Abstract
Obese/diabetic mothers present a higher risk to develop offspring with myelomeningocele (MM), evidence supporting the role of energy homeostasis-related genes in neural tube defects. Using polymerase chain reaction–restriction fragment length polymorphism, we have genotyped SLC2A1, HK1, and LEPR single-nucleotide polymorphisms in 105 Chilean patients with MM and their parents in order to evaluate allele–phenotype associations by means of allele/haplotype transmission test (TDT) and parent-of-origin effects. We detected an undertransmission for the SLC2A1 haplotype T-A (rs710218-rs2229682; P = .040), which was not significant when only lower MM (90% of the cases) was analyzed. In addition, the leptin receptor rs1137100 G allele showed a significant increase in the risk of MM for maternal-derived alleles in the whole sample (2.43-fold; P = .038) and in lower MM (3.20-fold; P = .014). Our results support the role of genes involved in energy homeostasis in the risk of developing MM, thus sustaining the hypothesis of diverse pathways and genetic mechanisms acting in the expression of such birth defect.
Keywords: myelomeningocele, energy homeostasis, SLC2A1, HK1, LEPR
Introduction
The closure of the neural tube occurs between the third and fourth week in human embryonic development. Total or partial failure in such process generates a group of birth defects known as neural tube defects (NTDs).1 When the failure affects the caudal portion of the neural tube, the malformation is denominated spina bifida (SB). Infants born with SB show relatively high survival rates and usually have variable degrees of physical disability.2–4 The NTDs affect between 0.5 and 2 cases per 1000 pregnancies worldwide.1 In Chile, the prevalence of NTDs is 0.5 to 0.8 per 1000 births, where over 50% account for SB and most of the cases correspond to myelomeningocele (MM).5,6
A multifactorial etiology has been postulated for SB. Both genetic and environmental factors interact in its expression.7 Genetic factors are postulated based on different prevalence rates according to ethnic origin and on the fact that first-degree SB relatives have a higher recurrence risk than second-degree relatives.8 In addition, several mouse lines carrying genetic mutations show SB among other defects.9 Consequently, association studies have shown a relation between SB and human genes that are mainly involved in metabolic pathways such as vitamin B12 and folate transport/metabolism and methylation.10
The association between obesity, diabetes mellitus, and NTDs has been also identified in humans. An increased risk (1.2- to 3.5-fold) has been observed in obese women to have offspring with NTDs when compared to nonobese ones.11 A similar figure has been described for pregestational and gestational diabetes.12 Animal studies have shown that high plasma glucose levels impair the expression of genes regulating embryonic development and normal organogenesis.13–17 Furthermore, Phelan et al18 reported that embryos of diabetic rats showed 3-fold higher rates of NTDs when compared to nondiabetic rats. These authors found that genes involved in apoptotic pathways were underexpressed in diabetic rat embryos, and that hyperglycemia affects proliferation and apoptosis of neural cell progenitors during the development of the caudal portion of the neural tube. Moreover, Yazdy et al19 recently demonstrated that diets with high dietary glycemic load may put the developing fetus at risk of NTDs in humans.
To address the hypothesis of the possible association between energy homeostasis genes and MM, Davidson et al20 analyzed the risk of MM and single-nucleotide polymorphisms (SNPs) within 12 genes which encode proteins involved in this network. In a sample of case–parent trios, these authors found a significant overtransmission of the coding variants Pro196 within glucose transporter 1 gene (SLC2A1), Lys481 of hexokinase 1 (HK1), and Arg109lys within the leptin receptor (LEPR). For this last SNP, Carter et al21 also reported an association with this malformation.
For complex diseases, association studies usually consider the functional capacity of maternal- and paternal-derived alleles as equivalent. The latter can sometimes be an incorrect assumption. Thus, parent-of-origin effects (POE) may be considered as a model of inheritance in this kind of genetic disorders.22 The POE can be directly tested by means of the case–parent trio design, stratifying the allele transmission test for mothers and fathers.23 These differential effects can be explained by epigenetic events such as imprinting, which is recognized as an important source of variation in complex traits.24 A small proportion (76) of human genes has been described as imprinted, many of which regulate placental and fetal growth (http://igc.otago.ac.nz/home.html). The POE is especially important for birth defects due to in utero environment control exerted by the maternal genotype.25 This is the case of a reduced maternal methylenetetrahydrofolate reductase activity that decreases fetal tetrahydrofolate availability, leading to birth defects such as NTDs.26,27
This report constitutes the first study in Chile attempting to find a relation between genes involved in energy homeostasis and MM. The aims of this study were (1) to examine the association between SNPs for SLC2A1, HK1, and LEPR with MM in a sample of Chilean patients and their parents and (2) to evaluate the presence of POE for the selected markers in the risk of developing MM in our sample.
Materials and Methods
Sample
Among patients followed at the Centers of the Sociedad Pro Ayuda del Niño Lisiado (TELETON-Chile) located in Santiago and Valparaiso and at the Centro de Rehabilitación Infantil del Ejército, patient carriers of SB born after January 1, 2001 (postfortification period) were invited to take part of this study. A total of 129 patients were recruited and assessed by a medical geneticist. Patient assessment included a structured survey addressing social, demographic, environmental, and medical data and a clinical assessment. Additionally, karyotypes were performed (with G-banding at 600 pb resolution) in patients considered by the Medical Geneticist to require such procedure. Following such assessment, 24 patients were excluded from further analysis. Reasons for exclusion were the following: 14 patients had occult SB, 8 patients had syndromic SB (caudal regression in 5 cases, Lenz microphthalmia in 1 case, Klippel-Feil Syndrome in 1 case, Spondylocostal Dysostosis in 1 case), 2 patients were born before the postfortification with folic acid period, and 1 patient only accepted to fill the sociodemographic survey. Therefore, the final sample included 105 isolated MM cases (86 case-parents trios and 19 case-mother duos) distributed according to lesion level in 8 upper (T-12 and above), 2 thoracolumbar, and 95 lower (L-1 and below) MM. Of all MM, 53.3% were girls. Patient age ranged between 2 months and 10 years. Patients belonged to all socioeconomic strata. Blood samples from patients and parents were obtained after signing an informed consent document. The institutional review board of the Hospital Clínico Universidad de Chile approved this study.
Molecular Analysis
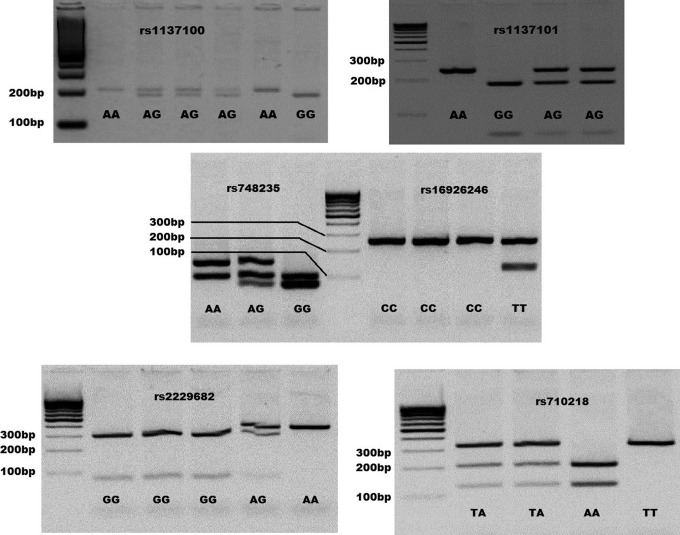
Genomic DNA was extracted from white blood cells, according to the method described by Chomczynski and Sacchi.28 Polymorphisms considered herein were selected due to their previous association with SB in other populations. This is the case of LEPR markers rs1137100 and rs1137101, SLC2A1 rs2229682, and HK1 Lys481 rs748235.20 In addition, 2 SNPs were included due to their association with conditions related to altered glucose homeostasis. Thus, we also analyzed SLC2A1 rs71021829 and HK1 rs16926246.30 Genotyping was performed using polymerase chain reaction followed by restriction fragment length polymorphism technique (PCR-RFLP) designed for this study, with the exception of SLC2A1 rs710218, which was developed by Hodgkinson et al.29 Primers were designed based on the SNP flanking sequence described in dbSNP (http://www.ncbi.nlm.nih.gov/snp/, build 135) and using the online tool Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Restriction endonucleases for each SNP were selected using the software DNA for Windows version 2.2 (http://www.dna-software.co.uk/). Primers, restriction endonucleases, and the length of digested fragments for each SNP are listed in Supplemental Table 1. Figure 1 shows restriction enzyme digestion fragment lengths for all SNPs.
Table 1.
Locations and Allele Frequencies for LEPR, SLC2A1, and HK1 SNPs.
| Gene | SNP | Physical location | Gene location | Allelesa | MAF cases mothers/fathers | |
|---|---|---|---|---|---|---|
| LEPR | rs1137100 | Chr1:66036441 | Exon 4 (Arg109Lys) | A/G | 0.242 | 0.269/0.213 |
| rs1137101 | Chr1:66058513 | Exon 6 (Arg223Gln) | A/G | 0.484 | 0.433/0.516 | |
| SLC2A1 | rs710218 | Chr1:43427218 | Promoter | A/T | 0.437 | 0.476/0.403 |
| rs2229682 | Chr1:43395635 | Exon 5 (Pro196) | G/A | 0.260 | 0.311/0.295 | |
| HK1 | rs748235 | Chr10:71142420 | Exon 10 (Lys481) | A/G | 0.381 | 0.316/0.331 |
| rs16926246 | Chr10:71093392 | Intron 12 | C/T | 0.095 | 0.121/0.118 | |
Abbreviations: LEPR, leptin receptor; MAF, minor allele frequency; SNP, single-nucleotide polymorphism.
a Major allele listed first.
Figure 1.
Restriction fragments lengths for LEPR, SLC2A1, and HK1 SNPs. Some samples and their genotypes are shown according to a 100-bp ladder and the fragment sizes described in Supplemental Table 1. Bp indicates base pair.
Statistical Analysis
All parental genotypes were considered for SNP allele frequencies estimation and to assess the Hardy–Weinberg equilibrium based on a chi-square test implemented in STATA 12 package. Transmission disequilibrium test (TDT)31 was used to detect allele/haplotype association, evaluating the pattern of allele/haplotype transmission from heterozygous parents to affected children. This test was performed using the UNPHASED 3.1.6 program which allows to include incomplete triads (case-one parent duos) by virtue of its robust capacity to estimate missing parental genotypes.32 The POE was evaluated using Clayton extension of the TDT,33 incorporated into STATA 12 package which considers 1 as the risk of the major allele. Additionally, Clayton extension includes a function that enables the detection of direct effects of maternal genotype on offspring risk. The 95% confidence intervals (CIs) for the maternal and paternal risks were estimated by applying the formula described by Bland and Altman34 based on standard error extracted from Clayton extension results.
Results
Allele frequencies for LEPR, SLC2A1, and HK1 SNPs are described in Table 1. For all markers, genotype frequencies were concordant with Hardy–Weinberg proportions (data not shown). The TDT analysis for single markers showed only borderline significance for both SLC2A1 SNPs. Thus, transmission rates from parents to affected progeny was less than expected for rs710218 T allele and rs2229682 A allele, but they did not reach statistical significance by themselves (odds ratio [OR] 0.66; 95% CI 0.42-1.03; P = .066 and OR 0.66; 95% CI 0.41-1.04; P = .072, respectively; Table 2). However, when haplotype TDT analysis was performed, a significant undertransmission was observed for the haplotype composed by these alleles (T-A: OR 0.57; 95% CI 0.34-0.97; P = .040; order of SNPs: rs710218-rs2229682; Table 2). Nevertheless, this significance disappeared when only lower MM cases (L1 and below, 90.4% of the cases) were considered (OR 0.64; 95% CI 0.37-1.12; P = .108, data not shown).
Table 2.
Results for Allele and Haplotype TDT for LEPR, SLC2A1, and HK1 SNPs.
| Gene | SNP | Allele | Allele transmission O/Ea | OR (95% CI) | P value | Haplotypesb | Haplotype transmission O/Ea | OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|
| LEPR | rs1137100 | G | 47/46 | 1.01 (0.63-1.59) | .987 | G-G | 95/97 | Ref. | .887 |
| G-A | 60/57 | 1.06 (0.65-1.73 | 0.753 | ||||||
| rs1137101 | G | 102/96 | 1.14 (0.75-1.73) | .528 | A-G | 9/12 | 0.81 (0.28-2.27) | .632 | |
| A-A | 37/36 | 1.04 (0.59-1.84) | .959 | ||||||
| SLC2A1 | rs710218 | T | 85/103 | 0.66 (0.42-1.03) | .066 | A-G | 110/95 | Ref. | .107 |
| A-A | 10/9 | 0.98 (0.26-3.72) | .938 | ||||||
| rs2229682 | A | 55/72 | 0.66 (0.41-1.04) | .072 | T-G | 39/37 | 0.87 (0.49-1.55) | .814 | |
| T-A | 44/62 | 0.57 (0.34-0.97) | .040 | ||||||
| HK1 | rs748235 | G | 73/58 | 1.43 (0.92-2.23) | .108 | A-C | 116/122 | Ref. | .529 |
| A-T | 15/24 | 0.55 (0.25-1.23) | .110 | ||||||
| rs16926246 | T | 21/28 | 0.69 (0.34-1.35) | .272 | G-C | 69/55 | 1.35 (0.85-2.12) | .162 | |
| G-T | 6/4 | 2.06 (0.40-10.5) | .479 |
Abbreviations: CI, confidence interval; LEPR, leptin receptor; OR, odds ratio; TDT, transmission disequilibrium test; SNP, single-nucleotide polymorphism.
a O/E: count for observed and expected allele/haplotype transmission from heterozygous parents.
bOrder of SNPs in haplotypes: LEPR rs1137100-rs1137101; SLC2A1 rs710218-rs2229682; HK1 rs748235-rs16926246.
The results of POE analysis for LEPR, SLC2A1, and HK1 SNPs and MM are described in Table 3. Although LEPR was not statistically significant in the overall allele transmission test, the mother–father-stratified TDT evidenced POE. Thus, the LEPR rs1137100 G allele showed a significant increase in the risk of developing an affected offspring when it was inherited from the mother (2.43-fold; 95% CI 1.01-5.86; P = .038). In addition, we intended to evaluate whether this result could be attributed to maternal genotype effects but found no significance (P = .130, data not shown). We repeated the analysis including only lower MM cases. In such situation, an increase in the risk of LEPR rs1137100 G allele was observed for maternal-derived alleles (3.20-fold increase; 95% CI 1.17-8.33; P = 0.014; data not shown) with a nonsignificant estimation for maternal genotype effect (P = .075; data not shown).
Table 3.
Maternal and Paternal Risks for Alleles of LEPR, SLC2A1, and HK1 SNPs.
| Gene | SNP | Allelea | Risk of maternally | Risk of paternally | P value |
|---|---|---|---|---|---|
| inherited allele (95% CI)b | inherited allele (95% CI)b | (likelihood ratio test) | |||
| LEPR | rs1137100 | G | 2.43 (1.01-5.86) | 0.41 (0.17-0.99) | .038 |
| rs1137101 | G | 0.68 (0.37-1.26) | 1.47 (0.79-2.72) | .220 | |
| SLC2A1 | rs710218 | T | 0.78 (0.39-1.56) | 1.29 (0.64-2.59) | .480 |
| rs2229682 | A | 1.22 (0.51-2.94) | 0.82 (0.34-1.97) | .650 | |
| HK1 | rs748235 | G | 1.29 (0.64-2.59) | 0.78 (0.39-1.56) | .480 |
| rs16926246 | T | 0.80 (0.32-2.03) | 1.25 (0.49-3.17) | .640 |
Abbreviations: CI, confidence interval; LEPR, leptin receptor; TDT, transmission disequilibrium test; SNP, single-nucleotide polymorphism.
a Allele with minor frequency (MAF). The highest frequency allele is considered as reference (risk =1) by the Clayton extension of TDT.
bConfidence intervals estimated according to Bland and Altman (2000).
Discussion
Despite the clear relationship between obesity/diabetes mellitus and increased risk of SB (and other NTDs), only one study has specifically addressed the involvement of genes related to energy homeostasis in such birth defect. In that study, Davidson et al15 described the variants Pro196 (rs2229682) for SLC2A1, Lys481 (rs748235) for HK1, and Arg109lys (rs1137100) within LEPR as MM risk factors. In addition, and considering several genes related to diverse molecular and metabolic pathways, Carter et al21 confirmed the aforementioned association for rs1137100.
Glucose is the main energy substrate for the placenta and embryo and is essential for their appropriate metabolism and growth. Glucose uptake by embryonic tissues is regulated by glucose transporters. In the brain and erythrocytes, glucose transporter 1 (GLUT1, encoded by SLC2A1 gene) is particularly responsible for glucose uptake.35 Moderate levels of GLUT1 expression are also observed in the placenta, the adipose tissue, muscle, and liver.36 Its expression has been demonstrated in preimplantation and postimplantation animal embryos36–39 and specifically during early embryogenesis in neural tube development in rats.39 Also, GLUT1-deficient mice have shown increased apoptosis levels and NTDs.40,41 Moreover, GLUT1 expression is inversely related to extracellular glucose concentration in the placenta.42 The hexokinase enzyme catalyzes the phosphorylation of glucose to glucose-6-phosphate at the initial phase of glucose metabolism. Among the 4 hexokinase isoforms, HK1 presents the highest activity in tissues with glucose dependence such as the brain and red blood cells in mammals.43 The LEPR, the receptor for the adipocyte hormone leptin, which is mainly expressed in the central nervous system, has been involved in food intake and energy expenditure processes. Leptin stimulates the expression of pro-opiomelanorcotin (POMC) in the hypothalamic arcuate nucleus. One of the posttranslational product of POMC, the α-melanocyte-stimulating hormone (α-MSH) binds to the melanocortin-4 receptor (MCR4) generating an anorexigenic stimulus. The absence of leptin increases the expression of other MC4R ligands neuropeptide Y (NPY) and Agouti-related protein (AGRP), which act by producing an orexigenic response.44 Leptin has been involved in the modulation of apoptosis in nonneural tissues.45,46 Such effect could be linked to the role of its receptor in NTDs.
The present study was based on the case–parent trio design that avoids the effect of population stratification, which can generate spurious association. This property of triads is based on the fact that the case is compared to an ethnically matched control (pseudo-control) constructed with the nontransmitted alleles.47 The genetic structure of the Chilean population presents a relation between ethnicity, Amerindian admixture, and socio-economic strata, generating a population.48,49 Thus, the case–parent trio design seems to be an excellent alternative to evaluate association by linkage disequilibrium in our sample.50
The first stage of our study searched for associations between genes related to glucose metabolism and the risk of MM in a sample of Chilean trios and duos. The results of this analysis showed that the SLC2A1 T-A haplotype (rs710218-rs2229682) could play a protective role in the whole sample, which disappears when only lower MM cases are considered. However, when these results are interpreted, the fact that lower MM is a subset of our sample (ie, with smaller sample size) should be considered. It has been postulated that NTDs can be subdivided according to lesion location, into “upper” (anencephaly and thoracic SB, which arise by failure of neurulation) or “lower” (lumbar and sacral SB, which represent errors in canalization).51 Moreover, some studies report that sibling recurrence, previous spontaneous abortions, association with other congenital malformations, and female cases are most frequent in patients with upper lesions.52–57 An EUROCAT Collaborative work studied differences between upper and lower NTDs specifically in order to look for differences in high prevalence (United Kingdom) and low prevalence (Continental Europe) areas. In the former areas, the proportion of upper SB was higher than in the latter areas (30.8% and 13.1% of all SB, respectively).58 On the other hand, the use of the antiepileptic drug valproic acid increases the risk of low SB.59–61 These differences may be the result of different embryogenesis modes or may just be a consequence of different positions in the body and the different timing of an insult. Clearly, further studies are required to assess whether these differences between upper and lower NTDs are real and how they relate to NTD incidence.
Some authors have postulated the use of haplotypes in order to improve the power to detect linkage disequilibrium (LD) in gene–disease association studies in comparison to single-marker tests.62,63 In this context, Akey et al64 have demonstrated that haplotypes-based studies can significantly improve the robustness and power of mapping complex disease genes. These authors postulate that single-marker-based methods may not capture all of the available LD information that is contained in 2 or more loci haplotypes. The SLC2A1 T-A haplotype significance could reflect underlying LD with an unknown protective variant located in the genomic segment delimitated by these SNPs. The absence of significant results for LEPR and HK1 in this stage, in comparison to previous reports, can be explained by a genetic heterogeneity phenomenon characteristic of complex diseases,65 which is related to ethnic origin differences between the analyzed populations by Davidson et al20 Carter et al,21 and the one herein considered.
We decided to present uncorrected P values for our results due to a lack of consensus about the most adequate strategy or method for correcting association P values for multiple tests.66,67 UNPHASED also includes a function that enables including incomplete triads, which was applied in our study. The authors of this software state that the program is robust in its capacity to estimate missing parental genotypes.32Nonetheless, this function seems to increase Type I error for the TDT test68 which, in addition to the modest sample size, might be a limitation for our study. In this context, applying the UNPHASED haplotype TDT considering only complete triads, the result for the SLC2A1 T-A haplotype remains significant (P = .019; data not shown) similar to the one observed using FBAT.69 This last program shows a Type I error lower than UNPHASED.68 Thus, taking into account all of the just mentioned elements, our results can be considered as significant.
The second stage of our study considered the possibility that the analyzed SNP alleles present a differential risk of MM depending on its parental origin. To our knowledge, previous reports have detected this relation for NTDs when gene–nutrient interactions are present such as those occurring in folate metabolism.26,27 As we previously mentioned in complex traits, POE can be explained by epigenetic phenomena or by the effect of maternal genotype over in utero environment.24,27 In our study, significant results were observed only for marker rs1137100 (Arg109Lys) within LEPR, where its G allele shows a higher risk when inherited from mothers (Table 3). Although, differences in allele frequency for this SNP are observed among mothers and fathers (see Table 1), these are not significant (P = .206, data not shown) a fact observed for all of the markers included herein. This result contributes to infer that the rs1137100 G allele shows a maternal preferential transmission not influenced by its higher frequency in mothers than fathers. This POE might have 2 alternative interpretations. It is possible that the presence of rs1137100 G allele in the maternal genotype might have any kind of effect over the fetus, that is, a maternal genotype effect. However, our analysis did not demonstrate that such differential transmission is attributable to this phenomenon. Thus, the second interpretation points out to the presence of epigenetic control by imprinting in the role of LEPR in MM. Nevertheless, this result is only the statistical evidence that requires biological support. In this context, at least in the Catalogue of Parent of Origin Effects (http://igc.otago.ac.nz/home.html), there are no records proving imprinting for LEPR gene. Consequently, further analyses are needed in order to elucidate whether this regulatory mechanism is present for the cited gene. Finally, we wish to emphasize the nonsignificant results obtained for rs1137100 when conventional TDT was applied. The TDT analysis does not consider the origin of the alleles that could debilitate the significance of a specific risk allele. On the other hand, the Clayton extension stratifies alleles according to their parental origin, discriminating the effects of markers depending on parents’ gender.
In summary, our results support the role of SCL2A1 and LEPR genes in the risk of developing MM since significance was found for allele/haplotype transmission regardless of considering their parental origin. These findings allow us to support the hypothesis of diverse genetic mechanisms acting in the expression of this birth defect as in other complex diseases. Although our findings are interesting, it is important to note that (1) our sample size is modest, (2) it corresponds to a specific population, and (3) we did not include a correction by multiple comparisons (which would decrease the significance especially for haplotype association). Therefore, to validate the relevance of our findings, it is important to replicate them in one or more independently ascertained MM samples (in family- and/or population-based studies).
Supplementary Material
Acknowledgments
We are grateful to all patients and their families. This work was supported by Sociedad Chilena de Pediatría (Grant No. 2010001) and Genetics Unit, Hospital Clínico Universidad de Chile (N°. OAIC 466/11). We also thank Sociedad Pro Ayuda del Niño Lisiado (TELETON- Chile), and Centro de Rehabilitación Infantil del Ejército de Chile (CRIE) for their logistic support.
Footnotes
Authors’ Note: This study was performed in Hospital Clínico Universidad de Chile and Pontificia Universidad Católica de Chile. Supplemental Table 1 is available at http://rs.sagepub.com/supplemental.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sociedad Chilena de Pediatría (Grant No. 2010001) and Sección de Genética, Hospital Clínico Universidad de Chile (No. OAIC 466/11).
References
- 1. Bassuk AG, Kibar Z. Genetic basis of neural tube defects. Semin Pediatr Neurol. 2009;16(3):101–110. [DOI] [PubMed] [Google Scholar]
- 2. Gutierrez FR, Woodard PK, Fleishman MJ. Normal anatomy and congenital anomalies of the spine and spinal cord. In: Osborn AG, Maack H, eds. Diagnostic Neuroradiology. St. Louis, MO: Mosby; 1994:785–819. [Google Scholar]
- 3. Melvin EC, George TM, Worley G, et al. Genetic studies in neural tube defects. NTD Collaborative Group. Pediatr Neurosurg. 2000;32(1):1–9. [DOI] [PubMed] [Google Scholar]
- 4. Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: a review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology. 2000;42(7):471–491. [DOI] [PubMed] [Google Scholar]
- 5. Cortés F, Mellado C, Pardo RA, Villarroel LA, Hertrampf E. Wheat flour fortification with folic acid: changes in neural tube defects rates in Chile. Am J Med Genet A. 2012;158A(8):1885–1890. [DOI] [PubMed] [Google Scholar]
- 6. López-Camelo JS, Castilla EE, Orioli IM; INAGEMP (Instituto Nacional de Genética Médica Populacional); ECLAMC (Estudio Colaborativo Latino Americano de Malformaciones Congénitas). Folic acid flour fortification: impact on the frequencies of 52 congenital anomaly types in three South American countries. Am J Med Genet A. 2010;152A(10):2444–2458. [DOI] [PubMed] [Google Scholar]
- 7. Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341(20):1509–1519. [DOI] [PubMed] [Google Scholar]
- 8. Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79(3):187–210. [DOI] [PubMed] [Google Scholar]
- 10. Green R. Is it time for vitamin B-12 fortification? What are the questions? Am J Clin Nutr. 2009;89(2):712S–716S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Racusin D, Stevens B, Campbell G, Aagaard KM. Obesity and the risk and detection of fetal malformations. Semin Perinatol. 2012;36(3):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carmichael SL, Rasmussen SA, Shaw GM. Prepregnancy obesity: a complex risk factor for selected birth defects. Birth Defects Res A Clin Mol Teratol. 2010;88(10):804–810. [DOI] [PubMed] [Google Scholar]
- 13. Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in preimplantation embryos through cell death effector pathways. Nature Med. 1998;4(12):1421–1424. [DOI] [PubMed] [Google Scholar]
- 14. Chang TI, Loeken MR. Genotoxicity and diabetic embryopathy: impaired expression of developmental control genes as a cause of defective morphogenesis. Semin Repord Endocrinol. 1999;17(2):153–165. [DOI] [PubMed] [Google Scholar]
- 15. Moley KH. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol Metab. 2001;12(2):78–82. [DOI] [PubMed] [Google Scholar]
- 16. Fine EL, Horal M, Chang TI, Fortin G, Loeken MR. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48(812):2454–2462. [DOI] [PubMed] [Google Scholar]
- 17. Fu J, Tay SS, Ling EA, Dheen ST. High glucose alters the expression of genes involved in proliferation and cell-fate specification of embryonic neural stem cells. Diabetologia. 2006;49(5):1027–1038. [DOI] [PubMed] [Google Scholar]
- 18. Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46(7):1189–1197. [DOI] [PubMed] [Google Scholar]
- 19. Yazdy MM, Liu S, Mitchell AA, Werler MM. Maternal dietary glycemic intake and the risk of neural tube defects. Am J Epidemiol. 2010;171(4):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davidson CM, Northrup H, King TM, et al. Genes in glucose metabolism and association with spina bifida. Reprod Sci. 2008;15(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carter TC, Pangilinan F, Troendle JF, et al. Evaluation of 64 candidate single nucleotide polymorphisms as risk factors for neural tube defects in a large Irish study population. Am J Med Genet A. 2011;155A(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guilmatre A, Sharp AJ. Parent of origin effects. Clin Genet. 2012;81(3):201–209. [DOI] [PubMed] [Google Scholar]
- 23. Weinberg CR. Methods for detection of parent-of-origin effects in genetic studies of case-parents triads. Am J Hum Genet. 1999;65(1):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hager R, Cheverud JM, Wolf JB. Maternal effects as the cause of parent-of-origin effects that mimic genomic imprinting. Genetics. 2008;178(3):1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkins JF, Haig D. What good is genomic imprinting: the function of parent-specific gene expression. Nat Rev Genet. 2003;4(5):359–368. [DOI] [PubMed] [Google Scholar]
- 26. Christensen B, Arbour L, Tran P, et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet. 1999;84(2):151–157. [DOI] [PubMed] [Google Scholar]
- 27. Dean JC, Moore SJ, Osborne A, Howe J, Turnpenny PD. Fetal anticonvulsant syndrome and mutation in the maternal MTHFR gene. Clin Genet. 1999;56(3):216–220. [DOI] [PubMed] [Google Scholar]
- 28. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. [DOI] [PubMed] [Google Scholar]
- 29. Hodgkinson AD, Page T, Millward BA, Demaine AG. A novel polymorphism in the 5' flanking region of the glucose transporter (GLUT1) gene is strongly associated with diabetic nephropathy in patients with Type 1 diabetes mellitus. J Diabetes Complications. 2005;19(2):65–69. [DOI] [PubMed] [Google Scholar]
- 30. Soranzo N, Sanna S, Wheeler E, et al. Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59(12):3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet. 1993;52(3):506–516. [PMC free article] [PubMed] [Google Scholar]
- 32. Dudbridge F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered. 2008;66(2):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cordell HJ, Barratt BJ, Clayton DG. Case/pseudocontrol analysis in genetic association studies: a unified framework for detection of genotype and haplotype associations, gene-gene and gene-environment interactions, and parent-of-origin effects. Genet Epidemiol. 2004;26(3):167–185. [DOI] [PubMed] [Google Scholar]
- 34. Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ. 2000;320(7247):1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo X, Geng M, Du G. Glucose transporter 1, distribution in the brain and in neural disorders: its relationship with transport of neuroactive drugs through the blood-brain barrier. Biochem Genet. 2005;43(3-4):175–187. [DOI] [PubMed] [Google Scholar]
- 36. Trocino R, Akazawa S, Takino H, et al. Cellular-tissue localization and regulation of the GLUT-1 protein in both the embryo and the visceral yolk sac from normal and experimental diabetic rats during the early postimplantation period. Endocrinology. 1994;134(2):869–878. [DOI] [PubMed] [Google Scholar]
- 37. Gao L, Lv C, Xu C, et al. Differential regulation of glucose transporters mediated by CRH receptor type 1 and type 2 in human placental trophoblasts. Endocrinology. 2012;153(3):1464–1471. [DOI] [PubMed] [Google Scholar]
- 38. Morita Y, Tsutsumi O, Oka Y, Taketani Y. Glucose transporter GLUT1 mRNA expression in the ontogeny of glucose incorporation in Mouse preimplantation embryos. Biochem Biophys Res Commun. 1994;199(3):1525–1531. [DOI] [PubMed] [Google Scholar]
- 39. Matsumoto K, Akazawa S, Ishibashi M, et al. Abundant expression of GLUT1 and GLUT3 in rat embryo during the early organogenesis period. Biochem Biophys Res Commun. 1995;209(1):95–102. [DOI] [PubMed] [Google Scholar]
- 40. Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem. 2000;275(51):40252–40257. [DOI] [PubMed] [Google Scholar]
- 41. Heilig CW, Saunders T, Brosius FC, 3rd, et al. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci U S A. 2003;100(26):15613–15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Illsley NP. Glucose transporters in the human placenta. Placenta. 2000;21(1):14–22. [DOI] [PubMed] [Google Scholar]
- 43. Bianchi M, Magnani M. Hexokinase mutations that produce nonspherocytic hemolytic anemia. Blood Cells Mol Dis. 1995;21(1):2–8. [DOI] [PubMed] [Google Scholar]
- 44. Farooqi IS, O'Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4(10):569–577. [DOI] [PubMed] [Google Scholar]
- 45. Mansour E, Pereira FG, Araújo EP, et al. Leptin inhibits apoptosis in thymus through a janus kinase-2-independent, insulin receptor substrate-1/phosphatidylinositol-3 kinase-dependent pathway. Endocrinology. 2006;147(11):5470–5479. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, Huang C. Targeting adipocyte apoptosis: a novel strategy for obesity therapy. Biochem Biophys Res Commun. 2012;417(1):1–4. [DOI] [PubMed] [Google Scholar]
- 47. Beaty TH, Hetmanski JB, Zeiger JS, et al. Testing candidate genes for non-syndromic oral clefts using a case-parent trio design. Genet Epidemiol. 2002;22(1):1–11. [DOI] [PubMed] [Google Scholar]
- 48. Valenzuela CY, Acuña MP, Harb Z. Sociogenetic gradient in the Chilean population. Rev Med Chil. 1987;115(4):295–299. [PubMed] [Google Scholar]
- 49. Valenzuela CY. On sociogenetic clines. Ethol Sociobiol. 1988;9(5):259–268. [Google Scholar]
- 50. Suazo J, Santos JL, Carreño H, Jara L, Blanco R. Linkage disequilibrium between MSX1 and non-syndromic cleft lip/palate in the Chilean population. J Dent Res. 2004;83(10):782–725. [DOI] [PubMed] [Google Scholar]
- 51. Toriello HV, Higgins JV. Possible causal heterogeneity in spina bifida cystica. Am J Med Genet. 1985;21(1):13–20. [DOI] [PubMed] [Google Scholar]
- 52. Toriello HV, Higgins JV. Occurrence of neural tube defects among first-, second-, and third-degree relatives of probands: results of a United States study. Am J Med Genet. 1983;15(4):601–606. [DOI] [PubMed] [Google Scholar]
- 53. Hall J, Solehdin F. Folic acid for the prevention of congenital anomalies. Eur J Pediatr. 1998;157(6):445–450. [DOI] [PubMed] [Google Scholar]
- 54. Drainer E, May HM, Tolmie JL. Do familial neural tube defects breed true? J Med Genet. 1991;28(9):605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garabedian BH, Fraser FC. Upper and lower neural tube defects: an alternate hypothesis. J Med Genet. 1993;30(10):849–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Källén B, Cocchi G, Knudsen LB, et al. International study of sex ratio and twinning of neural tube defects. Teratology. 1994;50(5):322–331. [DOI] [PubMed] [Google Scholar]
- 57. Seller MJ. Sex, neural tube defects, and multisite closure of the human neural tube. Am J Med Genet. 1995;58(4):332–336. [DOI] [PubMed] [Google Scholar]
- 58. Dolk H, De Wals P, Gillerot Y, et al. Heterogeneity of neural tube defects in Europe: the significance of site of defect and presence of other major anomalies in relation to geographic differences in prevalence. Teratology. 1991;44(5):547–559. [DOI] [PubMed] [Google Scholar]
- 59. Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2(8304):937. [DOI] [PubMed] [Google Scholar]
- 60. Van Allen MI, Kalousek DK, Chernoff GF, et al. Evidence for multi-site closure of the neural tube in humans. Am J Med Genet. 1993;47(5):723–743. [DOI] [PubMed] [Google Scholar]
- 61. Lindhout D, Omtzigt JG, Cornel MC. Spectrum of neural-tube defects in 34 infants prenatally exposed to antiepileptic drugs. Neurology. 1992;42(4 suppl 5):111–118. [PubMed] [Google Scholar]
- 62. Escamilla MA, McInnes LA, Spesny M, et al. Assessing the feasibility of linkage disequilibrium methods for mapping complex traits: an initial screen for bipolar disorder loci on chromosome 18. Am J Hum Genet. 1999;64(6):1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martin ER, Lai EH, Gilbert JR, et al. SNPing away at complex diseases: analysis of single-nucleotide polymorphisms around APOE in Alzheimer disease. Am J Hum Genet. 2000;67(2):383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Akey J, Jin L, Xiong M. Haplotypes vs single marker linkage disequilibrium tests: what do we gain? Eur J Hum Genet. 2001;9(4):291–300. [DOI] [PubMed] [Google Scholar]
- 65. Schork NJ. Genetics of complex disease: approaches, problems, and solutions. Am J Respir Crit Care Med. 1997;156(4 pt 2):S103–S109. [DOI] [PubMed] [Google Scholar]
- 66. Barroso I, Luan J, Middelberg RP, et al. Candidate gene association study in type 2 diabetes indicates a role for genes involved in b-cell function as well as insulin action. PLoS Biol. 2003;1(1):41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Perneger TV. Adjusting for multiple testing in studies is less important than other concerns. Brit Med J. 1999;318(7193):1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hiekkalinna T, Göring HH, Lambert B, et al. On the statistical properties of family-based association tests in datasets containing both pedigrees and unrelated case-control samples. Eur J Hum Genet. 2012;20(2):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(suppl 1):S36–S42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.