Abstract
A recent ground-breaking publication described hypothalamus-driven programmatic aging. As a Russian proverb goes “everything new is well-forgotten old”. In 1958, Dilman proposed that aging and its related diseases are programmed by the hypothalamus. This theory, supported by beautiful experiments, remained unnoticed just to be re-discovered recently. Yet, it does not explain all manifestations of aging. And would organism age without hypothalamus? Do sensing pathways such as MTOR (mechanistic Target of Rapamycin) and IKK-beta play a role of a “molecular hypothalamus” in every cell? Are hypothalamus-driven alterations simply a part of quasi-programmed aging manifested by hyperfunction and secondary signal-resistance? Here are some answers.
Keywords: senescence, geroconversion, rapamycin, diseases, molecular hypothalamus
New discovery
The secret of aging has been finally revealed [1]. As described by Cai and coworkers [2], the activity of the inflammatory pathway is increased with age in the hypothalamus, a place in the brain that connects sensory inputs of the nervous system with endocrine function [2, 3]. This small neuroendocrine region consists of neurons and glia cells and governs growth, metabolism and reproduction. With age, overactivated glia secrete proinflammatory cytokines, activating the IKK-beta/NF-kB pathway in neurons, leading to organismal aging [2-4]. Also, it was suggested that hypothalamic SIRT1 may regulate aging [5].
Well-forgotten Old
In the paper entitled “On the … Elevation of the Activity of Certain Hypothalamic Centers” (Dilman, 1958), Vladimir Dilman suggested that aging is caused by a progressive loss of sensitivity of the hypothalamus coupled with increased activity of its certain centers. This caused progressive alteration of homeostasis, metabolic disturbances, leading to age-related diseases [6-13]. In other words, there is an age-related loss of sensitivity by the hypothalamus to the negative feedback of certain hormones, such as estrogens and glucocorticoids [6-13]. This explains the development of age-related diseases, including metabolic disorders and menopause. Furthermore, several agents including phenformin [11, 13] and its analog metformin extended life span in rodents [14-18]. Unfortunately, the hypothalamic theory was far ahead of its time and too medical to be appealing to gerontologists. Also, it cannot clearly link cellular and organismal aging and diseases. On one hand, aging (as a process) was assumed to be caused by accumulation of molecular damage. On the other hand, manifestations of aging such as age-related diseases were described as driven by the hypothalamic activity. Finally, it seemed odd that such a universal process as aging has such a specific driver, making it difficult to extend the theory to invertebrates (at that time).
MTOR in geroconversion
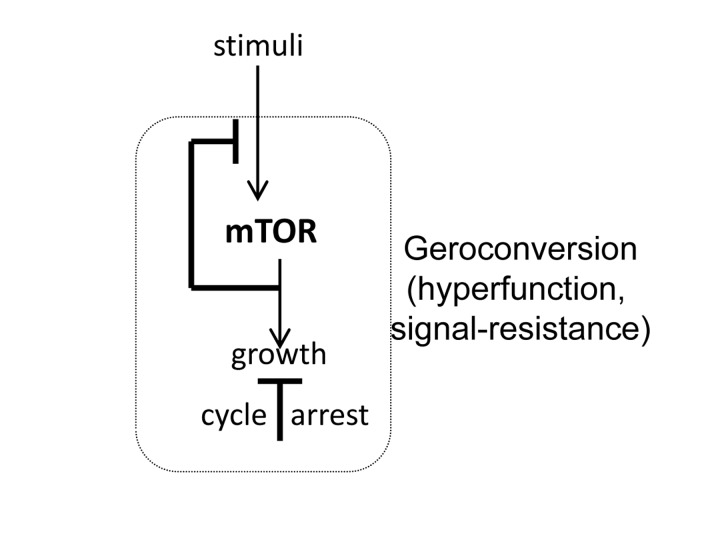
Meanwhile, in the field of cancer research, it was found that oncogenic/mitogenic signaling, such as Ras and Raf, can cause cellular senescence [19, 20]. Strong mitogenic signaling causes simultaneously (a) cell-cycle arrest and (b) hyper-activation of growth-promoting pathways, such as MEK/MAPK and MTOR (mechanistic target of rapamycin). The attention of investigators was attracted only to cell-cycle arrest because it is a barrier to cancer. Cellular senescence became almost a synonym of cell-cycle arrest. Yet, cell-cycle arrest is not senescence. It is growth-promoting pathways such as MTOR that cause the senescent phenotype (Fig. 1). When the cell cycle is arrested, still active MAPK and MTOR first force cell growth in size, followed by acquiring of hallmarks of senescence [21], a process that was named gerogenic conversion or geroconversion [22]. A senescent phenotype is characterized by hyperfunction (such as a hypersecretory and pro-inflammatory phenotype) and secondary signal resistance [21, 22]. Thus, pro-inflammatory and hypersecretory phenotypes are hallmarks of senescence [23-32]. Some other examples are contraction of smooth muscle cells, adhesion of platelets and bone resorption by osteoclasts, which lead to hypertension, thrombosis, and osteoporosis. Inhibition of MTOR suppresses geroconversion, preventing senescence, while maintaining cell cycle arrest [33-44]. Thus senescence is a continuation of cellular growth, when actual growth is constrained [33, 45, 46]. The MTOR is the primary cause of cellular aging, or senescence, leading to the well recognized diseases of aging [47-50].
Figure 1. MTOR-dependent geroconversion to senescence.
When cell cycle is arrested, MTOR drives geroconversion, leading to cellular hypertrophy, hyperfunction, loss replicative/regenerative potential and resistance to signals such as insulin.
MTOR-driven quasi-programmed aging
As cellular aging is a continuation of growth, similarly, organismal aging is a continuation of organismal growth. Aging is not programmed but quasi-programmed, an aimless continuation of organism growth [47, 51-55]. Since geroconversion is driven by in part by MTOR, it was predicted that rapamycin should prolong life span in mammals [47, 56]. In fact, fibroblasts from long-lived mutant mice exhibit lower TOR activity [57] and rapamycin extends life span in mice [58-65]. It also inhibits chronological senescence, which is self-poisoning by acetic and lactic acids in yeast [66, 67] and mammalian cells [68, 69], respectively. Inhibition of MTOR also extends replicative lifespan in yeast [66, 70, 71] and mammalian cells [72, 73].
MTOR as hypothalamus
Discovered by Michael Hall and co-workers in yeast [74], TOR (target of rapamycin) is a “molecular hypothalamus”. It integrates signals generated by insulin, mitogens, cytokines, oxygen, and nutrients [75-79]. Noteworthy, IKK-beta activates MTOR [80-83]. Given that IKK-beta is activated in aging hypothalamus, one may suggest that MTOR is activated too. In agreement, there is an age-dependent increase of MTOR signaling in hypothalamic neurons that express pro-opiomelanocortin. Systemic or intracerebral administra-tion of rapamycin causes weight loss in old mice [84].
Sensing and aging in invertebrates
Although the hypothalamus as an organ is absent in worm, single neurons are analogs of the hypothalamus. C. elegans senses environmental signals through ciliated sensory neurons and mutations that cause defects in sensory cilia extend lifespan [85]. To a great extend, life span of C. elegans is regulated by environmental cues [86-88]. However, there are other determinants of C. elegan lifespan. For example, signals from the reproductive system regulate the lifespan of C. elegans and germline removal extends lifespan [89, 90].
Hypothalamus is not (absolutely) necessary for aging
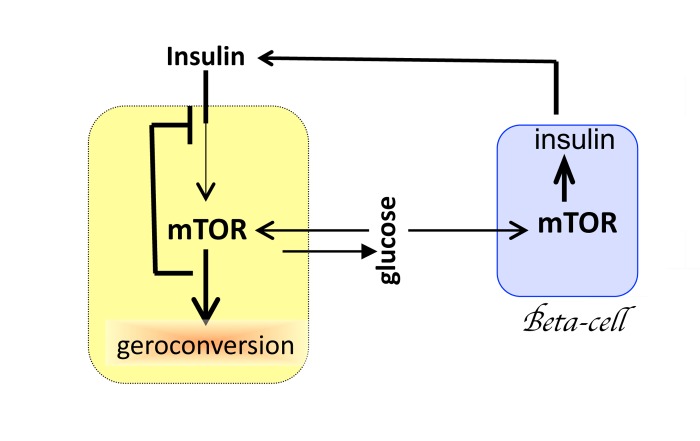
Despite its small size, the hypothalamus plays a disproportionally important role in aging. The reason is that the hypothalamus interacts with numerous organs and tissues, driving alterations of homeostasis. But aging would unfold without the hypothalamus. Mutual cell-cell stimulation, with positive feedback loops, causes overstimulation and geroconversion, manifested as hyperfunction and secondary signal-resistance. For example, glucose activates MTOR, causing resistance to insulin and IGF-1 (Fig. 2). In turn, activation of MTOR increases insulin production by beta-cells of the pancreas (Fig. 2). Increased production of, insulin further stimulates MTOR in fat, muscle and liver tissues, further promoting insulin-resistance. Insulin resistance increases levels of glucose, which over stimulates beta-cells to induce insulin and simultaneously making beta-cells resistant to survival signals (Fig. 2). The process may culminate in beta-cells failure and diabetes type II. This in turn will lead to renal failure, blindness and death. And the hypothalamus is not required (Fig. 2) because each cell contains a molecular “hypothalamus”.
Figure 2. Mutual overstimulation of MTOR in beta-cells and insulin-dependent tissues.
Mutual and reciprocal over-stimulation leads to cellular hyperfunctions and secondary signal-resistance. By negative feedback, overactivated MTOR blocks insulin signaling. See text for explanation. Yellow cell: hepotocyte, adipocyte or muscle cell. Blue cell: beta-cell of the pancreas.
REFERENCES
- 1.Gabuzda D, Yankner BA. Physiology: Inflammation links ageing to the brain. Nature. 2013;497:197–198. doi: 10.1038/nature12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging (Albany NY) 2012;4:98–115. doi: 10.18632/aging.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramadori G, Coppari R. Does hypothalamic SIRT1 regulate aging? Aging (Albany NY) 2011;3:325–328. doi: 10.18632/aging.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dil'Man VM. [Age-connected hypercholesterinemia as an index of increased activity of the hypothalamic centers] Ter Arkh. 1960;32:72–77. [PubMed] [Google Scholar]
- 7.Dilman VM, Berstein LM, Bobrov YF, Bohman YV, Kovaleva IG, Krylova NV. Hypothalamopituitary hyperactivity and endometrial carcinoma. Qualitative and quantitative disturbances in hormone production. Am J Obstet Gynecol. 1968;102:880–889. doi: 10.1016/0002-9378(68)90517-6. [DOI] [PubMed] [Google Scholar]
- 8.Dilman VM. Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageine, and disease. Lancet. 1971;1:1211–1219. doi: 10.1016/s0140-6736(71)91721-1. [DOI] [PubMed] [Google Scholar]
- 9.Anisimov VN, Ostroumova MN, Dil'man VM. Increase with age in the hypothalamo-hypophyseal threshold to the inhibitory action of estrogens and the effect of pineal extract on this process. Bull Exp Biol Med. 1974;77:440–442. doi: 10.1007/BF00798110. [DOI] [PubMed] [Google Scholar]
- 10.Dil'man VM, Anisimov VN. [An increase in hypothalamic sensitivity to the inhibiting effects of estrogens caused by use of L-DOPA, diphenine, epithalamin and phenformin in old rats] Biull Eksp Biol Med. 1975;80:96–98. [PubMed] [Google Scholar]
- 11.Dilman VM, Berstein LM, Zabezhinski MA, Alexandrov VA, Bobrov JF, Pliss GB. Inhibition of DMBA-induced carcinogenesis by phenformin in the mammary gland of rats. Arch Geschwulstforsch. 1978;48:1–8. [PubMed] [Google Scholar]
- 12.Dilman VM, Anisimov VN. Hypothalmic mechanisms of ageing and of specific age pathology--I. Sensitivity threshold of hypothalamo-pituitary complex to homeostatic stimuli in the reproductive system. Exp Gerontol. 1979;14:161–174. doi: 10.1016/0531-5565(79)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Dilman VM, Anisimov VN. Effect of treatment with phenformin, diphenylhydantoin or L-dopa on life span and tumour incidence in C3H/Sn mice. Gerontology. 1980;26:241–246. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- 14.Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med. 2005;139:721–723. doi: 10.1007/s10517-005-0389-9. [DOI] [PubMed] [Google Scholar]
- 15.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 16.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berstein LM. Metformin in obesity, cancer and aging: addressing controversies. Aging (Albany NY) 2012;4:320–329. doi: 10.18632/aging.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menendez JA, Cufi S, Oliveras-Ferraros C, Vellon L, Joven J, Vazquez-Martin A. Gerosuppressant metformin: less is more. Aging (Albany NY) 2011;3:348–362. doi: 10.18632/aging.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serrano M, Lim AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK1A. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 20.Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blagosklonny MV. Cell senescence and hypermitogenic arrest. EMBO Rep. 2003;4:358–362. doi: 10.1038/sj.embor.embor806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CoppŽ JP, Patil CK, Rodier F, Sun Y, Mu-oz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubackova S, Krejcikova K, Bartek J, Hodny Z. IL1- and TGFbeta-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘Bystander senescence’. Aging (Albany NY) 2012;4:932–951. doi: 10.18632/aging.100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodier F, Munoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppe JP, Campeau E, Beausejour CM, Kim SH, Davalos AR, Campisi J. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- 28.Vitale G, Brugts MP, Ogliari G, Castaldi D, Fatti LM, Varewijck AJ, Lamberts SW, Monti D, Bucci L, Cevenini E, Cavagnini F, Franceschi C, Hofland LJ, Mari D, Janssen J. Low circulating IGF-I bioactivity is associated with human longevity: findings in centenarians' offspring. Aging (Albany NY) 2012;4:580–589. doi: 10.18632/aging.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tominaga-Yamanaka K, Abdelmohsen K, Martindale JL, Yang X, Taub DD, Gorospe M. NF90 coordinately represses the senescence-associated secretory phenotype. Aging (Albany NY) 2012;4:695–708. doi: 10.18632/aging.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012;4:166–175. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pani G. From growing to secreting: new roles for mTOR in aging cells. Cell Cycle. 2011;10:2450–2453. doi: 10.4161/cc.10.15.16886. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 34.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 35.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A. 2012;109:13314–13318. doi: 10.1073/pnas.1205690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle. 2012;11:4642–4649. doi: 10.4161/cc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Deth Diff. 2013 Jul 12; doi: 10.1038/cdd.2013.86. doi: 10.1038/cdd.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair S, Ren J. Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle. 2012;11:2092–2099. doi: 10.4161/cc.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serrano M. Dissecting the role of mTOR complexes in cellular senescence. Cell Cycle. 2012;11:2231–2232. doi: 10.4161/cc.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dulic V. Be quiet and you'll keep young: does mTOR underlie p53 action in protecting against senescence by favoring quiescence? Aging (Albany NY) 2011;3:3–4. doi: 10.18632/aging.100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez JA, Vellon L, Oliveras-Ferraros C, Cufi S, Vazquez-Martin A. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging. Cell Cycle. 2011;10:3658–3677. doi: 10.4161/cc.10.21.18128. [DOI] [PubMed] [Google Scholar]
- 43.Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging (Albany NY) 2012;4:952–965. doi: 10.18632/aging.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iglesias-Bartolome R, Gutkind SJ. Exploiting the mTOR paradox for disease prevention. Oncotarget. 2012;3:1061–1063. doi: 10.18632/oncotarget.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demidenko ZN, Blagosklonny MV. Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential. Aging (Albany NY) 2009;1:1008–1016. doi: 10.18632/aging.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009;1:357–362. doi: 10.18632/aging.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- 48.Blagosklonny MV. mTOR-driven aging: speeding car without brakes. Cell Cycle. 2009;8:4055–4059. doi: 10.4161/cc.8.24.10310. [DOI] [PubMed] [Google Scholar]
- 49.Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol. 2012;181:1142–1146. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 50.Blagosklonny M. Answering the ultimate question “What is the Proximal Cause of Aging?”. Aging (Albany NY) 2012;4:861–877. doi: 10.18632/aging.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blagosklonny MV. Paradoxes of aging. Cell Cycle. 2007;6:2997–3003. doi: 10.4161/cc.6.24.5124. [DOI] [PubMed] [Google Scholar]
- 52.Blagosklonny MV. Calorie restriction: Decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–688. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- 53.Blagosklonny MV. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging (Albany NY) 2010;2:265–273. doi: 10.18632/aging.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011;3:1051–1062. doi: 10.18632/aging.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leontieva OV, Geraldine M. Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY) 2012;4:899–916. doi: 10.18632/aging.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Disc Today. 2007;12:218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Wang M, Miller RA. Fibroblasts from long-lived mutant mice exhibit increased autophagy and lower TOR activity after nutrient deprivation or oxidative stress. Aging Cell. 2012;11:668–674. doi: 10.1111/j.1474-9726.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blagosklonny MV. Rapamycin and quasi-programmed aging: Four years later. Cell Cycle. 2010;9:1859–1862. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- 59.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandezr E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature. 2009;460:392–396. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 62.Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging (Albany NY) 2012;4:709–714. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, Gudkov AV, Antoch MP. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53−/− mice by delaying carcinogenesis. Aging (Albany NY) 2012;4:715–722. doi: 10.18632/aging.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spong A, Bartke A. Rapamycin slows aging in mice. Cell Cycle. 2012;11:845. doi: 10.4161/cc.11.5.19607. [DOI] [PubMed] [Google Scholar]
- 66.Powers RWr, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011;3:1078–1091. doi: 10.18632/aging.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fabrizio P, Wei M. Conserved role of medium acidification in chronological senescence of yeast and mammalian cells. Aging (Albany NY) 2011;3:1127–1129. doi: 10.18632/aging.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaeberlein M, Powers RWr, K.K. S, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 71.Polymenis M, Kennedy BK. Chronological and replicative lifespan in yeast: do they meet in the middle? Cell Cycle. 2012;11:3531–3532. doi: 10.4161/cc.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–2401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pospelova TV, Leontieva OV, Bykova TV, Zubova SG, Pospelov VA, Blagosklonny MV. Suppression of replicative senescence by rapamycin in rodent embryonic cells. Cell Cycle. 2012;11:2402–2407. doi: 10.4161/cc.20882. [DOI] [PubMed] [Google Scholar]
- 74.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 75.Duran RV, Hall MN. Glutaminolysis feeds mTORC1. Cell Cycle. 2012;11:4107–4108. doi: 10.4161/cc.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013 doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Sarbassov dos D, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dan HC, Adli M, Baldwin AS. Regulation of mammalian target of rapamycin activity in PTEN-inactive prostate cancer cells by I kappa B kinase alpha. Cancer Res. 2007;67:6263–6269. doi: 10.1158/0008-5472.CAN-07-1232. [DOI] [PubMed] [Google Scholar]
- 81.Lee DF, Hung MC. All roads lead to mTOR: integrating inflammation and tumor angiogenesis. Cell Cycle. 2007;6:3011–3014. doi: 10.4161/cc.6.24.5085. [DOI] [PubMed] [Google Scholar]
- 82.Dan HC, Baldwin AS. Differential involvement of IkappaB kinases alpha and beta in cytokine- and insulin-induced mammalian target of rapamycin activation determined by Akt. J Immunol. 2008;180:7582–7589. doi: 10.4049/jimmunol.180.11.7582. [DOI] [PubMed] [Google Scholar]
- 83.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 75:425–436. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- 86.Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- 87.Lans H, Jansen G. Multiple sensory G proteins in the olfactory, gustatory and nociceptive neurons modulate longevity in Caenorhabditis elegans. Dev Biol. 2007;303:474–482. doi: 10.1016/j.ydbio.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 88.Lee SJ, Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol. 2009;19:715–722. doi: 10.1016/j.cub.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell. 2007;6:715–721. doi: 10.1111/j.1474-9726.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 90.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]