Abstract
We evaluated radiation-induced myocardial damage using iodine-123 β-methyl-iodophenyl pentadecanoic acid (I-123 BMIPP) scintigraphy. Between May 2010 and April 2011 we performed I-123 BMIPP scintigraphy for patients who had maintained complete response to curative radiotherapy (RT) for esophageal cancer for more than six months. We compared the area of the myocardium in the RT fields with that of reduced I-123 BMIPP uptake using a 15-segment model that is based on axial computed tomography (CT) images. We classified the segments into three categories: segments receiving 40 Gy (Segment 40 Gy), segments receiving 60 Gy (Segment 60 Gy) and segments out of the radiation fields (Segment 0 Gy). A segment with reduced uptake in the RT fields was defined as positive. A total of 510 segments in 34 patients were used for analysis. The median interval from completion of RT to I-123 BMIPP scintigraphy was 22 months (range, 6–103 months). The numbers of Segment 0 Gy, Segment 40 Gy and Segment 60 Gy were 324, 133 and 53, respectively. Reduced uptake was detected in 42.9% (57/133) of Segment 40 Gy, 67.9% (36/53) of Segment 60 Gy and 13.3% (43/324) of Segment 0 Gy. The odds ratios of 40 Gy and 60 Gy compared with regions out of the RT fields were 5.2 (95% confidence interval [CI]: 3.7–7.4) and 15.4 (95% CI: 6.9–34.6), respectively. Reduced myocardial I-123 BMIPP uptake in RT fields, suggesting RT-induced myocardial damage, was frequently observed. I-123 BMIPP myocardial scintigraphy may be useful for identifying RT-induced myocardial damage.
Keywords: radiotherapy, radiation-induced myocardial damage, I-123 BMIPP, esophageal cancer, heart
INTRODUCTION
Radiotherapy (RT) for malignant diseases may induce cardiac damage when the heart is included in the RT fields. Some studies have shown that RT-induced cardiac disease is manifested from several months to decades after RT for breast cancer and Hodgkin's lymphoma [1–8]. Recently, other studies have also shown that RT-induced cardiac disease in the late phase occurred in patients receiving RT for esophageal cancer [9, 10]. Therefore, patients successfully treated by RT for such malignant diseases should be carefully observed for the potential risk of late cardiac damage.
In some studies, myocardial perfusion scintigraphy, using 99m technetium (Tc-99m) sestamibi, Tc-99m tetrofosmin or thallium-201, demonstrated myocardial perfusion defects in the RT fields of some patients who had been treated for left breast cancer or esophageal cancer [11–15]. RT has been shown to cause endothelial cell damage in myocardial capillaries, with progressive obstruction of the microvascular circulation [16–18]. These studies may imply that RT induces damage of myocardial microvascular circulation. However, perfusion scintigraphy is limited for the evaluation of myocardial function.
It is known that the main energy source of the myocardium changes from free fatty acids to glucose and lactate under ischemic conditions [19]. Iodine-123 β-methyl-iodophenyl pentadecanoic acid (I-123 BMIPP) is a branched fatty acid analogue that enters myocardial cells and can show the degree of myocardial fatty acid metabolism [20]. I-123 BMIPP has been used for the evaluation of various cardiac diseases, such as ischemic cardiac disease and hypertrophic myocardiopathy. Impaired uptake indicates reduced fatty acid utilization, meaning that this myocardial region has possible cardiac dysfunction. Some studies have shown that I-123 BMIPP is superior to perfusion imaging for evaluating the extent and severity of damage to the myocardium in patients with acute and old myocardial infarctions [20, 21]. Therefore, we were interested in the change in myocardial metabolism after RT. We hypothesized that I-123 BMIPP would be useful for evaluating RT-induced myocardial damage. We previously demonstrated RT-induced myocardial damage using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), which showed high FDG uptake in the RT fields [22]. In that study, I-123 BMIPP scintigraphy was also performed as a pilot study in eight patients who had high FDG uptake corresponding to the RT fields. Five of those eight patients showed low I-123 BMIPP uptake in the myocardium corresponding to the FDG uptake regions. To the best of our knowledge, there are no other detailed reports on the evaluation of RT-induced myocardial damage using I-123 BMIPP. The purpose of this study was to detect RT-induced myocardial damage by investigating reduced uptake of I-123 BMIPP in the myocardium corresponding to the RT fields.
MATERIALS AND METHODS
Patients
We conducted this study between May 2010 and April 2011. Patients who had maintained complete response to curative RT for esophageal cancer for more than six months were included in this study and underwent I-123 BMIPP scintigraphy. This study was approved by a local institutional review board, and all of the patients gave written informed consent before enrolment.
Radiotherapy
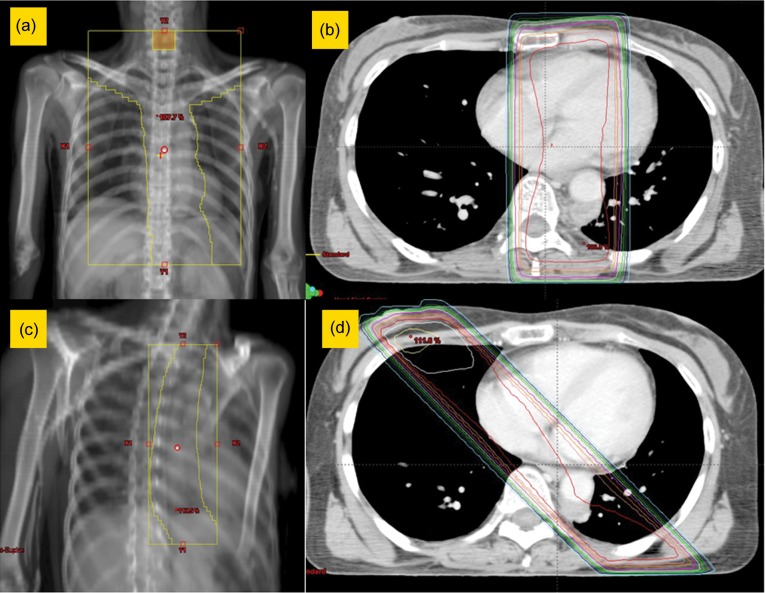
The initial target prophylactically included from the supraclavicular to the celiac lymph nodes and received 39.6–40 Gy, at 1.8–2 Gy per day, using parallel-opposed anterior-posterior fields. After that, the primary tumor and metastatic lymph nodes received 20–30 Gy, at 1.8–2 Gy per day, using parallel-oblique fields to avoid the spinal cord (Fig. 1). The mean total dose in all patients was 64.7 ± 4.7 Gy. A part of the left ventricle was included in the fields in all patients. In consideration of their performance state and age, we made the fields small (for example, by restricting the field to the supraclavicular lymph nodes). RT was delivered using photon beams of 10- or 15-megavoltage equipment with a multiple leaf collimator. The treatment planning was performed by computed tomography (CT) planning in all patients, and the dose distribution was determined by CADPLAN or ECLIPSE Varian Medical Systems (Palo Alto, CA) with Batho power law correction.
Fig. 1.
Main radiation field. (a, b) Irradiation was performed with 39.6–40 Gy for the initial target, which prophylactically included from the supraclavicular to the celiac lymph nodes, using anterior-posterior fields. (c, d) Irradiation was performed with 20–30 Gy for the primary tumor and metastatic lymph nodes, using parallel-oblique fields to avoid the spinal cord.
I-123 BMIPP scintigraphy (myocardial fatty acid imaging)
None of the patients received iodine premedication before the examination. Single photon emission computed tomography (SPECT) imaging was acquired 20 minutes after intravenous administration of 111 MBq of I-123 BMIPP (Cardiodine® Injectable, provided by Nihon Mediphysics Co., Sendai, Japan), using a dual-detector gamma camera combined with CT (Symvia T2, Siemens, Hoffman Estates, IL). The SPECT scan was performed with a low-energy, high-resolution collimator, a matrix of 64 × 64 (pixel size, 3.3 × 3.3 mm) and an acquisition time of ninety 25-s frames over 360°. The 159 keV photopeak for I-123 with a 24% window was selected for data acquisition. No downscatter correction was performed. The SPECT images were reconstructed iteratively using 3-dimensional ordered-subsets expectation maximization with 8 iterations and 5 subsets with a 3D spatial Gaussian filter (9.6 mm in full width at half maximum). We performed SPECT/CT in the condition of expiration to compare with the RT fields more easily and detect anatomical locations more clearly, especially in the basal septum.
Other examinations
Brain natriuretic peptide (BNP) was measured within weeks of I-123 BMIPP scintigraphy to evaluate the correlation with RT-induced myocardial damage. Echocardiography was performed to measure the left ventricular ejection fraction (LVEF) and the ratio of early peak flow velocity to atrial peak flow velocity (E/A).
Correlation of RT fields and SPECT images
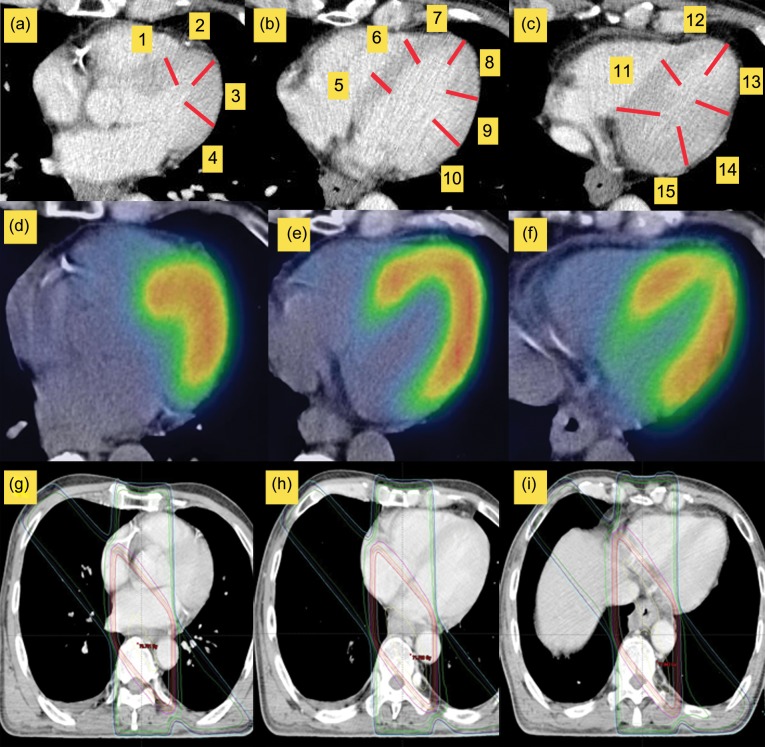
Two reviewers, through consensus agreement, evaluated the results of myocardial I-123 BMIPP scintigraphy in all of the patients. For evaluation of the myocardium, we developed a 15-segment model of the myocardium, using CT axial images for RT planning and CT axial images of SPECT/CT, as a guide for comparison of the RT fields and regional reduced I-123 BMIPP uptake. The 15 segments were defined as shown in Fig. 2. We set up four segments in a CT slice located in the aortic valve, six segments in a CT slice in which the left ventricle space was the largest, and five segments in a CT slice located under the left atrium. One reviewer (an experienced radiation oncologist) visually classified the segments into segments receiving 40 Gy (Segment 40 Gy), segments receiving 60 Gy (Segment 60 Gy) and segments out of the RT fields (Segment 0 Gy). Segments including at least a partial radiation field were defined as segments in radiation fields. The other reviewer (an experienced radiologist of nuclear medicine) visually evaluated the I-123 BMIPP scintigraphy. I-123 BMIPP uptake was qualitatively graded as ‘reduced’ or ‘normal’. The reviewer was blinded to the RT fields and the information about the patients. Using a workstation (Aquarius Net; TeraRecon, San Mateo, CA), fusion images of SPECT and CT in the axial, coronal and sagittal planes were reconstructed. Internal anatomic landmarks, such as the diaphragm and heart, that were visible on both CT and SPECT images were used for co-registration of the fused images. The translucency of SPECT and CT images on the fused images can be changed, and a common pointer for both images can exactly co-localize the morphological and functional areas of interest identified in either one of the two images. A segment including at least partially reduced uptake was defined as a segment of reduced uptake. A segment with reduced uptake corresponding to the RT fields was defined as a positive segment. A segment with reduced uptake out of the RT fields or without reduced uptake was defined as a negative segment.
Fig. 2.
15-segment model in a computed tomography (CT) axial image. (a) 4 segments in a CT slice located in the aortic valve. (b) 6 segments in a CT slice in which the left ventricle space was the largest. (c) 5 segments in a CT slice located under the left atrium. Based on this model, we compared single photon emission computed tomography (d–f) with RT fields (g–i).
Statistical analysis
We analyzed the dataset on both a segment basis and a patient basis.
On the segment basis, we evaluated the sensitivity and specificity for positive segments of I-123 BMIPP using the 2 × 2 chi-square test. The odds ratios of 40 Gy and 60 Gy to the regions out of the RT fields were calculated by logistic regression models. The logistic regression models were fitted using generalized estimating equations (GEE) with exchangeable correlation structures to adjust for the correlation of the repeated measures within a patient (i.e. 15 segments per patient).
On the patient basis, patients who had at least one positive segment were regarded as being positive for RT-induced myocardial damage. The differences in patients' demographic data and results of other examinations were analyzed between patients with and without RT-induced cardiac damage. The continuous variables (age, BNP, LVEF and E/A) were presented as the mean values ± standard deviation (SD). These variables were analyzed using an unpaired t-test or Mann-Whitney U test. The dichotomous variables (sex, history of smoking, hypertension, diabetes, hyperlipidemia, cardiac disease and chemotherapy) were analyzed using the 2 × 2 chi-square test. Statistical significance was defined as a value of P < 0.05. The statistical analysis was performed with PASW Statistics (SPSS) version 18.0 (PASW Statistics, SPSS Inc., Chicago, IL).
RESULTS
A total of 34 patients were enrolled in this study. The patient characteristics are shown in Table 1. The median interval from completion of RT to I-123 BMIPP scintigraphy was 22 months (range, 6–103 months). Concomitant chemotherapy with RT was performed in 30 patients. A combination of cisplatin or nedaplatin and 5-fluorouracil was used in 29 patients, and a combination of nedaplatin and docetaxel was used in the remaining patient. Three patients had cardiac diseases before RT. Two of those patients had unstable angina pectoris, and one patient had atrial fibrillation.
Table 1.
Patient characteristics
| Age at radiotherapy | 67.1 ± 9.0 |
| Sex | |
| Male | 29 |
| Female | 5 |
| Smoking | |
| + | 25 |
| – | 9 |
| Hypertension | |
| + | 14 |
| – | 20 |
| Diabetes | |
| + | 5 |
| – | 29 |
| Hyperlipidemia | |
| + | 6 |
| – | 28 |
| Cardiac disease | |
| + | 3 |
| – | 31 |
| Chemotherapy | |
| + | 30 |
| – | 4 |
| Brain natriuretic peptide (pg/ml) | 85.59 ± 83.06 |
| Electrocardiogram change | |
| + | 15 |
| – | 17 |
| Left ventricular ejection fraction (%) | 65.82 ± 5.81 |
| Early peak flow velocity to atrial peak flow velocity | 0.72 ± 0.25 |
Continuous variables are presented as mean values ± SD.
On the segment basis, we evaluated 510 segments in total. Of these, 324 segments were categorized as Segment 0 Gy, 133 segments were categorized as Segment 40 Gy, and 53 segments were categorized as Segment 60 Gy. The results for I-123 BMIPP are shown in Table 2. Reduced uptake was detected in 42.9% (57/133) of Segment 40 Gy, 67.9% (35/53) of Segment 60 Gy and 13.3% (43/324) of Segment 0 Gy (P < 0.001). The odds ratios of the 40 Gy and 60 Gy regions to the regions out of the RT fields were 5.2 (95% confidence interval [CI]: 3.7–7.4, P < 0.001) and 15.4 (95% CI: 6.9-34.6, P < 0.001), respectively. As shown in Fig. 3, reduced uptake corresponding to the RT field was remarkable in some patients. Thus, reduced uptake tended to appear in segments within the RT fields (especially segments receiving 60 Gy). An analysis of each segment showed that segments 5, 10, 11 and 15, located mainly in the anterobasal, posteriobasal and inferior regions, were included in RT fields in almost all cases. These segments tended to show reduced uptake (Table 3). Segment 15 especially demonstrated reduced uptake (28/34, 82.35%).
Table 2.
Results of 123I β-methyl-iodophenyl pentadecanoic acid in total
| Reduced uptake (–) | Reduced uptake (+) | Rate of reduced uptake (%) | Relative risk (95% CI) | |
|---|---|---|---|---|
| Segment 0 Gy | 281 | 43 | 13.3 | Reference |
| Segment 40 Gy | 76 | 57 | 42.9 | 5.2 (3.7–7.4) |
| Segment 60 Gy | 17 | 36 | 67.9 | 15.4 (6.9–34.6) |
Fig. 3.
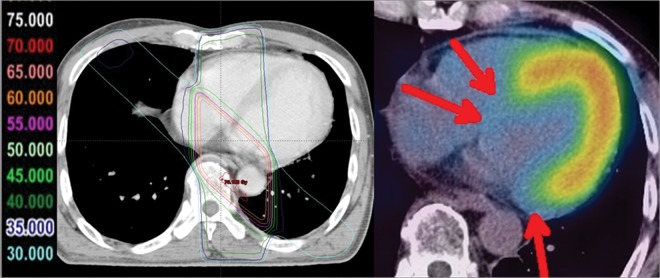
Images of a remarkable case (62-year-old male) at 29 months after radiotherapy for esophageal cancer. Reduced uptake corresponding to radiation fields was distinct in this case.
Table 3.
Results of 123I β-methyl-iodophenyl pentadecanoic acid in each segment
| Segment 0 Gy |
Segment 40 Gy |
Segment 60 Gy |
||||
|---|---|---|---|---|---|---|
| Segment number | Reduced uptake (+) | Total | Reduced uptake (+) | Total | Reduced uptake (+) | Total |
| 1 | 0 | 0 | 5 | 33 | 1 | 1 |
| 2 | 2 | 28 | 1 | 6 | 0 | 0 |
| 3 | 0 | 34 | 0 | 0 | 0 | 0 |
| 4 | 9 | 29 | 0 | 3 | 1 | 2 |
| 5 | 0 | 0 | 14 | 22 | 6 | 12 |
| 6 | 6 | 13 | 7 | 21 | 0 | 0 |
| 7 | 5 | 33 | 0 | 1 | 0 | 0 |
| 8 | 0 | 34 | 0 | 0 | 0 | 0 |
| 9 | 3 | 34 | 0 | 0 | 0 | 0 |
| 10 | 6 | 18 | 3 | 5 | 8 | 11 |
| 11 | 0 | 1 | 19 | 31 | 0 | 2 |
| 12 | 3 | 33 | 0 | 1 | 0 | 0 |
| 13 | 0 | 34 | 0 | 0 | 0 | 0 |
| 14 | 9 | 33 | 0 | 1 | 0 | 0 |
| 15 | 0 | 0 | 8 | 9 | 20 | 25 |
| Total | 43 | 324 | 57 | 133 | 36 | 53 |
On the patient basis, 28 of the 34 patients had at least one positive segment. The average number of positive segments in the 34 patients was 2.7 ± 1.9 (range, 0–7 segments). Among the patients' demographics and results of other examinations, BNP tended to be associated with RT-induced myocardial damage (P = 0.064) (Table 4). The mean values of BNP in patients with and without RT-induced myocardial damage were 94.6 ± 86.3 pg/ml and 43.6 ± 52.6 pg/ml, respectively. When patients were separated into two groups, with and without more than three positive segments (i.e. below or above the average number of positive segments in all patients), the mean values of BNP in patients with and without more than three positive segments were 112.4 ± 95.0 pg/ml and 47.3 ± 40.5 pg/ml, respectively (P = 0.015). Moreover, there was a significant correlation between the number of positive segments and the level of BNP (Spearman rank-correlation coefficient, P = 0.0497).
Table 4.
Analysis of the parameters associated with radiation-induced myocardial damage
| Myocardial damage (–) | Myocardial damage (+) | P value | |
|---|---|---|---|
| Age at radiotherapy | 61.8 ± 7.5 | 68.2 ± 9.0 | 0.115 |
| Sex | 0.353 | ||
| Male | 6 | 23 | |
| Female | 0 | 5 | |
| Smoking | 0.513 | ||
| + | 4 | 21 | |
| – | 2 | 7 | |
| Hypertension | 0.482 | ||
| + | 3 | 11 | |
| – | 3 | 17 | |
| Diabetes | 0.205 | ||
| + | 2 | 3 | |
| – | 4 | 25 | |
| Hyperlipidemia | 0.281 | ||
| + | 2 | 4 | |
| – | 4 | 24 | |
| Cardiac disease | 0.547 | ||
| + | 0 | 3 | |
| – | 6 | 25 | |
| Chemotherapy | 0.441 | ||
| + | 6 | 24 | |
| – | 0 | 4 | |
| Brain natriuretic peptide (pg/ml) | 43.57 ± 52.57 | 94.60 ± 86.28 | 0.064 |
| Electrocardiogram change | 0.267 | ||
| + | 4 | 11 | |
| – | 2 | 15 | |
| Left ventricular ejection fraction (%) | 63.17 ± 5.64 | 66.39 ± 5.79 | 0.223 |
| Early peak flow velocity to atrial peak flow velocity | 0.76 ± 0.33 | 0.72 ± 0.24 | 0.646 |
Continuous variables are presented as mean values ± SD.
None of the other variables were significantly associated with RT-induced myocardial damage (Table 4).
DISCUSSION
A patient whose heart was included in the RT fields may be at risk for RT-induced cardiac disease. However, a method for predicting RT-induced cardiac disease has not been established. Here, we demonstrated reduced uptake metabolism of the myocardium within RT fields by using I-123 BMIPP in patients who had completed RT for esophageal cancer.
In some previous studies, myocardial perfusion scintigraphy was performed to detect RT-induced myocardial damage [11–15]. Marks et al. found myocardial perfusion defects corresponding to RT fields in some patients six months after RT [14]. Some experimental studies have shown that RT to the myocardium resulted in progressive obstruction of the vessel lumen by damaging the endothelium of myocardial blood capillaries, leading to ischemia and, ultimately, fibrosis [16–18]. Generally, the onset of ischemia caused by an imbalance between myocardial oxygen supply and demand is followed by metabolic disorder, left ventricular dysfunction, ECG abnormalities and angina [23]. As myocardial perfusion imaging is limited for the evaluation of myocardial metabolic function, a modality that enables evaluation of myocardial metabolism would be useful for identifying RT-induced myocardial damage. It is known that the main energy source of the myocardium changes from free fatty acids to glucose and lactate under ischemic conditions [19]. We reported that FDG-PET often shows focal increased uptake in the basal myocardium after radiotherapy for esophageal cancer [22]. This finding indicates the possibility of increased glucose metabolism caused by RT. However, the limitation of FDG-PET is that FDG uptake is occasionally observed in the whole myocardium. In such cases, evaluation of RT-induced myocardial damage is difficult. Therefore, I-123 BMIPP may be more useful.
For evaluation of the myocardium, a 17-segment method, shown in the short axis and vertical long axis of the left ventricle, recommended by the American Heart Association is usually used [24]. However, the 17-segment method could not be applied to evaluate RT-induced myocardial damage in the present study because the basal septum, located at the edge of the myocardium, tended to be affected by an artifact of the cardiac nuclear examination. It may thus be more appropriate to evaluate the myocardium in an axis image of CT. We therefore developed a 15-segment model of the myocardium using CT axial images for RT planning and CT axial images of SPECT/CT.
In this study, reduced uptake corresponding to RT fields was present in 93 (50%) of 186 segments. As shown in Fig. 3, reduced uptake corresponding to RT fields was distinct in some patients, indicating that the myocardium was affected to some degree by RT, as expected. This reduction of fatty acid metabolism might result from obstruction of the microvascular circulation caused by RT. It is also possible that myocardial cells themselves were injured by RT and that the injured cells caused change in metabolism. Barjaktarovic et al. reported that mitochondria exposed to irradiation showed impairment in mitochondrial oxidative metabolism and caused the development of cardiovascular disease [25]. Of the 34 patients in the present study, 28 (82.4%) had at least one positive segment. Although comparison with results of previous studies might not be appropriate, I-123 BMIPP demonstrated higher sensitivity of abnormal uptake than myocardial perfusion scintigraphy and FDG-PET in patients with esophageal cancer (Table 5) [15, 22]. Considering this result, the change in myocardial metabolism might be caused both directly and indirectly by RT. Thus, I-123 BMIPP myocardial scintigraphy may be more useful for identifying RT-induced myocardial damage than myocardial perfusion scintigraphy and FDG-PET.
Table 5.
Comparison with myocardial perfusion scintigraphy studies in patients with esophageal cancer
Generally, the tolerance dose of the heart is estimated at about 40 Gy (whole organ), and it may be even higher for partial volume exposure [26]. In this study, reduced uptake was present in 57 (42.9%) of the 133 segments that received about 40 Gy, which may be accounted for by this tolerance dose. Segments that received about 60 Gy tended to have reduced uptake more frequently (67.9%). Gayad et al. reported that most of the myocardial perfusion defects were encompassed within isodose lines ≥45 Gy in the RT plane in patients receiving radiotherapy for esophageal cancer [15]. Marks et al. reported that RT-induced myocardial perfusion defects were correlated with the volume of irradiated myocardium (receiving > 50% of prescribed dose) in patients who received a total dose of 46–50 Gy for left-sided breast cancer [14]. Rutqvist et al. and Gyenes et al. also reported that the cumulative mortality from ischemic heart disease was significantly higher in patients with a high dose per volume of heart than in those without RT [27–29]. Based on those results, we may need to limit irradiation of more than 40 Gy (especially more than 60 Gy) to as small of a volume of the myocardium as possible in RT for esophageal cancer.
The LVEF of patients with at least one positive segment was within the normal limit and was similar to that of patients without any positive segments. This result may be due to the short follow-up period after completion of RT for cardiac dysfunction to become symptomatic. We intend to follow these patients for a longer period to evaluate whether reduced uptake of I-123 BMIPP has clinical significance. Some studies have also shown that abnormal I-123 BMIPP uptake in acute coronary syndrome and idiopathic dilated cardiomyopathy was significantly associated with cardiac events [30–33]. Therefore, I-123 BMIPP scintigraphy might be most useful as a prognostic index for RT-induced cardiac events.
BNP is released from the heart ventricle in response to volume expansion and pressure overload, and it is thought that BNP has a cardioprotective effect [34]. BNP concentration has become a widely accepted index of heart failure or heart remodeling. In 2007, we also reported that there was a significant difference between BNP values in patients without abnormal FDG accumulation in the irradiated myocardium and those in patients with abnormal FDG accumulation (BNP in patients without abnormal FDG accumulation: 61.337 ± 78.577 pg/ml; BNP in patients with abnormal FDG accumulation: 155.978 ± 115.29 pg/ml) [35]. There was also a significant correlation between the number of positive segments and BNP values in the present study. Although the high values in the present study might be caused by other cardiac factors, this result may indicate that higher values of BNP are correlated with larger volumes of RT-induced myocardial damage. We may need to avoid irradiation to a large volume of the myocardium.
There were some limitations in the present study. First, we could not evaluate RT-induced myocardial changes because we did not perform I-123 BMIPP before RT. This examination needs to be performed in a prospective study. Therefore, there may have already been past ischemia in a few of the positive segments before RT. Second, it was difficult to discriminate Segment 40 Gy or Segment 60 Gy from Segment 0 Gy, mainly in the basal segments (especially segments 4, 6 and 10). Even when these segments were judged as Segment 0 Gy, reduced uptake was present in some of these segments. One of the reasons why this mismatch occurred was the difference in respiratory conditions at the CT scan. The CT scans were performed in the conditions of expiration for the I-123 BMIPP examination and free breathing for the RT planning. Even if a segment was not included in the RT planning CT scan, the segment may, in fact, have been irradiated, resulting in reduced uptake. These limitations might be the reason why there was 13.3% reduced uptake in Segment 0 Gy.
CONCLUSIONS
We demonstrated that reduced I-123 BMIPP uptake in the myocardium within RT fields frequently occurred in patients who had been treated for esophageal cancer. I-123 BMIPP might be a useful modality for identifying RT-induced myocardial damage. We may need to avoid high-dose irradiation to a large volume of the myocardium. Although cardiac ejection function was not reduced in patients with reduced uptake in the RT fields in the present study, we intend to follow up on patients with reduced uptake in the RT fields carefully.
ACKNOWLEDGEMENTS
We thank all of the patients who participated in the present study and all of the personnel of the Department of Radiation Oncology and Diagnostic Radiology for support of the present study.
REFERENCES
- 1.Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145–52. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- 2.Borger JH, Hooning MJ, Boersma LJ, et al. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: the role of irradiated heart volume. Int J Radiat Oncol Biol Phys. 2007;69:1131–38. doi: 10.1016/j.ijrobp.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–6. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 4.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J Radiat Oncol Biol Phys. 1999;43:755–62. doi: 10.1016/s0360-3016(98)00412-x. [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 6.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–86. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 7.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol. 1993;11:1208–15. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 8.Adams MJ, Hardenbergh PH, Constine LS, et al. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 9.Ishikura S, Nihei K, Ohtsu A, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 10.Morota M, Gomi K, Kozuka T, et al. Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2009;75:122–28. doi: 10.1016/j.ijrobp.2008.10.075. [DOI] [PubMed] [Google Scholar]
- 11.Gyenes G, Fornander T, Carlens P, et al. Myocardial damage in breast cancer patients treated with adjuvant radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 1996;36:899–905. doi: 10.1016/s0360-3016(96)00125-3. [DOI] [PubMed] [Google Scholar]
- 12.Gustavsson A, Bendahl PO, Cwikiel M, et al. No serious late cardiac effects after adjuvant radiotherapy following mastectomy in premenopausal women with early breast cancer. Int J Radiat Oncol Biol Phys. 1999;43:745–54. doi: 10.1016/s0360-3016(98)00454-4. [DOI] [PubMed] [Google Scholar]
- 13.Seddon B, Cook A, Gothard L, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol. 2002;64:53–63. doi: 10.1016/s0167-8140(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 14.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–23. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Gayed IW, Liu HH, Yusuf SW, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47:1756–62. [PubMed] [Google Scholar]
- 16.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–65. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauk S, Kiszel Z, Buschmann J, et al. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–08. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 18.Stewart JR, Fajardo LF, Gillette SM, et al. Radiation injury to the heart. Int J Radiat Oncol Biol Phys. 1995;31:1205–11. doi: 10.1016/0360-3016(94)00656-6. [DOI] [PubMed] [Google Scholar]
- 19.Neely JR, Rovetto MJ, Oram JF. Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc Dis. 1972;15:289–329. doi: 10.1016/0033-0620(72)90029-1. [DOI] [PubMed] [Google Scholar]
- 20.Tateno M, Tamaki N, Yukihiro M, et al. Assessment of fatty acid uptake in ischemic heart disease without myocardial infarction. J Nucl Med. 1996;37:1981–85. [PubMed] [Google Scholar]
- 21.Mochizuki T, Murase K, Higashino H, et al. Ischemic “memory image” in acute myocardial infarction of 123I-BMIPP after reperfusion therapy: a comparison with 99mTc-pyrophosphate and 201Tl dual-isotope SPECT. Ann Nucl Med. 2002;16:563–68. doi: 10.1007/BF02988634. [DOI] [PubMed] [Google Scholar]
- 22.Jingu K, Kaneta T, Nemoto K, et al. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int J Radiat Oncol Biol Phys. 2006;66:845–51. doi: 10.1016/j.ijrobp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59:23–30C. doi: 10.1016/0002-9149(87)90192-5. [DOI] [PubMed] [Google Scholar]
- 24.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 25.Barjaktarovic Z, Schmaltz D, Shyla A, et al. Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS ONE. 2011;6:e27811. doi: 10.1371/journal.pone.0027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andratschke N, Maurer J, Molls M, et al. Late radiation-induced heart disease after radiotherapy. Clinical importance, radiobiological mechanisms and strategies of prevention. Radiother Oncol. 2010;100:160–66. doi: 10.1016/j.radonc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Rutqvist LE, Lax I, Fornander T, et al. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992;22:887–96. doi: 10.1016/0360-3016(92)90784-f. [DOI] [PubMed] [Google Scholar]
- 28.Gyenes G, Rutqvist LE, Liedberg A, et al. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother Oncol. 1998;48:185–90. doi: 10.1016/s0167-8140(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 29.Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76:S77–85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 30.Hatano T, Chikamori T, Usui Y, et al. Diagnostic significance of positive I-123 BMIPP despite negative stress Tl-201 myocardial imaging in patients with suspected coronary artery disease. Circ J. 2006;70:184–89. doi: 10.1253/circj.70.184. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto A, Nakata T, Tamaki N, et al. Serial alterations and prognostic implications of myocardial perfusion and fatty acid metabolism in patients with acute myocardial infarction. Circ J. 2006;70:1466–74. doi: 10.1253/circj.70.1466. [DOI] [PubMed] [Google Scholar]
- 32.Matsuki T, Tamaki N, Nakata T, et al. Prognostic value of fatty acid imaging in patients with angina pectoris without prior myocardial infarction: comparison with stress thallium imaging. Eur J Nucl Med Mol Imaging. 2004;31:1585–91. doi: 10.1007/s00259-004-1551-8. [DOI] [PubMed] [Google Scholar]
- 33.Yazaki Y, Isobe M, Takahashi W, et al. Assessment of myocardial fatty acid metabolic abnormalities in patients with idiopathic dilated cardiomyopathy using 123I BMIPP SPECT: correlation with clinicopathological findings and clinical course. Heart. 1999;81:153–59. doi: 10.1136/hrt.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–28. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 35.Jingu K, Nemoto K, Kaneta T, et al. Temporal change in brain natriuretic peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1417–23. doi: 10.1016/j.ijrobp.2007.05.054. [DOI] [PubMed] [Google Scholar]