Abstract
The purpose of this study is to examine risk factors for late rectal toxicity for localized prostate cancer patients treated with helical tomotherapy (HT). The patient cohort of this retrospective study was composed of 241 patients treated with HT and followed up regularly. Toxicity levels were scored according to the Radiation Therapy Oncology Group grading scale. The clinical and dosimetric potential factors increasing the risk of late rectal toxicity, such as age, diabetes, anticoagulants, prior abdominal surgery, prescribed dose, maximum dose of the rectum, and the percentage of the rectum covered by 70 Gy (V70), 60 Gy (V60), 40 Gy (V40) and 20 Gy (V20) were compared between ≤ Grade 1 and ≥ Grade 2 toxicity groups using the Student's t-test. Multivariable logistic regression analysis of the factors that appeared to be associated with the risk of late rectal toxicity (as determined by the Student's t-test) was performed. The median follow-up time was 35 months. Late Grade 2–3 rectal toxicity was observed in 18 patients (7.4%). Age, the maximum dose of the rectum, V70 and V60 of the ≥ Grade 2 toxicity group were significantly higher than in those of the ≤ Grade 1 toxicity group (P = 0.00093, 0.048, 0.0030 and 0.0021, respectively). No factor was significant in the multivariable analysis. The result of this study indicates that the risk of late rectal toxicity correlates with the rectal volume exposed to high doses of HT for localized prostate cancer. Further follow-up and data accumulation may establish dose–volume modeling to predict rectal complications after HT.
Keywords: prostate cancer, helical tomotherapy, late toxicity, intensity-modulated radiation therapy, image-guided radiation therapy
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) has been shown to reduce late rectal toxicity in high-dose external beam radiation therapy (EBRT) for prostate cancer [1], but essential issues remain to be solved. Factors increasing the risk of late rectal toxicity include not only the prescribed dose and radiation technique delivering the dose, but also some clinical characteristics. Major factors reportedly associated with rectal complication risks include diabetes mellitus [1, 2], advanced age [3], androgen deprivation therapy (ADT) [4], rectum size [5], and prior abdominal surgery [6]. In addition, acute rectal toxicity is now recognized to be associated with an increased risk of developing late rectal complications [7]. Rectum volumes at especially high-dose areas on the dose–volume histogram (DVH) also have an impact on late rectal toxicity. The following dose–volume constraints are provided as a conservative starting point for 3-dimensional conformal radiotherapy (3DCRT): V50 < 50%, V60 < 35%, V65 < 25%, V70 < 20%, and V75 < 15% [8], which have been derived from some 3DCRT experiences. However, such conventional dose–volume constraints may not be valuable in current clinical practices because the significance of IMRT has already been established in EBRT for localized prostate cancer [9]. IMRT planning yields DVH curves in distinctly different shapes from those of forward-planned 3DCRT. In fact, the ratio of IMRT vs 3DCRT increased from 0.15% in the year 2000 to 95.9% in the year 2008 in the United States [10]. The significance of image-guided radiation therapy (IGRT) has also been established in this category [9]. Thus, dose–volume modeling derived from non-image guided 3DCRT may inevitably be modified to predict complications derived from image-guided IMRT (IG-IMRT). Data are, however, still too poor or insufficient to address dose–volume constraints in this modern combination technique.
Helical tomotherapy (HT, TomoTherapy, Madison, WI) is a form of IMRT, and detectors within the tomotherapy system provide megavolt–age computed tomographic (MVCT) images of patients, which can be obtained immediately before processes for setup, registration, and repositioning (i.e. IGRT). Next, we examined the impact of patient clinical characteristics and DVH parameters on late rectal toxicity after HT treatment for non-metastatic prostate cancer. We report the results of the examinations. It is of particular interest to describe dose–volume modeling to predict rectal complications after HT.
MATERIALS AND METHODS
Patients and treatment methods
A total of 241 consecutive patients clinically diagnosed with non-metastatic prostate cancer, who were treated with HT between June 2006 and December 2010 and followed up regularly at our institution, were enrolled in this study. Written informed consent for the treatment and an anonymous data application were obtained from each of the patients before the treatment. Pretreatment evaluations, androgen deprivation therapy (ADT), and HT treatment were described circumstantially in our previous study [11]. In brief, the clinical target volume (CTV) was defined as the entire prostate and the proximal seminal vesicle. The planning target volume 1 (PTV1) included the CTV with a 6–8 mm margin except for the prostatorectal interface, where a 4–6 mm margin was used. Outside PTV1, PTV2 was defined as the seminal vesicle with a similar margin to that of PTV1. By our definition, only the rectum around the PTV1 area with a cranio-caudal 10-mm margin is delineated as an organ at risk. Prescribed doses were PTV1 D95 (i.e. dose delivered to 95% of PTV1): 74 Gy in the low-risk group, 78 Gy in the intermediate- and high-risk groups, and PTV2 D95: 64 Gy in all of the risk groups. Patients had a tube inserted or were encouraged to defecate when their rectums were dilated on daily MVCT, and were checked on MVCT again.
Follow-up evaluations and data collection
Follow-up evaluations after the treatment were performed at 3-month intervals. Toxicity levels were scored according to the Radiation Therapy Oncology Group (RTOG) morbidity grading scale [12]. In brief, Grade 1 toxicity represents minimal side effects not requiring medication for symptom control; Grade 2 toxicity indicates symptoms requiring medication; Grade 3 indicates complications requiring minor surgical intervention (i.e. laser coagulation); and Grade 4 requires hospitalization and major intervention. The time until the occurrence of late toxicity was represented as the period from the start date of HT.
Patient characteristics (e.g. age, T-stage, diabetes mellitus, anticoagulants, and history of abdominal surgery) and DVH parameters (prescribed dose, PTV volume, rectal volume, mean dose of the rectum, maximum dose of the rectum, the percentage of the rectum at least covered by 70 Gy [V70], 60 Gy [V60], 40 Gy [V40], or 20 Gy [V20]) were collected from the patients on their initial visits to our departments. Total ADT time and acute and late rectal toxicities were reviewed on the patients' charts in the analysis. The prescribed dose on the DVH and the practically delivered dose varied from one another in seven of the patients, because of HT cessation for a range of reasons such as acute rectal symptoms. Practically delivered doses were 74 Gy in six of the patients and 70 Gy in one patient, despite the prescribed dose of 78 Gy on the DVH. In these patients, the prescribed dose, the mean dose of the rectum, the maximum dose of the rectum, V70, V60, V40, and V20 were approximately shown by these values on the DVH × practically delivered dose (70 or 74 Gy)/prescribed dose on the DVH (78 Gy) in this analysis. Table 1 shows patient characteristics and DVH parameters for this patient cohort.
Table 1.
Patient characteristics and DVH parameters
| Characteristic | Total | (n = 241) |
|---|---|---|
| Age (years) | 69 | (49–81) |
| PSA level (ng/ml) | 15.17 | (1.40–502.00) |
| Gleason score | 7 | (5–10) |
| Tumor stage | ||
| T1–T2 | 109 | (45.2%) |
| T3–T4 | 132 | (54.8%) |
| Risk group | ||
| Low | 17 | (7.0%) |
| Intermediate | 53 | (22.0%) |
| High | 171 | (71.0%) |
| Diabetes (%) | 23 | (9.5%) |
| Anticoagulants (%) | 41 | (17.0%) |
| Abdominal surgery (%) | 21 | (9.4%) |
| ADT (month) | 27 | (4–92) |
| ≥ Grade 2 acute toxicity (%) | 27 | (11.2%) |
| PTV volume (cc) | 59.0 | (20.7–190.9) |
| Rectum volume (cc) | 41.9 | (21.8–113.7) |
| Prescribed dose (Gy) | 78.0 | (70.0–78.0) |
| Rectum mean dose (Gy) | 38.8 | (27.0–46.4) |
| Rectum max dose (Gy) | 80.2 | (70.1–83.8) |
| V70 (%) | 7.2 | (0.1–13.6) |
| V60 (%) | 15.5 | (1.9–25.6) |
| V40 (%) | 38.1 | (20.0–77.8) |
| V20 (%) | 90.0 | (45.0–100.0) |
DVH = dose-volume histogram, ADT = androgen deprivation therapy, V dose = the percentage of the rectum at least covered by each dose; PTV = planning target volume. Age, PSA, ADT and DVH parameters are represented as mean and ranges.
Statistical analyses
The impact of clinical and dosimetric factors on Grade 2 or higher late rectal toxicity was analyzed. The clinical and dosimetric potential factors increasing the risk of late rectal toxicity were compared between the ≤ Grade 1 and the ≥ Grade 2 toxicity groups and were then analyzed by the Student's t-test. The following factors were examined: the patient characteristics described above, total ADT time, the presence of Grade 2 or higher acute rectal toxicity, and the DVH parameters described above. Multivariable logistic regression analysis was carried out for the factors that previously appeared to be associated with the risk of late rectal toxicity by the Student's t-test (P < 0.10). Significance was determined at a P value of <0.05.
RESULTS
Late rectal toxicity
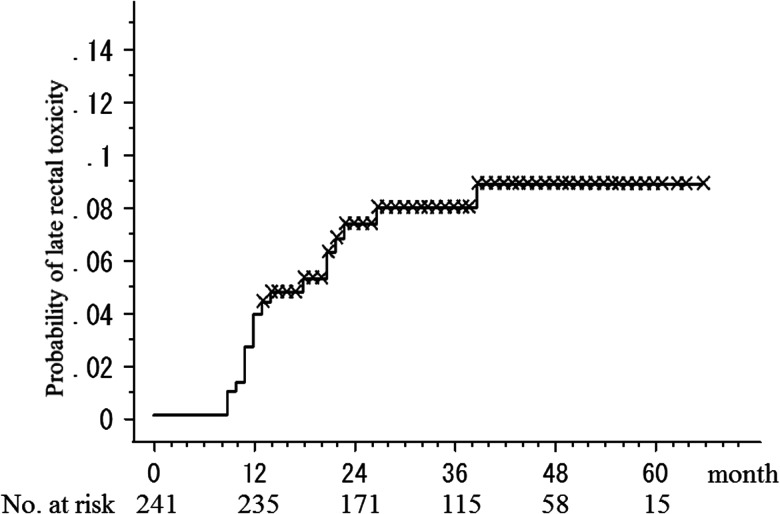
The median follow-up time from the start of HT was 35 months (range, 13–66 months). Rectal toxicity has been described in detail in our previous study [11]. Briefly, 18 (7.4%) of the patients developed late Grade 2 or 3 rectal toxicity. Of the 16 patients (6.6%) who developed late Grade 2 rectal toxicity, 13 developed Grade 2 rectal bleeding. Other Grade 2 symptoms were pain on defecation in two of the patients and subtle fecal incontinence in one of the patients. Two patients (0.8%) developed Grade 3 rectal bleeding requiring laser coagulation. No Grade 4 late rectal complications were observed. Figure 1 shows the rate of developing late Grade 2 or higher rectal toxicity in the time course after HT.
Fig. 1.
The rate of developing late ≥ Grade 2 rectal toxicity after helical tomotherapy.
Analysis of risk factors associated with late rectal toxicity
Table 2 shows the effects of patient characteristics and DVH parameters on Grade 2 or higher late rectal toxicity as analyzed by the Student's t-test. Age, maximum dose of the rectum, V70, and V60 were significantly variable between the ≤ Grade 1 and the ≥ Grade 2 toxicity groups as analyzed by the Student's t-test (P = 0.00093, 0.048, 0.0030 and 0.0021, respectively). To further evaluate the independent effects of the factors that displayed a P-value <0.10 by the Student's t-test, such as age, anticoagulants, the maximum dose of the rectum, V70 and V60 on ≥ Grade 2 late rectal toxicity, a multivariable logistic regression analysis was performed. None of the factors were found to be significantly correlated by this analysis, as shown in Table 3.
Table 2.
The effects of patient characteristics and DVH parameters on ≥ Grade 2 late rectal toxicity after helical tomotherapy, as analyzed by the Student's t-test
| Characteristic | ≤ Grade 1 (n = 223) | ≥ Grade 2 (n = 18) | P-value |
|---|---|---|---|
| Age (years) | 68.5 ± 6.1 | 71.2 ± 4.2 | 0.0093* |
| Tumor stage (≥T3) | 55.2% | 50.0% | 0.34 |
| Diabetes (%) | 9.4% | 11.1% | 0.42 |
| Anticoagulants (%) | 15.7% | 33.3% | 0.074 |
| Abdominal surgery (%) | 9.0% | 5.6% | 0.28 |
| ADT (≥27 months) | 48.4% | 27.8% | 0.26 |
| Acute toxicity (%) | 11.7% | 5.6% | 0.11 |
| PTV volume (cc) | 62.1 ± 22.9 | 66.6 ± 18.3 | 0.17 |
| Rectum volume (cc) | 44.5 ± 13.9 | 42.1 ± 16.4 | 0.28 |
| Prescribed dose (Gy) | 77.5 ± 1.4 | 77.3 ± 1.5 | 0.28 |
| Rectum mean dose (Gy) | 39.2 ± 5.0 | 38.6 ± 3.5 | 0.27 |
| Rectum max dose (Gy) | 79.2 ± 3.3 | 80.1 ± 2.0 | 0.048* |
| V70 (%) | 6.8 ± 3.5 | 9.0 ± 2.9 | 0.0030* |
| V60 (%) | 14.8 ± 5.0 | 17.6 ± 3.5 | 0.0021* |
| V40 (%) | 39.9 ± 8.7 | 39.5 ± 8.4 | 0.43 |
| V20 (%) | 87.1 ± 10.1 | 84.7 ± 10.3 | 0.18 |
DVH = dose-volume histogram, ADT = androgen deprivation therapy, V dose = the percentage of the rectum at least covered by each dose. Age and DVH parameters are represented as mean ± SD. *Statistically significant.
Table 3.
The effects of patient characteristics and DVH parameters on ≥ Grade 2 late rectal toxicity after helical tomotherapy, as analyzed by multivariable logistic regression analysis
| Characteristic | P-value | Hazard ratio (CI) |
|---|---|---|
| Age (years) | 0.10 | 1.08 (0.99–1.19) |
| Anticoagulants (%) | 0.12 | 2.18 (0.81–5.88) |
| Rectum max dose (Gy) | 0.87 | 0.99 (0.82–1.19) |
| V70 (%) | 0.16 | 1.30 (0.91–1.85) |
| V60 (%) | 0.85 | 0.98 (0.78–1.23) |
DVH = dose–volume histogram, ADT = androgen deprivation therapy, V dose = the percentage of the rectum at least covered by each dose, CI = 95% confidence interval, NA = not applicable.
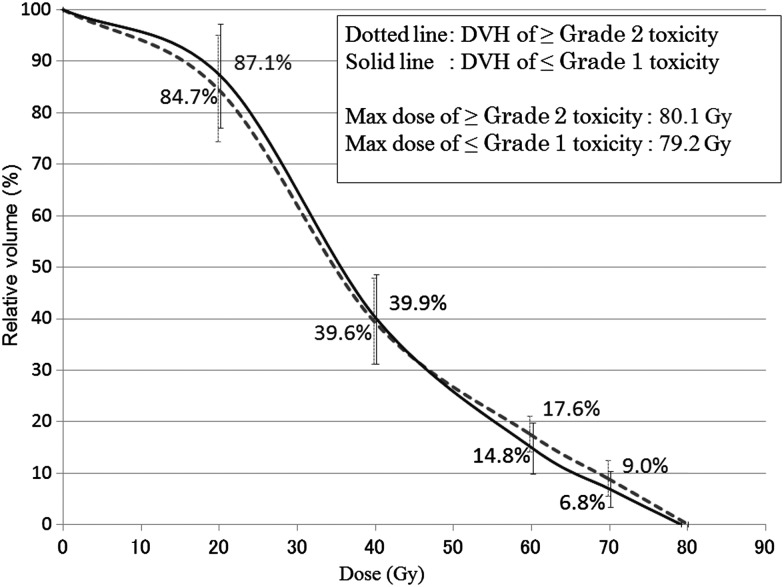
Figure 2 shows the mean DVH and standard deviations (SD) of patients with or without Grade 2 or higher late rectal toxicity after HT. The maximum dose of the rectum, V70, V60, V40 and V20 for patients with ≥ Grade 2 late rectal toxicity vs those with ≤ Grade 1 late rectal toxicity were 80.1 ± 2.0 Gy vs 79.2 ± 3.3 Gy, 9.0 ± 2.9% vs 6.8 ± 3.5%, 17.6 ± 3.5% vs 14.8 ± 5.0%, 39.6 ± 8.4% vs 39.9 ± 8.7%, and 84.7 ± 10.3% vs 87.1 ± 10.1%, respectively.
Fig. 2.
The mean dose–volume histograms and standard deviations (SD) of patients with or without ≥ Grade 2 late rectal toxicity after helical tomotherapy.
DISCUSSION
The DVH curves of IMRT are distinctly different from those of forward-planned 3DCRT. The combined use of IGRT may also have a possible impact on the DVH difference between IGRT and non-IGRT treatments because significant margin reduction between the prostate and PTV could be implemented clinically with the combined use of IGRT [13]. The results of the present study indicate that the risk of late rectal toxicity correlates with the rectal volume exposed to high doses in the HT treatment (i.e. IG-IMRT) for localized prostate cancer, although there were no significant factors in the multivariable logistic regression analysis. This suggestion is consistent with other reports derived from the 3DCRT data. Kuban et al. assessed the impact of 70 Gy vs 78 Gy doses on gastro-intestinal (GI) toxicity in 301 patients treated with 3DCRT. After a median follow-up period of 8.7 years, GI toxicity more severe than RTOG Grade 2 was often observed in high-dose patients (28% vs 15%; P = 0.013). DVH analysis showed that the incidence of complications could be significantly decreased by reducing the volume of the treated rectum. When <25% of the rectum was treated with >70 Gy, the Grade 2-or-greater complication incidence at 6 years post-treatment was much reduced, 16% as compared with 46% when this dose–volume cutoff point was exceeded [14]. Tucker et al. also analyzed DVH data from 1009 patients treated with 3DCRT on RTOG protocol 94-06. In these data, no evidence was found of any influence of the intermediate doses on the risk of ≥ Grade 2 late rectal toxicities. The critical dose for this endpoint seemed to be ≥75 Gy [15]. The results of our present study suggests that patients with advanced age are at risk of rectal complication. The routine medication of anticoagulants may be also associated with rectal bleeding, as shown in Table 2 and 3. These results are in line with the reports of Skwarchuk et al. [3] and Pederson et al. [16]. Even when optimal dose–volume constraints are applied, rectal complications can still occur due to clinical factors such as anticoagulant medications or advanced age.
Most of the mature published clinical data on dose-related rectal toxicity originate from 3DCRT. Some data derived from 3DCRT experiences recommended V60 < 35% and V70 < 20% as a conservative starting point for the dose–volume constraints for 3DCRT [8]. As shown in Table 1, the mean values of V70 and V60 were 7.2 (range, 0.1–13.6) and 15.5 (range, 1.9–25.6), respectively, in this patient cohort treated with HT. Although these values fulfill the terms of the conventional dose–volume constraints described above, we observed late Grade 2 or 3 rectal toxicities in 7.4% of the patients. This result indicates that tighter dose–volume constraints of the rectum would be necessary for IG-IMRT than the conventional constraints derived from the clinical data of 3DCRT. On the other hand, caution should be taken in interpreting this result, because Fig. 2 simply shows the mean DVH with or without Grade 2 or higher late rectal toxicity. In fact, the SDs of patients with or without late rectal toxicity overlapped considerably at each of the doses, as shown in Fig. 2. Further follow-up and data accumulation are needed to evaluate the clinical significance of the small absolute difference in the high-dose areas. To our knowledge, only one study has investigated dosimetric risk factors for late rectal toxicity after IMRT. Pederson et al. have reported that the incidence of ≥ Grade 2 rectal toxicity was 5% in 296 consecutive patients treated with IMRT with a median follow-up period of 41 months [16]. They found that 100% of men with rectal V70 ≤ 10%, V65 ≤ 20%, and V40 ≤ 40% were free from ≥ Grade 2 rectal toxicity; 92% of men with rectal V70 ≤ 20%, V65 ≤ 40%, and V40 ≤ 80% as well as 85% of men exceeding these criteria were also free from the toxicity. The results of their study together with those of our study also suggest that more stringent dose–volume constraints are necessary for IMRT compared with 3DCRT.
The reliability of this study resides in the use of IGRT involving MVCT. The position of the rectum at the time of the treatment planning CT scan is likely not fully representative of the position during RT because of intrafraction variations in rectal filling, intestinal gas, and bladder filling. We think that these uncertainties have little influence on the present study because we checked these situations carefully in both the CT simulation and the pretreatment MVCT, and because patients had a tube inserted or were encouraged to defecate as necessary. On the other hand, two essential points need to be considered when interpreting the results of this study. Firstly, we need to define the rectum. This study has specified rectal lengths only around the PTV1 area with a cranio-caudal 10-mm margin. However, DVH studies so far have used variable definitions for the rectum [8, 16]. The rectosigmoid flexure is an uncertainty as the superior limit in determining where the rectum starts. The inferior limit has been variably defined as being at the level of the anal verge, the ischial tuberosities, or above the anus. Our definition of the rectum has been reasonably accepted so far among physicians. It is frequently contoured as a solid, and we have adopted this definition in our study. Secondly, we need to consider the problem of the diversity of the toxicity. We brought together all late rectal symptoms in the analyses of factors associated with late rectal toxicity, including some types of sequelae such as rectal bleeding, pain on defecation, and fecal incontinence. Refined knowledge of the location of dose maximums in combination with separate scoring and modeling of the different aspects of rectal toxicity clarifies specific anatomic regions of dose sensitivity [8]. However, the symptoms were mostly rectal bleeding in this study (15 of 18 patients who developed late Grade 2 or 3 rectal toxicity). We considered that lumping all rectal symptoms had little influence on the results of this study.
The treatment of rectal bleeding is also a critical issue in high-dose EBRT for prostate cancer. Takemoto et al. evaluated the results of the treatment for hemorrhagic proctitis after IMRT for prostate cancer [17]. Among 403 patients treated with IMRT, 64 developed late rectal bleeding with a median follow-up time of 35 months. Most patients were ameliorated with the steroid suppositories as medication, or even without any treatment, but one patient treated with steroid enemas for 12 months developed septic shock and died of multiple organ failure. All of the 12 patients treated with Argon plasma coagulation (APC) were ameliorated in that study. They concluded that steroid suppositories/enemas and APC were effective, although a short duration of the administration with an appropriate steroid dosage is recommended. We also treated patients developing rectal bleeding with steroid suppositories. Two of these patients showed no response to steroid suppositories so they then received APC. All patients with Grade 2 or 3 rectal bleeding got an improvement with steroid suppositories or APC in our present study, as in the report by Takemoto et al.
In conclusion, we have demonstrated the impact of patient clinical characteristics and DVH parameters on late rectal toxicity in a large number of non-metastatic prostate cancer patients after HT treatment. Late Grade 2–3 rectal toxicities were observed in 7.4% of the patients. The result of this study indicates that the risk of late rectal toxicity correlates with increase in age and the rectal volume exposed to high doses in HT treatment for localized prostate cancer. Further follow-up and data accumulation may establish dose–volume modeling to predict rectal complications after HT.
ACKNOWLEDGEMENTS
This study was presented at the 25th Annual Meeting of the Japanese Society for Therapeutic Radiology and Oncology.
REFERENCES
- 1.Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151–61. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Vavassori V, Fiorino C, Rancati T, et al. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: results of a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2007;67:1401–10. doi: 10.1016/j.ijrobp.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Skwarchuk MW, Jackson A, Zelefsky MJ, et al. Late rectal toxicity after conformal radiotherapy of prostate cancer (I): multivariate analysis and dose-response. Int J Radiat Oncol Biol Phys. 2000;47:103–13. doi: 10.1016/s0360-3016(99)00560-x. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Pickles T, Agranovich A, et al. Impact of neoadjuvant androgen ablation and other factors on late toxicity after external beam prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:59–67. doi: 10.1016/s0360-3016(03)00777-6. [DOI] [PubMed] [Google Scholar]
- 5.Wachter S, Gerstner N, Goldner G, et al. Rectal sequelae after conformal radiotherapy of prostate cancer: dose-volume histograms as predictive factors. Radiother Oncol. 2001;59:65–70. doi: 10.1016/s0167-8140(01)00281-x. [DOI] [PubMed] [Google Scholar]
- 6.Valdagni R, Vavassori V, Rancati T, et al. Increasing the risk of late rectal bleeding after high-dose radiotherapy for prostate cancer: the case of previous abdominal surgery. Results from a prospective trial. Radiother Oncol. 2012;103:252–5. doi: 10.1016/j.radonc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–9. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Michalski JM, Gay H, Jackson A, et al. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76 doi: 10.1016/j.ijrobp.2009.03.078. S123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer V1. 2011 doi: 10.6004/jnccn.2010.0012. http://www.nccn.org/ (10 April 2012, data last accessed) [DOI] [PubMed] [Google Scholar]
- 10.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita N, Soga N, Ogura Y, et al. Preliminary results of intensity-modulated radiation therapy with helical tomotherapy for prostate cancer. J Cancer Res Clin Oncol. 2012;138:1931–6. doi: 10.1007/s00432-012-1277-0. [DOI] [PubMed] [Google Scholar]
- 12.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 13.Crehange G, Mirjolet C, Gauthier M, et al. Clinical impact of margin reduction on late toxicity and short-term biochemical control for patients treated with daily on-line image guided IMRT for prostate cancer. Radiother Oncol. 2012;103:244–6. doi: 10.1016/j.radonc.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Tucker SL, Dong L, Michalski JM, et al. Do intermediate radiation doses contribute to late rectal toxicity? An analysis of data from radiation therapy oncology group protocol 94-06. Int J Radiat Oncol Biol Phys. 2012;84:390–5. doi: 10.1016/j.ijrobp.2011.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pederson AW, Fricano J, Correa D, et al. Late toxicity after intensity-modulated radiation therapy for localized prostate cancer: an exploration of dose-volume histogram parameters to limit genitourinary and gastrointestinal toxicity. Int J Radiat Oncol Biol Phys. 2012;82:235–41. doi: 10.1016/j.ijrobp.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 17.Takemoto S, Shibamoto Y, Ayakawa S, et al. Treatment and prognosis of patients with late rectal bleeding after intensity-modulated radiation therapy for prostate cancer. Radiat Oncol. 2012;7:87. doi: 10.1186/1748-717X-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]