Summary
Patients with glioblastoma multiforme (GBM) are profoundly immunosuppressed and may benefit from restoration of an antitumor immune response in combination with conventional radiation therapy and temozolomide (TMZ). The optimal strategies to evaluate clinically relevant immune responses to treatment have yet to be determined. The primary objective of our study was to determine immunologic response to cervical intranodal vaccination with autologous tumor lysate-loaded dendritic cells (DCs) in patients with GBM after radiation therapy and TMZ. We used a novel hierarchical clustering analysis of immune parameters measured before and after vaccination. Secondary objectives were to assess treatment feasibility and to correlate immune response with progression-free survival (PFS) and overall survival. Ten eligible patients received vaccination. Tumor-specific cytotoxic T-cell response measured after vaccination was enhanced for the precursor frequency of CD4+ T and CD4+ interferon γ-producing cells. Hierarchical clustering analysis of multiple functional outcomes discerned 2 groups of patients according to their immune response, and additionally showed that patients in the top quintile for at least one immune function parameter had improved survival. There were no serious adverse events related to DC vaccination. All patients were alive at 6 months after diagnosis and the 6-month PFS was 90%. The median PFS was 9.5 months and overall survival was 28 months. In patients with GBM, immune therapy with DC vaccination after radiation and TMZ resulted in tumor-specific immune responses that were associated with prolonged survival. Our data suggest that DC vaccination in combination with radiation and chemotherapy in patients with GBM is feasible, safe, and may induce tumor-specific immune responses.
Keywords: dendritic cells, glioblastoma, vaccine, immune response
The median overall survival (OS) after diagnosis of glioblastoma multiforme (GBM) treated with surgery and radiation therapy (RT) is 12.1 months, improving to approximately 14.6 months with the addition of temozolomide (TMZ).1 Patients with GBM exhibit a systemically impaired immune response and pathologic examination shows, in most cases, little tumor-associated inflammatory infiltrate, suggesting that immune suppression plays a pivotal role in tumor progression. Lack of immune recognition of GBM may also be due to chemotherapy, limited access of immune cells to the central nervous system, and regulatory pathways. Thus, initial attempts to use immune therapies in patients with GBM have had marginal success.2–7
We designed a study to test the hypothesis that diminishing the activity of regulatory T cells (TREG) and improving immune cell access to the central nervous system would enhance response to immune therapy and consequently affect survival of patients with GBM. TMZ therapy and RT selectively reduce CD4+ T cells, the cell population that includes TREG,8,9 possibly minimizing their negative effects on immune therapy. Earlier studies showed that ex vivo exposure of dendritic cells (DCs) to prostaglandin E2 (PGE2) and tumor necrosis factor α (TNFα) contributed to maturation and induced high levels of interleukin 12 (IL-12) and immune activation.10,11 Animal and human data suggest that injecting DCs intranodally improves antitumor activity, probably by enhancing interactions between antigen-presenting cells and T cells.12,13 As the brain per se has no recognized lymphatic drainage, it is thought that the outflow of antigens and DCs escape along the cranial nerve sheets into the nasal lymphatics and drain into the cervical lymph nodes.14–17 In an animal model, it has been shown that antigen-pulsed DCs injected into the brain migrate to the cervical lymph nodes and recruit antigen-specific T cells with preferential homing to the brain.18 Accordingly, we chose to assess the potential benefit and safety of incorporating these strategies into the conventional treatment schema.
Earlier studies with therapeutic DC vaccinations have confirmed immune activation against GBM tumors and suggest an improvement in survival, but do not uniformly show a correlation between survival and immune response. 4–6,19,20 We hypothesized that in patients with newly diagnosed GBM, the combination of RT, TMZ, and cervical intranodal vaccination with autologous tumor lysate-loaded DCs matured ex vivo with PGE2 and TNFα would elicit a positive tumor-specific immunologic response. We used hierarchical cluster analysis of multiple immune function assay measures to evaluate the complex immune response in these patients, and examined whether these combined immune parameters correlated with clinical outcome.
MATERIALS AND METHODS
Patient Eligibility Criteria
To participate in this trial, patients must have been ≥18 years, able to undergo surgical resection for newly diagnosed GBM confirmed by neuropathology review, and have a resected tumor yield ≥8×107 tumor cells, after mechanical and enzymatic digestion of tumor tissue. After completing 6 weeks of conformal external beam RT (66 Gy in 33 fractions) with concomitant TMZ at 75 mg/m2/d, patients with a Karnofsky Performance Scale Index of ≥60, and adequate hematologic, hepatic, and renal function were eligible. Patients who had received systemic corticosteroids within 2 weeks of leukapheresis; had a history of autoimmune disease, HIV, hepatitis B or C infection; or had any other medical disorder that would impair their ability to receive study treatment were excluded. Eligible patients gave signed informed consent before enrollment. The study was approved by the Dartmouth College Committee for the Protection of Human Subjects, and the Food and Drug Administration (BB-IND 12903 and BB-IND 11162).
Vaccine Preparation and Administration
Tumor cells were isolated from surgical resection specimens by mechanical and enzymatic digestion and cryopreserved at −140°C as previously reported (BB-IND 11162).21 Mechanically prepared tumor cells were used for delayed-type hypersensitivity (DTH) tests. Tumor lysates were prepared for DC loading by irradiation (100 Gy) and freeze/thaw (5 cycles), and stored at −20°C. Three to seven weeks after completing 6 weeks of radiotherapy with concomitant daily TMZ, we obtained peripheral blood mononuclear cells from patients through leukapheresis and fractionated the product using elutriation. Monocyte-derived DCs were lysate loaded on day 5 of culture, matured with overnight treatment of TNFα and PGE2, and harvested on day 7 (DC vaccine).22 Lymphocytes fractionated from this apheresis product were the prevaccine (D-7) cells used for immune assays.
The total dose of 1×107 DC cells was injected in equal aliquots into 2 bilateral cervical lymph nodes using ultrasound guidance.21 Evaluable patients received 1 vaccination every 2 weeks on 3 occasions. Patients remained off systemic corticosteroids until the second leukapheresis after the third vaccination (postvaccine D42).
Delayed-type Hypersensitivity
Irradiated mechanically dissociated tumor cells were injected intradermally with a standard anergy panel in the forearm. Forty-eight hours later, DTH was assessed by measuring the area of erythema and induration using 2-dimensional measurements using calipers. A DTH reaction was considered positive if the area of erythema and induration was greater or equal to 10mm2.
Immunologic Assays
Tumor-specific cytotoxic T-cell response was measured by the dye dilution proliferation assay (DDPA)23,24 and interferon γ (IFNγ) enzyme-linked immunosorbent spot (ELISPOT) assay performed on prevaccination and postvaccination lymphocytes fractionated from apheresis product. The DDPA enabled us to simultaneously measure (1) T-cell proliferation, (2) precursor frequency (PF) (the proportion of cells in the parent population responding to GBM), (3) phenotype (CD4, CD8) of the responding T cells, and (4) T lymphocyte secretion of IFNγ after restimulation of T cells with freshly added DCs during the final 18 hours of coculture. The DDPA assay has been described and validated in comparison with tetramer and ELISPOT assays.23,25,26 The intra-assay coefficient of variation for tumor-specific PF values was 10%, and both time points from any one patient were tested in the same assay to eliminate interassay variation from the assessment of treatment-related changes.
A restimulation variation of the standard overnight ELISPOT assay was used to measure tumor-specific IFNγ production by lymphocytes. Prevaccination and postvaccination lymphocytes were cocultured with unloaded and GBM autologous lysate-loaded DCs (10:1) in the presence of IL-12 and IL-2 for 6 days before lymphocytes were harvested, washed, and plated for an overnight IFNγ capture assay with freshly added DCs. Each treatment condition was tested in triplicate, and both time points for each patient were tested simultaneously. The intra-assay coefficient of variation was 9% (range: 1% – 22%), and proficiency for ELISPOT by our Immune Monitoring Laboratory compared well with 36 reference laboratories participating in a panel conducted by the Cancer Vaccine Consortium.27
Other immunologic parameters measured before and after vaccination included phenotypic characteristics of vaccine DCs and peripheral blood T cells by flow cytometry. Phenotypic characteristics of peripheral blood T cells were also examined in a sample obtained after surgery but before RT and TMZ.
Clinical and Magnetic Resonance Imaging Evaluations
We monitored patients for adverse events at each visit and observed them for 2 hours after intranodal injections. Toxicities were graded using the Common Terminology Criteria for Adverse Events (version 3.0) and Common Toxicity Criteria (version 3.0). All patients had a magnetic resonance imaging (MRI) scan before and after completing therapy. Total tumor resection was defined as the absence of enhancement on postoperative MRI done within 48 hours after surgery. Subtotal resection was defined as removal of more than 90% of enhancing volume. Partial resection was defined as removal of 90% or less of enhancing volume. MRI tumor assessment was performed by an independent operator at the Brain Imaging Laboratory and tumor volumes were traced on the postgadolinium T1 scans using a semiautomated segmentation program based on a Sobel watershed filter (Alice, version 4.4.9, Parexel International Co., 1999). Approximately 4 weeks after vaccination, patients received maintenance cycles of TMZ1 for 12 cycles, unless the medication had to be stopped because of toxicity or tumor progression. Patients had MRI scans every 2 months to follow the effects of treatment on tumor volume. Progression-free survival was defined as the time from diagnosis until the patient reached objective disease progression.28 Survival was defined in the typical manner for patients with GBM as the time from diagnosis to death due to any cause. We censored patients without documented progression from their last objective tumor assessment, and followed all patients until death or for a minimum of 24 months after diagnosis (Fig. 1).
FIGURE 1.
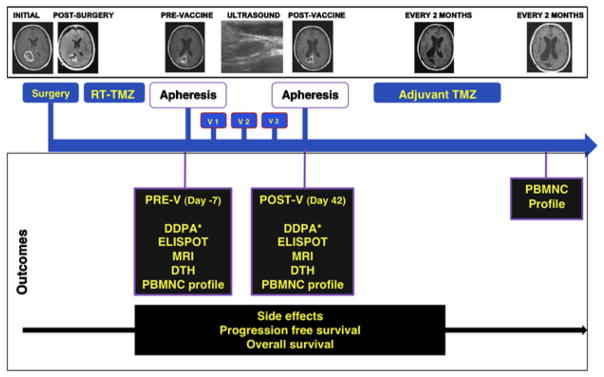
Study design. Four weeks after completing combined RT-TMZ, patients had a prevaccination (V) apheresis, DTH panel placement, and MRI. One week later, the first vaccination (V1) was administered, and 2 additional vaccinations were given 2 weeks apart. Two weeks after the third vaccine patients had a post-V apheresis, DTH panel placement, and MRI, followed by 12 cycles of adjuvant TMZ. DDPA indicates dye dilution proliferation assay; DTH, delayed-type hypersensitivity reaction; ELISPOT, enzyme-linked immunosorbent spot assay; PBMNC, peripheral blood mononuclear cells; POST-V, postvaccination; PRE-V, prevaccination; RT, radiation therapy; TMZ, temozolomide; v, vaccination.
Statistical Methods
Sample size calculation of 10 participants was based on the detection of a positive tumor DTH reactivity postvaccine therapy in 30% of the patients with a power of 80% and at 5% significance level. Median values for measurements of tumor-specific T-cell response from the DDPA and ELISPOT assays for day −7 (prevaccine) and day 42 (postvaccine) were compared using Wilcoxon signed-rank tests. As 5 primary comparisons were presented, statistical significance was defined as P<0.01 2-sided after Bonferroni correction. In a descriptive analysis, we visualized patterns of response in heatmaps showing hierarchical clustering by Euclidian distance and complete linkage using the heatmap.2 function available from the R Foundation for Statistical Computing (http://www.R-project.org, Vienna, Austria). Prevaccination and postvaccination immune assay results were summarized on a continuous scale using standard techniques. We confirmed the results of the hierarchical clustering using a Cox regression model on the first 3 principle immune parameters to calculate the estimated hazard ratio. We plotted Kaplan-Meier survival curves for immune responding and non-responding groups according to grouping defined by the hierarchical clustering results for the immune responses.
RESULTS
Accrual and Patient Characteristics
Between May 2006 and February 2008, 60 patients were diagnosed with GBM at our institution (Fig. 2). Of 11 patients who entered the study, 1 had a seizure with neurologic deterioration several weeks after leukapheresis and did not receive any DC vaccinations. Table 1 summarizes the demographics of the 10 patients who received 3 vaccinations with 1 × 107 DCs.
FIGURE 2.
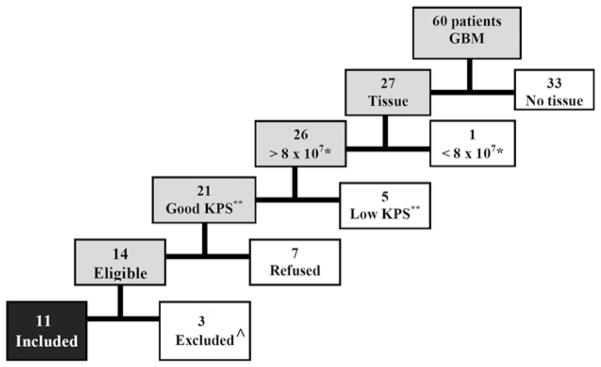
Eligibility of the 60 patients diagnosed with glioblastoma multiforme (GBM) during the study period. The reasons for exclusion of 49 patients were in 33 no tissue was available because only a biopsy was performed or patients did not sign informed consent to use the tissue; in 1 the tissue specimen yielded less than 8×107 tumor cells, 5 had a low performance status, 7 declined participation; and 3 although eligible were not included because enrollment was completed. One patient had initial leukapheresis but her performance status deteriorated after a seizure, therefore was not treated. KPS indicates Karnofsky Performance Scale.
TABLE 1.
Patient Demographic Characteristics and Treatment Outcomes
| No. | Sex | Age (y) | KPS | Surgery | Time From the Last Dose of Steroids and Vaccine 1 (mo) | No. CD4+ (×109/L) | No. CD8+ (×109/L) | CD4+/CD8+Ratio | PFS (mo) | Survival (mo) | Cluster* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 66 | 80 | Subtotal | 2.7 | 0.198 | 0.135 | 1.47 | 7 | 30 | 2 |
| 2 | F | 59 | 80 | Subtotal | 4.4 | 0.290 | 0.807 | 0.36 | 41 | 44† | 2 |
| 3 | F | 66 | 70 | Partial | 2.6 | 0.444 | 0.589 | 0.75 | 10 | 17 | 1 |
| 4 | M | 66 | 80 | Subtotal | 3.3 | 0.549 | 0.925 | 0.59 | 9 | 20 | 1 |
| 5 | M | 78 | 70 | Subtotal | No steroids | 0.588 | 0.097 | 6.05 | 7 | 26 | 1 |
| 6 | F | 54 | 80 | Subtotal | 1.0 | 0.225 | 0.320 | 0.70 | 5 | 15 | 1 |
| 7 | F | 61 | 90 | Subtotal | 2.7 | 0.921 | 0.976 | 0.94 | 14 | 17 | 1 |
| 8 | M | 57 | 70 | Partial | 1.0 | 0.555 | 0.223 | 2.49 | 7 | 29† | 2 |
| 9 | M | 48 | 90 | Total | 3.6 | 0.420 | 0.540 | 0.78 | 27† | 27† | 2 |
| 10 | M | 59 | 90 | Subtotal | 2.7 | 0.736 | 0.427 | 1.72 | 26† | 26† | 2 |
| Median | 60 | 80 | 2.7 | 0.496 | 0.4836 | 9.5 | 28 | ||||
| Range | 48–78 | 70–90 | 1.0–2.7 |
Clusters 1 and 2 determined by clustering analysis of immune response measures as observed in Figure 3.
Patient without progression and patient alive.
KPS indicates Karnofsky performance scale; PFS, progression-free survival.
Vaccine Preparation and Administration
DCs had a mature phenotype with upregulation of MHC Class II, CD83, costimulatory molecules CD80 and CD86 and a decrease in CD14 expression. All 60 injections were administered into the cervical lymph nodes except 1, which was administered into an inguinal lymph node.
Immunologic Endpoints
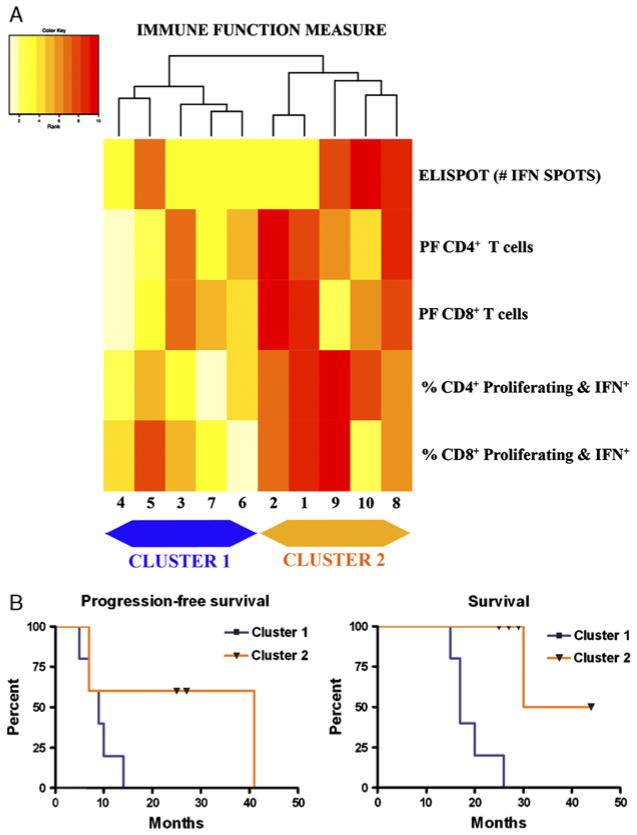
Pretreatment lymphocytes of most patients showed little CD4 or CD8 T-cell proliferation in response to tumor-loaded DCs. On the basis of a significance level corrected for 5 multiple comparisons, only the PF of tumor-specific CD4+ T cells was significantly enhanced postvaccination (P=0.004) (Table 2). The proportion of CD4+ IFNγ-producing cells, tumor-specific PF of CD8+ T cells, and proportion of CD8+ IFNγ-producing cells showed trends of an increase, but this did not reach statistical significance (Table 2). Increases in IFNγ production of lymphocytes (ELISPOT) related to treatment were observed in 4 patients (6-fold to 340-fold increase). Clustering of the postvaccine measures of 5 lymphocyte functional parameters yielded 2 distinct groups of patients based on the immunologic response to treatment (Fig. 3A). Patients on the right of the heatmap dendrogram (Fig. 3, cluster 2) survived longer than patients in cluster 1 (P=0.002). In cluster 1, the 5 patients with the lowest combined immune activation response had shorter survival (Fig. 3, cluster 1). Specifically, 4 of the 5 patients in the immune response group, cluster 2, are alive after a follow-up of at least 2 years, whereas all 5 patients in cluster 1 have died. The same clustering analysis for the prevaccination assay measurements of immune response to tumor did not distinguish different patient groups that correlated with survival (data not shown). Using OS as the endpoint, we found that the high-risk and low-risk groups defined by Cox regression analysis are the same as clusters 1 and 2, supporting the results of the cluster analysis.
TABLE 2.
Comparison of Tumor-specific T-cell Responses Prevaccination and Postvaccination for All 10 Patients
| Immunologic Assay | Median
|
Mean
|
P* | ||
|---|---|---|---|---|---|
| Prevaccination | Postvaccination | Prevaccination | Postvaccination | ||
| Precursor frequency of CD4+ T cells | 0.003 | 0.01 | 0.005 | 0.015 | 0.004 |
| Percentage of CD4+ proliferating and IFN+† | 0.15 | 0.25 | 0.380 | 0.880 | 0.016 |
| Precursor frequency of CD8+ T cells | 0.001 | 0.001 | 0.001 | 0.003 | 0.105 |
| Percentage of CD8+ proliferating and IFN+† | 0.27 | 0.25 | 0.45 | 0.92 | 0.105 |
| ELISPOT (# spots) | 0 | 0 | 1 | 44 | 0.188 |
Two-sided P-value from Wilcoxon signed-rank test for prevaccination to postvaccination difference in the median. With Bonferroni correction for 5 comparisons, only P values of less than 0.01 are statistically significant at the 0.05 significance level.
After 7 d culture with loaded DC.
DC indicates dendritic cell; ELISPOT, enzyme-linked immunosorbent spot; IFN, interferon.
FIGURE 3.
A, Heatmap of hierarchical clustering analysis of the postvaccination immune responses. Five patients with generally low ranks in immune function (pale yellow colors) formed cluster 1 on the left. Five other patients with higher ranks in immune function measures (dark red colors) formed cluster 2 on the right. B, Kaplan-Meier curves of progression-free survival and overall survival for the 2 clusters. The Kaplan-Meier progression-free survival curves for cluster 1 (median = 9 mo) and cluster 2 (median = 41 mo) are not statistically different based on a log-rank test (P = 0.09). The overall survival was significantly different between cluster 1 (median = 17 mo) and cluster 2 (median = not achieved) (P = 0.002). ELISPOT indicates enzyme-linked immunosorbent spot assay; IFN, interferon; PF, precursor frequency.
There was no DTH skin reactivity to autologous tumor prevaccination or postvaccination in any patient. Before the treatment no skin reactivity was detected for the control anergy panel in any patient, but after vaccination 2 patients developed a positive skin reaction to Candida.
Two weeks after vaccination, we observed an increase in the proportion of CD8+ T memory cells (CD45RO+CCR7−) (P=0.012) and in naive B cells (CD19+CD27−) (P=0.037). In addition, we observed posttreatment increases in the percent of circulating CD4+TREG cells (CD25+FoxP3+) (P=0.03) but not in the absolute numbers of CD4+TREG cells. Otherwise there was no significant difference in additional lymphocyte populations measured.
Clinical Outcomes
The median progression-free survival for the 10 study patients was 9.5 months and the median OS was 28 months. All 7 patients with evidence of tumor progression received second-line therapy with a bevacizumab-based regimen. There were no significant differences in patient tumor volumes between the prevaccination and postvaccination gadolinium-enhancing MRI studies (data not shown). The only adverse event attributed to DC vaccine was grade 2 unilateral neck pain after 1 cervical lymph node vaccine administration in 1 patient that persisted for several weeks.
DISCUSSION
Using a novel approach that combines multiple functional immune assays and hierarchical clustering analysis, we showed that autologous tumor lysate-DC vaccination after RT and TMZ triggered an antitumor immune response in patients with newly diagnosed GBM. Furthermore, cluster analysis of multiple assay immune measures showed a correlation of immune response and clinical outcome; even though single immune measures were poor predictors of survival. The use of multiple parameters may provide a more comprehensive assessment of immune system status after cancer immunotherapy. Vaccination in combination with RT and TMZ was feasible and associated with only a single, mild adverse event.
To date, only 41 patients treated with DC-based vaccination for newly diagnosed GBM have been reported in the literature.4,7,19,20,29,30 In most reports, the treatment before immune therapy was highly variable, preceded the routine addition of TMZ to radiation, and in some patients, RT was avoided because of concern that it could interfere with the efficacy of the immune therapy.7 A recently published pilot study included 8 patients with diagnosis of GBM who received 4 intradermal vaccinations of tumor lysate-loaded DC after RT and TMZ, followed by tumor lysate boosts, while on maintenance TMZ. An immune response measured by ELISPOT assay performed after the fourth vaccination was detected in 5 of the 8 (63%) patients.30 Preliminary data presented from a randomized phase 2 study suggested that patients with high-grade glioma receiving DC-based immunotherapy after radiation and chemotherapy had an improved survival compared with the control group who only received conventional treatment.31 This data combined with our current observations suggest that the immune suppressive effect associated with prior RT and chemotherapy does not prevent and, on the contrary, may predispose to, successful immune stimulation by a DC vaccine.
If the correlation between enhanced survival and immune response is the result of a positive interaction between RT and TMZ with DC vaccine therapy, it raises the paradox of how chemoradiotherapy immune suppression can facilitate immune activation. One hypothesis is that RT and TMZ may have a pronounced effect on the immune regulatory pathways. Elevated functional CD4+TREG cells have been reported in tumor-infiltrating and peripheral blood lymphocytes of GBM patients32–34; in vitro depletion of TREG cells restores T-cell immune function.33 RT and TMZ has been reported to cause a global CD4+ lymphopenia,8,9 which may change the regulatory environment in favor of enhanced tumor response. In contrast, we (unpublished data) and others have found that the CD4+TREG cell peripheral blood population is less affected than the general CD4+ population by RT and TMZ, thus the proportion of TREG in peripheral blood of patients with GBM increases immediately after treatment.35,36 Nonetheless, enhanced responses are reported with the combination of maintenance TMZ and immune therapy.35,37 Several other studies suggest that the addition of chemotherapy after vaccination in patients with GBM improves survival,4,38 although it is unclear what the precise mechanism of interaction is. We were able to show that the injection of intranodal DC matured with PGE2 and TNFα and primed with autologous GBM tumor does not expand the TREG population where others have shown that unprimed DCs matured with PGE2 and TNFα may induce TREG cells.39 Our observations imply that pathways other than those regulated by CD4+TREG may be at play in this complex interaction between RT-TMZ and immune therapy.
Although our patient population was carefully selected (maximum feasible surgical resection, good Karnofsky Performance Scale, and off corticosteroids before vaccination), only 50% of the patients developed a measurable immune response that associated with improved survival. The heterogeneity of patients and their immune system suggest that immune activation and regulatory pathways are highly individualized. This leads to impediments in using single immune parameter endpoints to identify relevant immune changes which correlate with outcome. The tumor antigen-specific DTH response after cancer vaccine has been used as a primary outcome in clinical trials and seems to correlate with in vitro assessment of peripheral blood antigen-specific T-cell responses.40 The DTH response to autologous tumor lysate reported in studies using DC vaccine for treatment of patients with high-grade glioma shows conflicting results,5,6,41 but a recent study19 in combination with our findings suggest that DTH is of limited use for immune monitoring of GBM DC vaccination studies. By use of hierarchical clustering of multiple parameters, we have been able to show that immune activation as a result of treatment correlates with enhanced outcome. Identification of more sensitive and specific biomarkers of immune activation would certainly lead to better prognostic and predictive capabilities, but more importantly, it could lead to a better understanding of the disease process and therefore to better treatments.42 Ideally, biomarkers that measure immune proficiency and predict immune response could help us identify patients who would benefit from immune therapy.19,43,44
Our investigation using immune monitoring hierarchical cluster analysis is not limited to GBM. Although there are many immunologic assays to monitor the response in an increasing number of cancer immune therapy trials, the variability in the methodology used, the lack of validation and harmonization of the different assays, and the unreliable association between individual assays and outcomes has often been an impediment to establishing the benefit of immune therapies.27,42,45 Even if these assays could be validated and standardized between laboratories, it is unlikely that a single assay could detect the subtle and complex immunologic shifts triggered by immunotherapies that translate to beneficial clinical endpoints. Our study was not designed to test the validity of cluster analysis as an early marker of immune response, but to examine the potential that by combining multiple assays one can measure immune response to cancer vaccines of clinical relevance. The absence of a correlation between prevaccination immune parameters and outcomes in our study could be the result of the small number of patients. Our results support further exploration and the need for validation of the correlation of immune response and improved survival in a larger study.
In our small cohort of patients, those capable of mounting a tumor-specific immune response to intranodal autologous tumor lysate-loaded DC after surgery and combined RT-TMZ had an improved outcome. We are encouraged that optimizing immune therapy with chemotherapy and radiation could enhance the lives of GBM patients. If our 2 patient clusters reflect 2 distinct patient phenotypes, only 1 of which is capable of effective immune activation, then immune competence may be a threshold effect, and small absolute differences in individual parameters could translate into differences in survival. Identification of those who are more likely to benefit, optimal preparation and administration of the DC product, and the addition of tolerance breaking strategies are important considerations in the design of future therapeutic vaccination trials.
Acknowledgments
Supported by the Norris Cotton Cancer Center, the Medical Oncology Immunotherapy Program, The Burdick Foundation, The Skip Matthews Memorial Run, CCSG NCI-CA-23108, P42 ES00737, RO1CA095648.
The authors thank the patients who participated in this study, Mary J. Robinson for assistance in the preparation of the manuscript and Linda Kingman for her support as clinical research coordinator.
Footnotes
All authors have declared there are no financial conflicts of interest in regard to this work.
Preliminary results of this study were presented as an abstract at the 12th Annual Scientific Meeting of the Society for Neuro-oncology in November 2007 [Neuro-oncology October 2007: 507 IM-25 (Abstract)].
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Weller M, Fontana A. The failure of current immunotherapy for malignant glioma. Tumor-derived. Brain Res Brain Res Rev. 1995;21:128–151. doi: 10.1016/0165-0173(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowski S, De Vleeschouwer S, Kaempgen E, et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 6.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Post-operative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 7.Walker DG, Laherty R, Tomlinson FH, et al. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114–121. doi: 10.1016/j.jocn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 9.Hughes MA, Parisi M, Grossman S, et al. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 10.Kalinski P, Schuitemaker JH, Hilkens CM, et al. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–2809. [PubMed] [Google Scholar]
- 11.Rieser C, Bock G, Klocker H, et al. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedrosian I, Mick R, Xu S, et al. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826–3835. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Lambert LA, Gibson GR, Maloney M, et al. Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res. 2001;61:641–646. [PubMed] [Google Scholar]
- 14.Weller RO, Galea I, Carare RO, et al. Pathophysiology of the lymphatic drainage of the central nervous system: implications for pathogenesis and therapy of multiple sclerosis. Pathophysiology. 2010;17:295–306. doi: 10.1016/j.pathophys.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Walter BA, Valera VA, Takahashi S, et al. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol Appl Neurobiol. 2006;32:388–396. doi: 10.1111/j.1365-2990.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann J, Kwidzinski E, Brandt C, et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 17.Hatterer E, Davoust N, Didier-Bazes M, et al. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood. 2006;107:806–812. doi: 10.1182/blood-2005-01-0154. [DOI] [PubMed] [Google Scholar]
- 18.Karman J, Ling C, Sandor M, et al. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173:2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 20.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 21.Schwaab T, Schwarzer A, Wolf B, et al. Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with Aldesleukin (Interleukin 2) and IFN-(alpha)2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res. 2009;15:4986–4992. doi: 10.1158/1078-0432.CCR-08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernstoff MS, Crocenzi TS, Seigne JD, et al. Developing a rational tumor vaccine therapy for renal cell carcinoma: immune yin and yang. Clin Cancer Res. 2007;13:733s–740s. doi: 10.1158/1078-0432.CCR-06-2064. [DOI] [PubMed] [Google Scholar]
- 23.Schwaab T, Fisher JL, Meehan KR, et al. Dye dilution proliferation assay: application of the DDPA to identify tumor-specific T cell precursor frequencies in clinical trials. Immunol Invest. 2007;36:649–664. doi: 10.1080/08820130701674760. [DOI] [PubMed] [Google Scholar]
- 24.Givan AL, Fisher JL, Waugh MG, et al. Use of cell-tracking dyes to determine proliferation precursor frequencies of antigen-specific T cells. Methods Mol Biol. 2004;263:109–124. doi: 10.1385/1-59259-773-4:109. [DOI] [PubMed] [Google Scholar]
- 25.Bercovici N, Givan AL, Waugh MG, et al. Multiparameter precursor analysis of T-cell responses to antigen. J Immunol Methods. 2003;276:5–17. doi: 10.1016/s0022-1759(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 26.Givan AL, Fisher JL, Waugh M, et al. A flow cytometric method to estimate the precursor frequencies of cells proliferating in response to specific antigens. J Immunol Methods. 1999;230:99–112. doi: 10.1016/s0022-1759(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 27.Janetzki S, Panageas KS, Ben-Porat L, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 29.Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 30.Ardon H, Van Gool S, Lopes IS, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol. 2010;99:261–272. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 31.Cho DY, Lee HC, Lin SZ. Autologous dendritic cell-based immunotherapy for maligant gliomas-A phase II prospective randomized clinical trial. Neuro-oncology. 2009;11:882. [Google Scholar]
- 32.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 33.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 34.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampson JH, Aldape KD, Gilbert MR, et al. Temozolomide as a vaccine adjuvant in GBM. J Clin Oncol (Meeting Abstracts) 2007;25:2020. [Google Scholar]
- 36.Chiba Y, Hashimoto N, Tsuboi A, et al. Effects of concomitant temozolomide and radiation therapies on WT1-specific T-cells in malignant glioma. Jpn J Clin Oncol. 2010;40:395–403. doi: 10.1093/jjco/hyp196. [DOI] [PubMed] [Google Scholar]
- 37.Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheeler CJ, Das A, Liu G, et al. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee DK, Dhodapkar MV, Matayeva E, et al. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Disis ML, Schiffman K, Gooley TA, et al. Delayed-type hypersensitivity response is a predictor of peripheral blood T-cell immunity after HER-2/neu peptide immunization. Clin Cancer Res. 2000;6:1347–1350. [PubMed] [Google Scholar]
- 41.Yamanaka R, Abe T, Yajima N, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16:1745–1755. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 43.Wheeler CJ, Black KL, Liu G, et al. Thymic CD8+ T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171:4927–4933. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
- 44.Kammula US, Marincola FM, Rosenberg SA. Real-time quantitative polymerase chain reaction assessment of immune reactivity in melanoma patients after tumor peptide vaccination. J Natl Cancer Inst. 2000;92:1336–1344. doi: 10.1093/jnci/92.16.1336. [DOI] [PubMed] [Google Scholar]
- 45.Britten CM, Janetzki S, van der Burg SH, et al. Toward the harmonization of immune monitoring in clinical trials: quo vadis? Cancer Immunol Immunother. 2008;57:285–288. doi: 10.1007/s00262-007-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]