Abstract
Background:
The first case of 2009 pandemic influenza A (H1N1) virus in Gujarat, India, was reported in August 2009. Oseltamivir was used for treatment of pandemic influenza in India. We discuss the clinical characteristics and outcome of the hospitalized patients with H1N1 infection during 2009 pandemic influenza season.
Materials and Methods:
Hospitalized patient with laboratory-confirmed H1N1 flu during August 2009 to February 2010 were included in this retrospective study. Data were collected from hospital ICU charts. Patients discharged from hospital were considered cured from swine flu. Data analysis was performed using CDC software EPI Info v3.5.3. Both univariate and multivariate analyses were conducted.
Results:
A total of 63 patients were included in the study, of them 41 (65%) males and 22 (35%) females. Median age was 34 (3-69) years and median duration of symptoms before hospitalization was 5 (2-20) days. Common presenting symptoms include fever 58 (92.06%), cough 58 (92.06%), breathlessness 38 (60.31%), common cold 14 (22.22%), vomiting 12 (19.04%), weakness 9 (14.28%), throat pain 7 (11.11%), body ache 5 (7.93%), and chest pain 4 (6.34%). Co-morbidities were seen in 13 (20.63%) patients. Steroids were used in 39 (61.90%) patients, and ventilatory support was required in 17 (26.98%) patients. On presentation chest x-ray was normal in 20 (31.74%) patients, while pulmonary opacities were seen in 43 (68.26%) patients. Forty-seven (74.60%) patients were cured and discharged from hospital, 14 (22.22%) patients died, and 2 (3.17%) patients were shifted to other hospital. Ventilatory requirement, pneumonia, and co-morbidities were the independent predictors of mortality, while age, sex, and steroid use were not associated with increased mortality.
Conclusion:
2009 pandemic influenza A had the same clinical features as seasonal influenza except vomiting. Mortality rate was high in 2009 H1N1-infected patients with pneumonia, co-morbid conditions, and patients who required ventilatory support.
Keywords: 2009 H1N1, Pandemic flu, Swine flu, Viral pneumonia
INTRODUCTION
Influenza is an acute, usually self-limited, febrile illness caused by infection with influenza type A or B viruses and occurs in outbreaks of varying severity almost every winter.[1] In April 2009, cases of human infection with a new variant of influenza A (H1N1) virus were identified in the United States and Mexico and shown to cause severe illness among several patients.[2,3] Virus spread rapidly to other parts of the world and on June 11, 2009, WHO raised the pandemic alert level to phase 6, indicating a global pandemic. The 2009 H1N1 virus is a triple-reassortant influenza virus containing genes from human, swine, and avian influenza viruses[4,5] and thus has been labeled “swine flu.” Most cases of pandemic influenza H1N1 infection have been mild or subclinical, some patients experienced severe illness and complications from H1N1 influenza infection.[6,7,8,9,10,11,12,13] The most common cause of death is respiratory failure; other causes of death are pneumonia, high fever leading to neurological problems, dehydration, and electrolyte imbalance. Persons at high risk for severe disease and complications secondary to 2009 pandemic H1N1 influenza A include patients with underlying pulmonary or cardiac co-morbid conditions, immunosuppressive states, pregnancy and post-partum states, diabetes mellitus, obesity and in children with prior neurological disabilities.[14,15,16,17]
Since the report of first case of “swine flu” in India in May 2009, there was a progressive increase in the number of swine flu cases all over the subcontinent. From May 2009 till February 28, 2010, samples from 128,627 persons were tested for 2009 pandemic H1N1 influenza A across the country and 29,652 (23.05%) of them were found to be positive. In the state of Gujarat, the first case of 2009 H1N1 influenza A was reported on 3rd August 2009, and by 28th February 2010, 1209 cases were reported and 289 patients died.[18] We experienced a spectrum of illness ranging from mildly symptomatic patients to severe illness. Here we report the clinical characteristics and outcomes of hospitalized patients in Gujarat with confirmed H1N1 infection during 2009 influenza season.
MATERIALS AND METHODS
This is a hospital-based retrospective study of the influenza cases admitted at Sterling hospital, Ahmedabad, during 2009 pandemic. Patients infected with H1N1 virus who were hospitalized from August 2009 to February 2010 were included in the study. Demographic data, history, and physical examination findings were recorded in the hospital history and on the examination sheet. Inpatient charts were used to collect patient's hospital course and treatment. Diagnosis of H1N1flu was confirmed by polymerase chain reaction (PCR) testing of respiratory secretions (throat swab, endotracheal secretion) conducted at B.J. Medical College, Civil Hospital, Ahmedabad. Patients without pneumonia were treated with oseltamivir, 75 mg PO bid, and those with pneumonia were treated with 150 mg PO bid. In pediatric patients, an appropriate weight-based dose of oseltamivir was used. All hospitalized patients were treated with antibiotics. Treating physician decided dosage and route of administration of antibiotics. Non-invasive and invasive ventilatory care was given to patients with respiratory failure. Methyl prednisolone 40 mg IV q8h for first week followed by q12h for second week and q24h for third week were used for hypoxic patients with pulmonary opacities.
The protocol for treatment and use of systemic steroids was developed after first few deaths of hypoxic patients with acute lung injury. A round table consensus meeting with pulmonologists, critical care experts and infectious diseases consultants resulted in this practice. As per the government directives during outbreak all the patients were admitted in isolation ward for at least first 10 days and discharged only after they had clinical response. For the sake of this review, patients discharged from hospital were considered cured from swine flu.
STATISTICAL ANALYSIS
Data analysis was performed using CDC software EPI Info v3.5.3. Both univariate and multivariate analyses were conducted. Outcome variable, “death”, was categorized as “Yes” and “No.” The logistic regression method was used for both univariate and multivariate analyses. Exposure variables, which were statistically significant in univariate analysis or deemed clinically important (age, sex, co-morbidities, ventilatory care, and pneumonia), were included in the multivariate analysis. Final variables in the model were adjusted for confounding by age and gender. We used 95% confidence limits with 5% alpha error. Continuous variables were summarized as medians (with interquartile ranges). Hospital's ethics committee approval was taken before data collection and analysis of this study.
RESULTS
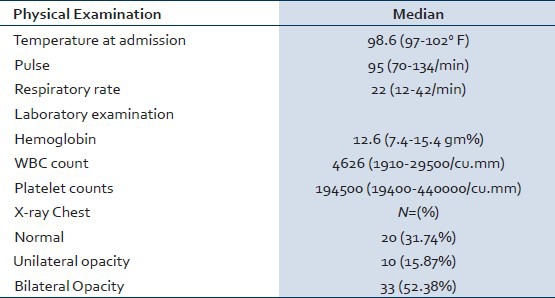
Sixty-three patients were hospitalized with confirmed swine flu by throat swab PCR methods during the study period. All the patients were included in the study for analysis, of them 41(65%) were males and remaining 22(35%) were females. The median age was 34 (3-69) years. History of travel or contact with a swine flu patient was present in 18 (28.57%) patients. Rest of the patients might have acquired infection from the community. The median duration of symptoms before hospitalization was 5 (2-20) days. Common presenting symptoms were fever 58 (92.06%), cough 58 (92.06%), breathlessness 38 (60.31%), common cold 14 (22.22%), vomiting 12 (19.04%), weakness 9 (14.28%), throat pain 7 (11.11%), body ache 5 (7.93%), and chest pain 4 (6.34%). None of the patient had hemoptysis. One patient had convulsion and altered sensorium. Various co-morbid conditions observed were diabetes mellitus 5 (7.93%), hypertension 10 (15.87%), ischemic heart disease 3 (4.76%), and human immunodeficiency virus infection 1 (1.58%). We have five pediatric patients with a median age 5 (3-8) years. Pediatric patients have high pulse rate 110 (96-128)/min and lower respiratory rate 20 (20-28)/min compared to adult patients. None of the pediatric patient died.
Physical findings and laboratory work up at the time of admission are shown in Table 1.
Table 1.
Baseline clinical and laboratory parameters
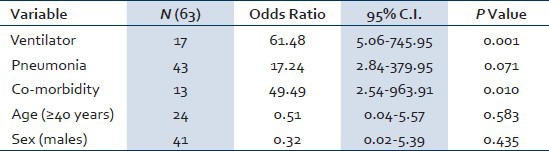
Steroids were used in 39 (61.90%) patients, of them 11 patients died. Steroids were not used in 24 (38.09%) patients, of them 3 patients died. Seventeen (26.98%) patient required ventilatory support, of them 2 patients were given non-invasive ventilator support and 15 were given invasive ventilator support. Of all the patients (n = 17) who required ventilatory support, 10 patients died, 5 patients survived, and 2 were shifted to other hospital. Forty-seven (74.60%) patients were cured and discharged from the hospital, 14 (22.22%) patients died, and 2 (3.17%) patients took discharge from hospital against medical advice or shifted to other hospital. One patient had encephalitis, myositis, and renal failure. The median duration of hospitalization was 7 (2-30) days. Cause of death was multi-organ failure in six patients and sepsis with adult respiratory distress syndrome in eight patients. The final multivariate model of factors associated with mortality in this study is shown in Table 2.
Table 2.
Multivariate analysis of factors associated with mortality
DISCUSSION
Seasonal influenza commonly starts during pre-winter period and ends as summer sets in, that is during the months of August to March in South Asian region. Diagnosis of influenza is confirmed by PCR from throat swab.[19] Common clinical symptoms of seasonal influenza include upper respiratory symptoms, cough, fever, body ache, throat pain, headache, and weakness. During the 2009 epidemic, swine flu patients also demonstrated gastrointestinal symptoms — vomiting and diarrhea apart from common symptoms. Fever and cough were the most common presenting symptoms in pandemic influenza, and in our study they were seen in 92.06% (n = 58) similar to reports from USA, Japan, and Mexico.[4,20,21,22] Vomiting and diarrhea were observed in nearly one third of patients in US series, but it was uncommon in hospitalized cases.[22] Vomiting was present in 12 (19.04%) patients but diarrhea was not observed on presentation in any patient in our series. Other symptoms observed in our study were breathlessness 38 (60.31%), common cold 14 (22.22%), weakness 9 (14.28%), throat pain 7 (11.11%), body ache 5 (7.93%), and chest pain 4 (6.34%). Breathlessness was seen in significant numbers of patients in our study, likely due to this study including only hospitalized patients.[22,23]
Seasonal influenza commonly affects old age people, while the 2009 H1N1 influenza significantly impacted young people. In our study, out of 63 patients, most were between 20 and 40 years of age (n = 34, 53.96%). Overall, 39 (61.90%) patients were below 40 years, while 24 (38.1%) were above 40 years of age, which suggest predilection for younger age in this 2009 H1N1 infection. Out of 14 patients who died in our study, 6 (42.58%) were <40 years of age, while 8 (57.14%) were ≥ 40 years of age. There was no statistically significant difference in mortality in-patient <40 or ≥40 years of age (P = 0.583) in our study. In our study, leucopenia (total WBC count <4000 cells/cu.mm) and thrombocytopenia (platelet count <100000/cu.mm) was seen in 23 (36.50%) and 7 (11.11%) patients, respectively, which is consistent with the findings in other studies.[24] Various co-morbid conditions were seen in 13 (20.63%) patients in our series. Co-morbid conditions were present in nearly 50% of hospitalized patients in USA and Mexico, which is significantly higher than our study. Morbid obesity and pregnant patients comprised a significant number of patients in their series, while in our study we did not have any pregnant and obese patients.[22] Six patients had more than one co-morbid condition. Most of the patients had either diabetes and/or cardiac disease as a co-morbid condition, while only one patient had immunosuppressive condition. In patients with co-morbidities, 8 (61.53%) patients died, while in patients without co-morbidity, 5 (10%) patients died. Co-morbidities were associated with increased risk of death in pandemic influenza 2009 patients in our study (P = 0.010). In series from United Kingdom, co-morbid conditions were also associated with increased risk of death, where obesity was present in significant number of patient as a co-morbidity and in another study from USA obesity was a risk factor for high mortality in H1N1 patients.[23,25] Out of 13 patients with co-morbid conditions, 5 required ventilatory support, while from remaining 50 patients, 12 patients required ventilatory support. Co-morbid conditions were not associated with increased risk of ventilatory requirement (P = 0.486).
Influenza is known to cause myositis, renal failure and neurologic complications; however, only one patient in our study had severe myositis, renal failure and encephalitis with residual debility.
Need for ventilatory care is associated with significantly increased mortality (P = <.0001), 10 patients out of 17 who required ventilatory support died, while 4 out of 46 patients who didn't required ventilatory support died.
Steroids were used in 39 (61.90%) patients, of them 11 patients died. Steroid was not used in 24 (38.09%) patients, of them 3 patients died. Steroid use had no effect on clinical outcome (P = 0.313). In our study use of steroids didn't affect mortality as compared to study by Quispe-Laime et al., where in ARDS patients, with and without confirmed H1N1 influenza, prolonged low-to-moderate dose corticosteroid treatment was well tolerated and associated with significant improvement in lung injury and multiple organ dysfunction scores and a low hospital mortality.[26] Two largest studies of severe H1N1 influenza A-associated respiratory failure reported the use of corticosteroids in 51-69% of patients, but its effect on outcome was not assessed, while in other studies steroids was either not useful or detrimental.[9,12,27] In our study steroids were used in patients with severe illness and need ventilator support compared to steroid non-recipient group. Subgroup analysis was not performed due to small number of subject. This might be a confounding factor for no statistically significant difference in clinical outcome seen with steroids use.
In our study, in the patients who died (n = 14), 12 had bilateral pulmonary opacities, while 2 had unilateral pulmonary opacities on chest radiograph on presentation. Bilateral opacities express possible adverse outcome. Any opacity on chest radiograph on presentation (unilateral or bilateral) was associated with increased mortality as compare to patient with normal chest radiograph (P = 0.071). In patients with bilateral pulmonary opacities (n = 33), 12 patients required mechanical ventilator support. In patient with unilateral pulmonary opacities (n = 10), only 1 patient required ventilator support. On multivariate analysis ventilatory requirement, pneumonia and co-morbidities were the independent predictors of mortality controlling for age and sex while steroid use was not adversely affecting mortality. In the state of Gujarat, western part of India, during the same time period 1209 patients with confirmed H1N1 infections were reported to the state health department, of them 289 patients died, having overall mortality rate of 23.90%. At our center, mortality was 22.22%, which is the same as overall mortality in our state.
CONCLUSIONS
In conclusion, clinical symptoms in 2009 pandemic influenza A were similar to seasonal influenza except vomiting. Mortality rate was higher in 2009 H1N1-infected patients with pneumonia, co-morbid conditions, and patients who required ventilatory support.
ACKNOWLEDGEMENTS
The authors acknowledge Drs. Mukesh Patel, Tushar Patel, Pratibha Dileep for their support during epidemic for development of consensus statement for management of swine flu patients at our hospital. They would like to acknowledge and thank Fogarty International Center, AIDS International Training and Research program faculty for their valuable technical support (AITRP Grant # D43 TW006793). They would also like to thank Dr. Patricia Emmanuel for their valuable comments and suggestions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Temte JL, Prunuske JP. Seasonal influenza in primary care settings: Review for primary care physicians. WMJ. 2010;109:193–200. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Swine influenza A (H1N1) infection in two children-Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:400–2. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection-Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–70. [PubMed] [Google Scholar]
- 4.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team; Emergence of a novel swine-origin influenza A (H1N1) virus inhumans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 5.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009A (H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilsdorf A, Poggensee G. Working Group Pandemic Influenza A(H1N1) v. Influenza A(H1N1)v in Germany: The first 10,000 cases. Euro Surveill. 2009;14 doi: 10.2807/ese.14.34.19318-en. pii:19318. [DOI] [PubMed] [Google Scholar]
- 7.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: A cross-sectional serological study. Lancet. 2010;375:1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 8.ANZIC Influenza Investigators. Webb SA, Aubron C, Bailey M, Bellomo R, Howe B, McArthur C, et al. Critical care services and the H1N1 (2009) influenza epidemic in Australia and New Zealand in 2010: The impact of the second winter epidemic. Crit Care. 2011;15:R143. doi: 10.1186/cc10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 10.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. California Pandemic (H1N1) Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 11.Zarychanski R, Stuart TL, Kumar A, Doucette S, Elliott L, Kettner J, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182:257–64. doi: 10.1503/cmaj.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, et al. Critically Ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–7. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 13.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A (H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 14.Hanshaoworakul W, Simmerman JM, Narueponjirakul U, Sanasuttipun W, Shinde V, Kaewchana S, et al. Severe human influenza infections in Thailand: Oseltamivir treatment and risk factors for fatal outcome. PLoS One. 2009;4:e6051. doi: 10.1371/journal.pone.0006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) Hospitalized patients with novel influenza A (H1N1) virus infection-California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–41. [PubMed] [Google Scholar]
- 16.Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lister P, Reynolds F, Parslow R, Chan A, Cooper M, Plunkett A, et al. Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet. 2009;374:605–7. doi: 10.1016/S0140-6736(09)61512-9. [DOI] [PubMed] [Google Scholar]
- 18.Government of India, Ministry of Health and Family Welfare. Consolidated Status of Influenza A H1N1. [Last accessed on 2010 Feb 28]. Available from: http://www.mohfw-h1n1.nic.in .
- 19.George KS. Diagnosis of influenza virus. Methods Mol Biol. 2012;865:53–69. doi: 10.1007/978-1-61779-621-0_4. [DOI] [PubMed] [Google Scholar]
- 20.Crum-Cianflone NF, Blair PJ, Faix D, Arnold J, Echols S, Sherman SS, et al. Clinical and Epidemiologic Characteristics of an Outbreak of Novel H1N1 (Swine Origin) Influenza A Virus among United States Military Beneficiaries. Clin Infect Dis. 2009;49:1801–10. doi: 10.1086/648508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Human infection with new influenza A (H1N1) virus: Clinical observations from a school-associated outbreak in Kobe, Japan, May 2009. Wkly Epidemiol Rec. 2009;84:237–44. [PubMed] [Google Scholar]
- 22.Human infection with new influenza A (H1N1) virus: Clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009;84:185–9. [PubMed] [Google Scholar]
- 23.Prasad HB, Puranik SC, Kadam DB, Sangle SA, Borse RT, Basavraj A, et al. Retrospective Analysis of Necropsy Findings in Patients of H1N1 and their Correlation to Clinical Features. J Assoc Physicians India. 2011;59:498–500. [PubMed] [Google Scholar]
- 24.Hospitalized patients with novel influenza A (H1N1) virus infection California, April-May, 2009. Morbidity and Mortality Weekly Report. 2009. [Last accessed on 2009 May 18]. p. 58. Available from: http://www.cdc.gov/mmwr/pdf/wk/mm58e0518.pdf . [PubMed]
- 25.Pebody RG, McLean E, Zhao H, Cleary P, Bracebridge S, Foster K, et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: Risk factors for death, April 2009 to March 2010. Euro Surveill. 2010:15. pii:19571. [PubMed] [Google Scholar]
- 26.Quispe-Laime AM, Bracco JD, Barberio PA, Campagne CG, Rolfo VE, Umberger R, et al. H1N1 influenza A virus-associated acute lung injury: Response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36:33–41. doi: 10.1007/s00134-009-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Hong SB, Yun SC, Choi WI, Ahn JJ, Lee YJ, et al. Korean Society of Critical Care Medicine H1N1 Collaborative. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: Analytic strategy using propensity scores. Am J RespirCrit Care Med. 2011;183:1207–14. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]


