Abstract
Neurons and other cells require intracellular transport of essential components for viability and function. Previous work has shown that while net amyloid precursor protein (APP) transport is generally anterograde, individual vesicles containing APP move bi-directionally. This discrepancy highlights our poor understanding of the in vivo regulation of APP-vesicle transport. Here, we show that reduction of presenilin (PS) or suppression of gamma-secretase activity substantially increases anterograde and retrograde velocities for APP vesicles. Strikingly, PS deficiency has no effect on an unrelated cargo vesicle class containing synaptotagmin, which is powered by a different kinesin motor. Increased velocities caused by PS or gamma-secretase reduction require functional kinesin-1 and dynein motors. Together, our findings suggest that a normal function of PS is to repress kinesin-1 and dynein motor activity during axonal transport of APP vesicles. Furthermore, our data suggest that axonal transport defects induced by loss of PS-mediated regulatory effects on APP-vesicle motility could be a major cause of neuronal and synaptic defects observed in Alzheimer's Disease (AD) pathogenesis. Thus, perturbations of APP/PS transport could contribute to early neuropathology observed in AD, and highlight a potential novel therapeutic pathway for early intervention, prior to neuronal loss and clinical manifestation of disease.
INTRODUCTION
Presenilin (PS) is a ubiquitously expressed, developmentally regulated, multi-pass transmembrane protein (1). PS is evolutionarily conserved and is found to interact with many proteins (1). While two PS genes (PS1 and PS2) are found in mammals, invertebrates such as Drosophila have only one PS gene, but alternative splice forms are seen (2–4). PS1 and PS2 null mutations in mice and PS null mutations in Drosophila (PSN) are lethal (prenatal in mice and early larval in Drosophila), indicating that PS plays essential roles during development (2,3).
There is considerable evidence to suggest that PS is the catalytic component of the gamma-secretase complex, which cleaves amyloid precursor protein (APP). More than 50 other membrane spanning substrates have also been identified to date (5). PS has been extensively studied in the context of Alzheimer's disease (AD) and more than 150 mutations in PS that cause Familial AD (FAD) (6) have been identified. Recent studies suggest that PS has multiple roles in addition to its function as a secretase (7–13). Among the many proposed intracellular and neuronal functions of PS, there is some evidence to suggest that PS may also have a role in the regulation of axonal transport.
Although early work suggested that PS1 can be transported bi-directionally in rat sciatic nerves (14,15), and from the entorhinal cortex to the hippocampus via axons of the perforant pathway (16), several recent observations support a direct role for PS in the modulation of axonal transport (17). Biochemical and immunolocalization experiments revealed that PS could be found within APP containing axonal vesicles (18). Recent work also suggests that PS mutations can change the nature of kinesin by affecting its phosphorylation state, thereby affecting the membrane binding of kinesin. In experiments done in neuronal cells, the presence of an FAD mutant PS1 or the absence of PS1 increased GSK3β activity with simultaneous increases in the levels of KLC phosphorylation (19). However, the amount of kinesin-1 bound to membranes was reduced (19). Further work suggested that anterograde transport of APP and Trk receptors was impaired in the sciatic nerves of transgenic mice expressing two independent FAD-linked PS1 variants (20). Together these observations lead to the hypothesis that PS may have an important role in regulating axonal transport. One suggestion is that FAD mutations in PS or reductions of PS decrease kinesin-1 function by stimulating GSK3β activity (19,20). This proposal implies that FAD PS mutations or genetic reduction of PS function should have similar phenotypic effects to reduction of kinesin-1. Here, we use genetic, in vivo imaging and high-resolution quantitative analysis in Drosophila to directly test the normal role of PS in APP-vesicle transport. We found that PS specifically represses APP-vesicle movement in axons via a pathway that not only requires kinesin-1, but also requires dynein.
RESULTS
Presenilin genetically interacts with Amyloid precursor protein
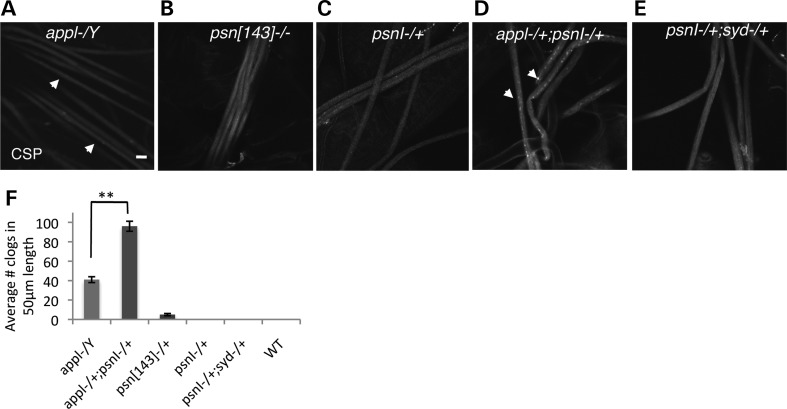
We expected that if PS is present within APP-containing vesicles, then PS and APP could have a functional interaction with each other. To test this hypothesis, we first used loss-of-function mutations of Drosophila PS and APP. Drosophila has an APP homolog with high-sequence similarity to mammalian APP throughout the length of the protein, except within the amyloid beta region (21). In addition, work has shown that APPL is also cleaved (22–24) at homologous sites similar to mammalian APP. Human APP is also cleaved in Drosophila AD models [Gunawardena unpublished, (25,26)]. Previously we found that a deletion of the Drosophila APP gene, appl, caused axonal transport defects (27). We confirmed this older finding in segmental nerves from mutant larvae that were homozygous for the appl mutation [appl-/Y, (27)]. These preparations contained accumulations of synaptic proteins such as synatotagmin (SYT) and cysteine string protein [CSP, (27), Fig. 1A].
Figure 1.
PS genetically interacts with APP. Loss of function of appl shows axonal transport defects [homozygous appl mutant larvae, appl-/Y, arrows, (A), while homozygous psn[143] (psn[143] −/−, hypomorphic allele, (B) or heterozygous psnI (psnI−/+, loss-of-function allele, (C) do not show axonal blockages when stained with the CSP antibody. (D) Larvae that have 50% reductions of Drosphila APP (appl) and PS (psn) show increased amounts of axonal blockages (appl−/+;psnI−/+, arrows) compared with homozygous appl mutants (appl−/Y) (A, arrows). Bar = 10 µm. (E) In contrast, PS does not genetically interact with SYD, although SYD transports another subclass of vesicles down the axon. Segmental nerves from larvae that have 50% reductions of Drosophila SYD (syd) and PS (psnI) are smoothly stained with the CSP antibody (syd−/+;psnI−/+). (F) Quantification analysis indicates that larvae that are appl-/Y; psnI contain twice as many axonal blockages compared with the homozygous mutation of appl (P < 0.01, n = 10 larvae).
Drosophila has a single PS-like gene (psn) with ∼50% amino acid sequence identity to human PS1 (28–30) and two alternatively spliced products (6,31). Loss-of-function psn mutations result in lethal Notch-like phenotypes (32,33). Similar to mammalian PS, two intramembranous aspartate residues are conserved in Drosophila PS and endoproteolysis also occurs. We used two loss-of-function mutations in the Drosophila PS gene, psnI and psn[143]. psnI is a null point mutation that generates a stop codon and produces a truncated product ending at W278 (33). psn[143] is a deletion and/or splice site mutation resulting in the deletion of amino acids between the first transmembrane domain and the middle of the fourth transmembrane domain and is thought to encode a loss-of-function allele (Annette Parks communication to Flybase). While psnI−/− larvae die at early larval stages (1st instar), psn[143]−/− larvae die at late larval stages (third instar). Using QPCR and two sets of primers to the Drosophila PS gene, we confirmed that heterozygous combinations of psnI−/+ and psn[143]−/+ larvae express reduced amounts of PS mRNA compared with wild-type larvae (Supplementary Material, Fig. S1A). Larvae homozygous for the psn[143] mutation (psn[143] −/−, Fig. 1B), or heterozygous larvae carrying either the psn[143]−/+ or the psnI−/+ genotypes did not show detectable levels of axonal accumulations (Fig. 1C) when stained for CSP, which is similar to wild-type larval nerves. Thus, loss or reduction of PS function alone does not cause any obvious axonal transport defect in Drosophila. To test whether reductions of PS enhanced phenotypes caused by reduction of kinesin-1 or dynein function, we generated heterozygous combinations between PS (psn) and motor protein genes, khc8, klc8ex94 (genes encoding subunits of kinesin-1) and roblk (a gene encoding dynein light chain, dlc). In all such heterozygous combinations, we failed to find axonal blockages (Supplementary Material, Fig. S1B–D) or adult viability changes (Supplementary Material, Table S1). Thus, PS does not show any obvious genetic interaction with kinesin-1 or dynein.
To test for genetic interactions between PS and APP in the context of axonal transport, we generated larvae that were double heterozygous for appl and psn mutations (appl−/+;psnI−/+). Strikingly we observed an enhancement of the number of axonal blockages in appl−/+; psnI−/+ larvae compared with what was observed in complete loss of APP larvae (Fig. 1D). Quantitative analysis of the average number of accumulations in a 50 µm larval segmental nerve length indicated that the extent of accumulations in appl−/+; psnI−/+ was approximately twice as frequent as in a homozygous APP mutant (appl-/Y, complete loss of function, Fig. 1F) and was comparable with what was seen in motor protein mutants [P < 0.01, N = 10, (27)]. These larvae also failed to eclose to adults indicating a strong genetic interaction between PS and APP that interfered with development (Supplementary Material, Table S1). In contrast, heterozygous combinations between the sunday driver (syd) mutant which functions in the movement of a different class of vesicles (34), and psn (syd−/+; psn−/+) did not cause axonal accumulations (Fig. 1E, Supplementary Material, Table S1), indicating that PS specifically interacts with APP.
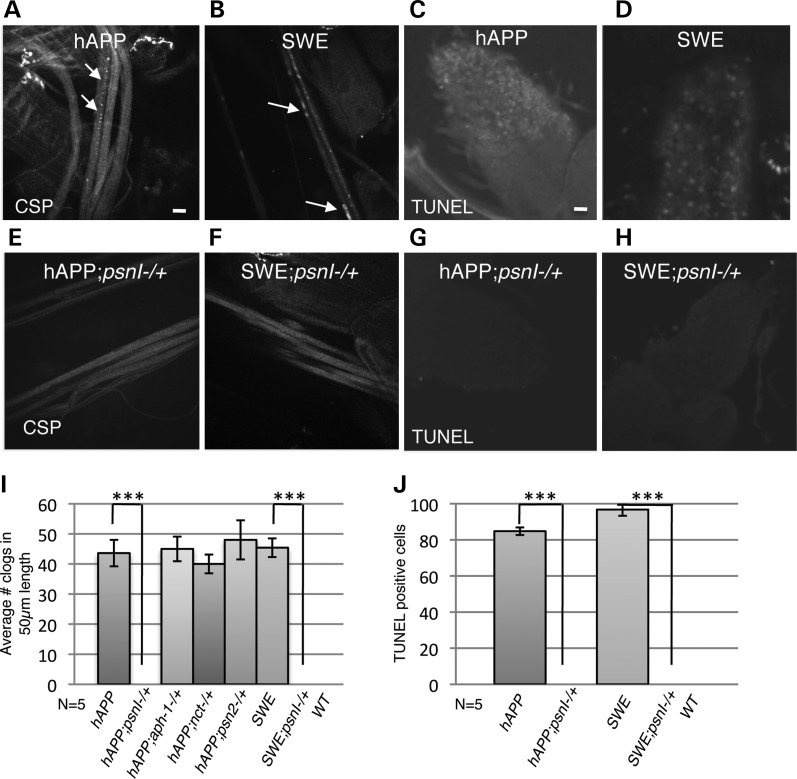
To further test the hypothesis that PS and APP functionally interact, we asked whether reduction of Drosophila PS function enhanced phenotypes caused by overexpression of human APP. Previously, we showed that excess wild-type human APP (hAPP) or human APP carrying the FAD Swedish mutation (hAPPSWE) caused axonal blockages within larval segmental nerves and neuronal cell death within larval brains [Fig. 2A–D, Table S2, (27)]. The extent of blockages seen in larvae expressing excess human APP was comparable with the extent of blockages observed in larvae carrying loss-of-function APPL mutations (Fig. 1F and 2I). Interestingly, 50% reduction of PS in the context of excess hAPP or hAPPSWE did not enhance the hAPP-induced axonal blockages but suppressed them as assayed by CSP antibody staining (compare Fig. 2A–E and Fig. 2B and F). Quantitative analysis revealed significant suppression of the frequency of axonal blockages in larvae expressing hAPP or hAPPSWE with 50% reduction of PS compared with larvae expressing hAPP or hAPPSWE alone (P > 0.001, Fig. 2I). We also found that 50% reduction of PS with excess hAPP or hAPPSWE suppressed hAPP-mediated neuronal cell death observed within larval brains as assayed by TUNEL analysis (compare Fig. 2C–G and Fig. 2D–H, P > 0.001, Fig. 2J). Adult viability was not affected when excess hAPP or hAPPSWE was combined with a 50% reduction of PS (Supplementary Material, Table S2).
Figure 2.
Reduction of PS rescues human APP-mediated axonal blockages and cell death phenotypes. (A, B) Neuronal expression of human APP (hAPP, A) or hAPP with a familial AD mutation (Swedish) (SWE, B) shows axonal blockages (arrows) as previously described in (27). Bar = 10 µm. (C, D) Neuronal expression of hAPP (C) or hAPP with a familial AD mutation (Swedish, D) also shows neuronal cell death as seen by TUNEL analysis (27). Note that these larvae are siblings from the cross APPL-GAL4;psnI/B3 crossed to UAS-hAPP (see methods). (E, F, G, H) Fifty percent reduction of PS with excess of hAPP (hAPP;psnI−/+,E) or hAPP with the Swedish mutation (SWE;psnI−/+, F) completely rescues axonal blockages and neuronal death (G,H). (I) Quantification analysis of the average amount of axonal blocks in 50 µm of larval segmental nerve length indicates that while expression of hAPP or SWE with 50% reduction of PS significantly suppresses axonal blockages, expression of hAPP with 50% reduction of APH-1 or NCT or PEN-2 does not (P < 0.001). (J) Quantification analysis of TUNEL-positive cells indicates that expression of hAPP or SWE with 50% reduction of PS significantly suppresses cell death (P < 0.001).
Since PS is part of the gamma-secretase complex, we tested whether the suppression of hAPP-mediated transport phenotypes is specific to PS alone, or whether reduction of other components of the gamma-secretase complex also had similar effects by generating larvae that had 50% reduction of APH-1, NCT and PEN2 (nctEY06883, aph-1D35 and a deficiency for pen-2) with excess hAPP. While homozygous mutations of NCT, APH or PEN-2 were larval lethal, larvae containing 50% reduction of these mutations alone were similar to wild-type and did not show any axonal transport defects (Supplementary Material, Table S2). In contrast to reductions of PS, 50% reduction of APH-1, NCT, or PEN2 in the context of excess hAPP did not affect the hAPP-mediated transport phenotypes (Supplementary Material, Fig. S2A–C). Quantitative analysis failed to identify any significant suppression of axonal blockages compared with larvae expressing hAPP alone (Fig. 2J). Adult viability was also not affected in these larvae (Supplementary Material, Table S2). Together, these results indicate that the suppression of APP-induced axonal phenotypes we observed with 50% reduction of PS is apparently preferential for PS reduction.
Reduction of PS increases anterograde and retrograde velocities of APP vesicles but not of SYNT vesicles
It is conceivable that the suppression phenotype we observed with 50% reduction of PS is due to an effect on APP-vesicle motility by PS within axons. To test this prediction, we expressed yellow fluorescent protein (YFP)-tagged human APP (APP-YFP) and tracked moving APP vesicles in living larvae using the GAL4 driver pGAL4-62B SG26-1, which expresses only in a small population of larval neurons (35,36). In this context, expression of APP-YFP does not cause axonal blockages or any other observable phenotype, thus APP-YFP movement can be visualized with single-axon resolution within larval segmental nerves. To determine how PS influences APP-vesicle dynamics, we expressed APP-YFP with 100% PS (Supplementary Material, Movie 1) or with 50% reduction of PS (Supplementary Material, Movie 2) using the heterozygous PS mutations (psnI−/+ and psn[143]−/+).
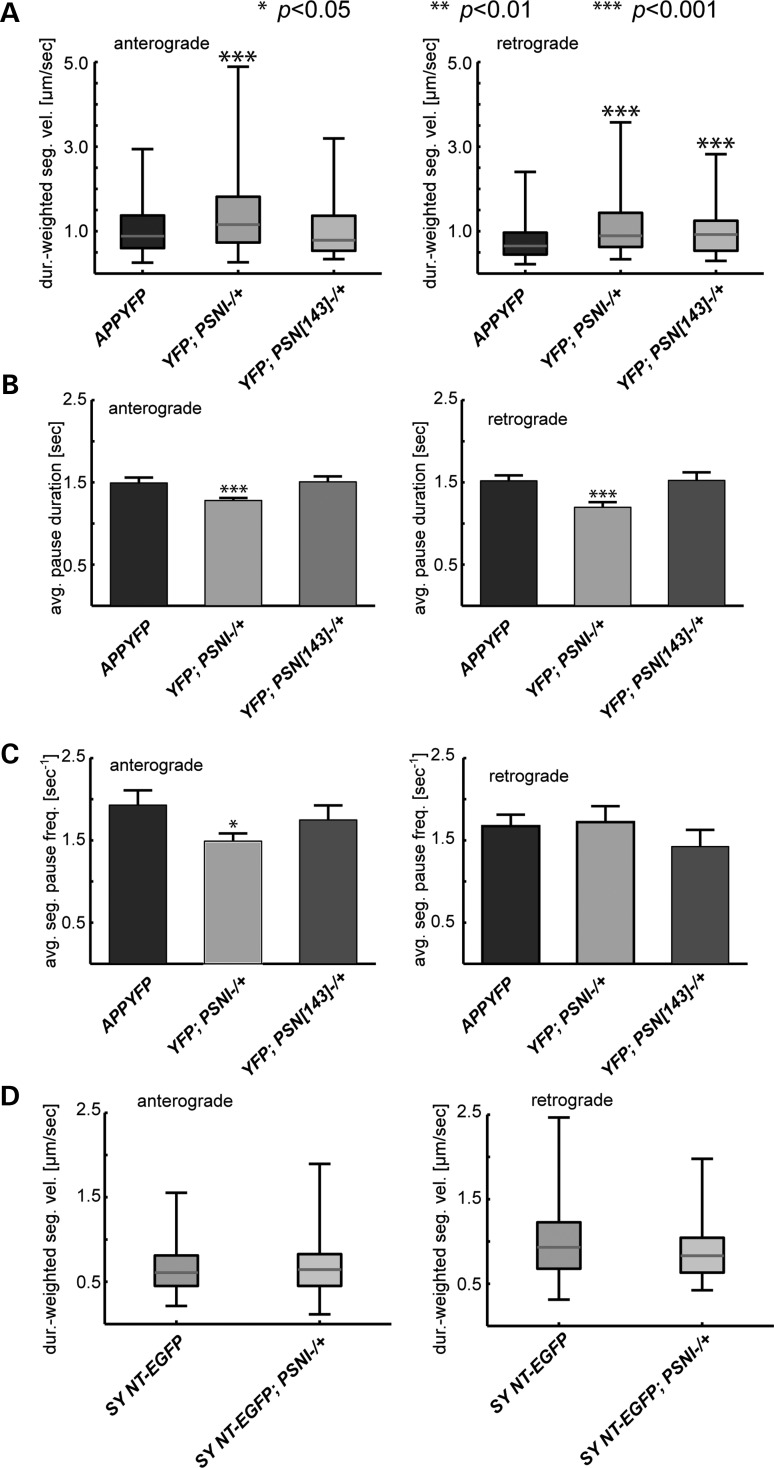
Larval axons containing APP-YFP with 100% PS exhibit many actively motile vesicles that moved at average velocities of 1.03 and 0.76 µm/s in the anterograde and retrograde directions, respectively (Fig. 3A, Supplementary Material, Table S3). To characterize APP-YFP movement within single-larval axons, we used a customized particle tracking software to analyze data collected at a spatial resolution of 0.126 µm and a temporal resolution of 0.15 s/frame (36). To quantitatively evaluate how PS affects APP-YFP vesicle movement, we expressed APP-YFP in heterozygous PS mutant larvae (psnI−/+, 50% reduction of PS) and tracked individual moving APP-YFP vesicles and compared the duration-weighted segmental velocities (see Methods) in these larvae with larvae expressing APP-YFP alone (with 100% PS). The duration-weighted segmental velocity reports the average velocity of vesicles during their time of movement (see Methods). Strikingly, we found significant increases in the average anterograde and retrograde duration-weighted segmental velocities of APP-YFP in the context of 50% reduction of PS (Fig. 3A, Supplementary Material, Table S3, P < 0.001). Similar changes were also observed in psnI−/+ and psn[143]−/+ segmental velocities, which report the velocity behavior independent of the duration of movement (Supplementary Material, Fig. S3A and B). Specifically, significant increases were observed in both anterograde and retrograde segmental velocities in psnI−/+, but only in retrograde segmental velocities in the weaker PS mutation, psn[143]−/+ (Fig. 3A, Supplementary Material, Fig. S3A and B, P < 0.001).
Figure 3.
Reduction of PS increases anterograde and retrograde velocities of APP vesicles but not SYNT vesicles. (A) Box plots of duration-weighted velocities of APP vesicles in larvae expressing hAPP-YFP alone or larvae expressing 50% reduction of PS with hAPP-YFP. Box plots outline the distribution of duration-weighted segmental velocities for each genotype. The red horizontal bar represents the median. The upper and lower box edges represent 75% percentile (i.e. upper quartile) and 25% percentile (i.e. lower quartile), respectively. Fifty percent genetic reduction of PS (psnI) with APP-YFP within single-larval axons significantly increases anterograde (P < 0.001, left) and retrograde (P < 0.001, right) velocities of APP-YFP vesicle movement, while 50% genetic reduction of psn[143], the hypomorphic allele, only increases retrograde velocities of APP-YFP (P < 0.001, right) compared with the expression of APP-YFP alone (data analyzed from 10 larvae and a total of 40 movies, 4 movies from each larvae, anterograde: control n = 229 tracks/vesicles; psnI−/+ n = 892; psn[143]−/+ n = 166; retrograde: control n = 204 tracks; psnI−/+ n = 302; psn[143]−/+ n = 124). (B) Significant reductions are observed in the average pause duration for both anterograde and retrograde vesicles from APP-YFP;psnI−/+ larvae compared with APP-YFP larvae (P < 0.001). No significant changes are observed for APP-YFP vesicles with the hypomorphic allele psn[143] (anterograde: control n = 275 pauses; psnI−/+ n = 739; psn[143]−/+ n = 246; retrograde: control n = 353 pauses; psnI−/+ n = 196; psn[143]−/+ n = 143). (C) Significant reductions in the average segment pause frequency is observed for anterograde vesicles from APP-YFP;psnI−/+ larvae compared with APP-YFP (P < 0.05). No significant changes are seen for retrograde average segment pause frequency. Further no significant changes are observed for APP-YFP vesicles with the hypomorphic allele psn[143] (anterograde: control n = 275 pauses; psnI−/+ n = 739; psn[143]−/+ n = 246; retrograde: control n = 353 pauses; psnI−/+ n = 196; psn[143]−/+ n = 143). (D) In control experiments, 50% genetic reduction of psnI has no effect on synaptotagmin vesicle movement as assayed by expression of SYNT-EGFP with 50% genetic reduction of psnI (anterograde: SYNT-EGFP n = 48 tracks; SYNT-EGFP, psnI−/+ n = 65; retrograde: SYNT-EGFP n = 79 tracks; SYNT-EGFP, psnI−/+ n = 61).
Further examination of vesicle movement in PS-reduced axons also revealed substantial changes in pause durations (Fig. 3B and C). Significant decreases in anterograde and retrograde pause duration were observed in psnI−/+ but not in psn[143]−/+ (Fig. 3B), while only a mild significant change was detected in the anterograde pause frequency in psnI−/+ larvae ((P < 0.05, Fig. 3C). Together, these findings suggest that, in addition to velocity, other parameters of anterograde and retrograde movements are stimulated by PS reduction.
Analysis of the vesicle populations revealed that in larvae containing a 50% reduction of PS (psnI−/+), 54.67% of the measured APP-YFP vesicles exhibited anterograde movement compared with 28.82% seen in control larvae (Supplementary Material, Fig. S3C, Supplementary Material, Table S3, P < 0.001). Surprisingly, the amount of retrograde vesicles was significantly reduced in larvae that had 50% reduction of psnI−/+; 8.77% compared with 24.24% in APP-YFP larvae (P < 0.01). The percentage of stationary vesicles was also reduced in larvae with a 50% reduction of PS in psnI−/+, 18.36% compared with 31.11% in APP-YFP larvae (P < 0.05). However, no significant changes were seen in the vesicles that reversed, 15.84% in APP-YFP compared with 18.2% in larvae with 50% reduction of psnI−/+. These observations indicate that reduction of PS causes significant changes in the balance of anterograde and retrograde APP vesicles, leading to a bias toward greater anterograde movement (Supplementary Material, Table S4).
To test whether the PS reduction effect on APP-YFP vesicles is specific to APP axonal vesicles or whether it is a general property of all moving axonal vesicles, we evaluated the motility of synaptotagmin vesicles using a transgenic line expressing synaptotagmin-EGFP (SYNT-EGFP). Similar to APP-YFP, we evaluated SYNT-EGFP behavior using the GAL4 driver pGAL4-62B SG26-1 in single-larval axons. Strikingly, we found no effect of PS reduction (psnI−/+) on anterograde or retrograde transport of synaptotagmin vesicles. No changes in anterograde and retrograde duration-weighted segmental velocities were observed when PS was reduced in the context of SYNT-EGFP (Fig. 3D, Supplementary Material, Table S3). Further, no changes were seen in the distribution of synaptotagmin vesicles for larvae that had 50% reduction of psnI−/+ in the context of SYNT-EGFP (Supplementary Material, Fig. S3D). Taken together, these observations indicate that PS reduction does not alter the transport of all axonal vesicles and thus, exhibits specificity for a subset that includes APP.
Since PS is thought to be the catalytic component of the gamma-secretase complex, it is possible that increased APP velocities caused by reduction of PS are due to reduction in gamma-secretase activity. To test this proposal, we blocked gamma-secretase activity using a potent and well-characterized gamma-secretase inhibitor compound E (Supplementary Material, Fig. S4A–D, see methods) (37–39). Using two in vitro assays, we first evaluated gamma-secretase activity in wild-type larvae and in mouse brains (see methods) and compared them with activity levels in loss-of-function mutations of PS (Supplementary Material, Fig. S4A). Compared with wild-type larvae, both psn[143]−/+ and psnI−/+ heterozygous larval brains showed reduced levels of gamma-secretase activity (green and blue, respectively, in Supplementary Material, Fig. S2B, compared with red), while psn[143]−/− homozygous larval brains showed greater reductions of gamma-secretase activity (Supplementary Material, Fig. S4B, orange). Consistent with these findings, 5, 10 and 50 µm concentrations of compound E also inhibited gamma-secretase activity in wild-type larvae (Supplementary Material, Fig. S4A). Similar concentrations of another potent gamma-secretase inhibitor DAPT (37,40) also inhibited gamma-secretase activity in Drosophila larvae.
Using compound E, we tested whether the increased velocities of APP vesicles we observed with 50% reduction of PS could be caused by inhibition of gamma-secretase activity. We assayed APP-YFP vesicle motility in APP-YFP expressing larvae that were fed on food laced with 1, 10 and 50 µm concentrations of compound E from the time they hatched until the third instar stage. Control APP-YFP expressing larvae were fed on food supplemented with 1DMSO:10H20 (the buffer used for compound E dilutions) and these larvae were indistinguishable from larvae fed on regular food. Strikingly, we observed significant changes in the retrograde APP-YFP velocities in larvae raised on 1, 10 or 50 µm compound E compared with control APP-YFP larvae raised on DMSO (Supplementary Material, Fig. S5A, Supplementary Material, Table S3). At all compound E concentrations, significant increases were seen in the retrograde (P < 0.001) duration-weighted segmental velocities similar to what was observed for 50% reduction of PS (Supplementary Material, Fig. S5A). However, anterograde APP-YFP velocities were significantly increased (P < 0.001) only in larvae raised on 1 and 10 µm compound E. Taken together, these observations indicate that gamma-secretase activity can influence the transport of APP vesicles.
Faster anterograde and retrograde APP velocities caused by reduction of PS depend on functional motor proteins
It is possible that PS may exert a regulatory function on motor proteins, and that the fast velocities we observed with loss of PS function might be a consequence of this regulation, although we do not observe any genetic interaction between PS and motor proteins and a direct biochemical interaction has not been reported. To test this prediction, we generated larvae with 50% reductions of PS (psnI−/+) and kinesin-1 (khc8−/+) in the context of APP-YFP (see Methods) and compared them with larvae with 50% reduction of PS in the context of APP-YFP but without kinesin-1 reduction. Previously, western blot analysis showed that removal of one functional KHC gene using genetics caused approximately a 50% reduction of both KHC and KLC proteins (36). Membrane flotation analysis indicated that 50% reduction of KHC leads to a reduction in the amount of kinesin-1 that is associated with vesicles (36). Thus, we expected that reduction of the KHC gene results in the reduction of the function of the entire kinesin-1 motor complex.
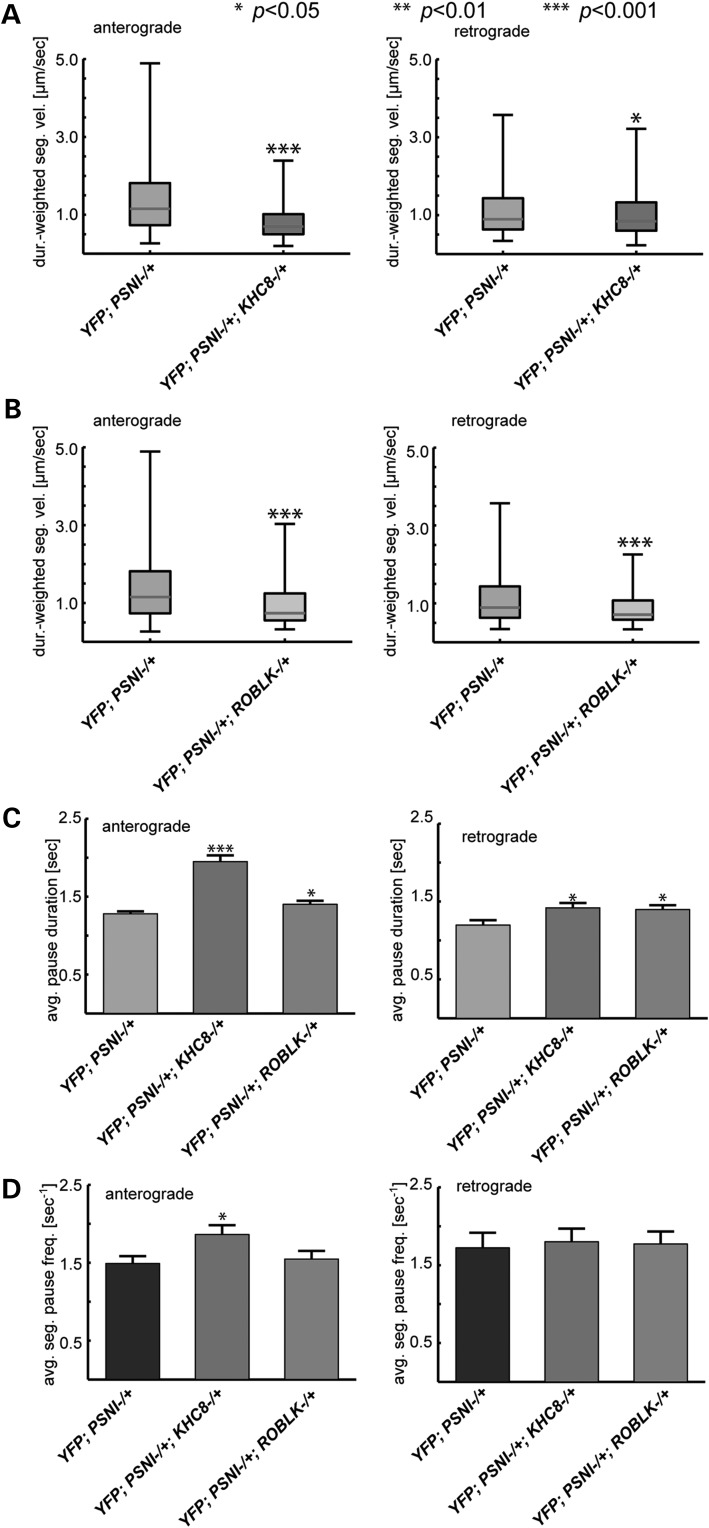
We found significant reductions in both the PS-mediated fast anterograde and retrograde APP-YFP vesicle velocities in larval axons that were expressing APP-YFP with psnI−/+ and khc8−/+ (Fig. 4A, Supplementary Material, Table S3). The anterograde duration-weighted segmental velocities were significantly reduced (P < 0.001) in psnI−/+; khc8−/+ compared to psnI−/+ alone. The retrograde duration-weighted segmental velocities were also reduced (P < 0.05). Consistent with the changes in duration-weighted segmental velocities, the anterograde (P < 0.001) and retrograde (P < 0.01) average segmental velocities were also significantly reduced (Supplementary Material, Fig. S6A). In addition, a significant increase in anterograde (P < 0.001) and retrograde (P < 0.01) pause duration was seen with simultaneous reduction of PS and KHC (Fig. 4C), while a significant increase was seen only in the anterograde pause frequency with simultaneous reduction of PS and KHC (P < 0.01, Fig. 4D). Analysis of vesicle population distribution revealed that reduction of kinesin decreases the percentage of anterograde (P < 0.001) vesicles and significantly increases the percentage of stationary APP vesicles (P < 0.001, Supplementary Material, Fig. S5C, Supplementary Material, Tables S3, S4). From these data, we conclude that there is a genetic interaction between PS and kinesin-1, and that the increases in velocity observed with PS reduction are due to kinesin-1. Thus, the fast anterograde velocities observed for APP vesicles in the context of reduction of PS are dependent on the amount of functional kinesin-1.
Figure 4.
Fast anterograde and retrograde APP velocities caused by reduction of PS are dependent on functional kinesin-1 and dynein motors. (A) Box plots of duration-weighted velocities of APP vesicles show that 50% genetic reduction of khc8, with 50% genetic reduction of psnI in the context of APP-YFP suppresses the fast anterograde velocities observed with 50% reduction of psnI in the context of APP-YFP (P < 0.001). Statistical comparisons are determined between APP-YFP;khc−/+;psnI−/+ and APP-YFP;psnI−/+. The fast retrograde APP-YFP velocities are also suppressed with 50% reduction of khc8 in APP-YPF;psnI−/+ larvae (APP-YFP;khc−/+;psnI−/+, P < 0.05). (anterograde: psnI−/+ n = 892 tracks; psnI−/+;khc−/+ n = 346; retrograde: psnI−/+ n = 302 tracks; psnI−/+;khc−/+ n = 244). (B) Fifty percent genetic reduction of the dynein light chain, roblk, with 50% genetic reduction of PS suppresses the fast retrograde velocities observed with 50% reduction of psnI in the context of APP-YFP (APP-YFP;roblk−/+;psnI−/+ P < 0.001). Surprisingly, suppression of the fast anterograde velocities of APP-YFP is also observed with 50% reduction of roblk and with 50% reduction of psnI (P < 0.001). Statistical comparisons are determined between APP-YFP;roblk−/+;psnI−/+ and APP-YFP;psnI−/+. (anterograde: psnI−/+ n = 892 tracks; psnI−/+;roblk−/+ n = 334; retrograde: psnI−/+ n = 302 tracks; psnI−/+;roblk−/+ n = 181). (C) A significant increase is observed in the average pause duration for anterograde vesicles in larvae with 50% genetic reduction of the kinesin heavy chain with APP-YFP; psnI−/+ (P < 0.001) and in larvae with 50% genetic reduction of the dynein light chain with APP-YFP; psnI−/+ (P < 0.05), compared with APP-YFP;psnI−/+ (anterograde: APP-YFP; psnI−/+ n = 739 pauses; psnI−/+;khc−/+ n = 536; psnI−/+;roblk−/+ n = 526;retrograde: APP-YFP;psnI−/+ n = 196 pauses; psnI−/+;khc−/+ n = 251; psnI−/+;roblk−/+ n = 230). (D) A significant increase is observed in the average segment pause frequency for anterograde velocities for larvae that have 50% genetic reduction of kinesin heavy chain with APP-YFP; psnI−/+ (P < 0.05) while no significant change is seen for larvae that have 50% genetic reduction of the dynein light chain with APP-YFP; psnI−/+ compared with APP-YFP;psnI−/+. Further, no significant changes are seen for the average pause frequency for retrograde vesicles (anterograde: APP-YFP;psnI−/+ n = 739 pauses; YFP;psnI−/+;khc−/+ n = 536; YFP;psnI−/+;roblk−/+ n = 526;retrograde: APP-YFP;psnI−/+ n = 196 pauses; YFP;psnI−/+;khc−/+ n = 251; YFP;psnI−/+;roblk−/+ n = 230).
To further evaluate whether PS also affects dynein function, we generated larvae that had 50% reductions of psnI and roblk (dynein light chain, DLC) in the context of APP-YFP. Previous western blot analysis indicated that removal of one functional DLC gene using genetics caused a reduction in the other components of dynein, DIC and DHC (36). Thus, reduction of the DLC gene results in the reduction of the function of the entire dynein motor complex. In larval axons expressing APP-YFP in the context of roblk−/+ and psnI−/+, we found significant reductions of both the fast anterograde and retrograde velocities of APP-YFP vesicles due to PS reduction (P < 0.001, Fig. 4B, Supplementary Material, Tables S3, S4). Consistent with these findings, reductions of dynein also significantly reduced both the anterograde and retrograde average segmental velocities (Supplementary Material, Fig. S6B, P < 0.001). Similar to simultaneous PS and KHC reduction, mild, but significant increases in pause duration (P < 0.05) were observed under simultaneous PS and DLC reduction. However, no change in pause frequency was detected. Analysis of vesicle populations revealed that reduction of dynein significantly decreased the percentage of anterograde vesicles while increasing the percentage of stationary APP vesicles (P < 0.001, Supplementary Material, Fig. S6D, Supplementary Material, Table S3). These data suggest that from a formal genetic perspective there is a functional interaction between PS and dynein. Thus, PS-induced retrograde velocity increases require functional dynein motors. In addition, our data also suggest that both PS and dynein motor functions are required for the regulation of anterograde velocities of APP-YFP vesicle movement.
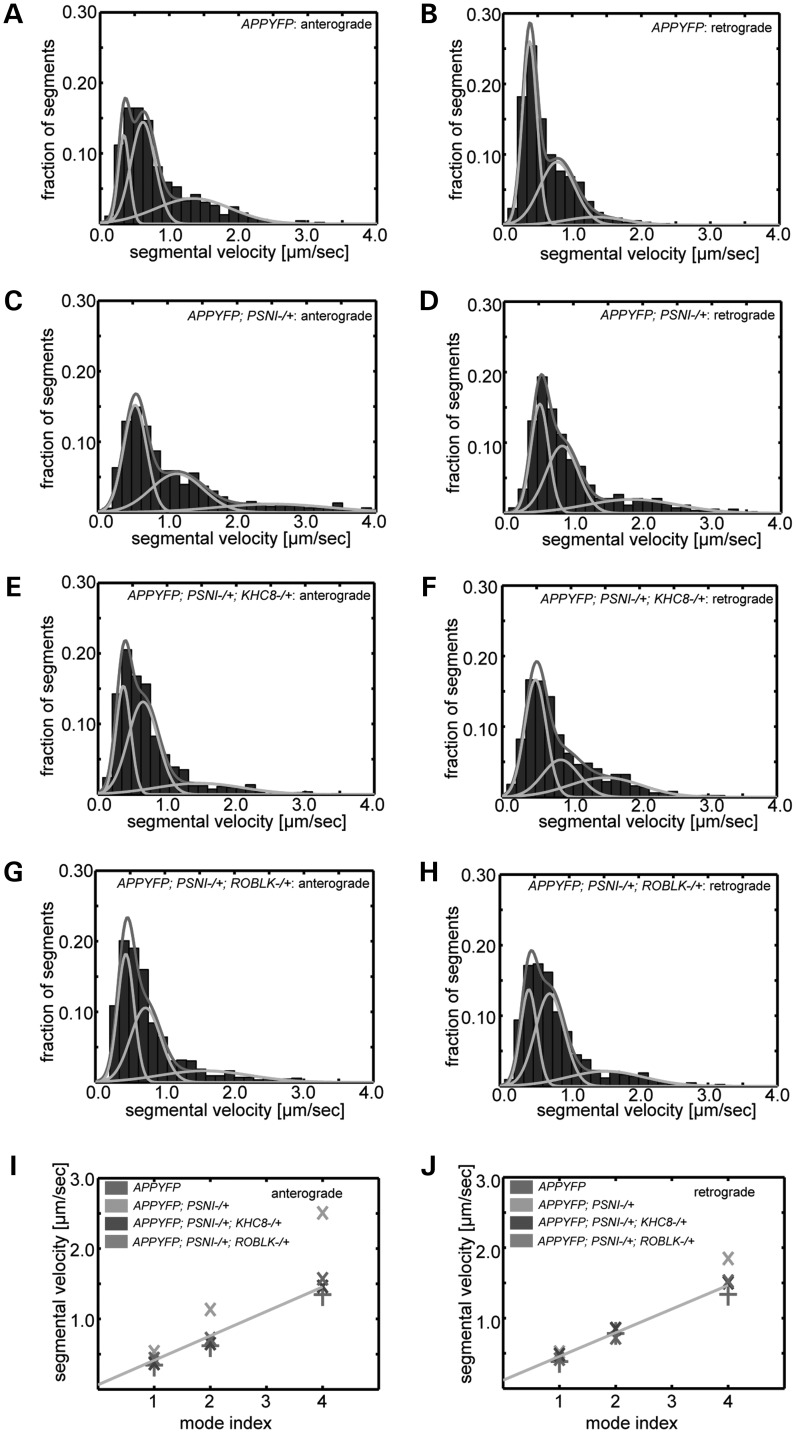
We hypothesize that if the number of active motors on APP vesicles determines the increased velocities we observe, then the distribution of velocities should show shifts in the multiple modal peaks that may reflect differential motor numbers on vesicles (36,41). Using statistical mode analysis of vesicle velocity distributions in both anterograde and retrograde populations (36), we further analyzed the APP velocities observed with 50% reduction of PS (see Methods). For the control (APP-YFP alone), the anterograde velocity distribution fitted three strong modes (Fig. 5A), while the retrograde distribution showed two strong modes and a third weak mode (Fig. 5B). Similar modal distributions were recently observed for APP (36) and PrPc protein (42). Strikingly, 50% reduction of PS shifted both the anterograde and retrograde mode distributions by almost a factor of 2. The anterograde modes changed from 0.3, 0.6 (2×) and 1.3 (4×) µm/s to 0.5, 1.1(2×) and 2.5 (4×) µm/s (Fig. 5C and Supplementary Material, Table S4). The retrograde modes changed from 0.3, 0.7 (2×) and 1.3 (4×) µm/s to 0.5, 0.8 (2×) and 1.8 (4×) µm/s (Fig. 5D, Supplementary Material, Table S4). In both cases, a 1:2:4 ratio of mode averages is preserved (Fig. 5I and J). Fifty percent reduction of kinesin with 50% reduction of PS completely rescued the three anterograde modes back to what was observed for APP-YFP alone (Fig. 5E and F). Similarly, 50% reduction of dynein with 50% reduction of PS also rescued the three retrograde modes back to what was observed for APP-YFP alone (Fig. 5G and H). Together, these observations indicate that the modal changes in velocity distributions we observe with reduction of PS are the result of changes in kinesin and dynein motors, suggesting that loss of regulation of motors can contribute to increased activity changes.
Figure 5.
Statistical mode analysis of APP-vesicle segmental velocities indicates that increased APP velocities are dependent on active motor numbers. (A–H) For different genotypes, normalized segmental velocity distributions in the anterograde and retrograde directions are decomposed into three modes (cyan). The red line depicts the fitting of the overall normalized distribution by the superposition of three modes. The total area under the red line equals one. The area under each mode (cyan line) represents its contribution to (fraction of) all segmental velocities. (I and J) Graphs show linear regression of anterograde and retrograde mode centers assembled from all genotypes analyzed. Y-axis depicts the segmental velocities in microns per second. X-axis depicts the mode index. Note that for each genotype, the three modes in both the anterograde and the retrograde directions approximately follow a mode index ratio of 1:2:4.
Reduction of PS increases the net amount of anterograde APP vesicles
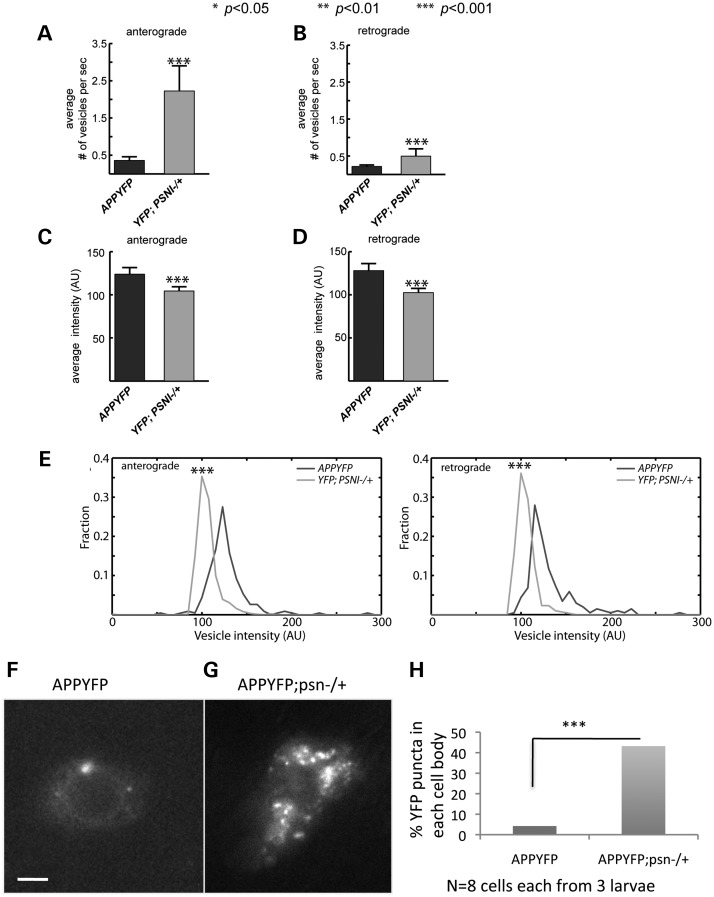
Since the reduction of PS increased the proportion of anterograde vesicles, and decreased the proportion of retrograde vesicles, we evaluated whether PS also influenced the net movement of APP axonal vesicles. To test this, we set equally distributed monitoring points along the axon and examined APP-YFP vesicle movement in two different ways. First, we checked APP-YFP vesicle movement as defined by the average number of vesicles passing per second in the anterograde and retrograde directions in different genetic backgrounds. Our data revealed significant increases in the number of both anterograde and retrograde vesicles with 50% PS reduction compared with wild-type. Specifically, the number of anterograde vesicles passing a fixed point increased by approximately 6-fold from 0.36 vesicles/s to 2.23 vesicles/s, whereas the number of retrograde vesicles passing a fixed point increased by approximately 2-fold from 0.22 vesicles/s to 0.50 vesicles/s (Fig. 6A and B).
Figure 6.
Reduction of PS increases the number of moving vesicles but decreases the amount of APP that is transported in vesicles. (A and B) Comparison of the average number of vesicles passing a total of 10 monitoring points distributed along each axon per second in the anterograde and retrograde directions. Significant increases in both anterograde and retrograde vesicle movements are seen with 50% reduction of PS compared with APP-YFP alone (P < 0.001). n = 10 animals. (C, D) Comparison of the average intensity of vesicles passing a defined boundary in the anterograde and retrograde directions. Assuming that the amount of APP-YFP scales linearly with total YFP intensity on individual vesicles, a significant decrease in APP intensity in both anterograde and retrograde vesicles is seen with 50% reduction of PS compared with APP-YFP alone (P < 0.001). n = 10 animals. (E) Comparison of vesicle intensity distributions of 50% reduction of PS compared with APP-YFP alone. Intensity histograms of both anterograde and retrograde vesicle intensities are much narrower with 50% reduction of PS compared with APP-YFP alone. (F,G,H) Reduction of PS also increases the accumulation of APP-YFP in single-cell bodies. (F) An image of a single-cell body from a larva expressing APP-YFP using the GAL4 driver pGAL4-62B SG26-1, which expresses only in a small population of larval neurons. (G) An image from a single-cell body from a larva expressing APP-YFP with 50% reduction of PS shows increased the accumulation of APP-YFP compared with the cell body from larvae expressing APP-YFP alone. (H) Quantification analysis of YFP puncta in each cell body indicates a significant increase in YFP with 50% reduction of PS. n = 8 cells from three larvae. (P < 0.001). Y-axis depicts percent YFP puncta in each cell body. X-axis depicts the genotypes.
Although PS reduction increased the proportion of anterograde and retrograde vesicles (Fig. 6A and B), it is unclear whether this translates to increased amounts of APP being transported in these vesicles. To test this, we estimated the amount of moving vesicular APP by measuring the average intensity of vesicles passing per second in the anterograde and retrograde directions in different genetic backgrounds. Assuming that the amount of APP scales with the level of YFP, our data revealed significant decreases in average intensity with 50% PS reduction, indicating that reduced amounts of APP are present in moving vesicles (Fig. 6C and D). We also found that the intensity distribution becomes narrower with 50% reduction of PS (Fig. 6E, P < 0.001). Since we could estimate the number of vesicles moving per second (Fig. 6A), and we could estimate the relative fluorescence intensities of the moving vesicles (Fig. 6B), it is possible to estimate the relative amounts of APP moving in anterograde and retrograde directions and how PS deficiency affected these amounts (Table 1). In control animals, there is an excess of anterograde relative to retrograde APP movement, such that there is a net anterograde movement of 17AU/s (AU = arbitrary units of fluorescence intensity) or a 1.64-fold excess of anterograde APP movement. Strikingly, when PS is reduced by 50% both anterograde and retrograde transport increase (Table 1), but the anterograde increase is larger and causes an excess of 183AU/s or a 4.5-fold excess of anterograde over retrograde APP movement. Another way to view these numbers is that PS reduction increases the anterograde APP-vesicle supply by 10-fold. If this is the case then the cell body sorting of APP to the axon must also increase 10-fold. Indeed strikingly, we observe that cell body fluorescence of APP-YFP is increased (Fig. 6E–G) with reduction of PS, indicating increased APP sorting. The cell body increases we observe were not due to variations in APP-YFP expression from larva to larva since no significant changes were observed (n = eight cell bodies from three larvae, Fig. 6H). Taken together, these observations indicate that reduction of PS increases the net amount of APP anterograde vesicles. Perhaps the enhanced supply of APP that is transported in the anterograde direction represents APP C-terminal fragments that are more stable owing to reduction in γ-secretase cleavage.
Table 1.
Summary of the net vesicle movement in axons
| APPYFP; +/+ | APPYFP;PSNI−/+ | |
|---|---|---|
| Anterograde vesicles per second | 0.36 | 2.23 |
| Arbitrary vesicle intensity per anterograde vesicle | 126.7 | 105.7 |
| Arbitrary anterograde vesicle intensity per second | 46 | 235 |
| Retrograde vesicles per second | 0.22 | 0.50 |
| Arbitrary vesicle intensity per retrograde vesicle | 131.1 | 104.7 |
| Arbitrary retrograde vesicle intensity per second | 28 | 52 |
| Net arbitrary anterograde vesicle intensity per second | 17 | 183 |
DISCUSSION
We have found a novel function for PS in axonal transport of APP vesicles using genetic analysis, in vivo imaging coupled with single particle tracking and high-resolution computational analysis. Specifically, our observations allow us to infer a role for PS in the repression of kinesin-1 and dynein activity on APP vesicles in axons. We report that PS reduction dramatically stimulates anterograde and retrograde movements with anterograde stimulation more dramatic than retrograde, such that there is a large increase in net axonal APP transport when PS is reduced. These findings have important implications for our understanding of the role PS plays in the regulation of APP-vesicle motility by kinesin-1 and dynein motors in vivo within axons.
PS normally represses kinesin-1 and dynein motor activity on APP axonal vesicles
Recent studies suggest that PS can influence GSK3β activity to affect kinesin-1 mediated axonal transport (6,19,20). These studies reported decreased vesicle movement in axons in fixed material according to the criteria of vesicle staining in cultured neurons or by decreased APP accumulation in sciatic nerve ligation experiments when a transgenic PS1 mutant was expressed. However, our genetic interaction and in vivo imaging studies showed that PS loss of function was not equivalent to kinesin-1 loss of function. Our direct in vivo observations of live APP-vesicle movement in larval neurons revealed that the role of PS in APP-vesicle motility might in fact be opposite to that originally suggested.
Previously, loss of function of PS1 or the PS1 M146V mutation was reported to increase activated GSK3β, which is thought to increase the phosphorylation of kinesin, thereby leading to reduced binding of kinesin to membranes (19). Furthermore, anterograde transport of APP and Trk receptors was apparently impaired in sciatic nerve ligation analyses of two transgenic FAD mutations of PS1 by immunofluorescence (20). However, we find that PS reduction specifically influences the bi-directional movement dynamics of a specific cargo, APP, such that both anterograde and retrograde velocities are increased together with the overall anterograde transport of APP substantially increased (Figs 3 and 6, Supplementary Material, Fig. S3, Supplementary Material, Table S4). The anterograde movement changes are clearly dependent upon kinesin-1 and the retrograde changes are dependent upon dynein (Fig. 4, Supplementary Material, Fig. S6). Evidence that these effects by PS on APP-vesicle transport are not global comes from the observation that PS reduction does not affect the behavior of synaptotagmin vesicles, which uses kinesin-3 (Fig. 3D, Supplementary Material, Fig. S3D). Thus, PS appears to normally regulate the transport of APP vesicles by repressing kinesin-1 and dynein motor activity.
Our results also demonstrate that PS influences both kinesin-1 and dynein motors, while previous work only showed a role for PS with kinesin-1. There are at least three possibilities to explain why our work differs from previous work. One possibility is that by directly studying movement behaviors in vivo in real-time, we can evaluate behavior that is simply missed by analysis of fixed material or by accumulation experiments in sciatic nerve ligations. A second possibility is that the sciatic nerve ligation experiments only evaluated full-length APP and did not resolve CTFs. It is possible that the movements we see are of full-length APP and CTFs, since the tag we used is on the C-terminus of APP. A third possibility is that the mutations studied in the previous work were not null mutations and so may have altered PS protein behavior in a manner different from the heterozygous null mutations that we studied. Finally, and perhaps most likely, it is possible that some combinations of these three possibilities are correct. Perhaps, these influences coupled to the intrinsic axonal injury that occurs during sciatic nerve ligations are responsible. Further work is needed to evaluate the contributions of each of these factors.
Studies also suggest that PS may control kinesin-1 motor activity on vesicles via GSK3beta, which is reported to be inactivated by phosphorylation, when PS is reduced or mutant (19,20). If this is the case then we expect that PS reduction will decrease anterograde vesicle movement. However, this possibility is rendered less likely by our in vivo finding that PS reduction leads to increases in anterograde APP-vesicle motility (Fig. 3 and Supplementary Material, Fig. S3). An alternative possibility is that PS reduction leads to increased APP in vesicles and therefore increased kinesin-1 recruitment to APP vesicles. This possibility also seems unlikely since we calculated that less APP was present on average in axonal vesicles when PS is reduced (Fig. 6, Table 1, arbitrary vesicle intensity per vesicle), yet the net amount of anterograde APP vesicles transported increased (Supplementary Material, Fig. S3C). A third possibility is that while APP may recruit kinesin-1 motors, PS reduction may lead to the activation of a larger fraction of kinesin-1 motors or to the greater activation of the kinesin-1 motors that have been recruited. Alternatively, the reduced levels of APP in each vesicle that we calculated when PS is reduced (Table 1) might reduce the vesicle size when less APP is present and therefore generate less drag in the potentially crowded axonal environment (43). Since many factors could contribute to the movement of APP vesicles under physiological conditions within an animal, perhaps a combination of these proposals might be true in vivo.
Differential regulation of motor function by presenilin in vivo
The in vivo velocities of kinesin-1 and dynein driven vesicles when PS is reduced are much greater than velocities seen in vitro. Although considerable biophysical work suggests that changes in kinesin-1 motor protein amounts do not generally alter velocities of cargoes (44–48), our work raises the possibility that the in vivo situation is considerably different, and that PS is part of the in vivo regulatory circuit generating these differences. However, consistent with our findings, several studies have previously documented comparable fast velocities for kinesin-1 and dynein driven transport in many different systems in vivo, in contrast to velocities observed in vitro. Ashkin et al. (49) found that mitochondria can move on microtubules as fast as 15 µm/s. Endosomes in cells can be moved by dynein at speeds as fast as 4 µm/s (50). Kural et al. (51) proposed that multiple kinesin or multiple dynein motors can work together to produce velocities up to 10 times the velocities observed in vitro (11.7 µ/s). Although the mechanisms of how such dramatic velocities can occur in vivo is not yet known, it is evident that motility rates in vivo can be greatly affected by higher-order regulatory processes (44). One such process might be the number of active motor proteins present on a single vesicle. The modal distribution of velocities we observe suggests that PS could affect the changes in motor number on APP vesicles to greatly influence the movement dynamics of APP (Fig. 6). The 1:2:4 ratio of modal velocities in both anterograde and retrograde movements could indicate the cooperative behavior of 1, 2 or 4 motor proteins [(36), Fig. 6I and J, Supplementary Material, Table S5]. Fifty percent reduction of PS dramatically increases each mode for both anterograde and retrograde velocities (Fig. 6I and J, Supplementary Material, Table S5), which is rescued by reducing the number of kinesin-1 and dynein motors. This observation not only indicates the close coupling between kinesin and dynein function, but also suggests that in vivo in axons kinesin-1 and dynein activity is dictated by PS. Consistent with this, previous structural studies show that multiple motors can attach to a single vesicle (52). Thus, our observations support a model in which PS acts as a ‘higher order’ regulatory factor, which controls the activity of functional kinesin-1 and dynein motors that are engaged on an APP vesicle under physiological conditions (36,53). A key question that must now be addressed is whether the differential regulation we observe with reduction of PS could occur in axonal vesicles containing different cleavage products of APP (54–56). Further work is required to elucidate this possibility.
Taken together, we suggest that axonal transport defects induced by loss of PS-mediated regulatory effects on APP-vesicle motility could contribute to the neuronal and synaptic defects observed in familial AD pathogenesis. Thus, our work may highlight a potential novel therapeutic pathway for early intervention, prior to neuronal loss and clinical manifestation of disease.
MATERIALS AND METHODS
Drosophila genetics
The loss-of-function Drosophila mutant for the amyloid precursor-like gene (APPL) was obtained from Dr Kalpana White (22). Two loss-of-function Drosophila PS mutants, psnI and psn [143], were obtained from Flybase at Bloomington. psnI is a null mutation, while psn[143] is a hypomorph. For genetic interaction tests, females carrying the APPL mutation were crossed to males that were psnI/TM6C or psn[143]/TM6C. As a control, females that were syd4/TM6B were crossed to males that were psnI/TM6C or psn[143]/TM6C. For genetic interaction tests with kinesin and dynein motors, females that were khc8/T(2:3) CyO TM6B, Tb, klc8ex94/TM6B or roblk/ T(2:3) CyO TM6B, Tb were crossed to males that were psnI/TM6C or psn[143]/TM6C. The chromosome carrying T(2:3) CyO TM6B, Tb is referred to as B3 and carries the dominant markers, Hu, Tb and Cyo. Drosophila transgenic lines of human APP (UAS-hAPP695) or human APP with the Swedish mutant (UAS-hAPPSWE) were expressed using the APPL-GAL4 driver at 29°C as previously done (27). Genetic interaction tests were performed as in (27,35). For genetic interaction experiments, APPL-GAL4/APPL-GAL4;T(2:3) CyO TM6B, Tb/Pin88K was generated. APPL-GAL4/APPL-GAL4;B3/Pin88K females were crossed to psnI/TM6C males. Males that were APPL-GAL4/Y;psnI//B3 were crossed to females from UAS-hAPP695 or UAS-hAPPSWE and only females were used for analysis. The Tb marker on the B3 chromosome enables us to identify larvae that express hAPP with 50% reduction of PS. To knockdown nicastrin, we used the mutant nctEY06883, to knockdown aph-1 we used the mutant aph-1D35 and to knockdown pen-2 we used the deficiency Df(2R)PC4 and genetic interaction crosses were generated as for psnI.
For in vivo microscopy, female third instar larvae were generated by crossing males that were UAS-hAPP-YFP with virgin females that were SG26.1GAL4. Males from this cross are considered as an internal control since they do not express APP-YFP. To evaluate the effect of PS reduction on APP-YFP vesicle movement, we first generated males that were UAS-hAPP-YFP;psnI/T(2:3) CyO TM6B, Tb which were then crossed to virgin females that were SG27.1GAL4 and female non-tubby third instar larvae that are SG27.1GAL4;UAS-hAPPYFP; psnI were selected for imaging. A similar scheme was used to evaluate the effect of the other genes on the gamma-secretase complex. Males that were UAS-hAPPYFP; nctEY06883/T(2:3) CyO TM6B, Tb, UAS-hAPPYFP; aph-1D35/T(2:3) CyO TM6B, Tb, or UAS-hAPPYFP; Df(2R)PC4/T(2:3) CyO TM6B, Tb, were then crossed to virgin females that were SG27.1GAL4. To evaluate the effect of motor protein reductions, we generated a stock that was SG27.1GAL4;khc8/T(2:3) CyO TM6B, Tb and SG27.1GAL4;roblk/T(2:3) CyO TM6B, Tb. Virgin females from this stock which were SG27.1GAL4;khc8/T(2:3) CyO TM6B, Tb or SG27.1GAL4;roblk/T(2:3) CyO TM6B, Tb were crossed to males that were UAS-hAPPYFP;psnI/T(2:3) CyO TM6B, Tb. Female non-tubby third instar larvae that were SG27.1GAL4;UAS-hAPPYFP/khc8;psnI or SG27.1GAL4;UAS-hAPPYFP/roblk;psnI were selected for imaging.
In vivo microscopy
Drosophila transgenic lines expressing human APP C-terminally tagged with a YFP was generated as previously described (35,57) and females from this line were crossed to SG27.1GAL4 which expresses only in a few neurons. Third instar larvae were dissected on a sylgard platform using Ca2+ free buffer containing the following, NaCl (128 mm), EGTA (1 mm), MgCl2 (4 mm), KCl (2 mm), HEPES (5 mm) and sucrose (36 mm) as described in Kuznicki and Gunawardena (58). Dissected animals were inverted onto a cover slip and imaged using a Nikon Eclipse TE 2000-U inverted microscope with a Coolsnap HQ camera and a 100×/1.40NA oil objective. One hundred fifty frames of videos were collected at 150 ms exposure for 22.5 s under the control of Metamorph software. For each genotype, four times lapse movies were collected for each animal; 10 animals were imaged for data analysis; a total of 40 movies.
Immunofluorescence and TUNEL assay: larval dissections, immunofluorescent staining of larval segmental nerves and the TUNEL assay of cell bodies in the ventral ganglia were performed as previously described (27,59). In brief, to examine axonal blockages, third instar larvae were dissected in dissection buffer (2× stock contains 128 mm NaCl, 4 mm CaCl2, 4 mm MgCl2, 2 mm KCl, 5 mm HEPES and 36 mm sucrose, pH 7.2). Dissected larvae were washed in 1XPBST (1XPBT and 0.1%triton) incubated for 1 h at room temperature with antibodies against cystein string protein (CSP, Developmental Studies Hybridoma Bank) or Drosophila synaptotagmin 2 (SYT, Dr H. Bellen) followed by the appropriate fluorophore-conjugated secondary antibodies and mounted using Vectashield (Vector Labs). Larval brains from different genotypes were dissected for TUNEL analysis and detection of apoptotic cells was preformed as previously described (27) using the fluorescein-based cell death kit (Roche). Images of segmental nerves and ventral ganglia were collected with an Optiphot inverted microscope (Nikon) coupled to an MRC1024 confocal imaging system (BioRad). Collected images of segmental nerves and ventral ganglia were analyzed using NIH Image software (RSB, NIH) as previously described (27). Student's t-test was used to determine significant changes.
Gamma-secretase inhibitor feeding
We utilized two commonly used gamma-secretase inhibitors (60–65). Compound E is a potent and well-characterized gamma-secretase inhibitor (37–39). Previous studies showed that compound E can completely block Aβ generation from APP C100, but only has a minor effect on Notch cleavage and NICD generation (37). We also use another potent gamma-secretase inhibitor DAPT (37,40) to inhibit gamma-secretase activity in Drosophila larvae. For compound E/DATP feeding experiments, eggs were collected from the cross containing SG27.1GAL4 females and UAS-hAPPYFP males for 12 h at room temperature. Using a brush, the eggs were collected into a fine sieve, washed and placed in a microcentrifuge tube in a suspension of water. Two hundred microliter of egg/water solution was placed in each vial that contained dry food with the three different concentrations or doses of compound E/DAPT or control conditions. Compound E/DAPT was diluted in DMSO to the final concentrations of 1, 10 and 50 µm and mixed with dry food. As a control, DMSO and water were mixed into dry food. Third instar larvae were dissected and examined as described above.
Gamma-secretase activity assay
We used two well-characterized commercially available methods [QCB and R&D, Supplementary Material, Fig. S4A, (63,66,67)] in which a quenched fluorescent group is released when the substrate is cleaved by gamma-secretase. psn[143] homozygous (psn[143]−/−), psn[143] and psnI heterozygous (psn[143]−/+, psnI−/+) and wild-type larval brains were dissected, homogenized in CHAPSO containing extraction buffer and assayed as directed in the two assay kits. The assay was carried out at 10, 20, 30, 40 and 50 µg protein concentrations. No lysate and no substrate negative controls were included. As a positive control and to validate that we can detect activity, extracts from mouse brains were examined. Intensity readings depicting the cleavage of the quenched substrate were taken at 580 nms. Data were graphed using EXCEL. Using both these methods, gamma-secretase activity was detected in both Drosophila and mice. In Drosophila, we found increasing activity with increasing protein concentrations from wild-type larval brain extracts (Supplementary Material, Fig. S4B, red). To test whether an inhibitor of gamma-secretase inhibits the observed activity, we used compound E at various concentrations. Thirty micrograms of wild-type larval brain extracts were incubated with 0, 5, 10 and 50 mm concentrations of compound E and assayed. Specific inhibition of fluorescence generation by compound E was observed demonstrating endogenous gamma-secretase activity in Drosophila (Supplementary Material, Fig. S4A). Similar results were seen using mouse brain extracts. Phenotypes specific to inhibition of Notch in Drosophila (such as wing and eye defects) were also found by inhibiting gamma-secretase activity using compound E, similar to phenotypes previously observed for another gamma-secretase inhibitor, DAPT (60). We found wing defects starting to occur in adult flies at 5 µm with 35% of adults showing wing defects at 50 µm concentration of compound E (Supplementary Material, Fig. S4C and D). Very pronounced eye defects were observed in adult flies at 300 µm concentration of compound E (Supplementary Material, Fig. S4E). Our observations confirm that gamma-secretase activity can be detected in Drosophila larval extracts, and that compound E/DAPT specifically inhibits this activity.
Image data analysis
Image data analysis was performed as described in (36). Briefly, APP-YFP vesicles in time-lapse movies were detected as single particles as described in (69). Their full trajectories were recovered using customized software based on a modification of the single-particle tracking technique described in (70). Since the particle tracking output contained mainly trajectory segments, a further computational process was performed to link these segments to full trajectories. Finally, a manual process was used to recover trajectories that the software was unable to recover. All recovered trajectories were manually inspected so that errors were either corrected or removed. For each genotype, individual cargoes were automatically classified as being either stationary, anterograde, retrograde or reversing. Cargo trajectories of each genotype were then analyzed by calculating different descriptors that characterize the overall distribution of cargo population and individual cargo behavior in terms of velocity, run length, pause and reversal (36). In particular, to determine the velocity of a specific cargo, its trajectory is first partitioned into segments that are uninterrupted by pause or reversal events. For a given direction, either anterograde or retrograde, duration-weighted segmental velocity of the cargo is defined by its total distance of movement divided by its total duration of movement in that direction. This definition effectively weights cargo velocities within different segments by their durations. This is in contrast to unweighted segmental velocity, which reports the average velocity of a cargo without considering different durations of different segments. Further details as well as other definitions of cargo velocities are provided in (36).
For vesicle content analysis, a total of 10 monitoring points are distributed along each axon. In a first definition, vesicle movement amounts are calculated as the average number of vesicles passing per monitoring point per second in the anterograde and retrograde directions. In a second definition, we calculated the average intensity of vesicles passing per monitoring point in the anterograde and retrograde directions. The second definition characterizes the amount of APP transported, assuming the amount of APP-YFP is proportional to YFP intensity on individual vesicles. All data analysis was conducted using customized software written in MATLAB (Mathworks) and C++.
The modes of transport velocities were determined using a standard statistical analysis method, which is described in Fraley et al. (41). Essentially the optimal collection of velocity modes that best fit the actual transport velocity distributions is determined from the experimental data. As detailed in (41), for a given velocity, the probability of this velocity belonging to every velocity mode is calculated and the calculated probabilities for all velocity modes are sorted. The given velocity is then assigned to the velocity mode that has the highest probability. For each genotype, different modes in cargo velocities were identified by model-based clustering of the collection of all cargo segmental velocities using the mclust package of R (41).
Statistics
Data were first checked for normality using three different tests implemented in the nortest package of R: the Lilliefors test, the Anderson–Darling test and the Shapiro–Francis test. For those that generally follow normal distributions, their means were compared using the two-sample two-sided Student's t-test. For those following non-normal distributions, their means were compared using the permutation t-test (71) or the Wilcoxon rank-sum test.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported in part by NIH grants GM35252 and AG032180 to L.S.B.G. S.G. was supported by an Ellison Medical Foundation Senior postdoctoral fellowship, a New Investigator Grant from the Alzheimer's Association and a grant from the John R. Oishei Foundation. G.Y. was supported by grants from Samuel and Emma Winters Foundation, NSF grants MCB-1052660 and DBI-1052925. L.S.B.G. is an Investigator of the Howard Hughes Medical Institute.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs White and Paro for providing Drosophila APPL and human APP stocks, Dr Todd Golde for providing the Compound E inhibitor; Drs Edward Koo and Bing Zhang for helpful comments; Dr Reis for communicating unpublished results, James Stamos for help with image conversion, Dr Karunaratne for constant support and Drs Szpankowski, Shah, Bache, Almenar-Queralt, Falzone, Weaver and Cheryl Herrera for helpful discussions and/or technical assistance.
REFERENCES
- 1.Laudon H., Hansson E.M., Melén K., Bergman A., Farmery M.R., Winblad B., Lendahl U., von Heijne G., Näslund J. A nine-transmembrane domain topology for presenilin 1. J. Biol. Chem. 2005;280:35352–35360. doi: 10.1074/jbc.M507217200. [DOI] [PubMed] [Google Scholar]
- 2.Donoviel D.B., Hadjantonakis A.K., Ikeda M., Zheng H., Hyslop P.S., Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukinova N.I., Roussakova V.V., Fortini M.E. Genetic characterization of cytological region 77A-D harboring the presenilin gene of Drosophila melanogaster. Genetics. 1999;153:1789–1797. doi: 10.1093/genetics/153.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marfany G., Del-Favero J., Valero R., De Jonghe C., Woodrow S., Hendriks L., Van Broeckhoven C., Gonzàlez-Duarte R. Identification of a Drosophila presenilin homologue: evidence of alternatively spliced forms. J. Neurogenet. 1998;12:41–54. doi: 10.3109/01677069809108554. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi T., De Strooper B. Presenilins: members of the gamma-secretase quartets, but part-time soloists too. Physiology. 2008;23:194–204. doi: 10.1152/physiol.00009.2008. [DOI] [PubMed] [Google Scholar]
- 6.De Strooper B., Annaert W. Novel research horizons for presenilins and γ-secretases in cell biology and disease. Annu. Rev. Cell Dev. Biol. 2010;26:235–260. doi: 10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- 7.Shen J., Bronson R.T., Chen D.F., Xia W., Selkoe D.J., Tonegawa S. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89:629–39. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 8.Handler M., Wines-Samuelson M., Shen J. Presenilins in the developing, adult, and aging cerebral cortex. Neuroscientist. 2005;11:441–51. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- 9.Kang D.E., Soriano S., Xia X., Eberhart C.G., De Strooper B., Zheng H., Koo E.H. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;6:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G., et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S.F., Hao Y.H., Serneels L., De Strooper B., Yu G., Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Repetto E., Yoon I.S., Zheng H., Kang D.E. Presenilin 1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J. Biol. Chem. 2007;282:31504–1516. doi: 10.1074/jbc.M704273200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M., Haapasalo A., Kim D.Y., Ingano L.A., Pettingell W.H., Kovacs D.M. Presenilin/gamma-secretase activity regulates protein clearance from the endocytic recycling compartment. FASEB J. 2006;20:1176–1178. doi: 10.1096/fj.05-5531fje. [DOI] [PubMed] [Google Scholar]
- 14.Papp H., Pakaski M., Kasa P. Presenilin-1 and the amyloid precursor protein are transported bidirectionally in the sciatic nerve of adult rat. Neurochem. Int. 2002;41:429–435. doi: 10.1016/s0197-0186(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 15.Kasa P., Papp H., Pakaski M. Presenilin-1 and its N-terminal and C-terminal fragments are transported in the sciatic nerve of rat. Brain Res. 2001;909:159–169. doi: 10.1016/s0006-8993(01)02679-8. [DOI] [PubMed] [Google Scholar]
- 16.Sheng J.G., Price D.L., Koliatsos V.E. The beta-amyloid-related proteins presenilin 1 and BACE1 are axonally transported to nerve terminals in the brain. Exp. Neurol. 2003;184:1053–1057. doi: 10.1016/j.expneurol.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Stokin G.B., Almenar-Queralt A., Gunawardena S., Rodrigues E.M., Falzone T., Kim J., Lillo C., Mount S.L., Roberts E.A., McGowan E., Williams D.S., Goldstein L.S. Amyloid precursor protein-induced axonopathies are independent of amyloid-beta peptides. Hum. Mol. Genet. 2008;17:3474–86. doi: 10.1093/hmg/ddn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamal A., Almenar-Queralt A., LeBlanc J.F., Roberts E.A., Goldstein L.S.B. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- 19.Pigino G., Morfini G., Pelsman A., Mattson M.P., Brady S.T., Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarov O., Morfini G.A., Pigino G., Gadadhar A., Chen X., Robinson J., Ho H., Brady S.T., Sisodia S.S. Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer's disease-linked mutant presenilin 1. J. Neurosci. 2007;27:7011–7020. doi: 10.1523/JNEUROSCI.4272-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo L., Tully T., White K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 1992;9:595–605. doi: 10.1016/0896-6273(92)90024-8. [DOI] [PubMed] [Google Scholar]
- 22.Torroja L., Packard M., Gorczyca M., White K., Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J. Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolkan B.J., Triphan T., Kretzschmar D. β-secretase cleavage of the fly amyloid precursor protein is required for glial survival. J. Neurosci. 2012;32:16181–16192. doi: 10.1523/JNEUROSCI.0228-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmine-Simmen K., Proctor T., Tschäpe J., Poeck B., Triphan T., Strauss R., Kretzschmar D. Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol. Dis. 2009;33:274–281. doi: 10.1016/j.nbd.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stempfle D., Kanwar R., Loewer A., Fortini M.E., Merdes G. In vivo reconstitution of gamma-secretase in Drosophila results in substrate specificity. Mol. Cell. Biol. 2010;30:3165–3175. doi: 10.1128/MCB.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo M., Hong E.J., Fernandes J., Zipursky S.L., Hay B.A. A reporter for amyloid precursor protein gamma-secretase activity in Drosophila. Hum. Mol. Genet. 2003;12:2669–2678. doi: 10.1093/hmg/ddg292. [DOI] [PubMed] [Google Scholar]
- 27.Gunawardena S., Goldstein L.S.B. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- 28.Hong C.S., Koo E.H. Isolation and characterization of Drosophila presenilin homolog. Neuroreport. 1997;8:665–668. doi: 10.1097/00001756-199702100-00017. [DOI] [PubMed] [Google Scholar]
- 29.Boulianne G.L., Livne-Bar I., Humphreys J.M., Liang Y., Lin C., Rogaev E., St George-Hyslop P. Cloning and characterization of the Drosophila presenilin homologue. Neuroreport. 1997;8:1025–1029. doi: 10.1097/00001756-199703030-00041. [DOI] [PubMed] [Google Scholar]
- 30.Ye Y., Fortini M.E. Characterization of Drosophila Presenilin and its colocalization with Notch during development. Mech. Dev. 1998;79:199–211. doi: 10.1016/s0925-4773(98)00169-5. [DOI] [PubMed] [Google Scholar]
- 31.Nowotny P., Gorski S.M., Han S.W., Philips K., Ray W.J., Nowotny V., Jones C.J., Clark R.F., Cagan R.L., Goate A.M. Posttranslational modification and plasma membrane localization of the Drosophila melanogaster presenilin. Mol. Cell. Neurosci. 2000;15:88–98. doi: 10.1006/mcne.1999.0805. [DOI] [PubMed] [Google Scholar]
- 32.Struhl G., Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 33.Ye Y., Lukinova N., Fortini M.E. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature. 1999;398:525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- 34.Cavalli V., Kujala P., Klumperman J., Goldstein L.S. Sunday driver links axonal transport to damage signaling. J. Cell. Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunawardena S., Her L., Brusch R.G., Laymon R.A., Neisman I.R., Gordesky-Gold B., Sintasath L., Bonini N.M., Goldstein L.S.B. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron. 2003;40:1–20. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- 36.Reis G.F., Yang G., Szpankowski L., Shah S.B., Robinson J.T., Hays T.S., Danuser G., Goldstein L.S.B. Molecular motor function in axonal transport in vivo probed by genetic and computational analysis in Drosophila. Mol. Biol. Cell. 2012;23:1700–1714. doi: 10.1091/mbc.E11-11-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang T., Arslanova D., Gu Y., Augelli-Szafran C., Xia W. Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein. Mol. Brain. 2008;1:15. doi: 10.1186/1756-6606-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran H.T., LaFerla F.M., Holtzman D.M., Brody D.L. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-β accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 2011;31:9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 40.Imbimbo B.P. Therapeutic potential of gamma-secretase inhibitors and modulators. Curr. Top Med. Chem. 2008;8:54–61. doi: 10.2174/156802608783334015. [DOI] [PubMed] [Google Scholar]
- 41.Fraley C., Raftery A.E. Model-based clustering, discriminant analysis and density estimation. J. Am. Stat. Assoc. 2002;97:611–631. [Google Scholar]
- 42.Encalada S.E., Szpankowski L., Xia C.H., Goldstein L.S. Stable kinesin and dynein assemblies drive the axonal transport of mammalian prion protein vesicles. Cell. 2011;144:551–565. doi: 10.1016/j.cell.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hancock W.O., Howard J. Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl Acad. Sci. USA. 1999;96:13147–13152. doi: 10.1073/pnas.96.23.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shubeita G.T., Tran S.L., Xu J., Vershinin M., Cermelli S., Cotton S.L., Welte M.A., Gross S.P. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klumpp S., Lipowsky R. Cooperative cargo transport by several molecular motors. Proc. Natl Acad. Sci. USA. 2005;102:17284–17289. doi: 10.1073/pnas.0507363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soppina V., Rai A.K., Ramaiya A.J., Barak P., Mallik R. Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc. Natl Acad. Sci. USA. 2009;106:19381–19386. doi: 10.1073/pnas.0906524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendricks A.G., Perlson E., Ross J.L., Schroeder H.W., 3rd, Tokito M., Holzbaur E.L. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr. Biol. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunwar A., Vershinin M., Xu J., Gross S.P. Stepping, strain gating, and an unexpected force-velocity curve for multiple-motor-based transport. Curr. Biol. 2008;18:1173–1183. doi: 10.1016/j.cub.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashkin A., Schütze K., Dziedzic J.M., Euteneuer U., Schliwa M. Force generation of organelle transport measured in vivo by an infrared laser trap. Science. 1990;348:346–348. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- 50.Lakadamyali M., Rust M.J., Babcock H.P., Zhuang X. Visualizing infection of individual influenza viruses. Proc. Natl Acad. Sci. USA. 2003;100:9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kural C., Kim H., Syed S., Goshima G., Gelfand V.I., Selvin P.R. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. [DOI] [PubMed] [Google Scholar]
- 52.Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J. Cell Biol. 1982;94:129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szpankowski L., Encalada S.E., Goldstein L.S.B. Sub-pixel colocalization reveals amyloid precursor protein dependent kinesin-1 and dynein association with axonal vesicles. Proc. Natl Acad. Sci. USA. 2012;109:8582–8587. doi: 10.1073/pnas.1120510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai D., Leem J.Y., Greenfield J.P., Wang P., Kim B.S., Wang R., Lopes K.O., Kim S.H., Zheng H., Greengard P., et al. Presenilin-1 regulates intracellular trafficking and cell surface delivery of beta-amyloid precursor protein. J. Biol. Chem. 2003;278:3446–3454. doi: 10.1074/jbc.M209065200. [DOI] [PubMed] [Google Scholar]
- 55.Muresan V., Varvel N.H., Lamb B.T., Muresan Z. The cleavage products of amyloid-beta precursor protein are sorted to distinct carrier vesicles that are independently transported within neurites. J. Neurosci. 2009;29:3565–3578. doi: 10.1523/JNEUROSCI.2558-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee E.B., Zhang B., Liu K., Greenbaum E.A., Doms R.W., Trojanowski J.Q., Lee V.M. BACE overexpression alters the subcellular processing of APP and inhibits Abeta deposition in vivo. J. Cell Biol. 2005;168:291–302. doi: 10.1083/jcb.200407070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaether C., Skehel P., Dotti C.G. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuznicki M.L., Gunawardena S. In vivo visualization of synaptic vesicles within Drosophila larval segmental axons. J. Vis. Exp. 2010;44:2151. doi: 10.3791/2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fye S., Dolma K., Kang M.J., Gunawardena S. Visualization of larval segmental nerves in 3rd instar Drosophila larval preparations. J. Vis. Exp. 2010;43:2128. doi: 10.3791/2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong H., Callaghan D., Jones A., Walker D.G., Lue L.F., Beach T.G., Sue L.I., Woulfe J., Xu H., Stanimirovic D.B., Zhang W. Cholesterol retention in Alzheimer's brain is responsible for high beta- and gamma-secretase activities and Abeta production. Neurobiol. Dis. 2008;29:422–37. doi: 10.1016/j.nbd.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kyriazis G.A., Wei Z., Vandermey M., Jo D.G., Xin O., Mattson M.P., Chan S.L. Numb endocytic adapter proteins regulate the transport and processing of the amyloid precursor protein in an isoform-dependent manner: implications for Alzheimer disease pathogenesis. J. Biol. Chem. 2008;283:25492–25502. doi: 10.1074/jbc.M802072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahn K., Shelton C.C., Tian Y., Zhang X., Gilchrist M.L., Sisodia S.S., Li Y.M. Activation and intrinsic gamma-secretase activity of presenilin 1. Proc. Natl Acad. Sci. USA. 2010;107:21435–21440. doi: 10.1073/pnas.1013246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y.M., Lai M.T., Xu M., Huang Q., DiMuzio-Mower J., Sardana M.K., Shi X.P., Yin K.C., Shafer J.A., Gardell S.J. Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state. Proc. Natl Acad. Sci. USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burns M., Gaynor K., Olm V., Mercken M., LaFrancois J., Wang L., Mathews P.M., Noble W., Matsuoka Y., Duff K. Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J. Neurosci. 2003;23:5645–5649. doi: 10.1523/JNEUROSCI.23-13-05645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crews L., Mizuno H., Desplats P., Rockenstein E., Adame A., Patrick C., Winner B., Winkler J., Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J. Neurosci. 2008;28:4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sastre M. Troubleshooting methods for APP processing in vitro. J. Pharmacol. Toxicol. Methods. 2010;61:86–91. doi: 10.1016/j.vascn.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Yin Y.I., Bassit B., Zhu L., Yang X., Wang C., Li Y.M. {gamma}-secretase substrate concentration modulates the Abeta42/Abeta40 ratio: implications for Alzheimer disease. J. Biol. Chem. 2007;282:23639–23644. doi: 10.1074/jbc.M704601200. [DOI] [PubMed] [Google Scholar]
- 68.Micchelli C.A., Esler W.P., Kimberly W.T., Jack C., Berezovska O., Kornilova A., Hyman B.T., Perrimon N., Wolfe M.S. Gamma-secretase/presenilin inhibitors for Alzheimer's disease phenocopy Notch mutations in Drosophila. FASEB J. 2003;17:79–81. doi: 10.1096/fj.02-0394fje. [DOI] [PubMed] [Google Scholar]
- 69.Ponti A., Vallotton P., Salmon W.C., Waterman-Storer C.M., Danuser G. Computational analysis of F-actin turnover in cortical actin meshworks using fluorescent speckle microscopy. Biophys. J. 2003;84:3336–3352. doi: 10.1016/S0006-3495(03)70058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang G., Matov A., Danuser G. Reliable tracking of large-scale dense particle motion for fluorescent live cell imaging. Proc. IEEE Int. Conf. Computer Vision Pattern Recognition. 2005;3:138. [Google Scholar]
- 71.Moore D.S., McCabe G.P. Introduction to the practice of statistics. 5th edn. New York: W. H. Freeman; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.