Abstract
This study sought to investigate the clinical outcome and the role of postoperative radiotherapy for patients with salivary duct carcinoma (SDC) who had undergone surgery and postoperative radiotherapy. We performed a retrospective analysis of 25 SDC patients treated between 1998 and 2011 with surgery and postoperative radiotherapy. The median prescribed dose was 60 Gy (range, 49.5–61.4 Gy). The clinical target volume (CTV) was defined as the tumor bed in four patients, the tumor bed and ipsilateral neck in 14 patients, and the tumor bed and bilateral neck in six patients. Local control (LC), disease-free survival (DFS) and overall survival (OS) were estimated using the Kaplan-Meier method, and prognostic variables were analyzed with the log-rank test. The 5-year LC, DFS and OS were 67%, 45% and 47%, respectively. Disease recurrence was found in 12 patients: seven as local, four as regional and eight as distant failure. Perineural and lymphovascular invasion was a significant prognostic factor for LC (P = 0.03). Local failure was common, and the presence of local recurrence significantly affected the OS (P < 0.05). We conclude that surgery and postoperative radiotherapy is expected to decrease the risk of local failure and contribute to good prognoses for patients with SDC. It might be advisable to have the CTV include the cranial nerves involved and the corresponding parts of the skull base in cases of pathologically positive perineural invasion.
Keywords: salivary duct caricnoma, postoperative radiotherapy, perinerual invasion
INTRODUCTION
Salivary duct carcinoma (SDC) is a rare and highly malignant tumor of the salivary glands. Of all salivary gland tumors, SDC is associated with one of the poorest prognoses [1, 2]. Surgery is the mainstay of treatment for SDC; however, SDC is notorious for its high rate of locoregional and distant recurrences [3, 4]. The clinical and pathologic findings of SDC have been well described, and several authors recommend aggressive multimodal approaches such as adjuvant radiotherapy or chemoradiotherapy [5–7]. Nevertheless, there are only a limited number of reports about the efficacy of postoperative radiotherapy for SDC [8]. It has also been difficult to evaluate the role of adjuvant radiotherapy for SDC, because the incidence of SDC is low [9].
In this study, we investigated the clinical outcomes and demonstrated the role of postoperative radiotherapy in patients with SDC who had undergone surgery and postoperative radiotherapy.
MATERIALS AND METHODS
Patients
This is a retrospective study of patients with SDC treated at Kyushu University and the National Kyushu Cancer Center. Between July 1998 and November 2011, 25 patients underwent surgical resection with a curative intent without macroscopic residual disease, followed by postoperative radiotherapy. The type of surgery performed depended on the primary tumor site. The patient characteristics of the 25 cases of SDC are presented in Table 1. Six patients presented with facial nerve paresis. Regarding primary tumors, pT4 was the most common T class (n = 11, 44%). A total of 15 patients (n = 15, 60%) had cervical lymph node metastasis, and the majority of those patients (n = 14) had pN2b disease. Perineural and lymphovascular invasion were observed pathologically in seven and five patients.
Table 1.
Characteristics of the 25 patients with salivary duct carcinoma (SDC)
| Characteristics | |
|---|---|
| Gender (male: female) | 19:6 |
| Age: median (range) | 59 (36–82) |
| Site: | |
| Parotid gland | 21 |
| Submandibular gland | 4 |
| Pathological TNM stage: | |
| T1/T2/T3/T4 | 4/6/4/11 |
| N0/N1/N2b | 10/1/14 |
| pStage: | |
| I/II/III/IV | 2/3/1/19 |
| Surgical margin status: | |
| Positive/ close/ negative | 9/6/10 |
| Perineural invasion (yes/no) | 7/18 |
| Lymphovascular invasion (yes/no) | 5/20 |
| Neck dissection (yes/no) | 21/4 |
| Adjuvant treatment: | |
| Chemoradiotherapy | 22 |
| Radiotherapy | 3 |
Of the 21 patients with tumors of the parotid gland, 17 patients underwent total parotidectomy and four patients underwent partial parotidectomy. Four patients with tumors of the submandibular gland underwent total submandibular gland resection. The surgical margin was negative in 10 patients, close in six patients, and positive in nine patients. Planned neck dissection was performed in 21 patients. SDC was staged according to the TNM staging system for head and neck cancer (revisions for the 6th edition of the American Joint Committee on Cancer).
Radiotherapy
All 25 patients received postoperative radiotherapy. The median time from surgery to the start of adjuvant radiotherapy was 20 days (range, 7–69 days). The clinical target volume (CTV) included the surgical bed of the primary tumor and/or the involved node with appropriate margin in nine patients, and a prophylactic neck lymph node area as necessary in 16 patients. In the two patients with pathologically positive perineural invasion, the CTV included the base of the skull along the involved cranial nerve. The planning target volume (PTV) included the CTV with a 5-mm margin for possible positioning errors. The median prescribed dose to the surgical bed was 60 Gy (range, 49.5–61.4 Gy), and that to the prophylactic area was 40 Gy (range, 40–41.4 Gy). Conventional fractionation using a daily dose of 1.5–2.0 Gy was used in all patients. All 25 patients completed the scheduled radiotherapy. A total of 22 patients underwent concurrent chemotherapy. The regimen of concurrent chemotherapy was S-1 (Taiho Pharmaceutical Co., Tokyo, Japan) in 12 patients, 5-fluorouracil (5-FU) in two patients and cisplatin-based regimen in eight patients. There were 11 patients who received adjuvant chemotherapy using S-1 or cisplatin-based regimen after chemoradiotherapy.
Statistics
The overall survival (OS) was measured from the time of surgery to the last follow-up or death. Disease-free survival (DFS) was measured from the time of surgery to the date of recurrence, death from any cause, or last follow-up. Local control (LC) was measured from the time of surgery to the date of local recurrence or last follow-up. The rates of OS, DFS and LC were calculated according to the Kaplan-Meier method. To identify prognostic factors that might influence LC and survival, log-rank tests were performed to examine the univariate associations between LC and survival and parameters of interest, which included age, gender, pathologic stage, pathological T (pT) and pathological N (pN) classifications, and pathological features (perineural invasion, lymphovascular invasion, surgical margins). All statistical calculations were performed using statistical analysis software (JMP, version 8.0.2, SAS, Cary, NC, and Prism, version 5.0, GraphPad, San Diego, CA).
RESULTS
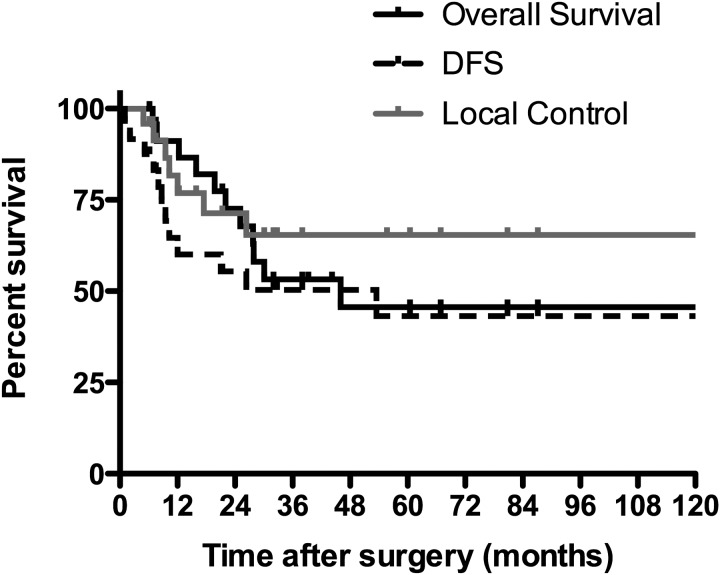
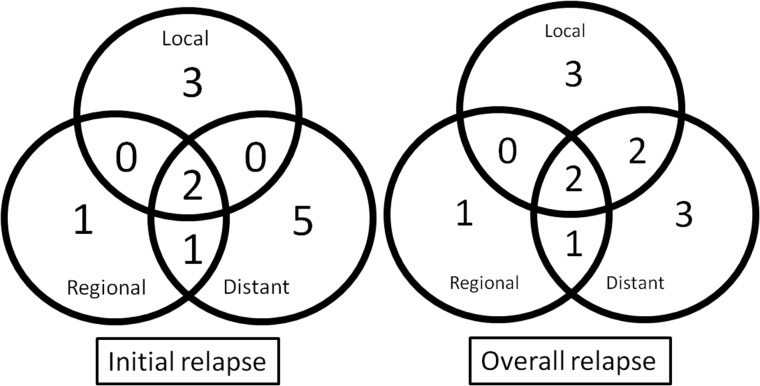
At the time of the analysis, 14 of the 25 patients were alive without disease and 11 patients had died of the disease. The median follow-up time for the survivors was 44 months (range, 5–127 months). The 5-year LC, locoregional control, DFS and OS were 67%, 54%, 45% and 47%, respectively (Fig. 1). Disease recurrence was found in 12 patients (48%). The sites of initial failure as well as overall failure are shown in Fig. 2. The common patterns of recurrence were both local and distant recurrences. Overall local recurrence occurred in seven patients (28%). Of the seven patients with local recurrence, five had pT1–3 tumors and two had pT4 tumors. The rates of local recurrence for pT1–3 and pT4 tumors were 36% and 18%, respectively.
Fig. 1.
Local control (LC) rate, disease-free survival (DFS) and overall survival (OS) of the 25 patients with SDC.
Fig. 2.
Initial and overall treatment failure (relapse) patterns in all 25 patients with SDC. Of the four patients with regional failure, three experienced distant failure simultaneously. In two patients with distant failure, local failure was detected afterward.
The details of the recurrent cases are summarized in Table 2. Of the seven patients with perineural invasion in pathological features, four experienced local recurrence in which intracranial invasion of the tumors was seen along the mandibular nerve or the facial nerve beyond the radiation field. Two of the four patients who had local recurrence with intracranial invasion died of the disease without distant metastasis. A representative image of a patient who developed intracranial tumor recurrence is shown in Fig. 3. Two patients irradiated with the radiation fields, including the base of the skull along the pathologically involved cranial nerve, did not experience local recurrence. Regional recurrence occurred in four patients (16%) with pN2b disease who received irradiation to the ipsilateral neck; contralateral lymph node recurrences were seen in two patients and ipsilateral recurrences in two patients.
Table 2.
Details of the recurrent cases (n = 12)
| Site | PStage | Pn | Lv | Field | Dose (Gy) | Overall failure site |
Status (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Local | Regional | Distant | ||||||||
| 1 | Parotid | T2N0 | − | − | tumor | 50.0 | + | − | − | DOD (28) |
| 2 | Parotid | T4aN0 | + | + | ipsilat | 60.0 | + | − | − | DOD (25) |
| 3 | Parotid | T4aN2b | − | − | ipsilat | 50.0 | − | + | + | DOD (12) |
| 4 | Parotid | T4bN2b | + | + | ipsilat | 50.0 | + | − | − | DOD (46) |
| 5 | Parotid | T1N2b | − | − | ipsilat | 50.0 | + | + | + | DOD (28) |
| 6 | Parotid | T3N2b | − | − | ipsilat | 60.0 | + | − | + | DOD (22) |
| 7 | Parotid | T3N1 | + | + | ipsilat | 66.0 | + | − | + | DOD (20) |
| 8 | Subman | T2N2b | + | + | ipsilat | 50.0 | + | + | + | DOD (8) |
| 9 | Parotid | T4aN0 | − | − | bilat | 60.0 | − | − | + | DOD (30) |
| 10 | Parotid | T4aN2b | + | + | bilat | 61.4 | − | − | + | DOD (7) |
| 11 | Subman | T2N2b | − | − | bilat | 49.5 | − | − | + | DOD (16) |
| 12 | Parotid | T4aN2b | − | − | ipsilat | 60.0 | − | + | − | NED (44) |
pn = perineural invasion, lv = lymphovascular invasion, subman = submandibular gland, posi = positive, tumor = tumor bed, ipsilat = ipsilateral, bilat = bilateral, DOD = dead of disease, NED = no evidence of disease.
Fig. 3.
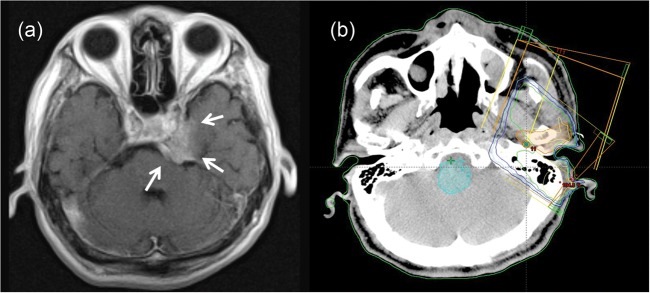
Intracranial recurrence in a 64-year-old male treated with 60 Gy in 30 fractions plus the administration of S-1. T1-weighted gadolinium-enhanced MRI (a) showed an enhanced mass in the parasellar region (arrow in Figure 3). This tumor progressed along the trigeminal nerve through the foramen ovale. In the radiotherapy plan (b), the PTV did not include the skull-base area, considering perineural invasion.
In the six patients who received irradiation to the bilateral neck, there was no regional recurrence. The cases of three of the four patients with regional recurrence were complicated by distant metastases, and the patients died of distant metastases. The remaining patient underwent salvage neck dissection and postoperative chemoradiotherapy, and is still alive without disease. Distant metastasis developed in eight patients (32%); the most common site was lung (six of the eight patients). Seven (47%) of the 15 patients with pathologically positive nodal metastases experienced distant failure after initial treatment, whereas only one (10%) of the 10 patients without nodal metastasis experienced distant failure.
The results of our univariate analysis for LC and OS are summarized in Table 3. Of the prognostic factors considered, perineural invasion and lymphovascular invasion were significantly correlated with LC (P < 0.05). Lymphovascular invasion and age were significant predictors of OS (P < 0.05). Although the significance was marginal (P = 0.07), perineural invasion also tended to affect OS. Moreover, the presence of local recurrence significantly affected OS (P = 0.0016). Gender, pT stage, pN stage, and surgical margin were not prognostic for LC or OS.
Table 3.
Clinicopathologic variables and univariate analysis for LC and OS
| Parameter |
n = 25 | 3y-LC | P-value | 3y-OS | P-value | |
|---|---|---|---|---|---|---|
| Gender | male | 19 | 60% | 44% | ||
| female | 6 | 83% | 0.413 | 83% | 0.113 | |
| Age | ≥60 | 14 | 74% | 27% | ||
| <60 | 11 | 57% | 0.300 | 82% | 0.014 | |
| pT | T1–3 | 14 | 58% | 50% | ||
| T4 | 11 | 77% | 0.233 | 60% | 0.914 | |
| pN | N0 | 10 | 78% | 65% | ||
| N1–2 | 15 | 57% | 0.396 | 48% | 0.144 | |
| pStage | I–III | 6 | 63% | 60% | ||
| IV | 19 | 68% | 0.54 | 53% | 0.533 | |
| pn | − | 18 | 80% | 61% | ||
| + | 7 | 36% | 0.031 | 57% | 0.070 | |
| lymphovascular invasion | − | 20 | 83% | 65% | ||
| + | 5 | 0% | 0.002 | 20% | 0.0014 | |
| Surgical margins | negative or close | 17 | 79% | 51% | ||
| Positive | 8 | 50% | 0.182 | 63% | 0.860 | |
LC = local control, OS = overall survival, pn = perineural invasion.
DISCUSSION
SDC is one of the rarest tumors in salivary glands. Although the number of reports concerning SDC has increased in recent years, only a limited few cases of SDC have been reported in the English literature, to our knowledge. In addition, the previous reports were case reports of one or several patients, or retrospective investigations of 10 to 30 cohorts. These past reports described SDC as a highly aggressive salivary gland tumor with a poor prognosis because of the high probability of local and distant recurrence. Locoregional recurrence rates have been reported as 17–67%, and distant recurrence rates as 33–66% [3, 4, 10].
The local and distant recurrence rates of our patients are similar to those of previous reports. In our series, locoregional recurrence occurred in 36% and distant metastasis occurred in 32% of the patients. Of the 12 patients with recurrences, 11 (92%) died of the disease. Of these 11 patients, three patients died of local recurrence with intracranial invasion along the cranial nerves without distant metastasis. Intracranial invasion along the cranial nerves might be one of the characteristics of the local failure pattern that is often life-threatening in patients with SDC [11]. In our study, perineural invasion was shown to be a significant prognostic factor for local failure. It is thus important to determine the most effective treatment strategy for SDC patients with perineural invasion.
Generally, postoperative radiotherapy has a role in reducing the risk of locoregional recurrence and contributes to good prognoses. In fact, most of the previous cases were treated with radiotherapy after surgical resection of SDC because of its aggressive clinical behavior. Roh et al. reported postoperative radiotherapy was a significant predictor of overall survival compared to surgery only [1]. In our study, three of the seven patients with perineural invasion did not experience local recurrence. Two of the three patients received postoperative radiotherapy with the field covering the skull base area along the course of the cranial nerves. In our patient series, the risk of perineural invasion was not necessarily taken into account in determining the radiation field. If the skull-base region had been included in the CTV in all of the patients with perineural invasion, local recurrence might have been prevented in more cases. Although to our knowledge there is no report in the English literature concerning the appropriate radiation field in patients with SDC, it might be advisable to have the CTV include the cranial nerves involved and the corresponding parts of the skull base in cases of pathologically positive perineural invasion, as is the case in the treatment of adenoid cystic carcinoma [12].
In agreement with previous findings, we found that one of the most common causes of treatment failure was distant metastasis [3, 10, 13]. In our series, eight (67%) of the 12 patients with recurrences had distant metastases. Chemotherapy should therefore be considered as an adjuvant therapy in addition to radiotherapy after surgery for patients with SDC. Systemic chemotherapy has been also selected for the treatment of distant disease. However, SDC shows poor response to chemotherapy, and there has been no consensus regarding the role of chemotherapy [14–16]. In our series, 22 patients (88%) received chemotherapy combined with radiotherapy, and 11 patients (44%) received adjuvant chemotherapy thereafter. A more intensive chemotherapy regimen may be needed for SDC. Several authors reported that the presence of androgen receptor or human epidermal growth factor receptor-2 is common in SDC [17–19]. Thus, androgen deprivation therapy or targeted therapy with trastuzumab in patients with recurrent or disseminated disease may be beneficial, but further prospective trials are needed to evaluate the efficacy of these new anticancer agents.
We found that 15 patients (60%) had cervical nodal metastasis at the time of diagnosis, and half of these patients experienced distant failure after treatment. Previous studies have indicated that the incidence of regional recurrence is 33%, that distant metastases appear to be correlated with lymphatic spread, and that prophylactic neck dissection has an important role [1, 10, 20]. In our series, neck dissection and neck irradiation were aggressively performed in 21 patients (84%). This might have contributed to the low incidence of nodal recurrence. Four patients (16%) experienced nodal recurrences at an extra-excision site, and two of the four experienced contralateral recurrences in the extra-irradiated field.
However, the incidence of regional recurrence in the contralateral neck was relatively low, and irradiation to the contralateral neck might cause exacerbating mucositis and dysfunction of the salivary glands or thyroid gland. When including the contralateral neck and/or skull-base area in the case of perineural invasion of the primary tumors, intensity-modulated radiotherapy should be useful for covering enough of the target volume and reducing the dose to organs at risk. The role of prophylactic contralateral neck irradiation needs further investigation.
This study had several limitations. A relatively small number of patients were studied, due to the rarity of SDC. Only a modest retrospective analysis was possible, which could limit the value of our statistical evidence. In addition, the irradiation technique and the regimen of chemotherapy varied somewhat in this study. Despite these limitations, our clinicopathological analysis of 25 patients provides clinical information about managing patients with SDC that may contribute to better treatment outcomes.
CONCLUSION
Local recurrence and distant metastasis were frequent causes of treatment failure in this patient series. Surgery and postoperative radiotherapy are expected to decrease the risk of locoregional failure and contribute to good prognoses in SDC. In patients with perineural invasion, the irradiation field may need to include the skull base along the cranial nerve(s) involved.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
FUNDING
This study was supported in part by KAKENHI (No. 23390302 and 23659589).
REFERENCES
- 1.Roh JL, Cho KJ, Kwon GY, et al. Prognostic values of pathologic findings and hypoxia markers in 21 patients with salivary duct carcinoma. J Surg Oncol. 2008;97:596–600. doi: 10.1002/jso.21045. [DOI] [PubMed] [Google Scholar]
- 2.Kashiwagi N, Takashima S, Tomita Y, et al. Salivary duct carcinoma of the parotid gland: clinical and MR features in six patients. Br J Radiol. 2009;82:800–4. doi: 10.1259/bjr/29600237. [DOI] [PubMed] [Google Scholar]
- 3.Colmenero Ruiz C, Patron Romero M, Martin P. Salivary duct carcinoma: a report of nine cases. J Oral Maxillofac Surg. 1993;51:641–6. doi: 10.1016/s0278-2391(10)80263-0. [DOI] [PubMed] [Google Scholar]
- 4.Weon YC, Park SW, Kim HJ, et al. Salivary duct carcinomas: clinical and CT and MR imaging features in 20 patients. Neuroradiology. 2012;54:631–40. doi: 10.1007/s00234-012-1014-z. [DOI] [PubMed] [Google Scholar]
- 5.Piao S, Zhao S, Guo F, et al. Increased expression of CD147 and MMP-9 is correlated with poor prognosis of salivary duct carcinoma. J Cancer Res Clin Oncol. 2012;138:627–35. doi: 10.1007/s00432-011-1142-6. [DOI] [PubMed] [Google Scholar]
- 6.Salovaara E, Hakala O, Back L, et al. Management and outcome of salivary duct carcinoma in major salivary glands. Eur Arch Otorhinolaryngol. 2012;270:281–5. doi: 10.1007/s00405-012-1997-4. [DOI] [PubMed] [Google Scholar]
- 7.Jamal AM, Sun ZJ, Chen XM, et al. Salivary duct carcinoma of the parotid gland: case report and review of the literature. J Oral Maxillofac Surg. 2008;66:1708–13. doi: 10.1016/j.joms.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Lee S, Cho KJ, et al. Treatment results of post-operative radiotherapy in patients with salivary duct carcinoma of the major salivary glands. Br J Radiol. 2012;85 doi: 10.1259/bjr/21574486. e947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mlika M, Kourda N, Zidi Y, et al. Salivary duct carcinoma of the parotid gland. J Oral Maxillofac Pathol. 2012;16:134–6. doi: 10.4103/0973-029X.92992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosal AS, Fan C, Barnes L, et al. Salivary duct carcinoma. Otolaryngol Head Neck Surg. 2003;129:720–5. doi: 10.1016/S0194-59980301386-X. [DOI] [PubMed] [Google Scholar]
- 11.De Riu G, Meloni SM, Massarelli O, et al. Management of midcheek masses and tumors of the accessory parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111 doi: 10.1016/j.tripleo.2011.01.005. e5–11. [DOI] [PubMed] [Google Scholar]
- 12.Garden AS, Weber RS, Morrison WH, et al. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–26. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 13.Ko YH, Roh JH, Son YI, et al. Expression of mitotic checkpoint proteins BUB1B and MAD2L1 in salivary duct carcinomas. J Oral Pathol Med. 2010;39:349–55. doi: 10.1111/j.1600-0714.2009.00835.x. [DOI] [PubMed] [Google Scholar]
- 14.Dimery IW, Legha SS, Shirinian M, et al. Fluorouracil, doxorubicin, cyclophosphamide, and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J Clin Oncol. 1990;8:1056–62. doi: 10.1200/JCO.1990.8.6.1056. [DOI] [PubMed] [Google Scholar]
- 15.Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759–69. doi: 10.1016/j.oraloncology.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Williams MD, Roberts DB, Kies MS, et al. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266–74. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaehne M, Roeser K, Jaekel T, et al. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103:2526–33. doi: 10.1002/cncr.21116. [DOI] [PubMed] [Google Scholar]
- 18.Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29 doi: 10.1200/JCO.2010.32.8351. e473–6. [DOI] [PubMed] [Google Scholar]
- 19.Nabili V, Tan JW, Bhuta S, et al. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29:907–12. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 20.Guzzo M, Di Palma S, Grandi C, et al. Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck. 1997;19:126–33. doi: 10.1002/(sici)1097-0347(199703)19:2<126::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]