Abstract
The purpose of this study was to assess the efficacy of 18F-fluoro-2-deoxy-glucose uptake positron emission tomography (FDG-PET) for the prediction of outcome in definitive chemoradiotherapy (CRT) for esophageal cancer. We enrolled 56 patients with esophageal cancer treated with definitive CRT and examined by FDG-PET before treatment. We examined the correlation of the maximum standardized uptake value (SUVmax) in FDG-PET of the primary tumor with overall survival (OS), progression-free survival (PFS), local control (LC) and response of the primary tumor. After definitive CRT, 30 patients had a clinical complete response (CR), making the CR rate 54%. For all 56 patients, the 2-year OS rate, PFS rate and LC rates were 64%, 38% and 51%, respectively. We divided the patients into two groups according to SUVmax: SUVmax < 10 (low-SUV) and ≥10 (high-SUV). The 2-year OS rates in the low- and high-SUV groups were 100% and 41%, the PFS rates were 73% and 19%, the LC rates were 71% and 39%, and the CR rates were 100% and 32%, respectively. A univariate analysis revealed significant differences between the low- and high-SUV group in OS, PFS, LC and response (P = 0.0005, 0.0002, 0.048, and <0.0001, respectively). SUVmax and T stage were significantly associated with OS, PFS, LC and response. A multivariate analysis showed significant differences between the SUVmax <10 and ≥10 groups in overall survival and response (P < 0.05). Our result suggests that the SUVmax in FDG-PET of the primary tumor before treatment may have prognostic value for esophageal cancer.
Keywords: FDG-PET, esophageal cancer, chemoradiotherapy, SUVmax
INTRODUCTION
The prognosis of esophageal cancer remains poor despite recent improvements in diagnosis and treatment. Definitive chemoradiotherapy (CRT) has become an accepted treatment for esophageal cancer. In Japan, the most common histologically confirmed esophageal cancer is squamous cell carcinoma (SqCC), and it is considered to have high radiosensitivity, though significant difference in treatment outcomes by histology has not been established. Definitive CRT has been investigated in advanced locoregional esophageal cancer to explore whether it can improve local control and survival rates. Definitive CRT has been considered a potentially curative treatment for locoregional esophageal cancer and may achieve the same survival benefit as surgical resection [1, 2].
18F-fluoro-2-deoxy-glucose positron emission tomography (FDG-PET) is a functional imaging technique that permits the characterization of tumor metabolism. FDG-PET has played an important role in the staging of various malignant tumors, including colorectal cancer, head and neck cancer, breast cancer, and non-small-cell lung cancer (NSCLC) [3–6]. For esophageal cancer, FDG-PET is often used for tumor staging before treatment, the evaluation of tumor response, locoregional recurrence, and/or distant metastases after treatment in patients treated with CRT. FDG-PET is expected to provide additional information to aid in the prediction of pathologic response with CRT. Some reports have suggested that esophageal carcinoma patient response to CRT by FDG-PET has a significant correlation with pathologic response and survival [7–9]. However, the value of FDG-PET before treatment for predicting the outcome of definitive CRT in esophageal cancer patients has not been established.
The purpose of the present study was to assess the efficacy of FDG-PET for predicting the outcome of definitive CRT for esophageal cancer.
MATERIALS AND METHODS
Patients
A total of 56 patients with esophageal cancer treated at Kyushu University Beppu Hospital with definitive CRT between April 2006 and December 2008 were retrospectively analyzed. FDG-PET was performed before treatment for all patients. The patients' characteristics are shown in Table 1. The median age of the patients was 68 years (range, 30–85 years); 49 were male and 7 were female. The histological type of tumors was confirmed to be SqCC in all 56 patients. Written informed consent was obtained from all patients enrolled.
Table 1.
Patient characteristics
| Characteristic | |
|---|---|
| Age | |
| Median | 68 years |
| Range | 30–85 years |
| Gender | |
| Male | 49 |
| Female | 7 |
| Pathology | |
| Squamous cell carcinoma | 56 |
| Portion | |
| Ce | 6 |
| Ut | 16 |
| Mt | 21 |
| Lt | 12 |
| Ae | 1 |
| Tumor length | |
| Median | 40 mm |
| Range | 5–100 mm |
| T stage | |
| T1 | 11 |
| T2 | 7 |
| T3 | 16 |
| T4 | 22 |
| N stage | |
| N0 | 18 |
| N1 | 38 |
| UICC stage | |
| I | 9 |
| II | 12 |
| III | 26 |
| IV | 9 |
Ce = cervical esophagus, Ut = upper thoracic esophagus, Mt = middle thoracic esophagus, Lt = lower thoracic esophagus, Ae = abdominal esophagus.
Pretreatment evaluation
The extent of disease was evaluated by physical examination, chest radiography, esophagoscopy, barium esophagography, computed tomography (CT) and FDG-PET/CT in all patients. Bronchoscopy was performed when tracheobronchial involvement was suspected. Endoscopic ultrasound was applied when the transducer could be passed through the tumor. The clinical stage was defined according to the criteria of the International Union against Cancer [10]. The tumor length was evaluated with esophagography and esophagoscopy. These characteristics are summarized in Table 1.
FDG-PET imaging
FDG-PET was performed for all patients before treatment. We obtained FDG-PET/CT images using an integrated PET/CT Discovery STE system (GE Medical systems, Milwaukee, WI), which integrates a PET system with bismuth germanate (BGO) crystal and 16-slice multidetector CT (MDCT).
All patients fasted for at least 4 h before FDG administration, and 185 MBq of FDG was intravenously administered to each patient. Images were acquired 1 h after FDG administration. Low-dose CT (tube voltage 120 kV; effective tube current 30–250 mA) was performed for attenuation correction and identifying the tumor's precise anatomical location before PET acquisition. The CT was reconstructed by filtered back projection (FBP) into 512 × 512 pixel images with a slice thickness of 5 mm to match the PET.
The standardized uptake value (SUV) was calculated as the regional radioactivity concentration divided by the injected amount of radioactivity normalized to body weight. The region of interest (ROI) for each SUV calculation was manually drawn as small as possible around a focal increased FDG uptake relative to the background on the transaxial image. The maximum standardized uptake value (SUVmax) was assigned to an abnormality in the primary tumor that had the highest SUV. If no accumulation of FDG was visible on the FDG-PET image, the ROI was determined with reference to the corresponding CT image.
Treatments
Radiation therapy was performed using 4-, 6- or 10-MV external photon beams delivered at a daily dose of 1.8–2 Gy, five times per week with a Clinac 21EX linear accelerator (Varian Medical Systems, Palo Alto, CA). The regional radiation therapy was delivered through anteroposterior portals in a T-shaped field including the bilateral supraclavicular, mediastinal and abdominal regional lymph nodes, or an I-shaped field including the mediastinal and abdominal regional lymph nodes, at a dose of 40–41.4 Gy, and the boost was delivered through parallel or nonparallel opposed oblique portals using 10-MV photon beams avoiding the spinal cord. The total dose ranged from 50–71.4 Gy (median 65 Gy). The concurrent chemotherapy consisted of cisplatin (CDDP) or carboplatin plus 5-fluorouracil (5-FU). The concurrent chemotherapy regimen for most patients consisted of a daily 24-h low-dose protracted infusion of 5 mg/m2/day of CDDP and 250–300 mg/m2/day of 5-FU on the days when the radiotherapy was performed.
Response evaluation
The response was determined within one month following the completion of treatment, using esophagography and esophagoscopy. The response of the primary tumor was evaluated using the criteria of the Japanese Society for Esophageal Diseases, which are based on findings from esophagograms and esophagoscopy [11]. A complete response (CR) was defined as the complete disappearance of the tumor lesion and ulceration from esophagography and esophagoscopy. A post-treatment biopsy was performed only if clinically indicated.
Statistical analysis
The survival rate and local control rate were calculated by the Kaplan-Meier method from the date of initiation of treatment. We estimated the correlation of the SUVmax in the FDG-PET of the primary tumor and other prognostic factors (age, gender, tumor length, T stage and N stage) with overall survival (OS), progression-free survival (PFS), local control (LC) and response of the primary tumor. The SUVmax of the primary tumor was compared with tumor response, and the SUVmax values were divided into two groups using the optimal cut-off value obtained from a receiver operating characteristic (ROC) analysis. The ROC curve was generated to assess the SUVmax in relation to tumor response and the optimal cut-off value was defined by the point on the ROC curve with the minimum distance between the 0% false-positive rate and the 100% true-positive rate. The OS, PFS and LC values were compared between the two groups using the log-rank test. The responses to treatment were compared between the two groups using the chi-square test. To assess the effect of the patients' characteristics and other prognostic factors on the outcome and response, a Cox proportional-hazards model and logistic regression model were used for multivariate analysis. Statistical significance was defined as a P-value <0.05.
RESULTS
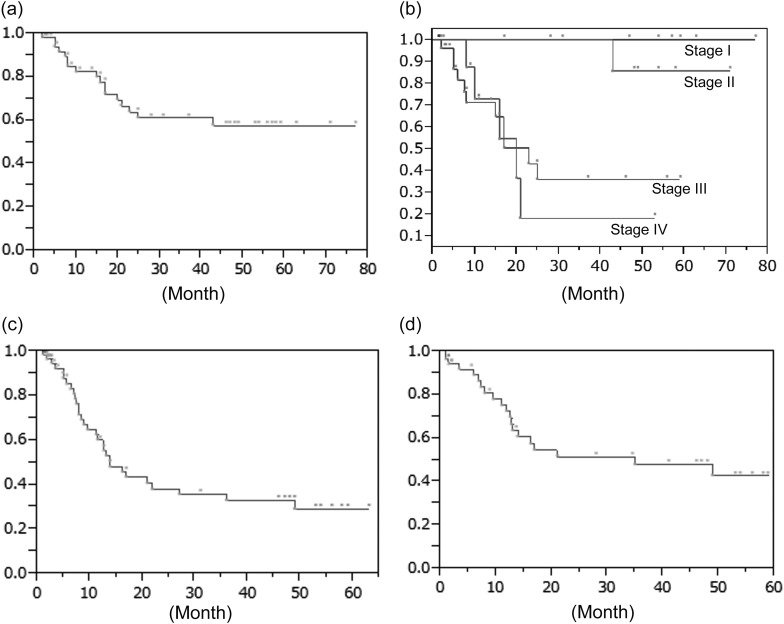
After definitive CRT, 30 patients had a clinical CR, and the other 26 patients had a non-CR, making the CR rate 54%. For all 56 patients, the 2-year OS was 64% (Fig. 1a). The 2-year OS rates of the Stage I, II, III and IV patients were 100%, 100%, 43% and 18%, respectively (Fig. 1b). The 2-year PFS was 38% (Fig. 1c), and the 2-year LC was 51% (Fig. 1d).
Fig. 1.
Survival probabilities of the 56 patients with esophageal cancer. (a) Overall survival (OS) probabilities. (b) Overall survival (OS) probabilities of patients with UICC Stage I–IV esophageal cancer. (c) Progression-free survival (PFS) probabilities. (d) Local control (LC) probabilities.
When residual or recurrent tumors were detected after definitive CRT, appropriate treatment was chosen by the attending physicians, taking into consideration the patient's general condition and the risk of salvage therapy. Salvage therapy was performed for five patients: two patients with surgery (one patient with residual tumor and one patient with recurrent tumor) and three patients with endoscopic therapy for recurrent tumor.
The median SUVmax in the FDG-PET images of the primary tumors was 12.6. The correlations between the SUVmax in FDG-PET of primary tumors and the various patient characteristics before treatment are shown in Table 2. The SUVmax of the primary tumor was significantly correlated with tumor length, T stage, N stage and UICC stage before treatment. The mean of the SUVmax in the CR patient group was 10.6 (95% CI, 8.0–13.3) and that in the non-CR group was 17.6 (95% CI, 14.7–20.5) (Table 3). The SUVmax of the CR patients was significantly less than that of the non-CR patients (P = 0.0002).
Table 2.
The correlation between patient characteristics before treatment and the SUVmax in FDG-PET of primary tumor
| Characteristics | Number of patients | SUVmax of primary tumor |
P-value | |
|---|---|---|---|---|
| Mean | 95% C.I. | |||
| Age | ||||
| <70 years | 32 | 13.3 | 10.4–16.2 | 0.53 |
| ≥70 years | 24 | 14.7 | 10.4–17.5 | |
| Gender | ||||
| male | 49 | 13.3 | 7.2–19.4 | 0.84 |
| female | 7 | 14.0 | 11.6–16.2 | |
| Tumor length | ||||
| <50 mm | 30 | 10.8 | 8.0–13.4 | 0.001* |
| ≥50 mm | 26 | 17.5 | 14.7–20.4 | |
| T stage | ||||
| T1–2 | 18 | 6.2 | 3.3–9.0 | <0.0001* |
| T3–4 | 38 | 17.5 | 15.6–19.5 | |
| N stage | ||||
| N0 | 18 | 8.2 | 4.9–11.5 | 0.0001* |
| N1 | 38 | 16.6 | 14.3–18.9 | |
| UICC stage | ||||
| I, II | 21 | 6.4 | 4.0–8.9 | <0.0001* |
| III, IV | 35 | 18.4 | 16.5–20.2 | |
C.I. = confidence interval.
Table 3.
The correlation between treatment response and the value of SUVmax
| Response | Number of patients | SUVmax of primary tumor |
P-value | |
|---|---|---|---|---|
| Mean | 95% C.I. | |||
| CR | 30 | 10.6 | 8.0–13.3 | 0.0008* |
| Non-CR | 26 | 17.6 | 14.7–20.5 | |
C.I. = confidence interval, CR = complete response.
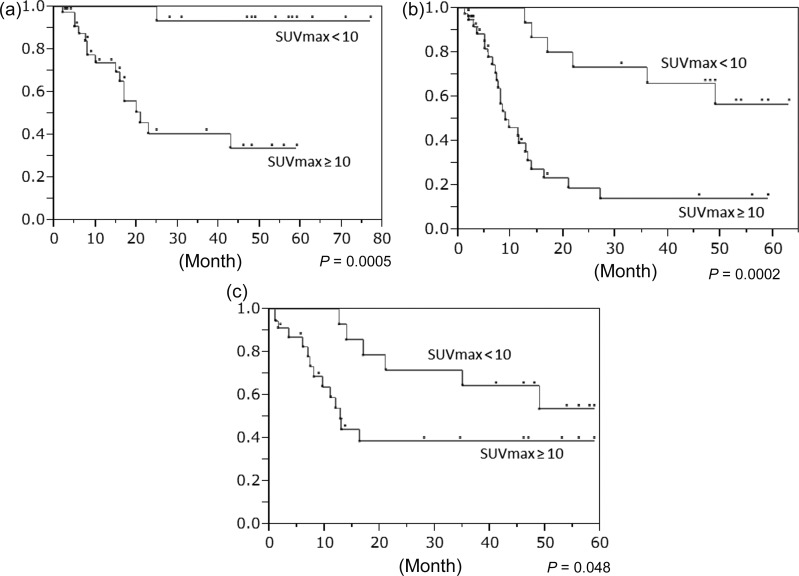
In response to this result, we divided the 56 patients into two groups according to their SUVmax values: <10 (low-SUV) and ≥10 (high-SUV) using a ROC analysis; in the low- and high-SUV groups, T1–2 patients were 15 and 3, and T3–4 patients were 3 and 35. In the low- and high-SUV groups, the 2-year OS, rates were 100% and 41%, the PFS rates were 73% and 19%, the LC rates were 71% and 39%, and the CR rates were 100% and 32%, respectively (Fig. 2a–c) and Table 4). The results of the univariate analysis are shown in Table 5: significant differences were revealed between the low- and high-SUV groups in OS, PFS, LC and response (P = 0.0005, 0.0002, 0.048, and <0.0001, respectively). SUVmax and T stage were significantly associated with OS, PFS, LC and response. The results of the multivariate analysis are shown in Table 6: SUVmax was significantly associated with both OS and response (P = 0.03, 0.0052) and was not associated with PFS or LC. Other characteristics were not associated with OS, PFS, LC or response.
Fig. 2.
Comparison of the survival and local control of the patient groups with SUVmax values <10 and ≥10. (a) Overall survival (OS) probabilities. (b) Progression-free survival (PFS) probabilities. (c) Local control (LC) probabilities.
Table 4.
The comparison between the groups of low- and high-SUV the group in response
| SUVmax | Number of patients | CR | Non-CR | CR rate (%) | P-value |
|---|---|---|---|---|---|
| <10 | 18 | 18 | 0 | 100 | <0.0001 |
| ≥10 | 38 | 12 | 26 | 32 |
C.I. = confidence interval.
Table 5.
Result of univariate analysis for the correlation with treatment outcome
| Characteristics |
P-value |
||||
|---|---|---|---|---|---|
| OS | PFS | LC | Response | ||
| Age | <70/70 ≤ | 0.25 | 0.77 | 0.56 | 0.24 |
| Gender | male/female | 0.26 | 0.80 | 0.82 | 0.54 |
| SUVmax | <10/10 ≤ | 0.0005* | 0.0002* | 0.048* | <0.0001* |
| Tumor length | <50 mm/50 mm ≤ | 0.45 | 0.55 | 0.96 | 0.034* |
| T stage | T1–2/T3–4 | 0.0016* | 0.0003* | 0.011* | <0.0001* |
| N stage | N0/N1 | 0.015* | 0.002* | 0.056 | 0.0014* |
OS = overall survival, PFS = progression free survival, LC = local control, Response = response of the primary tumor.
Table 6.
Result of multivariate analysis for the correlation with treatment outcome
| Characteristics |
P-value |
||||
|---|---|---|---|---|---|
| OS | PFS | LC | Response | ||
| Age | <70/70 ≤ | 0.14 | 0.82 | 0.56 | 0.36 |
| Gender | male/female | 0.28 | 0.97 | 0.56 | 0.79 |
| SUVmax | <10/10 ≤ | 0.03* | 0.17 | 0.73 | 0.0052* |
| Tumor length | <50 mm/50 mm ≤ | 0.85 | 0.46 | 0.38 | 0.61 |
| T stage | T1–2/T3–4 | 0.10 | 0.07 | 0.06 | 0.24 |
| N stage | N0/N1 | 0.52 | 0.77 | 0.58 | 0.72 |
OS = overall survival, PFS = progression free survival, LC = local control, Response = response of the primary tumor.
DISCUSSION
Definitive CRT is currently used for the treatment of locoregional esophageal cancer, as well as in conjunction with surgical resection and endoscopic resection [12]. In the Japan Clinical Oncology Group (JCOG) 9906 trial, the 5-year survival rate of resectable esophageal cancer treated with definitive CRT was 36.4% [13]. In the JCOG 9907 trial, the 5-year survival rates of the patients treated by surgical resection combined with preoperative and postoperative chemotherapy were 60.1% and 38.4%, respectively [14].
In light of the results of the JCOG trials, surgical resection combined with neoadjuvant chemotherapy has been a standard treatment for locoregional resectable esophageal cancer in Japan. However, Stahl et al. reported the results of a randomized trial in Germany, in which it seems that CRT alone offers equivalent survival to CRT followed by surgery [1]. In 172 patients with esophageal cancer treated with CRT with and without additional surgery, the median survival times were 16.4 months and 14.9 months and the 2-year survival rates were 39.9% and 35.4%, respectively. In a French multicenter trial, Fédération Francophone de Cancérologie Digestive (FFCD) 9102, neoadjuvant CRT of 40 Gy with surgery and definitive CRT of 60 Gy without surgery had the same impact on survival and quality of life for responders [2]. This suggests that CRT, which can preserve organ function, is equally as effective as surgery for responders. In light of these findings, it may be desirable to choose an appropriate treatment option on an individual patient basis and to identify responders to CRT, who might then avoid surgery and its risk. In our study, the SUVmax values of the CR patients were significantly lower than those of the non-CR patients, and all 18 patients in the low-SUV group (SUVmax < 10) had a CR. These results suggest that the SUVmax could be useful for identifying responders.
It is well known that malignant cells have an altered metabolic activity with increased uptake of FDG, and thus FDG-PET can provide better assessments of active primary tumors or significant metastases. In our study, the SUVmax of the primary tumor was significantly correlated with tumor length, T stage, N stage and UICC stage before treatment. In clinical settings, FDG-PET has commonly been used as an essential element in initial staging to exclude metastases in otherwise apparently localized cancer, and in continuing assessments after therapy for esophageal cancer.
Many studies have indicated the usefulness of FDG-PET for predicting the prognosis of patients, or the response to CRT and/or surgery for esophageal cancer [15–23]. These reports suggest that a higher SUV is associated with poorer prognosis or response in esophageal cancer patients, and that the SUV has a prognostic value in esophageal cancer patients treated with CRT and/or surgery. However, to our knowledge, there are few such studies of Japanese esophageal cancer patients. Suzuki et al. reported that the initial SUV is an independent prognostic variable for OS in a multivariate analysis in the USA [15]. In our present study, the high-SUV group (SUVmax ≥ 10) had poorer prognoses in OS, PFS, LC and response. The SUVmax could be an independent prognostic variable for OS and response in a multivariate analysis. Our results agree with those obtained by the above-mentioned studies, and they suggest that the SUVmax for esophageal cancer treated by definitive CRT in Japan is similar to that in other countries' populations. In our present study, recurrences or distant metastases were identified by esophagoscopy, barium esophagography, CT or PET/CT. Because of the retrospective nature of our study, it was difficult to obtain sufficient evaluations for several patients with a low performance status or bad general condition, and thus the SUVmax may not be significantly associated with PFS and LC in a multivariate analysis. In our study, tumor length, T stage and N stage did not reach statistical significance in multivariate analysis. They are simply morphological information that have been estimated from esophagoscopy, barium esophagography and CT. On the other hand, the SUVmax of the primary tumor in FDG-PET provides information about glucose metabolism and is thought to reflect the biological viability of the tumor. Additionally, the ROC curve, which was used to determine the optimal cut-off value of SUVmax, was generated to assess the relationship of SUVmax to tumor response, and thus whether the SUVmax may be more associated with OS and tumor response than the other factors.
The SUV as a semiquantitative parameter of glucose uptake can be affected by various factors such as patient size, ROI definition, the partial-volume effect, image resolution, reconstruction methods, noise, time between tracer injection and imaging, attenuation correction, normalization factor, and plasma glucose level, among others. In our study, a cutoff SUV of 10 was defined according to the ROC analysis of patient responses. In other studies, various cut-off SUV values have been used [15–23]. Because of differences in patient characteristics, the use of different sets of protocols and many other factors such as those described above, it is difficult to define an arbitrary cutoff value. To identify an appropriate cutoff value for predicting prognosis or response, each institution using PET should carefully consider several factors and conditions.
The SUVmax is considered the simplest and the most widely accepted functional biomarker derived from FDG-PET. Several parameters other than pretreatment SUVmax from FDG-PET with potential prognostic value have been reported. Some researchers suggested that a metabolic decrease in the FDG uptake of the tumor between pre- and post-CRT is correlated with a histopathologic response of the tumor [18, 23–25], but other studies did not find a significant correlation between a decrease in the SUV and response to treatment [26, 27]. The difficulty of differentiating radiation esophagitis or other radiation-induced inflammatory changes from residual esophageal cancer by FDG-PET after radiation therapy has been noted in some reports [26, 28, 29].
The best timing for FDG-PET after CRT for primary esophageal cancer is not yet known. In our study, SUVmax from pre-treatment FDG-PET was significantly associated with OS and response, which are not affected by other factors such as esophagitis. In other studies, volumetric parameters from FDG-PET such as the metabolic tumor volume or diameter have been proposed as more valuable for predicting prognosis or tumor response than SUVmax for the primary tumor in patients with esophageal cancer [30, 31]. The metabolic tumor volume and diameter are volumetric or quantitative measurements of tumor cells with high glycolytic activity. However, the volumetric parameters of FDG-PET have not yet been established. This report suggests that recently developed and commercially available volumetric analysis tools provide automatic ROI with an isocontour threshold method, making volumetric measurements applicable to routine practice. Further study is required before volumetric parameters are widely accepted.
CONCLUSION
In conclusion, the results of our study suggest that the SUVmax from FDG-PET of primary tumors is significantly associated with OS, PFS, LC and response. For OS and response, SUVmax is an independent prognostic variable. An SUVmax from FDG-PET of the primary tumor may have prognostic value regarding definitive CRT for esophageal cancer. For patients with higher SUV values, more aggressive adjuvant treatments should be considered.
FUNDING
This work was supported by Grants-in-aid for Scientific Research (24791313) from Japan Society for the promotion of Science.
REFERENCES
- 1.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–7. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 2.Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 3.Strauss LG, Clorius JH, Schlag P, et al. Recurrence of colorectal tumors: PET evaluation. Radiology. 1989;170:329–32. doi: 10.1148/radiology.170.2.2783494. [DOI] [PubMed] [Google Scholar]
- 4.Lapela M, Grenman R, Kurki T, et al. Head and neck cancer: detection of recurrence with PET and 2-[18F]-fluoro-2-deoxy-D-glucose. Radiology. 1995;197:135–9. doi: 10.1148/radiology.197.1.7568825. [DOI] [PubMed] [Google Scholar]
- 5.Crippa F, Agresti R, Seregni E, et al. Prospective evaluation of fluorine-18-FDG PET in presurgical staging of the axilla in breast cancer. J Nucl Med. 1998;39:4–8. [PubMed] [Google Scholar]
- 6.Silvestri GA, Tanoue LT, Margolis ML, et al. American College of Chest Physicians. The noninvasive staging of non-small cell lung cancer: the guidelines. Chest. 2003;123:147–56. doi: 10.1378/chest.123.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan FL, Dehdashti F, Siegel BA, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. Am J Roentgenol. 1997;168:417–24. doi: 10.2214/ajr.168.2.9016218. [DOI] [PubMed] [Google Scholar]
- 8.Couper GW, McAteer D, Wallis F, et al. Detection of response to chemotherapy using positron emission tomography in patients with oesophageal and gastric cancer. Br J Surg. 1998;85:1403–6. doi: 10.1046/j.1365-2168.1998.00963.x. [DOI] [PubMed] [Google Scholar]
- 9.Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–85. doi: 10.1002/cncr.20585. [DOI] [PubMed] [Google Scholar]
- 10.International Union Against Cancer. TNM Classification of Malignant Tumors. 6th edn. NewYork: Springer-Verlag; 2002. [Google Scholar]
- 11.Japanese Society for Esophageal Disease. Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus. 8th edn. Tokyo: Kanehara; 1992. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura Y, Mitsumori M, Hiraoka M, et al. A randomized phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer: short-term infusion versus protracted infusion chemotherapy (KROSG0101/JROSG021) Radiother Oncol. 2009;92:260–5. doi: 10.1016/j.radonc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II–III esophageal squamous cell carcinoma: JCOG Trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81:684–90. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Xiao L, Hayashi Y, et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer. 2011;117:4823–33. doi: 10.1002/cncr.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato H, Nakajima M, Sohda M, et al. The clinical application of 18-F-fluorodeoxyglucose positron emission tomography to predict survival in patients with operable esophageal cancer. Cancer. 2009;115:3196–203. doi: 10.1002/cncr.24399. [DOI] [PubMed] [Google Scholar]
- 17.Hong D, Lunagomez S, Kim EE, et al. Value of baseline positron emission tomography for predicting overall survival in patient with nonmetastatic esophageal or gastroesophageal junction carcinoma. Cancer. 2005;104:1620–6. doi: 10.1002/cncr.21356. [DOI] [PubMed] [Google Scholar]
- 18.Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–60. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Konski AA, Cheng JD, Goldberg M, et al. Correlation of molecular response as measured by 18-FDG positron emission tomography with outcome after chemoradiotherapy in patients with esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:358–63. doi: 10.1016/j.ijrobp.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara R, Yamamoto S, Iishi H, et al. Predicting the effects of chemoradiotherapy for squamous cell carcinoma of the esophagus by induction chemotherapy response assessed by positron emission tomography: toward PET-response-guided selection of chemoradiotherapy or esophagectomy. Int J Clin Oncol. 2012;17:225–32. doi: 10.1007/s10147-011-0278-3. [DOI] [PubMed] [Google Scholar]
- 21.Fukunaga T, Okazumi S, Koide Y, et al. Evaluation of esophageal cancers using fluorine-18-fluorodeoxyglucose PET. J Nucl Med. 1998;39:1002–7. [PubMed] [Google Scholar]
- 22.Yasuda T, Higuchi I, Yano M, et al. The impact of (18)F-fluorodeoxyglucose positron emission tomography positive lymph nodes on postoperative recurrence and survival in resectable thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19:652–60. doi: 10.1245/s10434-011-1928-4. [DOI] [PubMed] [Google Scholar]
- 23.Wieder HA, Beer AJ, Lordick F, et al. Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med. 2005;46:2029–34. [PubMed] [Google Scholar]
- 24.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;80:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 26.Song SY, Kim JH, Ryu JS, et al. FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1053–9. doi: 10.1016/j.ijrobp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Brink I, Hentschel M, Bley TA, et al. Effects of neoadjuvant radio-chemotherapy on 18F-FDG-PET in esophageal carcinoma. Eur J Surg Oncol. 2004;30:544–50. doi: 10.1016/j.ejso.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Flamen P, Van Cutsem E, Lerut A, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13:361–8. doi: 10.1093/annonc/mdf081. [DOI] [PubMed] [Google Scholar]
- 29.Wieder HA, Brücher BL, Zimmerman F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–8. doi: 10.1200/JCO.2004.07.122. [DOI] [PubMed] [Google Scholar]
- 30.Roedl JB, Halpern EF, Colen RR, et al. Metabolic tumor width parameters as determined on PET/CT predict disease-free survival and treatment response in squamous cell carcinoma of the esophagus. Mol Imaging Biol. 2009;11:54–60. doi: 10.1007/s11307-008-0169-9. [DOI] [PubMed] [Google Scholar]
- 31.Hyun SH, Choi JY, Shim YM, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–22. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]