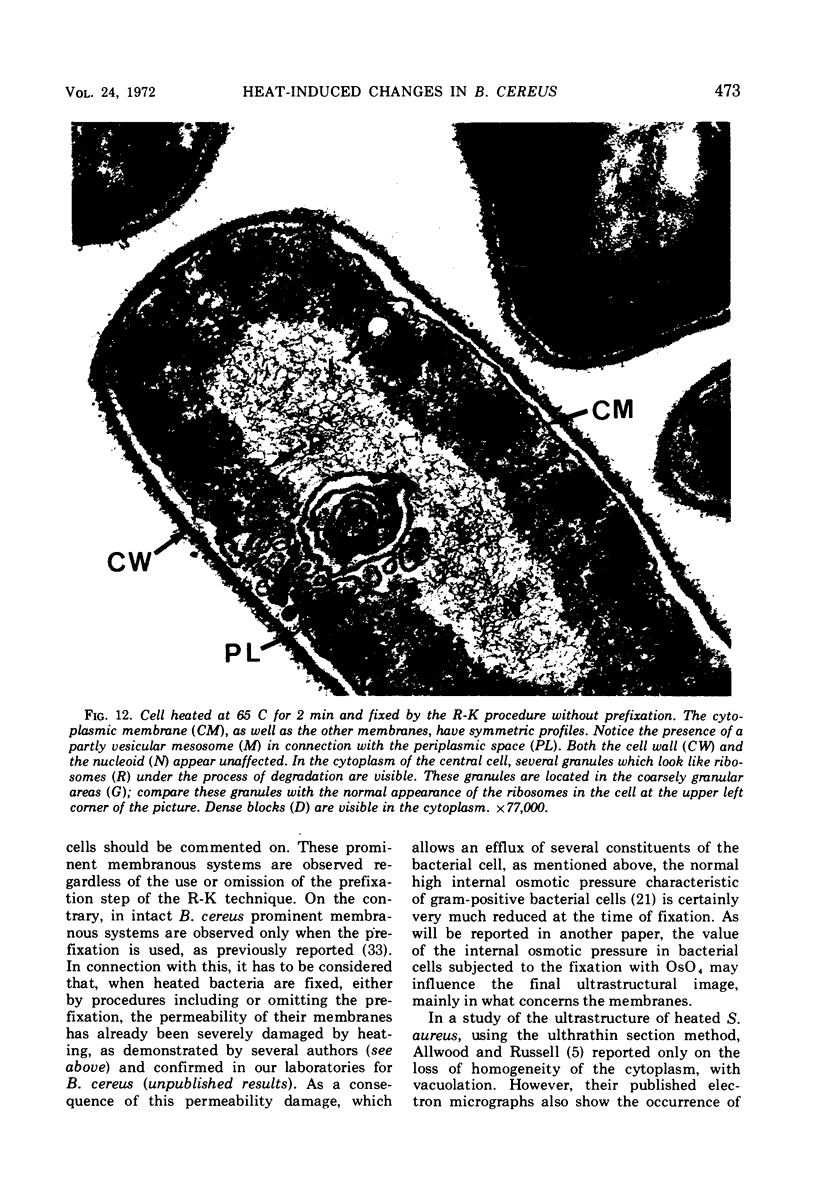
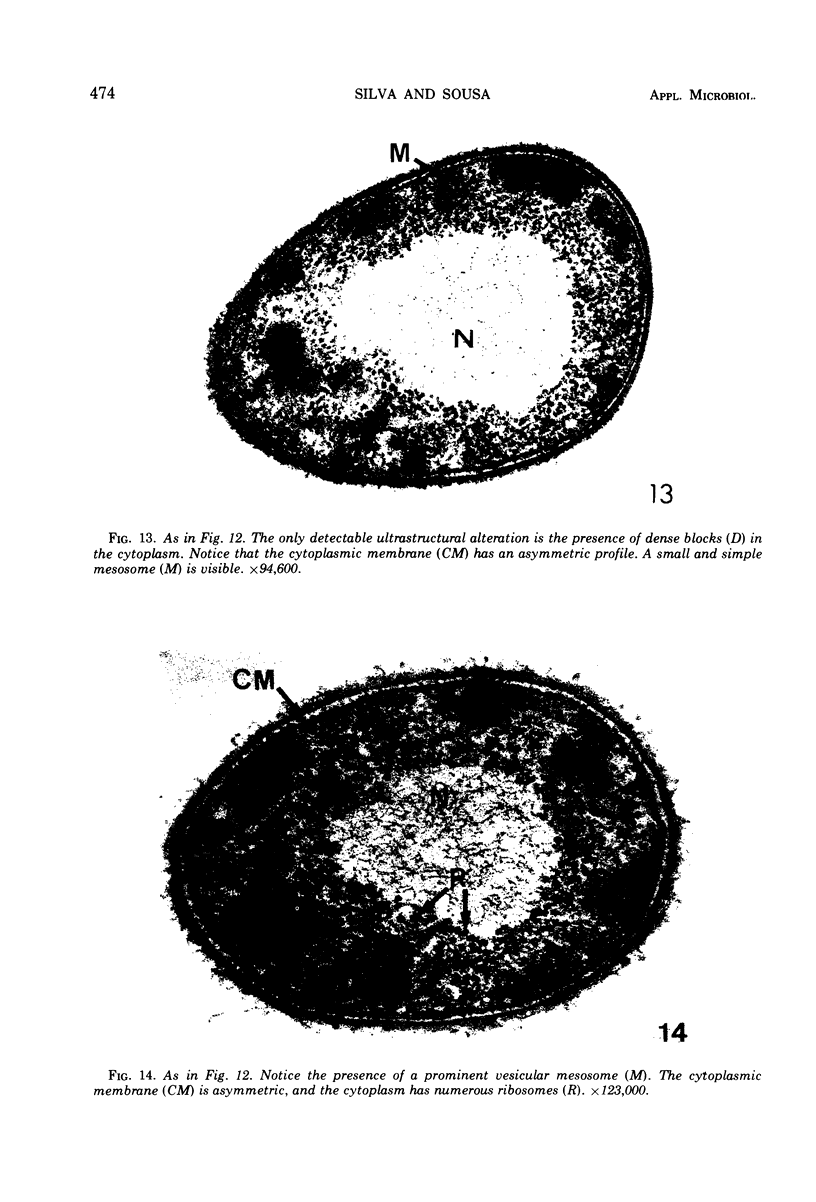
The ultrastructural alterations induced in vegetative, exponentially growing Bacillus cereus cells suspended in broth, by heating at 65 C for 2 and 15 min and 100 C for 5 min, were studied by electron microscopy of thin sections. The following alterations were observed: (i) change in the triple-layered profile of the membranes from the normal asymmetric to a symmetric geometry, appearance of fractures in the membranes, occurrence of prominent myelin-like systems of concentric membranes; (ii) disappearance of the ribosomes in most cells heated at 65 C and in all cells heated at 100 C; (iii) cytoplasmic precipitation resulting in the appearance of dense blocks of Pronase-sensitive material. The cell wall appears unaffected in most preparations. No signs of deoxyribonucleic acid damage are observed. These ultrastructural data are discussed in relation to the alterations in the chemistry and physiology of heated bacteria described in the literature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allwood M. C., Hugo W. B. The leakage of cations and amino acids from Staphylococcus aureus exposed to moist heat, phenol and dinitrophenol. J Appl Bacteriol. 1971 Jun;34(2):369–375. doi: 10.1111/j.1365-2672.1971.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. Mechanism of Thermal Injury in Staphylococcus aureus: I. Relationship Between Viability and Leakage. Appl Microbiol. 1967 Nov;15(6):1266–1269. doi: 10.1128/am.15.6.1266-1269.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. Mechanisms of thermal injury in nonsporulating bacteria. Adv Appl Microbiol. 1970;12:89–119. doi: 10.1016/s0065-2164(08)70583-5. [DOI] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. The leakage of intracellular constituents from heated suspensions of Staphylococcus aureus. Experientia. 1967 Oct 15;23(10):878–879. doi: 10.1007/BF02146908. [DOI] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. Thermally induced changes in the physical properties of Staphylococcus aureus. J Appl Bacteriol. 1969 Mar;32(1):68–78. doi: 10.1111/j.1365-2672.1969.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Allwood M. C., Russell A. D. Thermally induced ribonucleic acid degradation and leakage of substances from the metabolic pool in Staphylococcus aureus. J Bacteriol. 1968 Feb;95(2):345–349. doi: 10.1128/jb.95.2.345-349.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYRNER P., CHAPMAN D. LIQUID CRYSTALLINE NATURE OF PHOSPHOLIPIDS. Nature. 1964 Jun 6;202:987–988. doi: 10.1038/202987a0. [DOI] [PubMed] [Google Scholar]
- Beuchat L. R., Lechowich R. V. Effect of salt concentration in the recovery medium on heat-injured Streptococcus faecalis. Appl Microbiol. 1968 May;16(5):772–776. doi: 10.1128/am.16.5.772-776.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Rogers H. J. Modification of the appearance of mesosomes in sections of Bacillus licheniformis according to the fixation procedures. J Ultrastruct Res. 1970 Feb;30(3):354–367. doi: 10.1016/s0022-5320(70)80068-5. [DOI] [PubMed] [Google Scholar]
- CALIFANO L. Libération d'acide nucléique par les cellules bactériennes sous l'action de la chaleur. Bull World Health Organ. 1952;6(1-2):19–34. [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P., Hancock R. The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol. 1965 Aug;26(2):657–667. doi: 10.1083/jcb.26.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedén C. G., Wyckoff R. W. THE ELECTRON MICROSCOPY OF HEATED BACTERIA. J Bacteriol. 1949 Aug;58(2):153–160. [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod R. A., Light M., White L. A., Currie J. F. Sensitive rapid detection method for viable bacterial cells. Appl Microbiol. 1966 Nov;14(6):979–984. doi: 10.1128/am.14.6.979-984.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Corner T. R. Permeability changes associated with osmotic swelling of bacterial protoplasts. J Bacteriol. 1967 Mar;93(3):1177–1178. doi: 10.1128/jb.93.3.1177-1178.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETHICA B. A. Lysis by physical and chemical methods. J Gen Microbiol. 1958 Apr;18(2):473–480. doi: 10.1099/00221287-18-2-473. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Ray P. H., Brock T. D. Thermal lysis of bacterial membranes and its prevention by polyamines. J Gen Microbiol. 1971 May;66(2):133–135. doi: 10.1099/00221287-66-2-133. [DOI] [PubMed] [Google Scholar]
- Russell A. D., Harries D. Some aspects of thermal injury in Escherichia coli. Appl Microbiol. 1967 Mar;15(2):407–410. doi: 10.1128/am.15.2.407-410.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. Cell structure and the enzymic lysis of bacteria. J Gen Microbiol. 1953 Dec;9(3):512–523. doi: 10.1099/00221287-9-3-512. [DOI] [PubMed] [Google Scholar]
- Santos Mota J. M., Silva M. T., Carvalho Guerra F. Ultrastructural and chemical alterations induced by dicumarol in Streptococcus faecalis. Biochim Biophys Acta. 1971 Oct 12;249(1):114–121. doi: 10.1016/0005-2736(71)90088-5. [DOI] [PubMed] [Google Scholar]
- Silva M. T. Changes induced in the ultrastructure of the cytoplasmic and intracytoplasmic membranes of several Gram-positive bacteria by variations in OsO 4 fixation. J Microsc. 1971 Jun;93(3):227–232. doi: 10.1111/j.1365-2818.1971.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Silva M. T. Electron microscopic aspects of membrane alterations during bacterial cell lysis. Exp Cell Res. 1967 May;46(2):245–251. doi: 10.1016/0014-4827(67)90062-6. [DOI] [PubMed] [Google Scholar]
- Silva M. T. Electron microscopic study on the effect of the oxidation of ultrathin sections of Bacillus cereus and Bacillus megaterium. J Ultrastruct Res. 1967 May;18(3):345–353. doi: 10.1016/s0022-5320(67)80123-0. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Santos Mota J. M., Melo J. V., Guerra F. C. Uranyl salts as fixatives for electron microscopy. Study of the membrane ultrastructure and phospholipid loss in bacilli. Biochim Biophys Acta. 1971 Jun 1;233(3):513–520. doi: 10.1016/0005-2736(71)90151-9. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLS B. A. The resistances of vegetative bacteria to moist heat. J Pharm Pharmacol. 1957 Dec;9(12):864–876. doi: 10.1111/j.2042-7158.1957.tb12348.x. [DOI] [PubMed] [Google Scholar]