Abstract
Background
The prevalence of peanut allergy has increased in developed countries, but little is known about developing countries with high peanut consumption and widespread parasitic infections.
Objective
We sought to investigate peanut allergy in Ghana.
Methods
In a cross-sectional survey among Ghanaian schoolchildren (n = 1604), data were collected on reported adverse reactions to peanut, peanut sensitization (serum specific IgE and skin reactivity), consumption patterns, and parasitic infections. In a subset (n = 43) IgE against Ara h 1, 2, 3, and 9 as well as cross-reactive carbohydrate determinants (CCDs) was measured by using ImmunoCAP. Cross-reactivity and biological activity were investigated by means of ImmunoCAP inhibition and basophil histamine release, respectively.
Results
Adverse reactions to peanut were reported in 1.5%, skin prick test reactivity in 2.0%, and IgE sensitization (≥0.35 kU/L) in 17.5% of participants. Moreover, 92.4% of those IgE sensitized to peanut (≥0.35 kU/L) had negative peanut skin prick test responses. Schistosoma haematobium infection was positively associated with IgE sensitization (adjusted odds ratio, 2.29; 95% CI, 1.37-3.86). In the subset IgE titers to Ara h 1, 2, 3, and 9 were low (<1.3 kU/L), except for 6 moderately strong reactions to Ara h 9. IgE against peanut was strongly correlated with IgE against CCDs (r = 0.89, P < .0001) and could be almost completely inhibited by CCDs, as well as S haematobium soluble egg antigen. Moreover, IgE to peanut showed poor biological activity.
Conclusions
Parasite-induced IgE against CCDs might account largely for high IgE levels to peanut in our study population of Ghanaian schoolchildren. No evidence of IgE-mediated peanut allergy was found.
Key words: Peanut allergy, skin prick testing, IgE, Sub-Saharan Africa, IgE cross-reactivity, cross-reactive carbohydrate determinants, helminth infections, basophil histamine release, EuroPrevall
Abbreviations used: aOR, Adjusted odds ratio; BHR, Basophil histamine release; CCD, Cross-reactive carbohydrate determinant; CRD, Component-resolved diagnostics; SEA, Soluble egg antigen; SPT, Skin prick test
Recent studies report a significant increase in the incidence of peanut allergy, particularly in Europe and North America, where self-reported peanut allergy is approximately 1% among subjects less than 18 years of age.1,2 According to a 5-year follow-up survey among children in Montreal, Canada, peanut allergy prevalence (confirmed by skin prick tests [SPTs] and oral food challenges) increased from 1.34% in 2000-2002 to 1.62% in 2005-2007,3 and a population-based study conducted in Australia among infants aged 12 months found the prevalence of challenge-proved peanut allergy to be 3.0%.4
Although extensive peanut allergy research has been conducted in Western countries, there are only a few published studies from other areas of the world where peanut consumption is high, such as Southeast Asia. A population-based questionnaire survey in children of both 4 to 6 years and 14 to 16 years of age in 2 Asian populations indicates that self-reported adverse reactions to peanut in this region might vary between 0.43% and 0.64%.5 Additionally, a food allergy study among children 6 to 11 years old in China, India, and Russia described peanut allergy to be uncommon in all 3 countries.6 For Sub-Saharan Africa, no published data are available to date.
One reason proposed to explain the lower prevalence of allergic disorders in many developing countries is the possible suppressive role of chronic infections on the development of allergies.7 Infections, especially parasitic ones, are highly prevalent in Africa, Asia, and South America, particularly in rural areas or in poor sections of urban communities.8-10 One mechanism by which helminth infections are believed to protect against allergies is by activating regulatory networks that involve the induction of regulatory T and B cells, as well as the modulation of innate immune cells.11,12 Another mechanism of recent interest has been how cross-reactivity between parasite/helminth antigens and allergens can affect IgE sensitization patterns and their translation into clinical symptoms.13,14
Because there is little information on peanut allergy in Sub-Saharan Africa and on associated risk factors, we set out to investigate the epidemiology of peanut allergy in schoolchildren in Ghana, a country where peanut consumption is estimated to be high. In 2009 alone, the per capita consumption of peanuts in Ghana was approximately 12 kg15 compared with a per capita estimate of 6.6 kg for the United States in the same year.16 Our objective was to identify factors associated with peanut sensitization and reported symptoms, such as parasitic infections, peanut consumption patterns, and peanut preparation methods. We also sought to characterize IgE reactivity to peanut in our population.
Methods
Study design and population
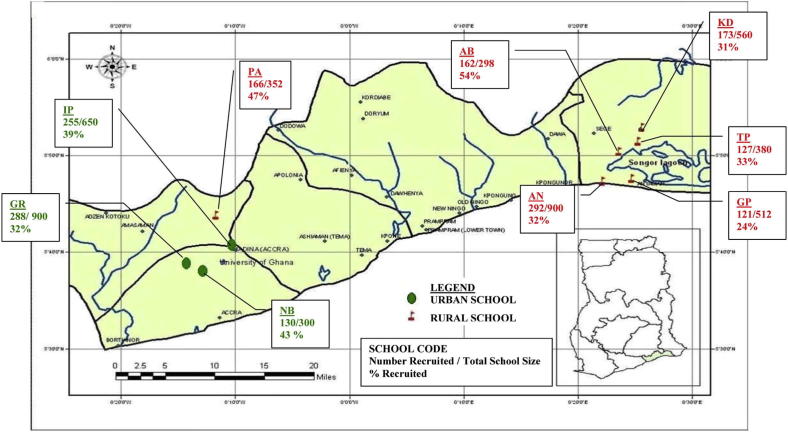
We conducted a cross-sectional study between March 2006 and March 2008 that was part of a larger investigation into allergic sensitization and parasitic infections in schoolchildren in Southern Ghana. This investigation was carried out within the framework of the European Union–funded EuroPrevall17 and GLOFAL18 projects (see details in the Methods section in this article's Online Repository at www.jacionline.org). Outcome parameters of interest were (1) reported adverse reactions to peanut and (2) peanut sensitization based on serum specific IgE levels and SPT reactivity. The study was approved by the Noguchi Memorial Institute for Medical Research Institutional Review Board, Ghana (NMIMR-IRB CPN 012/04-05). Three districts in the Greater Accra Region were selected for the investigation. Within these districts, schools were randomly selected and approached to participate in the study (see sampling methodology in the Methods section in this article's Online Repository).
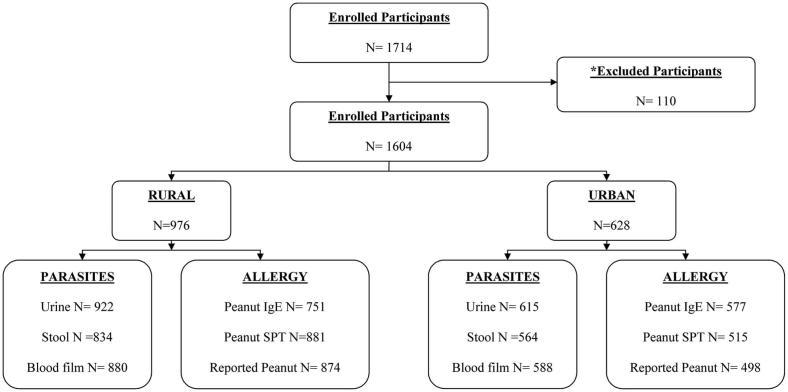
We recruited children between 5 and 16 years old attending 6 rural and 3 urban schools. Approximately 35% (1714/4852) of all children attending targeted schools agreed to participate in the study (see Fig E1 in this article's Online Repository at www.jacionline.org). The overall participation rate in the rural schools was 34.7% compared with 36.4% in the urban schools. There was no information available on nonparticipants. Of 1714 children enrolled, 59 subjects were ultimately unavailable for data collection, and 51 were excluded for being outside of the age range (see Fig E2 in this article's Online Repository at www.jacionline.org), leaving a total study population of 1604 children. Parameters measured were IgE serology (n = 1328), SPT reactivity (n = 1396), questionnaire results (n = 1372), urinary schistosomiasis (n = 1537), intestinal helminths (n = 1398), and malaria blood films (n = 1468).
Component-resolved diagnostics (CRD) could only be performed for a maximum of 50 subjects because of budgetary limitations. Subjects for whom a sufficient serum volume (≥350 μL) was available were included based on reported adverse reactions to peanut (n = 8), peanut SPT response positivity (n = 15), and randomly selected subjects with IgE levels to peanut of greater than 1.5 kU/L (n = 15). This threshold was chosen to increase the sensitivity for measuring IgE levels against individual peanut allergens. Five randomly selected negative control subjects with no reported adverse reactions to peanut and no peanut sensitization were also included. The detailed selection procedure for the CRD subset can be found in the Methods section in this article's Online Repository and also see Fig E3.
Parasitological examinations
One stool sample per subject was collected for the detection of intestinal helminth eggs by using the Kato-Katz technique19 with 25 mg of stool. A urine sample was also collected to determine Schistosoma haematobium infection by using the standard filtration method20 in which 10 mL of urine is filtered through a nylon nucleopore filter (pore size, 12 μm). For each subject, a small quantity of blood was collected to prepare a Giemsa-stained thick smear slide to detect malaria.
Questionnaire
A standard questionnaire (for a copy of the questionnaire, see this article's Online Repository at www.jacionline.org) was administered to the parents or guardians of study subjects to collect information on demographic and socioeconomic parameters, as well as information on established risk factors for the development of allergy. Questions on the symptoms of adverse reactions to food were included in the questionnaire. These were adapted from the validated EuroPrevall survey questionnaire.21 The questionnaire was administered by trained interviewers who were fluent in the local language of each participant. It was pretested in a pilot study under field conditions to ensure understanding and acceptability.
SPTs
SPT reactivity to a commercially available whole peanut extract (kindly provided by ALK-Abelló, Madrid, Spain) was assessed by using the standard protocol,22,23 as has been described in detail elsewhere.24 We defined peanut SPT response positivity as a mean wheal diameter of 3 mm or greater.25
IgE antibody measurements
ImmunoCAP (Thermo Fisher Scientific, Uppsala, Sweden) measurements were carried out according to the manufacturer’s instructions. IgE levels to peanut were assessed in all participants, and 0.35 kU/L was used as the sensitization cutoff. A cutoff of 15 kU/L or greater, which is reported to have a positive predictive value of 95% for clinical peanut allergy,26 was also examined.
For the CRD subset (n = 43), specific IgE to recombinant peanut allergens (rAra h 1, 2, 3, and 9), profilin (rPhl p 12), and bromelain, a marker for cross-reactive carbohydrate determinants (CCDs), was assessed by using ImmunoCAP. Bet v 1–homologous Ara h 8 was excluded from the analysis because there is no exposure to Fagales tree pollen in Ghana.
IgE inhibition assays
Titrated ImmunoCAP inhibition assays were conducted to establish the degree of cross-reactivity of peanut-specific IgE. To this end, 75 μL of pooled serum comprised of equal volumes of 17 sera (all with peanut-specific IgE levels ≥5.5 kU/L and similar IgE responses to peanut, as well as to bromelain) was mixed with 75 μL of inhibitor. Inhibitors used were either bromelain, S haematobium soluble egg antigen (SEA), S haematobium adult worm antigen, or Ascaris lumbricoides antigen. For 3 subjects, 2 with high and 1 with low IgE titers to Ara h 9, individual sera were also tested by using ImmunoCAP inhibition. Each serum pool (or individual sera) was preincubated with an inhibitor at room temperature for 1 hour. Subsequently, samples were analyzed for peanut-specific IgE, as described above. Results were expressed as percentages of an uninhibited control (PBS).
Basophil histamine release assays
Basophil histamine release (BHR) assays were performed with stripped basophils from a nonallergic donor that were sensitized with sera of subjects selected from the CRD subset (n = 43) to assess the biological activity of peanut-specific IgE in our population. Two sera with similar IgE levels against peanut and CCDs were selected. In addition, 2 sera with higher IgE levels against peanut than against CCDs in combination with high IgE levels against Ara h 9 were also evaluated (see full characteristics in Table E1 in this article's Online Repository at www.jacionline.org). BHR assays were performed, as described elsewhere.27,28
Statistical analysis
Analysis was carried out with STATA version 10 software (StataCorp, College Station, Tex). Urban-rural differences in subjects' characteristics, as well as in peanut sensitization (IgE and SPT) and reported adverse reactions, were examined by using the Pearson χ2 test with 1 df. To assess factors associated with peanut sensitization (IgE levels and SPT responses) and reported adverse reactions, multivariable random effects logistic regression models were fitted that took into account possible correlations among observations within each school by modeling school as a random effect. This approach was used because children attending the same school were likely to share common characteristics, as well as exposures. Models were adjusted for age, sex, and urban-rural area (as a priori confounders) along with other variables significant from crude analysis.
Results
Characteristics of the study population
The characteristics of the study participants stratified by area are given in Table I. There were no significant differences in sex distribution and age group when comparing the 2 areas, although urban children had a slightly higher median age. In addition, rural subjects had significantly more helminth infections and malaria.
Table I.
Characteristics of the study population stratified by area
| Factor | Area |
P value∗ | ||
|---|---|---|---|---|
| All, n/N (%) | Rural, n/N (%) | Urban, n/N (%) | ||
| Sex | ||||
| Male | 757/1604 (47.2) | 465/976 (47.6) | 292/628 (46.5) | .65 |
| Female | 847/1604 (52.8) | 511/976 (52.4) | 336/628 (53.5) | |
| Age | ||||
| ≤11 y | 785/1604 (48.9) | 496/976 (50.8) | 289/628 (46.0) | .06 |
| ≥11 y | 819/1604 (51.1) | 480/976 (49.2) | 339/628 (54.0) | |
| Parasitic infections | ||||
| Any intestinal helminth† (positive) | 248/1398 (17.7) | 236/834 (28.3) | 12/564 (2.1) | <.001 |
| S haematobium (positive) | 103/1537 (6.7) | 83/922 (9.0) | 20/615 (3.3) | <.001 |
| Plasmodium species‡ (positive) | 349/1468 (23.8) | 310/880 (35.2) | 39/588 (6.6) | <.001 |
| Peanut consumption | ||||
| Daily (yes) | 365/1372 (26.6) | 316/874 (36.2) | 49/498 (9.8) | <.001 |
| Weekly (yes) | 760/1372 (55.4) | 438/874 (50.1) | 322/498 (64.7) | <.001 |
| Monthly (yes) | 183/1372 (13.3) | 70/874 (8.0) | 113/498 (22.7) | <.001 |
| Every 6 mo (yes) | 21/1372 (1.5) | 12/874 (1.4) | 9/498 (1.8) | .52 |
| Never (yes) | 35/1372 (2.6) | 35/874 (4.0) | 0/498 (0.0) | <.001 |
| Missing consumption information | 8/1372 (0.6) | 3/874 (0.3) | 5/498 (1.0) | |
| Exclusive peanut preparation methods | ||||
| Boiled only (yes) | 61/1372 (4.4) | 56/874 (6.4) | 5/498 (1.0) | <.001 |
| Fried only (yes) | 19/1372 (1.4) | 19/874 (2.2) | 0/498 (0.0) | .001 |
| Roasted only (yes) | 277/1372 (20.2) | 276/874 (31.6) | 1/498 (0.2) | <.001 |
| Other peanut preparation methods | ||||
| Raw (yes) | 22/1372 (1.6) | 3/874 (0.3) | 19/498 (3.8) | <.001 |
| Peanut oil§ | ||||
| Use of peanut oil (yes) | 33/1370 (2.4) | 32/872 (3.7) | 1/498 (0.2) | <.001 |
P values were calculated by using Pearson χ2 test with 1 df. Values in boldface indicate significance.
Any intestinal helminth = Ascaris lumbricoides, hookworm (Ancylostoma duodenale or Necator americanus), Trichuris trichiura, or Schistosoma mansoni.
Plasmodium species = Plasmodium falciparum or Plasmodium malariae (the 2 malaria parasite species detected in our study population).
Peanut oil use information missing for 2 participants.
Although peanut consumption was high in both areas, reported daily consumption was considerably higher among rural schoolchildren (36.2%) compared with their urban counterparts (9.8%). Furthermore, in the rural area both “boiled-only” and “roasted-only” peanut preparation methods were reported more frequently than in the urban area, where the combination of roasting and then boiling peanuts in soup preparation was more common. Topical exposure to peanut, as assessed based on the use of peanut oil as a skin ointment, was higher in rural compared with urban schools.
Reported adverse reactions and sensitization (IgE levels and SPT responses) to peanut
Adverse reactions were reported in 1.5% (n = 21/1372) of participants (Table II), most of whom were rural schoolchildren. The distribution pattern of the characteristics of those reporting adverse reactions (see Table E2 in this article's Online Repository at www.jacionline.org) did not differ significantly from the rest of the study population (statistical test data not shown). About 67% of those reporting adverse reactions to peanut had gastrointestinal complaints, and 43% had complaints described as itching of the mouth or difficulty swallowing. Only 4 of 21 subjects reported a reaction time “within minutes” (see Table E3 in this article's Online Repository at www.jacionline.org).
Table II.
Prevalence of adverse reactions to peanut and peanut sensitization (SPT responses and IgE levels) stratified by area
| Factor | Area |
P value∗ | ||
|---|---|---|---|---|
| All, n/N (%) | Rural, n/N (%) | Urban, n/N (%) | ||
| Adverse reactions to food | ||||
| Any food | 154/1372 (11.2) | 115/874 (13.2) | 39/498 (7.8) | .003 |
| Peanut | 21/1372 (1.5) | 18/874 (2.1) | 3/498 (0.6) | .035 |
| SPT reactivity | ||||
| Peanut positive | 28/1396 (2.0) | 17/881 (1.9) | 11/515 (2.1) | .79 |
| Peanut-specific IgE | ||||
| ≥0.35 kU/L | 233/1328 (17.5) | 177/751 (23.6) | 56/577 (9.7) | <.001 |
| ≥15 kU/L | 12/1328 (0.9) | 8/751 (1.1) | 4/577 (0.7) | .48 |
P values were calculated by using the Pearson χ2 test with 1 df. Values in boldface indicate significance.
The percentage of subjects with a positive peanut SPT response was 2.0% (n = 28/1396), and this was not significantly different between the 2 areas (Table II). Positive wheal sizes for peanut ranged from 3.0 to 6.5 mm and did not vary between areas (data not shown).
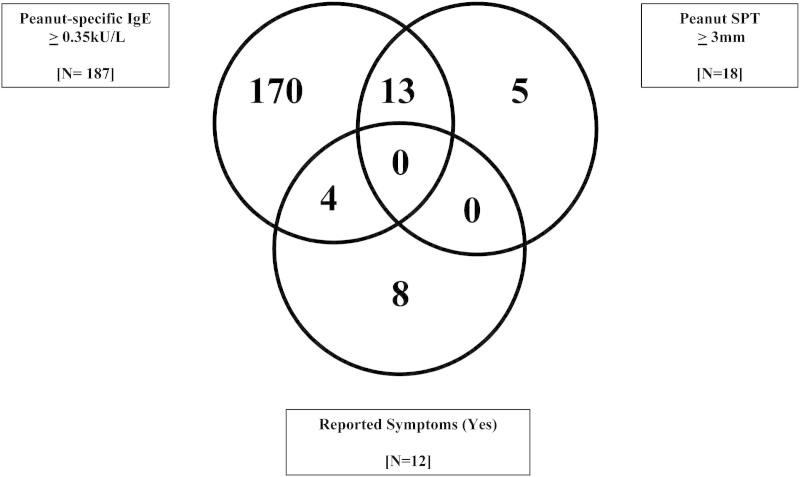
Peanut IgE sensitization (≥0.35 kU/L) was observed in 17.5% (n = 233/1328) of the study population, with 23.6% of rural children being sensitized compared with 9.7% of urban participants (P < .001). However, 92.4% (n = 194/210) of those IgE sensitized to peanut (≥0.35 kU/L) had negative peanut SPT responses. Interestingly, 0.9% (n = 12/1328) of the study subjects were highly sensitized when using the IgE cutoff of 15 kU/L or greater, which is reported to have a positive predictive value of 95% for clinical peanut allergy,26 but only 1 of them reported reactions. Fig 1 shows the overlap between the peanut-related outcomes for study subjects with complete allergy data (reported reactions, SPT levels, and IgE responses). No subjects had positive results for all 3 parameters.
Fig 1.
Overlap between reported adverse reactions to peanut and peanut sensitization (IgE levels and SPT responses) for subjects with complete data for allergy-related parameters (n = 1004).
Factors associated with peanut sensitization (IgE levels and SPT responses) and reported adverse reactions to peanut
In multivariable analysis area was strongly associated with peanut IgE sensitization of 0.35 kU/L or greater, with urban subjects having a reduced odds of increased IgE levels relative to their rural counterparts (adjusted odds ratio [aOR], 0.41; 95% CI, 0.25-0.67; P < .001; Table III). S haematobium infection was also associated with peanut IgE sensitization (aOR, 2.29; 95% CI, 1.37-3.86; P = .002), whereas intestinal helminth infection was not.
Table III.
Factors associated with reported adverse reactions to peanut and peanut sensitization (IgE levels and SPT responses)
| Factors | Peanut-specific IgE (≥0.35 kU/L vs <0.35 kU/L) |
Positive peanut SPT response (+ vs −) |
Reported adverse reactions to peanut (yes vs no) |
|||
|---|---|---|---|---|---|---|
| aOR (95% CI) | Wald test P value | aOR (95% CI) | Wald test P value | aOR (95% CI) | Wald test P value | |
| Peanut-specific IgE (≥0.35 kU/L vs <0.35 kU/L) | 17.09 (6.30-46.36) | <.001 | 1.94 (0.57-6.63) | .29 | ||
| Positive peanut SPT response (+ vs −) | 2.82 (0.35-22.70) | .33 | ||||
| Age (≥11 y vs <11 y) | 1.07 (0.78-1.47) | .67 | 1.36 (0.55-3.36) | .51 | 0.58 (0.24-1.42) | .23 |
| Sex (male vs female) | 1.12 (0.83-1.51) | .47 | 1.65 (0.67-4.03) | .27 | 0.68 (0.28-1.65) | .39 |
| Area (urban vs rural) | 0.41 (0.25-0.67) | <.001 | 2.94 (1.03-8.40) | .044 | 0.30 (0.09-1.01) | .052 |
| Any intestinal helminth∗ (+ vs −) | 1.01 (0.66-1.55) | .97 | 0.69 (0.17-2.84) | .61 | 0.35 (0.08-1.56) | .17 |
| S haematobium (+ vs −) | 2.29 (1.37-3.86) | .002 | 0.41 (0.05-3.42) | .41 | 0.65 (0.08-4.95) | .67 |
| Plasmodium species† (+ vs −) | 1.10 (0.77-1.56) | .61 | 0.49 (0.13-1.82) | .28 | 0.59 (0.16-2.20) | .44 |
Peanut-specific IgE models were adjusted for age, sex, area, and S haematobium infection. Peanut SPT models were adjusted for age, sex, area and peanut-specific IgE levels. Reported peanut reaction models were adjusted for age, sex, and area.
Any intestinal helminth = Ascaris lumbricoides, hookworm (Ancylostoma duodenale or Necator americanus), Trichuris trichiura, or Schistosoma mansoni.
Plasmodium species = Plasmodium falciparum or Plasmodium malariae (the 2 malaria parasite species detected in our study population).
Although the majority of peanut IgE–sensitized subjects did not have positive peanut SPT responses, almost all subjects with positive peanut SPT responses were IgE sensitized. Thus in multivariable analysis IgE sensitization was associated with peanut SPT reactivity (aOR, 17.09; 95% CI, 6.30-46.36; P < .001). In addition, although not observed in crude analyses, residing in an urban area was associated with a significantly higher chance of having a positive SPT response to peanut after adjusting for confounders (Table III). No other factors, including helminth infection, had an effect on SPT responses to peanut (Table III).
Data on peanut consumption and preparation methods as risk factors for peanut-related outcomes are shown in Table E4 in this article's Online Repository at www.jacionline.org. “Never” consuming peanuts, as a proxy for avoidance, was associated with reported symptoms (aOR, 5.40; 95% CI, 1.47-19.80; P < .05). Raw peanut consumption was also linked to reported adverse reactions to peanut (aOR, 17.14; 95% CI, 2.93-100.45; P < .01). However, numbers were low, as reflected in the wide CI. All other factors, including helminth infection, were not significantly associated with reported adverse reactions to peanut (Table III and see Table E4).
Component-resolved IgE testing
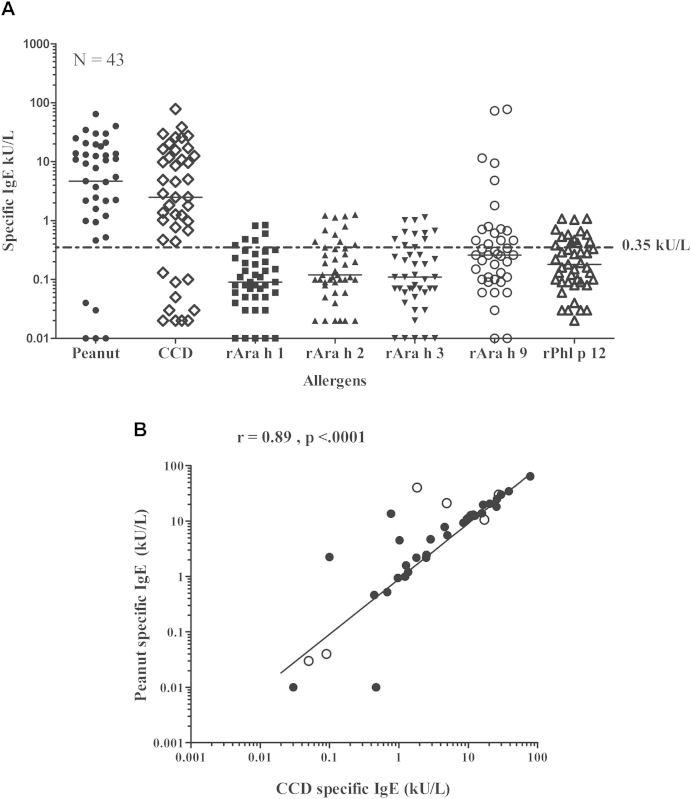
Fig 2, A, shows the results of CRD performed in a subset (n = 43) to better characterize peanut-specific IgE. Those with IgE levels to peanut of greater than 1.5 kU/L (median, 12.5 kU/L) had high levels of IgE to CCD but low IgE responses (<1.3 kU/L) to rAra h 1 to 3 and rPhl p 12. A strong correlation was seen between peanut-specific IgE and CCD-specific IgE levels (r = 0.89, P < .0001; Fig 2, B). For some subjects, IgE levels against peanut were significantly higher than those to CCDs, and in 6 of these subjects, high titers of IgE to the lipid transfer protein rAra h 9 were observed (Fig 2, A). Of note, 4 of 6 of these subjects had positive peanut SPT responses (see Table E1).
Fig 2.
A, Measurement of specific IgE levels to whole peanut extract, recombinant peanut allergens, profilin, and the CCD marker bromelain in a subset (n = 43). Median specific IgE levels are indicated by black lines. The dotted line shows an IgE sensitization cutoff of 0.35 kU/L. B, Correlation between peanut-specific IgE and CCD-specific IgE levels. Open circles indicate subjects with IgE to rAra h 9 of greater than 1.5 kU/L.
Inhibition of IgE binding to peanut by CCDs and schistosome egg antigen
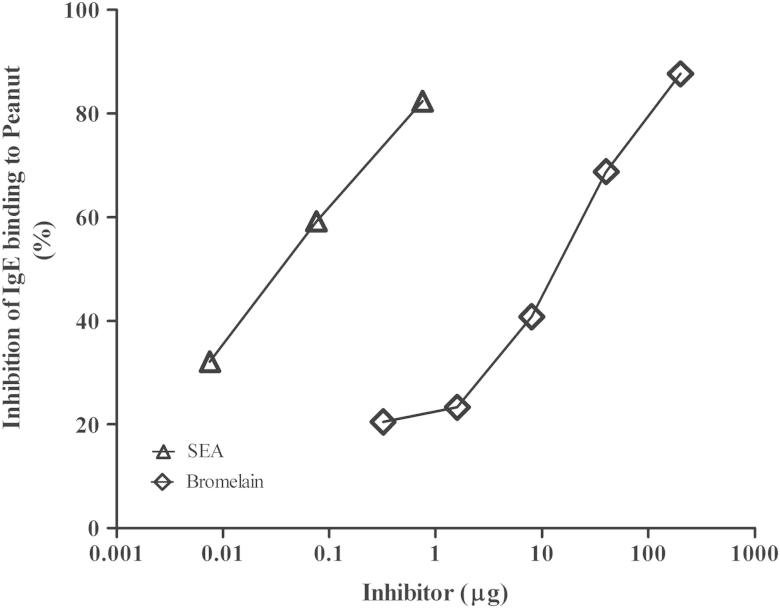
Titrated CAP inhibition assays demonstrated that binding of IgE from a serum pool of subjects (n = 17) with similar IgE titers to peanut as to CCDs was almost completely inhibited by CCDs, as well as by S haematobium SEA (Fig 3). Interestingly, SEA, a glycoprotein preparation of S haematobium eggs, inhibited at a greater than 100-fold lower protein concentration than the plant-derived glycoprotein marker for CCDs, bromelain. Individual inhibitions for 2 subjects with high IgE levels to peanut and Ara h 9, as well as low IgE levels to CCDs, showed less than 10% inhibition by SEA (see Table E1). In addition, S haematobium adult worm antigen and A lumbricoides antigen did not inhibit binding significantly (data not shown).
Fig 3.
Inhibition of IgE binding to whole peanut by bromelain and S haematobium SEA by using pooled sera (n = 17). The figure shows that IgE binding to whole peanut extract was almost completely inhibited by bromelain (diamonds) and S haematobium SEA (triangles), respectively.
BHR assays
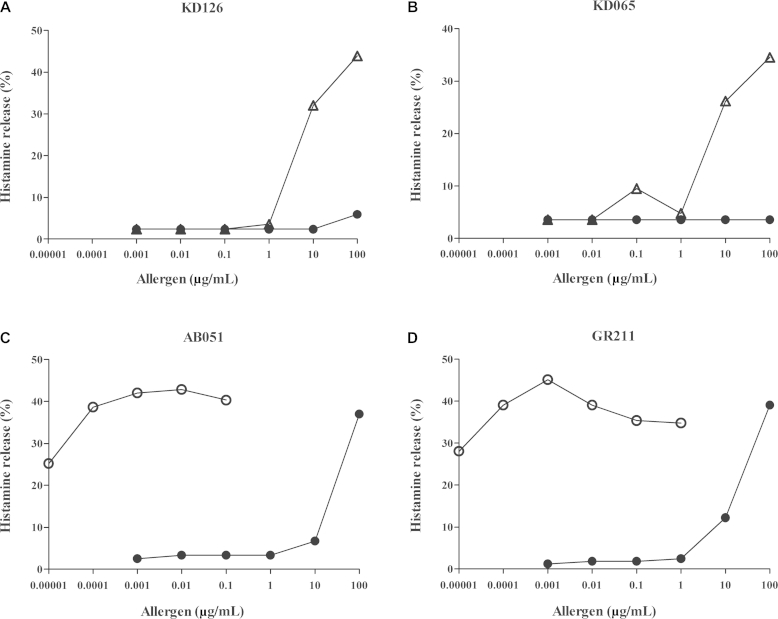
Peanut extract induced little histamine release when basophils were sensitized with IgE from subjects with similar IgE reactivity to peanut as to CCDs (Fig 4, A and B). For these subjects, the ability of S haematobium SEA to induce histamine release was tested, and only at a concentration of 10 μg/mL was release observed. For 2 subjects with titers of IgE against Ara h 9 of greater than 70 kU/L (Fig 4, C and D), Ara h 9 induced significant histamine release starting at 10 pg/mL, reaching maximum release at approximately 1 ng/mL, whereas with peanut extract, release was seen starting from a concentration of 10 μg/mL.
Fig 4.
BHR assay results. BHR induced by peanut extract (solid circles), S haematobium SEA (open triangles), and Ara h 9 (open circles). A and B, Results for 2 subjects with high IgE titers against peanut and CCD. C and D, Results for 2 subjects with high IgE titers against peanut and Ara h 9 but low IgE titers against CCDs.
Discussion
Our study is the first investigation of reported adverse reactions to peanut and peanut sensitization based on serum specific IgE measurements, as well as SPT reactivity, in Sub-Saharan Africa among an unselected group of children. We confirmed that there was a high frequency of daily peanut consumption in Southern Ghana, particularly among rural schoolchildren. We also observed an association between reported peanut-related adverse reactions and peanut avoidance. The percentage of reported peanut-related adverse reactions among schoolchildren in our survey was 1.5%. However, the majority of these reported reactions occurred within hours/days, whereas IgE-mediated peanut allergy is typically associated with symptoms appearing within minutes or up to 2 hours.29
Among study participants, 2.0% had positive peanut SPT responses. Although 17.5% of all subjects had increased IgE levels to peanut (≥0.35 kU/L), 92.4% of these had negative peanut SPT responses. One explanation for the discrepancy between specific IgE levels and SPT responses could be the suppression of IgE-induced inflammation by immunologic regulatory networks30 that might be operative during chronic helminth infections. However, we did not observe any association between helminth infection and SPT responses to peanut.
Notably, 12 of 1328 participants had peanut-specific IgE levels of 15 kU/L or greater, a cutoff reported to have a positive predictive value of 95% for clinical peanut allergy in a European study population26 but was virtually unaccompanied by reported symptoms in our study. This highlights the limitations in applying cutoff values determined in one population to other populations.
Analysis by CRD in a subset indicated that the majority of those with high IgE titers against peanut (median, 12.5 kU/L) had low responses (<1.3 kU/L) against the major peanut allergens (Ara h 1, 2, and 3) commonly associated with peanut allergy.31-33 Recently, IgE responses to Ara h 2 in particular have been used to differentiate between clinical peanut allergy and asymptomatic peanut sensitization,34 as well as to improve diagnostic accuracy.35 One study observed that an IgE cutoff to rAra h 2 of greater than 0.23 kU/L had a specificity of 97% and sensitivity of 93% among patients with peanut allergy and control subjects in France.32 Taken together, sensitization to peanut storage proteins in Ghana appears weak and rare compared with that in European or US patients with peanut allergy. The lack of clinical reactivity among study participants with increased IgE responses to Ara h 2 would have to be explored further.
The most dominant molecular component recognized by IgE in peanut-sensitized subjects in our subset was the CCD. A strong correlation was observed between IgE levels to peanut and to CCDs. CCDs are N-glycans in plants and invertebrate glycoproteins that result in a high degree of cross-reactivity between pollen and foods.36 CCDs have negligible in vivo biological activity, as well as clinical relevance.37-39 Grass pollen was found to be of minor importance in Ghanaian schoolchildren, as was established in a pilot study preceding the present survey. In our study population we observed that current S haematobium infection was associated with increased IgE levels to peanut. Moreover, among our subset, the results of the ImmunoCAP inhibition assays showed that plant-derived CCDs (bromelain) inhibited IgE binding to peanut but that a Schistosoma species–derived glycoprotein was a far more potent inhibitor. These observations suggest that carbohydrate-specific IgE is induced by glycoproteins from the eggs of S haematobium that are different from but cross-reactive with those on bromelain. Interestingly, Schistosoma species adult worm glycoproteins were not effective as inhibitors, indicating the importance of stage-specific N-glycans in this cross-reactivity. The importance of cross-reactivity might also explain the residual effect of the rural area on IgE to peanut, which was seen after adjusting for current S haematobium infection. Past infections in subjects residing in the rural area might have led to cross-reactive IgE to peanut.
Interestingly, in the studied subset IgE responses to Ara h 9 were increased in 6 children, with 2 having IgE titers of greater than 70 kU/L. Furthermore, IgE antibodies against Ara h 9 were biologically active at low allergen concentrations (picogram per nanogram range), as determined by using BHR assays. The observation that 4 of 6 subjects with high IgE levels to Ara h 9 had positive peanut SPT responses is in line with these BHR results. However, none of these reported immediate adverse reactions to peanut. Altogether, the data suggest that sources other than CCDs could contribute to increased IgE levels to peanut extract. The origin of sensitization to this lipid transfer protein is unknown, and whether a locally consumed fruit is at the basis of this sensitization, as is commonly reported in Europe in relation to peach,31,40,41 remains to be determined for Ghana.
Our study had a number of limitations, such as a low participation rate, but given our observation that IgE-mediated peanut allergy in Ghanaian schoolchildren is rare (if existing at all), it is unlikely that selection bias is affecting our findings in this respect. However, the borderline significant difference in age between rural and urban children, as well as the fact that the rural population is from areas that are endemic for helminth infections, need to be taken into account when considering the generalizability of our findings. The absence of a gold standard for peanut allergy (oral food challenges) is another limitation, but given that reported adverse reactions to peanut were largely not accompanied by immediate reactions, this is less likely to be an issue. An additional study weakness is the use of a questionnaire as a measurement tool for adverse reactions, as well as other self-reported parameters. Furthermore, our school-based study design meant that children less than 5 years of age were excluded from the investigation, which might bias the results by omitting an important age group affected by peanut allergy. However, given the persistent nature of peanut allergy among most subjects, the effect of an older age cutoff of 5 years is likely to be minimal. The fact that CRD was conducted in a relatively small subset of our larger study population is another limitation, although the subset did not differ from the wider study population on key demographic factors and parasitic infections.
Despite these limitations, our study provides new insight into the nature of peanut sensitization and reported adverse reactions to peanut in Ghana, a Sub-Saharan African country in which peanut consumption is high but does not appear to translate into true peanut sensitization, let alone peanut allergy. Overall, our observations suggest that IgE-mediated peanut allergy in Ghanaian schoolchildren is rare. Among a subset, we found a role for N-glycans, particularly related to Schistosoma species, in inducing cross-reactivity, resulting in increased IgE levels to peanut without skin reactivity or reported symptoms. This study once more highlights the poor biological activity of CCD-specific IgE. Interestingly, IgE to Ara h 9 demonstrated normal biological activity, suggesting that lack of biological activity is not the only explanation for the lack of clinical peanut allergy. Future studies on the characteristics of cross-reactive IgEs and the pathways behind their development might be essential to the ongoing investigation of immune regulatory mechanisms in an effort to curtail strong allergic inflammation.
Clinical implications.
Peanut-specific IgE antibodies in Ghana, a Sub-Saharan African country, show cross-reactivity with clinically irrelevant carbohydrate determinants and therefore might reduce the diagnostic value of this parameter in establishing peanut allergy.
Acknowledgments
We thank Drs Domingo Barber and Lucia Jimeno (ALK-Abelló, Madrid, Spain) for providing SPT material. Our appreciation goes to Mrs Yvonne Kruize-Hoeksma for technical expertise, Mr Dziedzom DeSouza for the design of the database, Mr Richard A. Akuffo for data entry, Ms Linda Tamatey for technical assistance in parasitology, and Dr Ron Wolterbeek for assistance with statistical analysis. We would also like to express our most sincere gratitude to the national service personnel involved in the study, community leaders, school authorities, and teachers of all participating schools for all their assistance. Finally, we are most indebted to the study participants and their families for their time and commitment.
Footnotes
Supported by EuroPrevall (FOOD-CT-2005-514000), GLOFAL (FOOD-CT-2005-517812), and the Wellcome Trust (075791/Z/04/Z).
Disclosure of potential conflict of interest: A. S. Amoah has received grants from the European Union and the Wellcome Trust. B. B. Obeng, I. A. Larbi, S. A. Versteeg, Y. Aryeetey, J. Akkerdaas, L. Zuidmeer, F. C. Hartgers, D. A. Boakye, and M. Yazdanbakhsh have received grants from the European Union. J. Lidholm is employed by Thermo Fisher Scientific. M. Fernández-Rivas has received a grant and travel support from the European Commission, is employed by the Hospital Clinico San Carlos, has received grants from the European Commission and Instituto de Salud Carlos III, has received payment for lectures from ALK-Abelló, and has received travel support from the European Academy of Allergology and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. R. van Ree has received grants from the European Union and has consultant arrangements with HAL Allergy BV.
Supplementary data
Fig E1.
Fig E2.
References
- 1.Ben-Shoshan M., Turnbull E., Clarke A. Food allergy: temporal trends and determinants. Curr Allergy Asthma Rep. 2012;12:346–372. doi: 10.1007/s11882-012-0274-3. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer S.H., Leung D.Y.M. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2011. J Allergy Clin Immunol. 2012;129:76–85. doi: 10.1016/j.jaci.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shoshan M., Kagan R.S., Alizadehfar R., Joseph L., Turnbull E., St Pierre Y. Is the prevalence of peanut allergy increasing? A 5-year follow-up study in children in Montreal. J Allergy Clin Immunol. 2009;123:783–788. doi: 10.1016/j.jaci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Osborne N.J., Koplin J.J., Martin P.E., Gurrin L.C., Lowe A.J., Matheson M.C. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–676.e2. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Shek L.P.-C., Cabrera-Morales E.A., Soh S.E., Gerez I., Ng P.Z., Yi F.C. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331.e7. doi: 10.1016/j.jaci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Wong G. Patterns of food allergy outside Europe. Clin Transl Allergy. 2011;1(suppl):S6. [Google Scholar]
- 7.Yazdanbakhsh M., Kremsner P.G., van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 8.Belyhun Y., Medhin G., Amberbir A., Erko B., Hanlon C., Alem A. Prevalence and risk factors for soil-transmitted helminth infection in mothers and their infants in Butajira, Ethiopia: a population based study. BMC Public Health. 2010;10:21. doi: 10.1186/1471-2458-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flohr C., Tuyen L.N., Lewis S., Quinnell R., Minh T.T., Liem H.T. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: a cross-sectional study. J Allergy Clin Immunol. 2006;118:1305–1311. doi: 10.1016/j.jaci.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Cooper P.J., Alexander N., Moncayo A.-L., Benitez S., Chico M., Vaca M. Environmental determinants of total IgE among school children living in the rural Tropics: importance of geohelminth infections and effect of anthelmintic treatment. BMC Immunol. 2008;9:33. doi: 10.1186/1471-2172-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smits H.H., Everts B., Hartgers F.C., Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10:3–12. doi: 10.1007/s11882-009-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussaarts L., van der Vlugt L.E.P.M., Yazdanbakhsh M., Smits H.H. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011;128:733–739. doi: 10.1016/j.jaci.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Acevedo N., Caraballo L. IgE cross-reactivity between Ascaris lumbricoides and mite allergens: possible influences on allergic sensitization and asthma. Parasite Immunol. 2011;33:309–321. doi: 10.1111/j.1365-3024.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 14.Santiago H.C., Bennuru S., Boyd A., Eberhard M., Nutman T.B. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: Implications for the hygiene hypothesis. J Allergy Clin Immunol. 2010;127:479–486. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Food and Agriculture . Statistics. Ministry of Food and Agriculture; Accra (Ghana): 2010. Agriculture in Ghana fact and figures (2009) [Google Scholar]
- 16.US Department of Agriculture . Food Availability (per capita) Data System. US Department of Agriculture; Washington (DC): 2012. Food availability. Spreadsheets. [Google Scholar]
- 17.Institute of Food Research. The prevalence cost and basis of food allergy. Available at: http://www.europrevall.org. Accessed October 16, 2012.
- 18.Global view of food allergy. Available at: http://www.glofal.org. Accessed October 16, 2012.
- 19.Katz N., Chaves A., Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 20.Peters P.A., Mahmoud A.A., Warren K.S., Ouma J.H., Siongok T.K. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ. 1976;54:159–162. [PMC free article] [PubMed] [Google Scholar]
- 21.Kummeling I., Mills E.N.C., Clausen M., Dubakiene R., Pérez C.F., Fernández-Rivas M. The EuroPrevall surveys on the prevalence of food allergies in children and adults: background and study methodology. Allergy. 2009;64:1493–1497. doi: 10.1111/j.1398-9995.2009.02046.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein I.L., Storms W.W. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1995;75:543–625. [PubMed] [Google Scholar]
- 23.Dreborg S. The skin prick test in the diagnosis of atopic allergy. J Am Acad Dermatol. 1989;21:820–821. doi: 10.1016/s0190-9622(89)70256-5. [DOI] [PubMed] [Google Scholar]
- 24.Obeng B.B., Amoah A.S., Larbi I.A., Yazdanbakhsh M., van Ree R., Boakye D.A. Food allergy: sensitization and reported symptoms in Ghanaian school-children. Int Arch Allergy Immunol. 2011;155:63–73. doi: 10.1159/000318704. [DOI] [PubMed] [Google Scholar]
- 25.Zarei M., Remer C.F., Kaplan M.S., Staveren A.M., Lin C.K., Razo E. Optimal skin prick wheal size for diagnosis of cat allergy. Ann Allergy Asthma Immunol. 2004;6:604–610. doi: 10.1016/S1081-1206(10)61425-1. [DOI] [PubMed] [Google Scholar]
- 26.Roberts G., Lack G., Avon Longitudinal Study of Parents and Children Study T Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol. 2005;115:1291–1296. doi: 10.1016/j.jaci.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Kleine Budde I., de Heer P.G., van der Zee J.S., Aalberse R.C. The stripped basophil histamine release bioassay as a tool for the detection of allergen-specific IgE in serum. Int Arch Allergy Immunol. 2001;126:277–285. doi: 10.1159/000049524. [DOI] [PubMed] [Google Scholar]
- 28.Mari A., Ooievaar-de Heer P., Scala E., Giani M., Pirrotta L., Zuidmeer L. Evaluation by double-blind placebo-controlled oral challenge of the clinical relevance of IgE antibodies against plant glycans. Allergy. 2008;63:891–896. doi: 10.1111/j.1398-9995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 29.Burks A.W. Peanut allergy. Lancet. 2008;371:1538–1546. doi: 10.1016/S0140-6736(08)60659-5. [DOI] [PubMed] [Google Scholar]
- 30.Macaubas C., Sly P.D., Burton P., Tiller K., Yabuhara A., Holt B.J. Regulation of T-helper cell responses to inhalant allergen during early childhood. Clin Exp Allergy. 1999;29:1223–1231. doi: 10.1046/j.1365-2222.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 31.Vereda A., van Hage M., Ahlstedt S., Ibañez M.D., Cuesta-Herranz J., van Odijk J. Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127:603–607. doi: 10.1016/j.jaci.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Codreanu F., Collignon O., Roitel O., Thouvenot B., Sauvage C., Vilain A.C. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011;154:216–226. doi: 10.1159/000321108. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaou N., Murray C., Belgrave D., Poorafshar M., Simpson A., Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–685. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Hong X., Caruso D., Kumar R., Liu R., Liu X., Wang G. IgE, but not IgG4, antibodies to Ara h 2 distinguish peanut allergy from asymptomatic peanut sensitization. Allergy. 2012;67:1538–1546. doi: 10.1111/all.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eller E., Bindslev-Jensen C. Clinical value of component-resolved diagnostics in peanut-allergic patients. Allergy. 2013;68:190–194. doi: 10.1111/all.12075. [DOI] [PubMed] [Google Scholar]
- 36.van Ree R. Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int Arch Allergy Immunol. 2002;129:189–197. doi: 10.1159/000066770. [DOI] [PubMed] [Google Scholar]
- 37.van der Veen M.J., van Ree R., Aalberse R.C., Akkerdaas J., Koppelman S.J., Jansen H.M. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100:327–334. doi: 10.1016/s0091-6749(97)70245-8. [DOI] [PubMed] [Google Scholar]
- 38.Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129:286–295. doi: 10.1159/000067591. [DOI] [PubMed] [Google Scholar]
- 39.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- 40.Krause S., Reese G., Randow S., Zennaro D., Quaratino D., Palazzo P. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009;124:771–778.e5. doi: 10.1016/j.jaci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Lauer I., Dueringer N., Pokoj S., Rehm S., Zoccatelli G., Reese G. The non-specific lipid transfer protein, Ara h 9, is an important allergen in peanut. Clin Exp Allergy. 2009;39:1427–1437. doi: 10.1111/j.1365-2222.2009.03312.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.