Abstract
Natural helper (NH) cells, a member of Lin−IL-2R+IL-7R+IL-25R+IL-33R+GATA3+ group 2 innate lymphoid cell subset, are characterized by the expression of transcription factors GATA3 and RORα and production of large amounts of Th2 cytokines such as IL-5, IL-6 and IL-13 upon IL-33 stimulation or a combination of IL-2 and IL-25. We studied in this paper the signal transduction pathway(s) critical for the cytokine expression and development of NH cell. Either stimulation with IL-33 or a combination of IL-2 and IL-25 induced p38 activation and phosphorylation of GATA3 in NH cells, and the phosphorylated form of GATA3 bound to the IL-5 and IL-13 promoters. All of these events were blocked by SB203580, a p38 inhibitor. Inhibition of p38 also blocked IL-6 production. The mature NH cells lacking Gata3 were impaired in the proliferation and production of IL-5 and IL-13 but not IL-6, indicating that both p38 and GATA3 are critical for the proliferation and production of IL-5 and IL-13 and that the mechanisms downstream of p38 differ between IL-6 and IL-5/IL-13. In contrast, the NH cells lacking RORα showed no impairment in the proliferation and cytokine production, indicating that GATA3 but not RORα plays a pivotal role in the effector functions of mature NH cell. However, deletion of either GATA3 or RORα in hematopoietic stem cells severally blocked the development into NH cells. Our results demonstrate the important roles of p38 and GATA3 in NH cell functions.
Introduction
We have previously identified an Id2-dependent novel innate lymphocyte subset named natural helper (NH)2 cells present in a novel lymphoid tissue, fat-associated lymphoid cluster (FALC), in mouse, rat and human adipose tissues (1). Recent reports showed NH cells also exist in the lung, small and large intestines (2–4). NH cells do not express lineage (Lin) markers but express IL-2R, IL-7R, IL-25R and IL-33R. IL-7 is critical for the differentiation of NH cells as well as NH cell survival. IL-2 induces proliferation of NH cells and IL-33 or a combination of IL-2 and IL-25 (IL-2+25) activates NH cells to produce large amounts of Th2 cytokines IL-5, IL-6 and IL-13. NH cells play important roles in innate immune reactions against helminth infections (1, 4–8). A distinct Id2-dependent innate lymphocyte subset, retinoic acid receptor-related orphan receptor γt (RORγt)+ lymphoid tissue inducer (LTi)-related cells present in the gut regulates intestinal homeostasis by producing IL-17 and IL-22 (9–11).
IL-33 is a member of the IL-1 family and is constitutively expressed in the nuclei of a variety of cells including fibroblasts, epithelial cells, endothelial cells and adipocytes (12, 13). The IL-33 receptor consists of T1/ST2 and IL-1RAcP and receptor binding of IL-33 activates NF-κB transcription factors and the MAP kinase family, including JNK and p38, through MyD88, IRAK, TRAF6 and TAK1 (14, 15). Administration of IL-33 in vivo induces Th2 cytokine production and associated physiological changes in mice including airway hyper-responsiveness, eosinophilia and goblet cell hyperplasia (16). Previous studies have shown that polymorphisms of IL-33 and T1/ST2 are associated in asthma in human, demonstrating that IL-33 and T1/ST2 have a role in human allergic diseases (17). The levels of IL-33 are increased in smooth muscle cells in the airways of severe asthma patients compared to healthy individuals (18). It is thus likely that NH cells play a major role in those IL-33-mediated responses.
Transcription factors GATA3 and retinoic acid receptor-related orphan receptor α (RORα) but not RORγt are highly expressed in NH cells and play important roles in the differentiation of NH cells (1, 3, 6, 19–21). GATA3 selectively activates the IL-4, IL-5 and IL-13 promoters through chromatin remodeling in Th2 cells (22). Interestingly, GATA3 is required for the continuous production of IL-5 and IL-13, but dispensable for maintaining the expression of IL-4 by Th2 cells (23). RORα is induced in Th17 cells and functions together with RORγt to induce IL-17 expression in Th17 cells (24). Although IL-33 induces Th2 cytokine production by various cells, roles of GATA3 and RORα in IL-33 signaling have been obscure.
We demonstrate here that a p38 inhibitor strongly suppresses IL-33-induced production of IL-5, IL-6 and IL-13 by NH cells. Inhibition of p38 blocks both GATA3 phosphorylation and GATA3 binding to the IL5 and IL13 promoters. GATA3 deletion in mature NH cells impairs the expression of IL-5 and IL-13 without affecting IL-6 production. Deletion of GATA3 significantly decreases proliferation of NH cells by cytokine stimulation. Contrary to GATA3, the mutation of RORα showed no effect on the proliferation and Th2 cytokine production of NH cells.
Materials and Methods
Mice
Mice used in this study were on a C57BL/6 background and were maintained in our animal facility under specific pathogen-free conditions. Wild-type (WT) C57BL/6 mice, WBB6F1-KitW/+ and WBB6F1-KitW/Wv mice were obtained from Japan SLC (Tokyo, Japan). B6.SJL-Rag2−/− mice and γc−/−Rag2−/− mice were obtained from Taconic Farms (Germantown, NY). Cre-Ert2 transgenic mice and Rorasg/+ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Gata3flox/flox mice were reported previously (23) and were crossed with Cre-Ert2 transgenic mice to generate Cre-Ert2:Gata3flox/flox mice. Conserved GATA3 response element (Cgre)−/− mice (25) and E4bp4−/− mice (26) were reported previously. All experiments were approved by the Animal Care and Use Committee of Keio University and RIKEN, and were performed in accordance with the institutional guidelines.
Antibodies and Reagents
Antibodies were purchased from BD Pharmingen (San Diego, CA), eBioscience (La Jolla, CA), MD Biosciences (St. Paul, MN), BioLegend (San Diego, CA), Santa Cruz Biotechnology (Santa Cruz, CA), Cell Signaling Technology (Danvers, MA), Abcam (Cambridge, MA), Millipore (Temecula, CA), Invitrogen (Carlsbad, CA) and GE Healthcare (Waukesha, WI). Recombinant mIL-3 and mIL-7 were purchased from PeproTech (Rocky Hill, NJ), hIL-2 was purchased from Shionogi Pharmaceutical Co., Ltd. (Osaka, Japan), mIL-25 and mIL-33 were purchased from R&D Systems (Minneapolis, MN), PMA and 4-hydroxytamoxyfen (4-OHT) were purchased from SIGMA (St. Louis MO), and ionomycin was purchased from Calbiochem (San Diego, CA). SB203580, SP600125 and BAY11-7082 were purchased from Calbiochem (Darmstadt, Germany). Corn oil was purchased from Ajinomoto (Tokyo, Japan).
Preparation of Cells
NH cells were isolated from mesentery as previously described (1) except that we used 10μg/ml Liberase DH from Roche (Tokyo, Japan) and gentle MACS Dissociator (Miltenyi Biotec, Auburn, CA) for the digestion of mesentery. Bone marrow-derived mast cells (BMMCs) and Th2 cells were prepared as described previously (1, 27). To isolate lymphocytes from the small intestinal lamina propria, gut fragments without Peyer’s patches were treated with 1 mM EDTA for 20 min to remove epithelial cells. Tissues were cut into small pieces and incubated for 60 min at 37 °C in DMEM containing dispase (1 U/ml; GIBCO) and DNase I (50 μg/ml; Roche). After pressing through 100-μm mesh, mononuclear cells were purified using discontinuous Percoll (GE Healthcare) gradients (44 and 70%) centrifugation for 20 min at 1500 r.p.m. at room temperature. Lin−RORγ+IL-7Rα+ cells in the collected lamina propria lymphocytes were analyzed by flow cytometry using FOXP3 Fix/Perm Buffer Set (BioLegend).
Culture of NH Cells
NH cells were cultured at 37°C under 5% CO2/95% air in RPMI-1640 (SIGMA) containing 10 ng/ml hIL-2, 10% FCS, 50 μM 2-mercaptoethanol (GIBCO, Grand Island, NY), 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO), 50 μg/ml Gentamycin (Nacalai, Kyoto, Japan), 1 x non-essential amino acids (SIGMA), 10 mM Hepes (SIGMA), and 1 mM sodium pyruvate (GIBCO).
Measurement of Cytokines
Sorted NH cells were seeded at 5 × 103 cells/well into 96 well round bottom tissue culture plates with various stimulants including IL-2 (10 ng/ml), IL-7 (10 ng/ml), IL-25 (10 ng/ml), IL-33 (10 ng/ml), SCF (50 ng/ml), and PMA (30 ng/ml) plus ionomycin (500 ng/ml). Supernatants were collected after 3–96 hrs. Amounts of cytokines in culture supernatants were determined by ELISA using Quantikine kits (R&D) for IL-5, IL-6 and IL-13. Cell numbers and viability were examined using a Countess Automated Cell Counter (Invitrogen). Cytokine production was calculated and presented as pg production per 1 × 103 cells.
Flow Cytometry
Flow cytometry was performed on a FACSAriaII (BD Bioscience, San Jose, CA) and data analyzed using FlowJo Software (Tree Star, Ashland, OR). For intracellular cytokine staining, cells were pretreated with Brefeldin A (eBioscience) for 3 hrs before harvest. Cells were fixed and permeabilized with IntraPrep (Beckman Coulter, Marseille, France) and then stained intracellularly with the indicated antibodies.
Immunoblot Analysis
After stimulation, cells were washed once with PBS and lysed with NE-PER Nuclear and Cytoplasmic Extraction Reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol. Lysates were separated by SDS-PAGE, followed by transfer to polyvinylidene difluoride membranes (Pall Corporation, Washington, NY). Membranes were probed using the designated antibodies and visualized with an ECL Advance™ western blotting detection kit (GE Healthcare, Buckinghamshire, UK).
Quantitative Real-time Polymerase Chain Reaction
Total RNA was prepared using RNeasy Mini Kit (QIAGEN, Hilden, Germany), and cDNA was synthesized with Ready-To-Go T-Primed First-Strand kit (GE Healthcare). A CFX96 Real Time PCR system, C1000 Thermal Cycler and SsoFast EvaGreen Supermix (both from Bio-Rad, Hercules, CA) were used to evaluate gene expression. The expression levels of Gata3 were normalized to 18s rRNA expression. PCR cycling was as follows: 95°C for 3 minutes for 1 cycle, 98°C for 5 seconds, 60°C for 5 seconds for 40 cycles, and 98°C for 10 seconds. Primer pair sequences, specific for Gata3 were (forward, 5′-AGAACCGGCCCCTTATGAA-3′, reverse, 5′-AGTTCGCGCAGGATGTCC-3′), those for Rora were (forward, 5′-TTACGTGTGAAGGCTGCAAG-3′, reverse, 5′-GGAGTAGGTGGCATTGCTCT-3′), and those for 18s rRNA were (forward, 5′-CGCCGCTAGAGGTGAAAATTCT-3′, reverse, 5′-CGAACCTCCGACTTTCGTTCT-3′).
Bone Marrow Transplantation
Whole bone marrow cells (BMCs) were transferred into sublethally irradiated (2 Gy) γc−/−Rag2−/− or B6.SJL-Rag2−/− mice. Cre-mediated recombination was induced on day 1–3. Mesenteric Lin−Thy1.2highCD25+T1/ST2+c-Kit+ or bone marrow (BM) Lin−CD25+T1/ST2+IL-7Rα+ NH cells were analyzed by flow cytometry after indicated time periods. IN the case of transfer into B6.SJL-Rag2−/− mice, donor-derived cells were gated with anti-CD45.2 mAb.
Cre-mediated recombination
For in vivo assays, 4-hydroxytamoxifen (4-OHT) (1 mg/25 g mouse body weight) was diluted with a combination of 90% corn oil (Ajinomoto, Tokyo, Japan) and 10% EtOH and injected intraperitoneally daily for 3 consecutive days. For in vitro assays, recombination was induced by incubation of NH cells cultured in IL-2 (10 ng/ml) with 100 nM 4-OHT or 0.01% EtOH for 48 hrs.
ChIP Assays
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (26). Primer pair sequences specific for each locus were as follows: Il5 (forward, 5′-TCGCCTTTATTAGGTGTCCTC-3′, reverse, 5′-GGCCTTCAGCAAAGGAAGAG-3′), Cgre (forward, 5′-GTCCTCTTATCGACCCCATC-3′, reverse, 5′-AAAGGCTTGGGGAAACAC-3’). Results are presented as the ratio of the cycling threshold value of immunoprecipitated DNA to that of input DNA. (2CT(input)−CT(IP), where ‘CT’ indicates the cycling threshold and ‘IP’ is immunoprecipitated chromatin.)
Statistical Analysis
Data are shown as the mean and s.e.m. Statistical analysis was performed using the repeated measures one way ANOVA. Bonferroni’s multiple comparison adjustment for preplanned contrasts was applied to secure overall type I error, whereas a false discovery rate method for multiple comparison adjustment was applied for pairwise comparisons to avoid over-adjustment.
Results
p38-mediated signaling pathway is critical for the IL-33-induced cytokine production by NH cells
NH cells in mesentery (Fig. S1) produce large amounts of IL-5, IL-6 and IL-13 upon stimulation by IL-33 (1). After stimulation with IL-33 for 96 hrs, NH cells produced ~1 pg/cell of IL-5 and IL-13 while BMMCS produced much lower amounts (Fig. 1A). In contrast, production of IL-6 was more comparable between NH cells and BMMCS (Fig. 1A). Although NH cells express the SCF receptor, c-Kit, c-Kit is dispensable for IL-33-medicated proliferation (Fig. 1B) and cytokine production (Fig. 1C) by NH cells and SCF had little effect on the production of IL-6 by NH cells while SCF and IL-33 had a synergistic effect on IL-6 production by mast cells (Fig. 1D and data not shown) as reported previously (28). While IL-6 production by BMMCS reached a plateau by 24 h after IL-33 stimulation, cytokine production by NH cells continued to increase over time (Fig. 1E).
FIGURE 1. NH cells produce IL-5, IL-6 and IL-13 upon stimulation by IL-33 in a different manner from BMMCS.
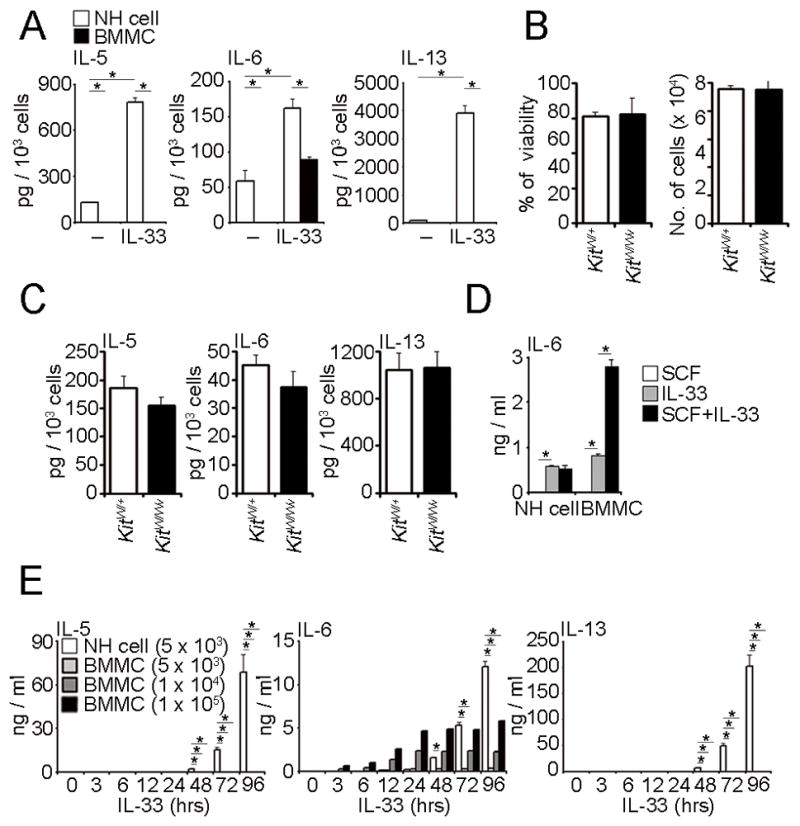
(A) Five thousand mesenteric NH cells or BMMCS were stimulated with IL-33 (10 ng/ml) for 96 hrs. The amounts of IL-5, IL-6 and IL-13 in the supernatants were detected by ELISA and are presented as pg production per 1 × 103 cells. Error bars show s.e.m. (n = 2–3). *, P < 0.05. (B, C) Five thousand mesenteric NH cells from KitW/+ or KitW/Wv mice were stimulated with IL-33 (10 ng/ml) for 96 hrs. Amounts of IL-5, IL-6 and IL-13 in the supernatants were detected by ELISA and are presented as pg production per 1 × 103 cells. Cell numbers and viability at the end of culture are also shown. Error bars show s.e.m. (n = 3). (D) Five thousand mesenteric NH cells or a one hundred thousand BMMCS were stimulated with SCF (50 ng/ml), IL-33 (10 ng/ml) or SCF and plus IL-33 for 96 hrs. The amounts of IL-6 in the supernatants were detected by ELISA. Error bars show s.e.m. (n = 3). *, P < 0.05. (E) Mesenteric NH cells or BMMCS were stimulated by IL-33 (10 ng/ml) and supernatants harvested at the indicated times. The amounts of IL-5, IL-6 and IL-13 were detected by ELISA. Error bars show s.e.m. (n = 3). *, P < 0.05. All results are representatives of two or three independent experiments with similar results.
We then examined the signal transduction pathways downstream of the IL-33 receptor in NH cells and BMMCS. IL-33 induced phosphorylation of NF-κB p65, JNK and p38 MAPK in the cytosol of both BMMCS and NH cells (Fig. S2A). Intriguingly, NH cells have phosphorylated NF-κB p65, JNK and p38 MAPK in the nucleus even before stimulation. IL-33 induced biphasic phosphorylation of NF-κB p65, c-Jun and ATF2 in the nucleus of NH cells (Fig. S2B). Phosphorylation of these molecules peaked 30–60 min after stimulation by IL-33, decreased somewhat and then increased again after 48 hr of activation. Stimulation-dependent complete degradation of an adopter molecule, IRAK1 was not observed in NH cells in contrast to BMMCS (Fig. S2C). In addition, 24 h after stimulation by IL-33, expression of Gfi-1, c-Maf and NFATc1, molecules highly expressed in Th2 cells, was strongly induced in NH cells (Fig. S2D).
To determine signaling pathways important for IL-33-induced Th2 cytokine production in NH cells, we examined the effects of various inhibitors. Among them SB203580, a p38 inhibitor, significantly suppressed IL-33-induced production of IL-5, IL-6 and IL-13 in NH cells while JNK and NF-κB inhibitors (SP600125 and BAY11-7082, respectively) had little effect (Fig. 2A, S3A). Inhibition of p38 during early phase of stimulation reduced IL-5, IL-6 and IL-13 production by IL-33 whereas inhibition in late phase showed little effect (Fig S3B). SB203580 and BAY11-7082 suppressed the growth of NH cells without affecting their viability (Fig. 2B) or the expression level of T1/ST2 (Fig. 2C). Intracellular staining confirmed that SB203580 significantly inhibited the amounts of these Th2 cytokine in NH cells (Fig. 2D), indicating that SB203580 suppressed NH cell production of IL-33-induced Th2 cytokines rather than the secretion step. Because IL-2+25 also induces Th2 cytokine production and cell expansion of NH cells (1), we examined the effect of p38 inhibitor upon IL-2+25 stimulation. In this case, too, SB203580 significantly inhibited IL-2+25-induced Th2 cytokine production and cell proliferation of NH cells in a dose dependent manner without affecting cell viability (Fig 2E and 2F).
FIGURE 2. p38 MAPK is important for cytokine production in NH cells.
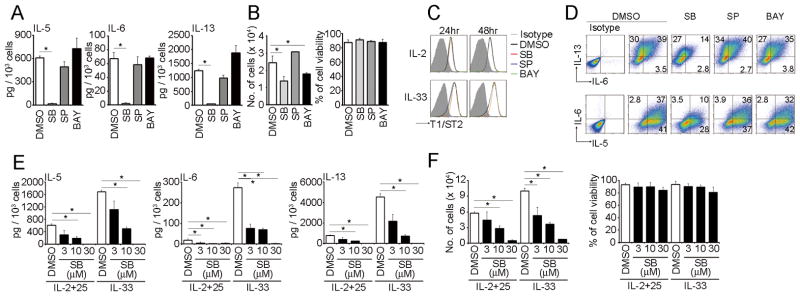
(A) Five thousand mesenteric NH cells were pretreated with DMSO (0.1%), SB203580 (10 μM), SP600125 (3 μM) or BAY11-7082 (100 nM) for 1 hr prior to IL-33 (10 ng/ml) stimulation for 96 hrs. Amounts of IL-5, IL-6 and IL-13 in the supernatants were detected by ELISA and are presented as pg production per 1 × 103 cells. (B) Cell numbers and viability at the end of culture are also shown. Error bars show s.e.m. (n = 3). *, P < 0.05. (C) Mesenteric NH cells were pretreated as in (A) and cultured with IL-2 (10 ng/ml) or IL-33 (10 ng/ml) for the indicated time periods and examined the expression levels of T1/ST2. (D) Mesenteric NH cells were pretreated as in (A) and stimulated IL-33 (10 ng/ml) for 24 hrs. Cells were incubated with Brefeldin A for the last 3 hrs of IL-33 treatment. IL-5, IL-6 and IL-13 expression levels were detected by intracellular cytokine staining. Numbers indicate the percentage of each population within the gate. (E) Five thousand mesenteric NH cells were pretreated with DMSO (0.1%) or SB203580 (3–30 μM) for 1 hr prior to IL-2+25 (10 ng/ml each) or IL-33 (10 ng/ml) stimulation for 96 hrs. Amounts of IL-5, IL-6 and IL-13 in the supernatants were detected by ELISA and are presented as pg production per 1 × 103 cells. (F) Cell numbers and viability at the end of culture are also shown. Error bars show s.e.m. (n = 3). *, P < 0.05. All results are representatives of two or three independent experiments with similar results.
GATA3 regulates IL-5 and IL-13 expression in NH cells
Although recent studies have shown that GATA3 is critical for cytokine production by NH cells (6, 19, 20), mechanisms how GATA3 is involved in the cytokine expression of NH cells have been obscure. As reported previously (1), mesenteric naïve NH cell express high amounts of GATA3 in the nucleus whose levels were similar to those in differentiated Th2 cells (Fig. 3A). IL-33 stimulation induced the phosphorylation of GATA3 and enhanced GATA3 expression (Fig. 3B). SB203580 inhibited IL-33-induced phosphorylation of GATA3 in the nucleus of NH cells (Fig. 3C) while JNK and NF-κB inhibitors showed little effect (Fig. 3D). These data collectively indicate that IL-33 activates the p38-mediated phosphorylation of GATA3 in NH cells. IL-25 is also known to activate TRAF6, TAK1 and p38 (29). In fact, stimulation of NH cells by IL-2+25 induced the activation of p38 and phosphorylation of GATA-3 (Fig. 3E). Furthermore, although phosphorylation of p38 and GATA3 in the nucleus of NH cell was observed even 96 hrs after IL2+25 or IL-33 stimulation, phosphorylation of p38 and GATA3 peaked at 30 min after stimulation (Fig. 3E), suggesting that the early phase of signal transduction is important for Th2 cytokine production in NH cells. Phosphorylation of GATA3 was induced in the order of IL-33, IL2+25 and IL-2, all of which were significantly inhibited by SB203580 (Fig. 3F).
FIGURE 3. IL-33-induced GATA3 phosphorylation by p38 MAPK in NH cells.
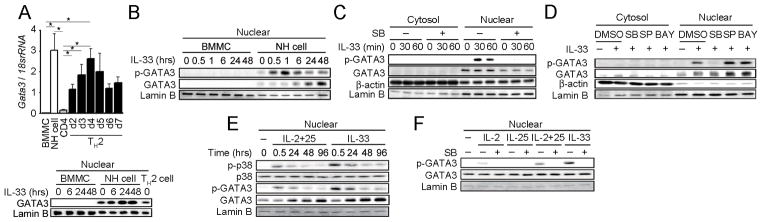
(A) Total RNA was extracted from the indicated cells and Gata3 mRNA levels were detected by real-time PCR (upper panel). Error bars show s.e.m. (n = 3). *, P < 0.05. Nuclear fractions were prepared from IL-33 stimulated BMMCS and mesenteric NH cells as well as Th2 cells and GATA3 levels examined by western blotting (lower panel). (B) Purified mesenteric NH cells and BMMCS were stimulated with IL-33 (10 ng/ml) for the indicated periods. Phosphorylation and expression levels of GATA3 in nuclear extracts were detected by immunoblot analysis. NH cells were pretreated with DMSO (0.1%) or SB203580 (10 μM) (C), or DMSO (0.1%), SB203580 (10 μM), SP600125 (3 μM) or BAY11-7082 (100 nM) (D) prior to IL-33 (10 ng/ml) stimulation for 1 hr. Phosphorylation and expression levels of GATA3 in cytoplasmic and nuclear extracts were detected by immunoblot analysis. (E) NH cells were stimulated with IL-2+25 (10 ng/ml each) or IL-33 (10 ng/ml) for the indicated periods. Phosphorylation and expression levels of p38 and GATA3 in nuclear extracts were detected by immunoblot analysis. We used anti-pGATA3 (phosphor-S308) antibody purchased from Abcam (Cat No.ab61052). (F) NH cells were pretreated with DMSO (0.1%) or SB203580 (10 μM) prior to IL-2 (10 ng/ml), IL-25 (10 ng/ml), IL-2+25 (10 ng/ml each) or IL-33 (10 ng/ml) stimulation for 30 min. Phosphorylation and expression levels of GATA3 in nuclear extracts were detected by immunoblot analysis. All results are representative of two or three independent experiments with similar results.
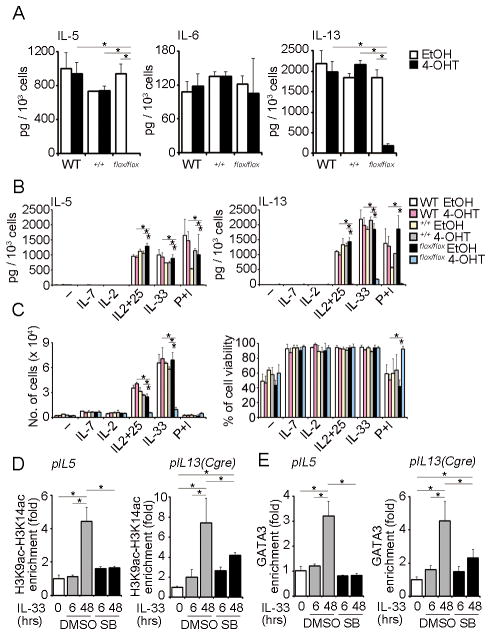
We next determined the role of GATA3 in IL-33-induced Th2 cytokine production using NH cells from Cre-Ert2:Gata3flox/flox mice. NH cells derived from WT and control Cre-Ert2:Gata3+/+ mice with or without 4-OHT treatment produced large amounts of IL-5, IL-6 and IL-13 in response to IL-33 (Fig. 4A). In contrast, upon 4-OHT treatment NH cells derived from Cre-Ert2:Gata3flox/flox mice produced significantly less IL-5 and IL-13 upon IL-33 induction while IL-6 production was unaffected (Fig. 4A). Production of IL-5 and IL-13 in response to a combination of either IL-2 and IL-25 or phorbol-12-myristate-13-acetate (PMA) and ionomycin was also impaired upon deletion of GATA3 (Fig. 4B). Intriguingly, at the same time, deletion of GATA3 inhibited the expansion of NH cells in response to IL-33 and IL-2+IL-25 without affecting cellular viability, while responses to IL-2 and IL-7 were not significantly affected (Fig. 4C).
FIGURE 4. IL-33 induced p38-mediated GATA3 phosphorylation is critical for IL-5 and IL-13 but not IL-6 production in NH cells.
(A) Mesenteric NH cells (5 × 103 cells) isolated from WT, Cre-Ert2:Gata3+/+ or Cre-Ert2:Gata3flox/flox mice were cultured in media containing IL-2 (10 ng/ml) with EtOH (0.01%) or 4-OHT (100 nM) for 48hr. After deleting Gata3, cells were stimulated with IL-33 (10 ng/ml) for 96 hrs. Amounts of IL-5, IL-6 and IL-13 in the supernatants were detected by ELISA and are presented as pg production by 1 × 103 cells. Error bars show s.e.m. (n = 3). *, P < 0.05. (B, C) After deleting Gata3 as in (A), cells were cultured with IL-7 (10 ng/ml), IL-2 (10 ng/ml), IL-2 and 25 (10 ng/ml each), IL-33 (10 ng/ml) or PMA (30 ng/ml) plus ionomycin (500 ng/ml) for 96 hrs. (B) Amounts of IL-5, IL-6 and IL-13 in the supernatants were detected by ELISA and are presented as pg production by 1 × 103 cells. (C) Cell numbers and viability at the end of culture are shown. Error bars show s.e.m. (n = 2–3). *, P < 0.05. (D, E). WT mesenteric NH cells were pretreated with DMSO (0.1%) or SB203580 (10 μM) for 1 hr prior to IL-33 (10 ng/ml) stimulation for the indicated time periods. ChIP analysis for the acetylation of histone H3 at Lys9 and Lys14 (H3K9ac-H3K14ac) (D) or GATA3 (E) binding to the Il5 and Il13 loci were detected by quantitative real-time PCR. Results are presented as relative enrichment compared to input DNA prepared from untreated cells. Error bars show s.e.m. (n = 3–4). *, P < 0.05. All results are representative of two or three independent experiments with similar results.
GATA3 directly binds to Cgre, which is located 1.6 kbp upstream from the transcriptional start site and functions as an enhancer for IL-13 expression (25). We examined the involvement of CGRE in IL-33-triggered IL-13 production in NH cells. IL-33-dependent induction of IL-13, but not IL-5, was severely impaired in NH cells derived from Cgre−/− mice, indicating that Cgre is critical for the expression of IL-13 in NH cells (Fig. S4A). The transcription factor E4BP4 or NF-IL3 is the initiator of IL-10 and IL-13 production in Th1 cells (26). However, IL-13 production was unaffected in NH cells derived from E4bp4−/− mice (Fig. S4A). Neither Cgre nor E4BP4 was required for IL-5 production (Fig. S4A).
To examine whether GATA3 binds Il5 and Il13 loci and induces transcription of these cytokine genes in NH cells, we performed ChIP assays (Fig. 4D and E). We first examined the histone H3 acetylation at lysine positions 9 and 14, which is commonly found in genes undergoing active transcription (30, 31). IL-33 stimulation enhanced histone H3 acetylation at lysine position 9 and 14 in both the Il5 locus and the Cgre site in the Il13 locus (Fig. 4D). We next investigated whether GATA3 directly binds to these loci. IL-33 strongly increased GATA3 binding to the promoter of the Il5 locus and the Cgre site of the Il13 locus and such GATA3 binding was strongly suppressed by SB203580 (Fig. 4E). SB203580 also decreased the acetylation of histone H3 (Fig. 4D). These results demonstrate that in IL-33-stimulated NH cells, GATA3 activates Il5 and Cgre-containing loci by inducing histone H3 acetylation. In addition, GATA3 binds to the Il5 promoter and the Cgre site of the IL-13 promoter in a manner dependent on p38.
GATA3 and RORα is critical for the differentiation of NH cells
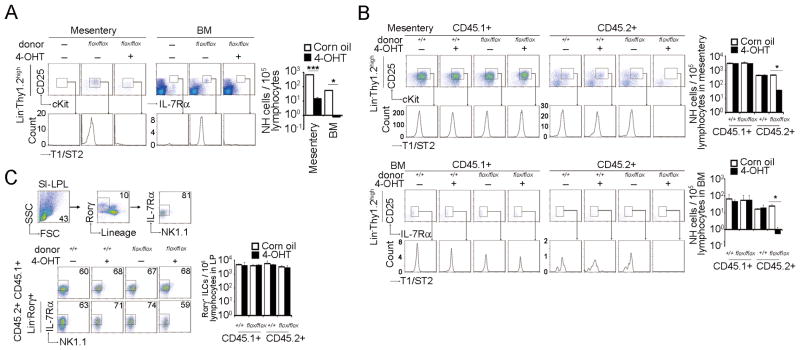
It has recently reported that GATA3 is critical for the differentiation of NH cells by deleting Gata3 in Id2+ cell lineage (6). NH cells are derived from lymphoid progenitor cells within the HSC population (32). To investigate the roles of GATA3 in mesenteric NH cell differentiation from hematopoietic stem cell (HSC), we examined the effect of GATA3 deletion using 4-OHT treatment of Cre-Ert2:Gata3flox/flox and Cre-Ert2:Gata3+/+ mice. Although Cre recombinase expression sometimes results in cellular toxicity (33–35), no significant differences between lymphocyte populations of Cre-Ert2:Gata3flox/flox and Cre-Ert2:Gata3+/+ mice were observed after administration of 40 μg/g body weight 4-OHT (data not shown). BMCs from Cre-Ert2:Gata3flox/flox or Cre-Ert2:Gata3+/+ mice were transplanted into γc−/−Rag2−/− mice lacking NH cells. We then injected 4-OHT and analyzed mesenteric as well as BMCs one month after 4-OHT treatment. Lin−Thy1.2highT1/ST2+CD25+ NH cells were readily observed in mesentery of γc−/−Rag2−/− mice transplanted with BMCs from Cre-Ert2:Gata3flox/flox mice but were absent in 4-OHT-treated γc−/−Rag2−/− mice transplanted with BMCs from Cre-Ert2:Gata3flox/flox mice (Fig. 5A). NH cells were present in 4-OHT-treated γc−/−Rag2−/− mice transplanted with BMCs from Cre-Ert2:Gata3+/+ mice. NH cells are also present in the bone marrow (BM) (3, 6, 36) and the differentiation of NH cells in the BM was also impaired in the absence of GATA3 (Fig. 5A). Essentially the same results were obtained by transplantation of hematopoietic stem cell fraction (data not shown). Similar results were also obtained when we transplanted BMCs into Rag2−/− mice expressing a congenic marker (Fig. 5B), indicating that the defect of NH cell differentiation is cell intrinsic and the effect of other γc-dependent cells is minimal. Deletion of GATA3 (Fig. 5C) or administration of 4-OHT into Cre-Ert2:Gata3flox/flox mice (data not shown) had no effect on RORγ+ LTi-related populations in the gut.
FIGURE 5. GATA3 is critical for the differentiation of NH cells.
BMCs from Cre-Ert2:Gata3+/+ or Cre-Ert2:Gata3flox/flox mice (2.0 × 107 cells) were transferred intravenously into γc−/−Rag2−/− mice (A) or B6.SJL-Rag2−/− mice (B, C). One day after transfer, corn oil or 4-OHT (1 mg/25 g mouse body weight) was injected intraperitoneally for 3 consecutive days and mice were analyzed on day 30. (A) Mesenteric Lin−Thy1.2highc-Kit+CD25+ and BM Lin−IL-7Rα+CD25+ NH cells were analyzed by flow cytometry. Histograms in the lower panels show the expression levels of T1/ST2 on NH cells. Graphs indicate the numbers of NH cells per 1 × 105 lymphocytes in mesentery or BM. (B) Mesenteric (upper panels) and BM (lower panels) NH cells from CD45.1+ recipient (left panels) and CD45.2+ donor (right panels) mice were analyzed by flow cytometry as in (A). (C) Lin−IL-7Rα+RORγ+ LTi-like cells in the small intestine were analyzed by flow cytometry and cell numbers examined per 1 × 105 lymphocytes. Error bars show s.e.m. (n = 2). * and ***, P < 0.05 and 0.005, respectively. Results are representative of two or three independent experiments with similar results.
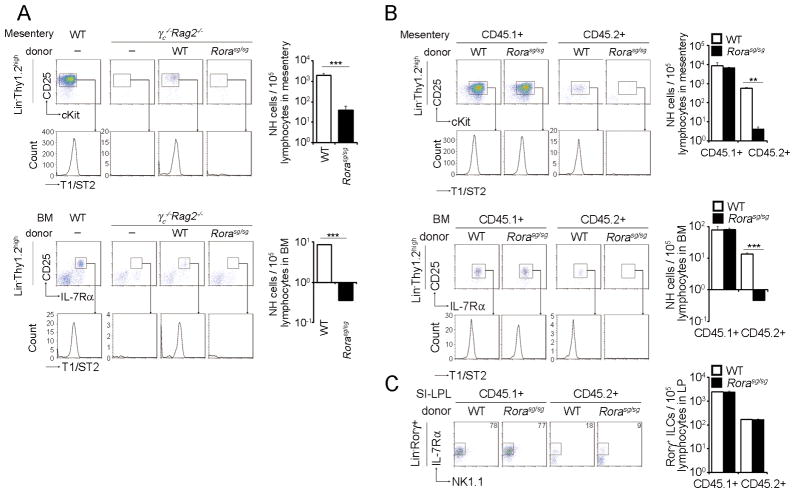
In contrast to the deletion of GATA3, deletion of E4bp4 encoding E4BP4 or NF-IL3, a transcription factor critical for NK cell differentiation (37, 38), or Cgre encoding a GATA3-binding site within the Il13 promoter (25), did not affect NH cell differentiation (Fig. S4B). NH cells express a high level of RORα (1, 3, 21), which is important for the differentiation of NH cells in the lung, small and large intestines (3, 21). We thus examined the role of RORα on mesenteric NH cell differentiation using Rorasg/sg (staggerer, sg) mice carrying a mutation in the Rora locus. We transplanted BMCs of Rorasg/sg mice into γc−/−Rag2−/− as well as Rag2−/− mice. The differentiation of NH cells in both the mesentery and BM was impaired in γc−/−Rag2−/− as well as Rag2−/− mice (Fig. 6A and 6B) without affecting the differentiation of RORγ+ LTi-related populations in the gut (Fig. 6C).
FIGURE 6. RORα is important for the differentiation of NH cells.
(A–C) BMCs from WT or Rora sg/sg mice (6 × 106 (A) or 5 × 106 (B) cells) were transferred intravenously into γc−/−Rag2−/− mice (A) or B6.SJL-Rag2−/− mice (B, C) and analyzed 30 days after transfer. Mesenteric (upper panels) and BM (lower panels) NH cells (A, B) and Lin−IL-7Rα+RORγ+ LTi-like cells in the small intestine (C) were analyzed by flow cytometry. Graphs indicate the numbers of NH cell per 1 × 105 lymphocytes in mesentery, bone marrow or small intestine. Error bars show s.e.m. (n = 2–4). ** and ***, P < 0.01 and 0.005, respectively. Results are representative of two or three independent experiments with similar results.
RORα is dispensable for Th2 cytokine production in NH cells
We next examined the effect of GATA3 deletion in the steady state to clarify the role of GATA3 in the maintenance of NH cells. When we administered 4-OHT into Cre-Ert2:Gata3flox/flox mice, the number of NH cells in the mesentery or BM were significantly decreased and the expression levels of T1/ST2 and IL-2Rα (CD25) on NH cells were greatly reduced (Fig. 7A). Deletion of GATA3 in purified NH cells by treatment with 4-OHT also downregulated T1/ST2 and CD25 but the expression level of RORα and IL-7Rα (CD127) was unaffected (Fig. 7A–C, data not shown). These results collectively indicate that GATA3 positively regulates the expression of IL-33 and IL-2 receptors in NH cells and is important in the maintenance of NH cells in vivo.
FIGURE 7. RORα is dispensable for Th2 cytokine production in NH cells.
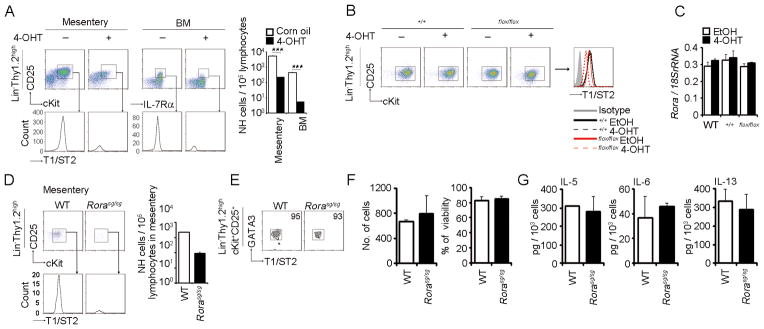
(A) Corn oil or 4-OHT (1 mg/25 g mouse body weight) was injected intraperitoneally for 3 consecutive days to Cre-Ert2:Gata3+/+ or Cre-Ert2:Gata3flox/flox mice and analyzed 4 days after the last injection Mesenteric Lin−Thy1.2highc-Kit+CD25+ and BM Lin−IL-7Rα+CD25+ NH cells were analyzed by flow cytometry. Histograms in the lower panels show the expression levels of T1/ST2 on NH cells. Graphs indicate the numbers of NH cells per 1 × 105 lymphocytes in mesentery or BM. Error bars show s.e.m. (n = 2). ***, P < 0.005. (B, C) Sorted NH cells from the mesentery of WT, Cre-Ert2:Gata3+/+ or Cre-Ert2:Gata3flox/flox mice were cultured in media containing IL-2 (10 ng/ml) and treated with EtOH (0.01%) or 4-OHT (100 nM) for 48 hrs before flow cytometric analysis. Expression levels of T1/ST2 (B) and Rora mRNA (C) were examined. Error bars in (C) show s.e.m. (n = 3). (D, E) Mesenteric NH cells from WT or Rora sg/sg mice were analyzed by flow cytometry as in (A). Error bars show s.e.m. (n = 2). The expression level of GATA3 was analyzed by flow cytometry (E). (F, G) Mesenteric NH cells isolated from WT or Rora sg/sg mice were stimulated with IL-33 (10 ng/ml) for 15 days. Cell numbers and viability at the end of culture (F) and amounts of IL-5, IL-6 and IL-13 in the supernatants (G) were examined. Error bars show s.e.m. (n = 3). All results are representatives of two or three independent experiments with similar results.
While RORα is important for the differentiation of NH cells (Fig. 6 and refs. 3, 21), Rorasg/sg mice have significant number of NH cells albeit the numbers are reduced (Fig. 7D). Mesenteric NH cells isolated from Rorasg/sg mice expressed T1/ST2 and GATA3 at same levels to control WT-derived NH cells (Fig. 7E). Furthermore, we observed that isolated Rorasg/sg NH cells normally responded to IL-33 to proliferate and to produce Th2 cytokines as WT NH cells (Fig. 7F and 7G), indicating that GATA3 and RORα independently function in NH cells and that RORα is dispensable for the function of mature NH cells.
Discussion
Lin−IL-2R+IL-7R+IL-25R+IL-33R+GATA3+ NH cells were originally discovered in lymphoid cluster located along the blood vessels in the mouse and human adipose tissues such as mesentery named ‘fat-associated lymphoid clusters’ or FALCs (1). Upon stimulation with IL-33, NH cells produce large amounts of IL-5 and IL-13 and the amounts produced by 5,000 cells for 5 days reach micro gram quantities (1). NH cells were also found in the lung and gut (3, 4). In human, CRTH2+ NH cells expressing high levels of GATA3 were found in nasal polyps (39). These cells are now classified as a member of group 2 innate lymphoid cells (40).
NH cells express high levels of GATA3 and RORα (1, 3, 6, 19–21) and importance of those transcription factors for the differentiation of NH cells have recently been reported (3, 6, 19–21). We confirmed that both GATA3 and RORα play critical role for the differentiation of NH cells from HSCs in vivo using transplantation of BMCs (Fig. 5 and 6). Contrary to NH cells, development of intestinal RORγ+ LTi-related population, a member of group 3 innate lymphoid cells (40), was unaffected by the deletion of GATA3 or RORα. Id2 is an essential transcription factor for the differentiation of all innate lymphoid cells (40, 41). It has been reported that the expression of both Id2 and GATA-3 is controlled by E4BP4 in NK cells (37) and NK cell differentiation is dependent on E4bp4 (37, 38). As demonstrated here, the differentiation of NH cells was intact in the absence of E4bp4, suggesting differences in the regulatory mechanisms of Id2 and GATA-3 expression between NK cells and NH cells.
NH cells are well known to produce large amounts of Th2 cytokine such as IL-5, IL-6 and IL-13 upon IL-33 or IL-2+25 stimulation and play important roles in anti-helminth immunity (1,5). Helminth infection resulted in the production of IL-33 in the body fluid that activates NH cells to produce IL-5 and IL-13, which leads to eosinophilia and goblet cell hyperplasia, critical for anti-helminth immunity (1, 5, 42). NH cells are thus important population governing the innate immune response against helminth In addition to anti-helminth immunity, NH cells are involved in allergic inflammation associated with eosinophilia and goblet cell hyperplasia that are induced by IL-5 and IL-13 produced by NH cells, respectively (2, 4, 39–41, 43).
The mechanisms of signal transduction for the cytokine expression have been elusive. Both IL-33 and IL-25 activate NF-κB and MAPK pathways through MyD88, IRAK, TRAF6 and TAK1 (13, 14, 29). We demonstrated that IL-33 stimulation induced strong and sustained activation of NF-κB and MAPK pathways in NH cells compared to BMMCS by IL-33. IRAK1 induces a negative feedback loop downstream of Toll-like receptors (44, 45), complete degradation of this adapter molecular was not observed in NH cells. In addition, IL-33 strongly enhanced the expression of Th2-related molecules such as GATA3, Gfi-1, c-Maf and NFATc1. Sustained expression of these molecules could be the reasons for the strong and sustained activation of cytokine production in NH cells. In T cells, TCR-stimulated phosphorylation of GATA3 by p38 is critical for the nuclear translocation of GATA3 and enhanced Th2 cytokine production (46, 47), Interestingly, NH cells expressed GATA3 protein in the nucleus without stimulation and IL-33 strongly increased GATA3 binding to the Il5 promoter and the Cgre site of the Il13 promoter in a manner dependent on phosphorylation by p38. Although the production of IL-5, IL-6 and IL-13 was accelerated 48 hrs after IL-33 stimulation, our data indicate that the early phase rather than late phase of IL-33-mediated p38 signal transduction is important for Th2 cytokine production because the inhibition of p38 at later time points was less effective. Consistent with these data, the phosphorylation of GATA3 peaked at 30 min to 6 hrs after IL-33 stimulation. Our data also indicate that IL2+25-induced Th2 cytokine production was also regulated by GATA3 in a p38-dependent manner. It should be determined in future experiments if phosphorylation of GATA3 by p38 directly regulates the binding of GATA3 to the promoters of Il5 and Il13 and the expression of IL-5 and IL-13 in NH cells. It should be noted that GATA3 deletion in mature NH cells impaired the production of IL-5 and IL-13 upon IL2+25, IL-33 or PMA+ionomycin induction while IL-6 production was unaffected, indicating that IL-6 expression is dependent on p38 but independent of GATA3. It has been shown that deletion of GATA3 in IL-13 expressing cells in worm-infected mice impaired Th2 cytokine expression in innate helper type 2 cells (19). Our results indicate the importance of GATA3 in regulating the expression of IL-5 and IL-13 in naïve NH cells.
Surprisingly, deletion of GATA3 in cultured NH cells resulted in growth retardation of NH cells in response to IL-33 and IL-2+IL-25 without affecting cellular viability, while responses to IL-2 and IL-7 were not significantly affected. Because IL-6 production in response to IL-33 or IL-2+IL-25 was not affected by GATA3 deletion, impaired production of IL-5 and IL-13 in the absence of GATA3 is unlikely due to the growth retardation. GATA3 deletion in mature NH cells in vivo also significantly decreased NH cells in the mesentery and BM, which is consistent with a recent report regarding lamina propria NH cells (6), collectively indicating that GATA3 is important in the maintenance of NH cells in vivo. GATA3 deletion caused downregulation of T1/ST2 and CD25 on mature NH cells (Fig. 7 and ref. 6) and overexpression of GATA3 induced T1/ST2 expression in human NH cells (20). Because NH cell differentiation is not impaired in IL-2−/−, IL-2Rβ−/−, IL-25−/−, IL-33−/− or T1/ST2−/− mice (ref. 1 and data not shown), the reduction of NH cells after deletion of GATA3 is unlikely due to the reduction of T1/ST2 or IL-2R expression. On the other hand, IL-7 is critical for the differentiation and maintenance of NH cells (1) but the expression level of IL-7 receptor is unaffected by the deletion of GATA3. It is unknown at the moment how GATA3 controls the maintenance or survival of NH cells. Contrary to GATA3, RORα is not involved in the expression of IL-2 or IL-33 receptors as T1/ST2 and CD25 expression levels of NH cells from Rorasg/sg mice were equal to those of WT NH cells. In addition, isolated NH cells from Rorasg/sg mice were not affected in terms of proliferation and Th2 cytokine production, suggesting that RORα is dispensable for the function of mature NH cells.
The present results demonstrate the critical role of p38 and GATA3 in IL-5 and IL-13 expression in NH cells. GATA3 is also critical for the differentiation, maintenance and proliferation of NH cells. The lack of RORα impairs the differentiation but not cytokine production and proliferation of NH cells, suggesting that RORα mainly plays a role in the differentiation process of NH cells but is dispensable for the function of mature NH cells. It is important in future studies to clarify how GATA3 and RORα regulate differentiation or maintenance of NH cells. GATA3 has been known to associate with allergic diseases such as asthma (48–50). As NH cell-derived IL-5 and IL-13 mediate eosinophilia and goblet cell hyperplasia, respectively, which are important for pathophysiology of asthma, GATA3 in NH cells could be a good target for new strategy to treatment asthmatic disease.
Supplementary Material
Acknowledgments
We thank M. Mochizuki, N. Takeno and S. Wada for their technical assistance; K. Hidaka, T. Higashide, M. Kikuchi and M. Ohno for animal care.
Footnotes
This work was supported in part by JST, PRESTO, a Grant-in Aid for Young Scientist (A) (22689013) to K. M. and a Grant-in-Aid for Scientific Research (S) (22229004) to S. K. from JSPS, a Grant-in-Aid for Scientific Research on Innovative Areas (23118526) to K. M. and a Scientific Frontier Research Grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan. J. Z. is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Abbreviations used in this paper: NH, natural helper; FALC, fat-associated lymphoid cluster; Lin, lineage; RORγt, retinoic acid receptor-related orphan receptor γt; RORα, retinoic acid receptor-related orphan receptor α; LTi, lymphoid tissue inducer; WT, wild-type; CGRE, conserved GATA3 response element; BMMC, bone marrow-derived mast cell; BMC, bone marrow cell; BM, bone marrow; 4-OHT, 4-hydroxytamoxifen; ChIP, chromatin immunoprecipitation; PMA, phorbol-12-myristate-13-acetate; HSC, hematopoietic stem cell.
Disclosure
S.K. is a consultant for Medical and Biological Laboratories, Co. Ltd. The authors otherwise have no financial conflicts of interest.
References
- 1.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 2.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Koyasu S, Moro K, Tanabe M, Takeuchi T. Natural helper cells: a new player in the innate immune response against helminth infection. Adv Immunol. 2010;108:21–44. doi: 10.1016/B978-0-12-380995-7.00002-1. [DOI] [PubMed] [Google Scholar]
- 6.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanos SL, V, Bui L, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin-22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105–109. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 14.Andrade MV, Iwaki S, Ropert C, Gazzinelli RT, Cunha-Melo JR, Beaven MA. Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur J Immunol. 2011;41:760–772. doi: 10.1002/eji.201040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 17.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 18.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 19.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, Te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemoto N, Arai K, Miyatake S. Cutting edge: the differential involvement of the N-finger of GATA-3 in chromatin remodeling and transactivation during Th2 development. J Immunol. 2002;169:4103–4107. doi: 10.4049/jimmunol.169.8.4103. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in TH1-TH2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 24.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka S, Motomura Y, Suzuki Y, Yagi R, Inoue H, Miyatake S, Kubo M. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in TH2 cells. Nat Immunol. 2011;12:77–85. doi: 10.1038/ni.1966. [DOI] [PubMed] [Google Scholar]
- 26.Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, Atarashi K, Hori S, Watarai H, Zhu J, Taniguchi M, Kubo M. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12:450–459. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata J, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 28.Drube S, Heink S, Walter S, Lohn T, Grusser M, Gerbaulet A, Berod L, Schons J, Dudeck A, Freitag J, Grotha S, Reich D, Rudeschko O, Norgauer J, Hartmann K, Roers A, Kamradt T. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood. 2010;115:3899–3906. doi: 10.1182/blood-2009-10-247411. [DOI] [PubMed] [Google Scholar]
- 29.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 31.Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naiche LA, V, Papaioannou E. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis. 2007;45:768–775. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 35.Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T, Murphy AJ, Rojas J, Heike T, Nakahata T, Kawamoto H, Kita T, Yanagita M. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J Immunol. 2009;182:5633–5640. doi: 10.4049/jimmunol.0802413. [DOI] [PubMed] [Google Scholar]
- 36.Brickshawana A, V, Shapiro S, Kita H, Pease LR. Lineage−Sca1+c-Kit−CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–5804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 38.Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, Akashi K, Lind EF, Haight JP, Ohashi PS, Look AT, Mak TW. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 40.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells - a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 41.Koyasu S, Moro K. Role of innate lymphocytes in infection and inflammation. Front Immunol. 2012;3:101. doi: 10.3389/fimmu.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, Taki Y, Futatsugi-Yumikura S, Tsutsui H, Ishii KJ, Yoshimoto T, Akira S, Nakanishi K. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci U S A. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawal T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubo-Murai M, Hazeki K, Nigorikawa K, Omoto T, Inoue N, Hazeki O. IRAK-4-dependent degradation of IRAK-1 is a negative feedback signal for TLR-mediated NF-κB activation. J Biochem. 2008;143:295–302. doi: 10.1093/jb/mvm234. [DOI] [PubMed] [Google Scholar]
- 45.Yamin TT, Miller DK. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem. 1997;272:21540–21547. doi: 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- 46.Chen CH, Zhang DH, LaPorte JM, Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J Immunol. 2000;165:5597–5605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- 47.Maneechotesuwan K, Xin Y, Ito K, Jazrawi E, Lee KY, Usmani OS, Barnes PJ, Adcock IM. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J Immunol. 2007;178:2491–2498. doi: 10.4049/jimmunol.178.4.2491. [DOI] [PubMed] [Google Scholar]
- 48.Liberman AC, Druker J, Refojo D, Holsboer F, Arzt E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. FASEB J. 2009;23:1558–1571. doi: 10.1096/fj.08-121236. [DOI] [PubMed] [Google Scholar]
- 49.Xinxin C, Chi C, Xiao C, Xue X, Yongjun Y, Junqing C, Xuming D. Florfenicol inhibits allergic airway inflammation in mice by p38 MAPK-mediated phosphorylation of GATA 3. Clin Immunol. 2011;138:231–238. doi: 10.1016/j.clim.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Barnes PJ. Role of GATA-3 in allergic diseases. Curr Mol Med. 2008;8:330–334. doi: 10.2174/156652408785160952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.