Abstract
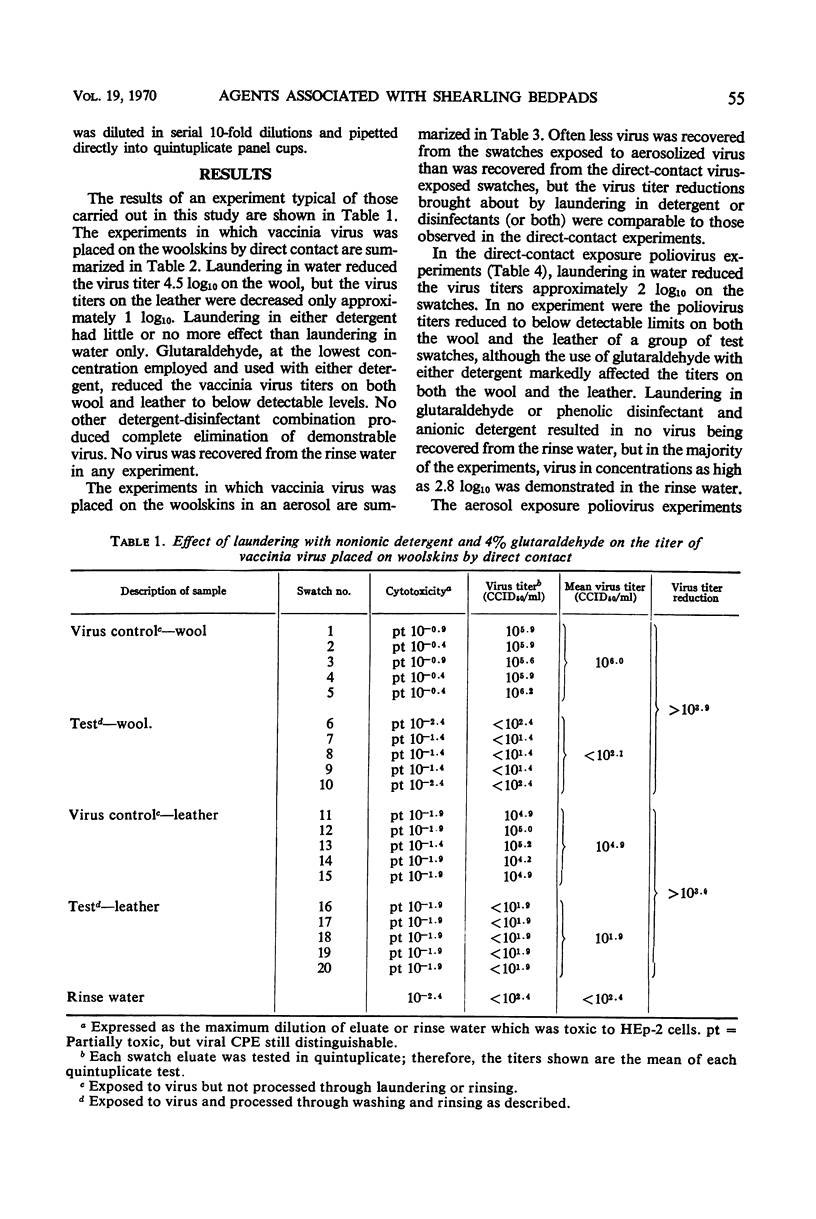
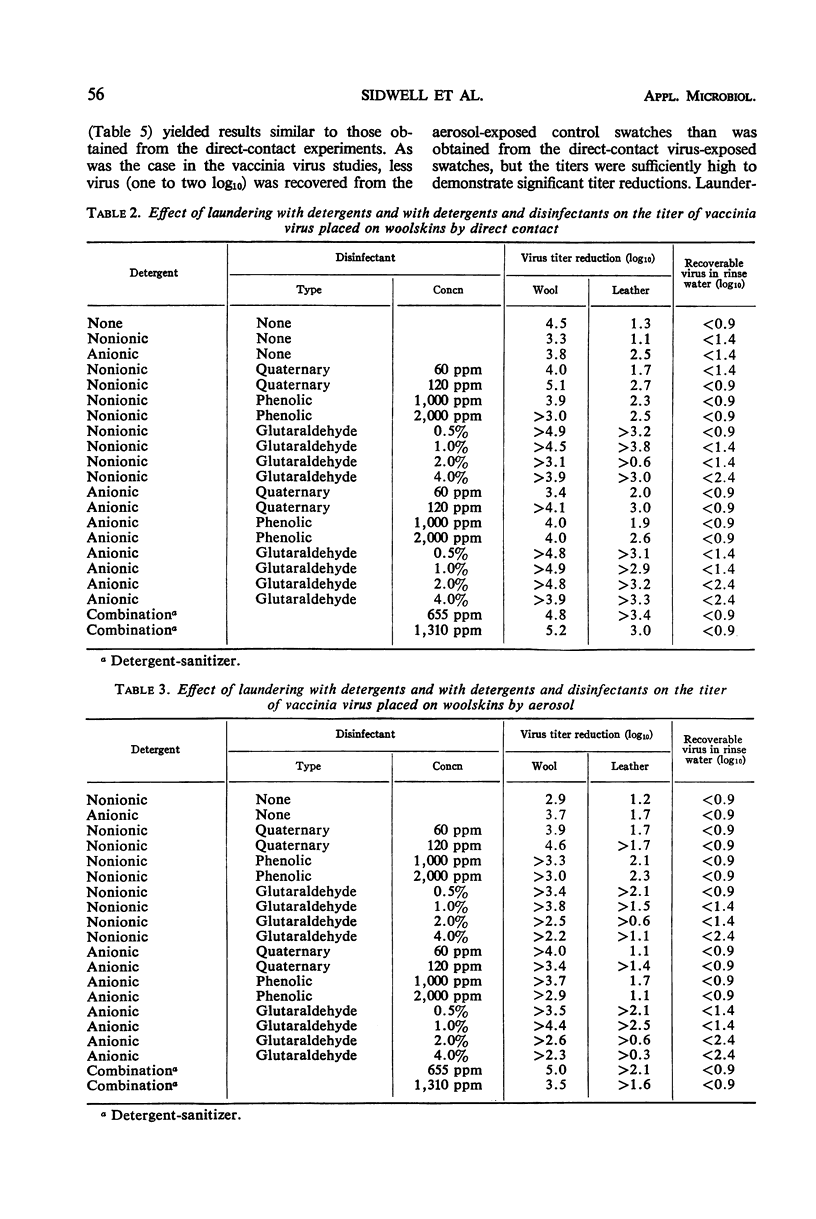
Glutaraldehyde-tanned woolskin pads which are used for the prevention of decubitus ulcers in bed patients were experimentally contaminated with polio or vaccinia viruses. Two methods of exposure, direct contact and aerosol, were used in separate experiments. Attempts were made to remove or inactivate these virus contaminants by laundering the woolskins in a quaternary ammonium disinfectant, a phenolic disinfectant, or alkalinized glutaraldehyde, in combination with an anionic detergent or a nonionic detergent. The effect of a commercial detergent-sanitizer was also studied. The virus titers were significantly reduced in all experiments, but only laundering in glutaraldehyde in combination with either detergent lowered the vaccinia virus titers to below detectable limits. High concentrations of glutaraldehyde altered the texture of the wool and leather apparently by precipitating a component of the detergent onto the fibers. In all the poliovirus experiments, the virus was still detectable on either or both the wool and the leather of the pads after laundering. The rinse water from each experiment was tested for the presence of virus. No vaccinia virus was recovered, but poliovirus was demonstrated in titers up to 103 cell culture 50% infectious doses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORICK P. M., DONDERSHINE F. H., CHANDLER V. L. ALKALINIZED GLUTARALDEHYDE, A NEW ANTIMICROBIAL AGENT. J Pharm Sci. 1964 Oct;53:1273–1275. doi: 10.1002/jps.2600531041. [DOI] [PubMed] [Google Scholar]
- Dixon G. J., Sidwell R. W., McNeil E. Quantitative studies on fabrics as disseminators of viruses. II. Persistence of poliomyelitis virus on cotton and wool fabrics. Appl Microbiol. 1966 Mar;14(2):183–188. doi: 10.1128/am.14.2.183-188.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The minimum vitamin requirements of the L and HeLa cells in tissue culture, the production of specific vitamin deficiencies, and their cure. J Exp Med. 1955 Nov 1;102(5):595–600. doi: 10.1084/jem.102.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. L., Jaeger R. F. Inactivation of yellow fever virus by glutaraldehyde. Appl Microbiol. 1968 Jan;16(1):177–177. doi: 10.1128/am.16.1.177-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- RIGHTSEL W. A., SCHULTZ P., MUETHING D., MCLEAN I. W., Jr Use of vinyl plastic containers in tissue cultures for virus assays. J Immunol. 1956 Jun;76(6):464–474. [PubMed] [Google Scholar]
- Rubbo S. D., Gardner J. F., Webb R. L. Biocidal activities of glutaraldehyde and related compounds. J Appl Bacteriol. 1967 Apr;30(1):78–87. doi: 10.1111/j.1365-2672.1967.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Sidwell R. W., Dixon G. J., McNeil E. Quantitative studies on fabrics as disseminators of viruses. 3. Persistence of vaccinia virus on fabrics impregnated with a virucidal agent. Appl Microbiol. 1967 Jul;15(4):921–927. doi: 10.1128/am.15.4.921-927.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R. W., Dixon G. J., McNeil E. Quantitative studies on fabrics as disseminators of viruses. I. Persistence of vaccinia virus on cotton and wool fabrics. Appl Microbiol. 1966 Jan;14(1):55–59. doi: 10.1128/am.14.1.55-59.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R. W., Dixon G. J., Westbrook L., Dulmadge E. A. Procedure for the evaluation of the virucidal effectiveness of an ethylene oxide gas sterilizer. Appl Microbiol. 1969 Jun;17(6):790–796. doi: 10.1128/am.17.6.790-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



