Abstract
Despite significant advances in prevention and treatment strategies, graft-versus-host disease remains the most significant cause of morbidity and nonrelapse mortality after allogeneic hematopoietic cellular transplantation. Corticosteroids remain the standard frontline therapy for graft-versus-host disease; however, a considerable number of patients will not respond adequately and others will be significantly affected by adverse effects. Extracorporeal photopheresis is one of several secondary therapies which have shown promise in the clinical setting. While the procedure itself has been around for over 20 years, our understanding of the mechanisms from which therapeutic benefits are seen, and the population they are seen in, remains limited. In this article, we review the use of extracorporeal photopheresis for the treatment of graft-versus-host disease including details covering the procedure’s mechanism of action, safety profile and clinical efficacy data.
Keywords: Graft-versus-host disease, hematopoietic stem-cell transplantation, immunomodulation, photopheresis
Introduction
Allogeneic hematopoietic cellular transplantation (AHCT) offers the potential for cure for the 25,000 patients worldwide with various forms of malignant and nonmalignant diseases who undergo this therapy each year. Risks associated with AHCT have been reduced through advancements in the prevention and treatment of infections, more precise human leukocyte antigen (HLA) typing of donors, improvement in conditioning regimens and better supportive care. Despite these advances, treatment-related mortality related to graft-versus-host disease (GVHD) remains a significant problem and hampers wider application. For patients who develop GVHD, corticosteroids remain the standard initial therapy; however, a sizable fraction of patients will not respond adequately and will require secondary therapy. Extracorporeal photopheresis (ECP; also referred to as extracorporeal photochemotherapy or photopheresis) is one such therapy commonly performed. ECP has been performed for the treatment of GVHD for over two decades; however, a complete understanding of which patients might best benefit remains to be established. The goal of this review is to provide the reader with an overview of ECP with particular attention to its use in the treatment of GVHD. In addition, a critical assessment of the current body of literature related to its efficacy and proposed mechanisms of action will be provided.
Overview of GVHD
The close association between GVHD and the derived benefit resulting from a graft-versus-tumor (GVT) effect is a major paradox of this complication. Mature donor T cells contained within the infused graft mediate both GVHD and GVT with both being reduced when T cells are depleted (referred to as T-cell-depleted transplants). GVHD is clinically separated into an acute and chronic form which was historically defined as GVHD before or after day 100. As practices have changed such as the use of less-intense conditioning regimens and the greater use of immune-modulating strategies, acute GVHD manifestations have increasingly occurred after day 100. As such, this time point is now viewed as an artificial distinction and instead, clinical manifestation and histologic findings are the sole factors used in defining these entities [Filipovich et al. 2005].
The incidence of acute GVHD varies from 20% to 70% depending upon the extent of histocompatibility differences between donor and patient, the age of the recipient, the stage of primary disease and the intensity of the conditioning regimen [Flowers et al. 2011; Hahn et al. 2008]. The primary target organs of acute GVHD are the skin, liver, and gastrointestinal (GI) tract which are graded clinically using a standardized system which allows for quantitative estimates of disease severity and response to therapy with scoring consisting of grades I to IV [Przepiorka et al. 1995]. Corticosteroids are the standard initial treatment but even with prompt initiation, response is suboptimal with less than 50% of patients achieving a durable response and ultimately requiring secondary treatment [Hings et al. 1994; MacMillan et al. 2002]. Outcomes for patients with steroid-refractory GVHD are poor with mortality rates as high as 70% and deaths commonly resulting from organ failure and infections related to poor immune reconstitution [Weisdorf et al. 1990].
Chronic GVHD affects approximately 50–70% of patients who receive an allogeneic transplant [Lee et al. 2003]. This number varies widely based on the presence or absence of risk factors with one large series demonstrating a 5-year cumulative incidence for chronic GVHD as low as 9% or as high as 75% based on the presence or absence of various risk factors [Carlens et al. 1998]. The consequences of chronic GVHD for patients can be profound with its presence being the most significant determinant of long-term survival and quality of life [Lee et al. 2006]. Chronic GVHD has protean manifestations and involvement of virtually every potential organ has been reported. The skin is the most frequently affected site with involvement in 75% of cases at the time of initial diagnosis followed by the mouth, liver, and eyes [Lee et al. 2003]. Less frequently, the GI tract (weight loss), lung, esophagus, female genital tract, or joints may be affected.
GVHD pathophysiology
Animal models designed to study the pathophysiology of acute GVHD suggest that it occurs in a three-phase process [Ferrara and Reddy, 2006]. In this model, GVHD is initiated when tissue damage occurs as a result of toxicity from the conditioning regimen which in turn results in release of inflammatory cytokines. The second phase occurs with infusion of mature donor lymphocytes contained within the graft into this inflamed environment promoting activation and expansion of these lymphocytes when contact is made with antigen-presenting cells (APCs) expressing disparate host antigens (minor histocompatibility antigens in the case of HLA-matched transplants). In the final effector phase, these alloreactive T cells expand into cytotoxic effector T cells which cause further tissue injury and release of more inflammatory cytokines. While this model is a simplification of the complex and varied pathophysiology of this disorder, it provides a framework for evaluating potential therapeutic interventions. Unfortunately, the immunobiology of chronic GVHD is less understood in large part due to the absence of animal models that are able to fully reproduce the complexity of the disorder [Chu and Gress, 2008; Toubai et al. 2008]. Instead, animal models of chronic GVHD are often based on disease-specific autoimmune models which only simulate certain organ involvement and narrowly test hypotheses. Clinical support for autoimmune animal models comes from the shared clinical manifestations between patients with chronic GVHD and various autoimmune disorders such as systemic sclerosis (scleroderma), dermatomyositis and the sicca syndrome. In addition, patients with chronic GVHD have a high incidence of autoantibodies and disease-related gene polymorphisms common to patients with autoimmune disorders [Quaranta et al. 1999; Shimada et al. 2007]. While these clinical similarities support consideration of chronic GVHD as an autoimmune disorder, this implies that the target antigens are not disparate antigens (as in acute GVHD), but rather nonpolymorphic antigens common to both donor and recipient. In the autoimmune model of chronic GVHD, it is postulated that failure of immune tolerance mechanisms results in activation and expansion of T cells directed against self-antigens. Murine models have suggested that pathogenic T cells may arise when maturation of donor precursor cells occurs in a damaged recipient thymus allowing escape from central negative selection [Fukushi et al. 1990; Hess et al. 1985, 1997; Hollander et al. 1994; Sakoda et al. 2007; Teshima et al. 2003]. In support of this, investigators have identified T cells in animal models of chronic GVHD which are specific for common (shared between host and donor) antigens and are thus considered true ‘autoreactive’ clones [Hess et al. 1985, 1997]. These pathogenic T cells can then escape tolerance mechanisms operating in the periphery [Fukushi et al. 1990; Hollander et al. 1994; Sakoda et al. 2007; Teshima et al. 2003]. Recent interest has focused on the role of T-regulatory cells (Tregs) in loss of tolerance in chronic GVHD. These cells have been found to be deficient in number and function in various autoimmune disorders, which led investigators to examine their role in GVHD [Ehrenstein et al. 2004; Miyara et al. 2005]. While data is conflicting, some investigators have suggested that an imbalance between Tregs and alloreactive effector T cells results in increased risk and worsening of chronic GVHD [Chen et al. 2007; Clark et al. 2004; Rieger et al. 2006; Zorn et al. 2005]. The infusion of Tregs has been successful in either preventing or treating acute and chronic GVHD in both animal models [Giorgini and Noble, 2007; Hoffmann et al. 2002; Mutis et al. 2006; Zhao et al. 2008] as well as in early clinical trials [Brunstein et al. 2011; Di Ianni et al. 2011]. While experimental and clinical evidence support the concept of chronic GVHD as an autoimmune disorder, there has been an inability to isolate in humans donor-derived T-cell clones that recognize nonpolymorphic antigens expressed in donor and recipient. Accordingly, some investigators have postulated that chronic GVHD could simply be the result of T cells responding to chronic antigen stimulation due to the presence of ubiquitous disparate antigens [Toubai et al. 2008].
National Institute of Health GVHD classification
In 2005 the National Institute of Health (NIH) Consensus group published a series of papers addressing the classification, diagnosis and assessment of chronic GVHD [Filipovich et al. 2005; Martin et al. 2006; Pavletic et al. 2006; Shulman et al. 2006]. The net result of these publications was the creation of GVHD categories based on specific manifestations and the time point for which they occur along with the categorization of chronic GVHD into progressive, quiescent and de novo forms (summarized in Tables 1 and 2). In addition, a chronic GVHD severity index was created to document the degree of individual organ involvement and assess organ-specific response to therapy. Prior to these guidelines, determining the efficacy for chronic GVHD therapy was less precise and based on subjective assessment by the clinician. Unfortunately, the NIH response criteria were created by expert opinion and lack evidence with respect to impact on long-term outcomes or patient assessed quality of life.
Table 1.
Categories of acute and chronic GVHD.
| Acute GVHD* | |
| Classic acute GVHD | Features which include maculopapular rash, nausea, vomiting, anorexia, profuse diarrhea, ileus or cholestatic hepatitis occurring within 100 days after transplant or DLI and without diagnostic or distinctive signs of chronic GVHD |
| Persistent, recurrent or late-onset acute GVHD | Features of classic acute GVHD without diagnostic or distinctive manifestations of chronic GVHD occurring beyond 100 days of transplant or DLI |
| Chronic GVHD | |
| Classic chronic GVHD | Classic chronic GVHD without features characteristic of acute GVHD |
| Overlap syndrome | Classic chronic GVHD along with features characteristic of acute GVHD |
DLI, donor lymphocyte infusion; GVHD, graft-versus-host disease.
In the absence of histologic or clinical signs or symptoms of chronic GVHD, the persistence, recurrence or new onset of characteristic skin, GI tract, or liver abnormalities should be classified as acute GVHD regardless of the time after transplant.
Table 2.
Onset types for chronic GVHD.
| De novo | Chronic GVHD appearing in a patient with no previous acute GVHD |
| Quiescent | Chronic GVHD occurring after a complete resolution of acute GVHD |
| Progressive | Chronic GVHD following unresolved acute GVHD |
GVHD, graft-versus-host disease.
ECP overview
ECP is an apheresis-based immunomodulatory therapy which received US Food and Drug Administration (FDA) approval in the late 1980s for the palliative treatment of skin manifestations in patients with cutaneous T-cell lymphoma (CTCL) unresponsive to other forms of treatment based on a study by Edelson and colleagues which demonstrated a response rate of greater than 70% [Edelson et al. 1987]. Recently, a meta-analysis was performed demonstrating an over-all response rate of greater than 50% for ECP in patients with CTCL and long-term follow-up data suggesting a survival advantage for patients receiving ECP versus other therapies [Edelson et al. 1987; Lim and Edelson, 1995; Zic, 2003]. While CTCL remains ECP’s sole FDA-approved indication, its use has been studied in a number of autoimmune-mediated disorders and in solid organ allograft rejection (presented in Table 3).
Table 3.
Other experimental indications for ECP therapy.
| Disease | Number of patients | Trial and results | Study |
|---|---|---|---|
| Systemic sclerosis | 64 | Multicenter, RCT of ECP versus sham with significant improvement in skin scores compared with baseline for ECP group | Knobler et al. [2006] |
| Solid-organ transplant rejection | 61 | Phase II RCT in cardiac transplantation of conventional ISP versus ISP + ECP with a significant reduction in acute rejection episodes for ECP group | Barr et al. [1998] |
| Crohn’s disease | 28 | Multicenter single-arm study of ECP in moderate–severe, refractory Crohn’s with 50% responding according to the Crohn’s disease activity index score | Abreu et al. [2009] |
| Atopic dermatitis | 35 | Bicenter single-arm study of ECP in severe, refractory patients with 73% responding and a significant overall reduction in atopic dermatitis score | Radenhausen et al. [2004] |
| Type I diabetes | 40 | RCT of ECP versus sham in newly diagnosed patients with significantly lower insulin requirements for ECP group | Ludvigsson et al. [2001] |
| Pemphigus vulgaris | 4 | Case series of treatment refractory patients with three of four experiencing complete remission and able to wean other ISP | Rook et al. [1989] |
| Rheumatoid arthritis | 7 | Open-label pilot study with four apparent responders | Malawista et al. [1991] |
| Systemic lupus erythematosus | 10 | Open-label pilot study with seven of eight patients completing the trial responding with a significant decrease in clinical activity score | Knobler et al. [1992] |
| Nephrogenic systemic fibrosis | 3 | Case series with all patients showing clinical response including softening of skin plaques and improved range of movement in all four limbs | Mathur et al. [2008] |
ECP, extracorporeal photopheresis; ISP, immunosuppression; RCT, randomized controlled trial.
The procedure of ECP therapy involves ultraviolet A (UVA) irradiation of autologous peripheral blood mononuclear cells (PBMCs) which have been collected by leukapheresis and exposed to the photosensitizing drug 8-methoxypsoralen (8-MOP). More specifically, whole blood is leukapheresed from the patient and centrifuged to separate out the leukocyte-enriched buffy coat. A sufficient amount of whole blood must be removed to produce approximately 240 ml of buffy coat which usually requires 3–6 cycles of apheresis [Foss et al. 2002]. The buffy coat layer is then injected with 20 µg/ml of soluble 8-MOP (Uvadex®) and irradiated for 25–45 minutes with UVA light providing an approximate exposure of 1.5 J/cm2 [Foss et al. 2002]. The photoactive buffy coat is subsequently re-infused into the patient. The technology and equipment involved with ECP therapy was developed by Therakos, Inc. (Westchester, PA, USA) with a majority of the procedures in the United States involving the Therakos Uvar XTS system (second-generation model). This machine uses a discontinuous separation process, which is problematic in patients weighing under 40 kg or those with a preprocedural hematocrit <30% because of the volume of extracorporeal blood required. More recently (in 2009), the third-generation Therakos Cellex device has become available with advances including continuous flow separation technology, shorter treatment times (1.5 versus 3 hours) and reduced extracorporeal blood volumes allowing for lower-weight patients to safely undergo the procedure.
The standard schedule for CTCL typically involves ECP treatment on 2 consecutive days every 2–4 weeks which is continued for approximately 6 months followed by a maintenance schedule tailored to patient response. While initial studies in patients with GVHD utilized a similar treatment schedule, an accelerated regimen consisting of 2–3 weekly treatments tapered to an every 2- to 4-week regimen has gained more popularity.
Mechanism of action
One of the most fascinating attributes of ECP therapy is that it is beneficial in both CTCL and GVHD, two different immune disorders. ECP is believed to be capable of eliciting two completely opposite immune effects: immunostimulatory effects against neoplastic cells in CTCL and immunosuppressive effects against T-cell-mediated disorders such as GVHD. During ECP, PBMCs are exposed to 8-MOP which covalently binds and cross-links DNA upon exposure to UVA light irradiation resulting in apoptosis [Berger et al. 2002; Bladon and Taylor, 2006; Cimino et al. 1985; Gasparro et al. 1989; Marks et al. 1990]. However, apoptosis of lymphocytes is unlikely to be the direct mechanism through which ECP exerts immunomodulatory affects as only 5–10% of circulating leukocytes are treated and prolonged responses have been observed well past what could be ascribed directly to apoptosis [Knobler et al. 2009]. Rather, the clinical benefits are likely to occur as a result of the processing of these apoptotic cells and the subsequent immune responses that this generates.
Dendritic cells (DCs) are specialized APCs that are capable of controlling immunity, triggering immune responses and maintaining tolerance [Steinman et al. 2000]. It is believed that ECP- induced lymphocyte apoptosis stimulates the differentiation of monocytes into immature DCs that can then become active APCs and cytokine producers [Foss et al. 2002]. Reports of cytokine responses post-ECP have demonstrated both an increased production of immunostimulatory [tumor necrosis factor (TNF)-α and interleukin (IL)-6] as well as immunosuppressive (IL-10 and IL-1Ra) cytokines in CTCL and GVHD patients, respectively [Berger et al. 2002; Fimiani et al. 2004; Vowels et al. 1992].
It has been shown that through the uptake of apoptotic cells, DCs are able to induce peripheral tolerance to self. This is likely to occur in humans during processing of apoptotic cells generated by normal cell turnover in the GI tract or in the processing of damaged host tissues following viral infections [Huang et al. 2000; Morelli et al. 2003; Steinman et al. 2000]. A similar process is likely to occur as a result of lymphocyte apoptosis induced by ECP. When ECP-treated lymphocytes are re-infused, APCs recognize the cell surface markers of apoptosis and modify APC function. APCs that have phagocytized apoptotic T cells present antigen to T cells in the lymph node and spleen which results in the generation of (antigen-specific) Tregs which are specific for the pathogenic T-cell clones. As the majority of T cells in the ECP-treated product are from expanded clones predominating in the blood stream, ECP is suppressive for the cellular immune responses active in the patient at the time of the procedure (in this case GVHD). The immune response resulting from ECP treatment may be further modulated by the immune environment present in the patient at the time of therapy. This may account for the seemingly divergent immune responses in patients with CTCL and GVHD.
Murine models provide further evidence implicating Tregs in ECP-induced immunosuppression. In one such study, splenocytes treated with 8-MOP and UVA irradiation were injected into syngeneic mice who then received a cardiac allograft [George et al. 2008]. Recipients of the treated splenocytes experienced improved graft survival when compared with mice receiving untreated splenocytes. Furthermore, adoptive transfer of CD4(+)CD25(+) splenocytes from ECP-treated graft recipients to untreated cardiac allograft recipients resulted in extended graft survival compared with animals that received the same number of CD4(+)CD25(+) splenocytes from cardiac allograft recipients not treated with ECP. Similarly, in an accepted mouse models of GVHD, transfer of cells treated with ECP reversed established GVHD by increasing donor Tregs and indirectly reducing the number of donor effector lymphocytes that themselves had never been exposed to psoralen and UVA radiation (PUVA) [Gatza et al. 2008]. These experiments would suggest that ECP reverses GVHD through a reduction in responses of donor effector T cells and generation of donor Tregs. The fact that this process occurs in donor cells that had not been directly exposed to PUVA offers additional support excluding direct apoptosis of effector cells as a mechanism for which ECP exerts its beneficial effects in GVHD. In support of these mouse models, Tregs have been shown to be increased in patients receiving ECP for the treatment of GVHD [Biagi et al. 2007].
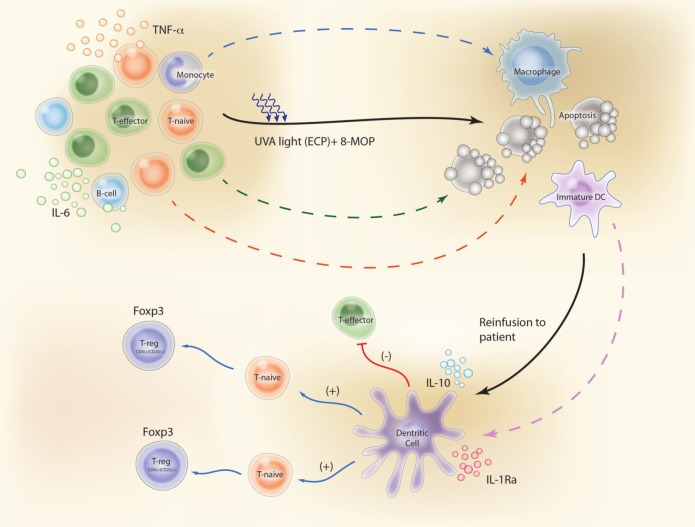
A summary of the key steps through which ECP is believed to reduce T-cell-mediated immune responses in patients with GVHD are: (1) apoptosis of white blood cells (WBCs); (2) phagocytosis of these apoptotic lymphocytes by APCs; (3) a switch in APC activity in favor of anti-inflammatory cytokines and away from pro-inflammatory cytokines; and (4) production of antigen-specific immunosuppressive Tregs (Figure 1).
Figure 1.
Proposed mechanisms of action of ECP in GVHD patients. (1) Apoptosis of white blood cells. (2) Phagocytosis of these apoptotic lymphocytes by APCs (DCs). (3) DCs secrete significant amounts of anti-inflammatory cytokines (IL-10 and IL-1Ra). (4) DCs generate antigen-specific immunosuppressive Tregs instead of activating naïve T-lymphocytes (T-naïve) to effector T-lymphocytes (T-effector). Illustration courtesy of Alessandro Baliani. Copyright © 2013. (Adapted from Goussetis et al. [2012] with permission from Elsevier.) ECP, Extracorporeal photopheresis, APCs, antigen-presenting cells; IL, interleukin; TNF, tumor necrosis factor; DC, dendritic cell; 8-MOP, 8-methoxypsoralen; UVA, ultraviolet A.
Safety of ECP
The development of ECP arose, in part, from concerns with the safety of another commonly employed skin-directed treatment, PUVA. PUVA, introduced in the early 1970s as an effective treatment for many skin conditions, is associated with a number of adverse effects. PUVA is performed by giving patients oral 8-MOP capsules followed 1–2 hours later by exposure to a UVA light source. Interpatient pharmacokinetic variability following administration of oral 8-MOP has been shown to be as high as 18-fold [Jansen et al. 1983]. Variable GI absorption and extensive hepatic metabolism, including first-pass hepatic elimination, contribute to this variability. As a result, blood concentrations for the oral formulation of 8-MOP are unpredictable and may result in inconsistent UVA exposure leading to toxicity or a diminished therapeutic effect. Potential toxicities of activated 8-MOP are linked to its photosensitizing properties which include direct DNA damage and the production of reactive oxygen species. As a result of excessive photosensitivity, photo-aging of the skin and an increase in dermatological malignancies have been reported when 8-MOP is administered in the oral form. In addition, aphakia has been described in older studies as the action spectrum of 8-MOP peaks in the long UV range which is strongly absorbed by the lens [Cloud et al. 1961]. In contrast, with ECP, 8-MOP is mixed ex vivo with the patient’s buffy coat cell layer, which is then re-infused back into the patient. The half-life of the drug administered in this fashion is extremely short resulting in substantially lower and much more predictable drug exposure. ECP utilizing the Therakos system has an established safety profile with over 500,000 treatments performed to date [Therakos, 2001]. The only absolute contraindication for ECP is in patients who cannot tolerate extracorporeal volume loss (heart failure, hypotension, sepsis) or patients with coagulation disorders. In addition, methoxsalen (Oxsoralen-Ultra®), the FDA-approved 8-MOP compound used with the Therakos device, is contraindicated in patients with idiosyncratic reactions to psoralen compounds, those with a history of light-sensitive disease (such as patients with systemic lupus erythematous with photosensitive disease), or those with aphakia. Serious side effects with the procedure itself have rarely been reported, most of which are related to hypotension secondary to changes in extracorporeal volume. A large, multicenter survey focusing on immediate adverse effects of therapeutic apheresis reported no adverse events from a total of 79 ECP procedures with the majority of these procedures done using the Therakos system [McLeod et al. 1999]. More recently, a phase II, randomized, controlled trial did not show significant differences in safety outcomes between the arm receiving ECP therapy and the control-arm. The overall rates of infections (53.1% versus 44%), diarrhea (20.4% versus 20%), and nausea (18.4% versus 12%) were not significantly different between those receiving ECP and steroids versus steroids alone, while anemia occurred more frequently in those receiving ECP treatment (24.5% versus 6%; p = 0.02) [Flowers et al. 2008]. In clinical practice, anemia in patients receiving ECP therapy can be seen and is most likely attributed to small volume blood loss over repeated procedures. In contrast to other GVHD therapies, studies have found that ECP therapy does not have immunosuppressive effects and therefore does not add to the risk for opportunistic infections [Couriel et al. 2006; Flowers et al. 2008; Greinix et al. 1998, 2000]. Secondary malignancies have not been associated with ECP; however, photosensitivity may occur and patients are instructed to take standard precautions as it relates to sun exposure. Fatigue, pruritus and fevers have also been reported during or after ECP treatment. Because intravenous (IV) access is required, patients are at risk for catheter-related complications including infection and thrombosis. When citrate is used as an anticoagulant for the procedure, hypocalcemia may result requiring electrolyte monitoring.
ECP therapy in the treatment of GVHD
The evaluation of ECP in patients with GVHD has made its way from small, uncontrolled case series to multicenter, randomized controlled studies. One of the biggest challenges in evaluating the available literature has been the marked heterogeneity of the patients involved in these reports. This is most evident in the treatment of chronic GVHD where definitions or criteria for the diagnosis, staging and assessment of response were unavailable prior to the NIH Chronic GVHD Consensus guidelines [Pavletic et al. 2006]. In addition, the role of previous or concomitant immunosuppressive therapies, different ECP treatment schedules and variable times from GVHD onset to initiation of ECP preclude a generalization of data across studies.
Chronic GVHD
ECP for the treatment of GVHD has been most extensively evaluated in the setting of steroid-refractory chronic GVHD where more than 20 studies have been conducted since Owisanowski and colleagues published their original case report in 1994 [Owsianowski et al. 1994]. While these reports have all concluded ECP to be feasible and efficacious in the treatment of patients with chronic GVHD, broad application of the results has been limited by the inclusion of small number of patients with heterogeneous forms of chronic GVHD (progressive, quiescent, de novo), organ involvement, disease duration and concurrent and prior therapies. Table 4 outlines the response rates of the largest of these studies.
Table 4.
Clinical trials with ECP in chronic GVHD.
| Study | Number of patients | Skin | Lung | Oral | Liver |
|---|---|---|---|---|---|
| Sniecinski et al. [1998] | 26 | OR=80% | OR=33% | OR=88% | OR=42% |
| Smith et al. [1998] | 18 | OR=40% | OR=None | OR=29% | OR=23% |
| Greinix et al. [1998] | 15 | OR=100% | OR=100% | OR=80% | |
| Child et al. [1999] | 11 | OR=100% | OR=40% | OR=17% | |
| Zic et al. [1999] | 11 | OR=75% | OR=20% | OR=40% | |
| Salvaneschi et al. [2001] | 14 | OR=83% | OR=100% | OR=67% | OR=67% |
| Apisarnthanarax et al. [2003] | 32 | OR=59% | |||
| Seaton et al. [2003] | 28 | OR=48% | OR=None | OR=50%* | OR=32% |
| Foss et al. [2005] | 25 | OR=80% | OR=100% | OR=46% | OR=None |
| Rubegni et al. [2005] | 32 | OR=81% | OR=40% | OR=92% | OR=77% |
| Couriel et al. [2006] | 71 | OR=59% | OR=55% | OR=78% | OR=71% |
| Dignan et al. [2012] | 82 | OR=92% | OR=91% | ||
| Tsirigotis et al. [2012] | 58 | OR=60% | OR=14% | OR=67% | OR=50% |
CR, complete response; ECP, extracorporeal photopheresis; GVHD, graft-versus-host disease; OR, overall response (complete + partial response).
Responses were not reported for all patients (14), 3 of 6 patients with severe ulcerative disease responded.
In the first of these reports, Sniecinski and colleagues documented organ-specific response rates of 80% for cutaneous, 88% oral, 42% liver and 33% for lung manifestations of chronic GVHD [Sniecinski et al. 1998]. Greinix and colleagues reported similar findings in a small series of 15 patients, with responses seen in multiple organ systems including complete responses achieved in all patients with oral mucosal ulcerations, 80% of patients with cutaneous involvement, 70% of those with liver disease and 17% of patients with ocular symptoms [Greinix et al. 1998]. Most of these patients had failed at least two prior immunosuppressive agents and 53% were receiving corticosteroids at the time of ECP initiation. In a large uncontrolled case series conducted by Couriel and colleagues, objective responses were noted in 61% of patients (n = 71) and complete responses in 20% [Couriel et al. 2006]. The best responses were observed in chronic GVHD affecting the oral mucosa (77%), liver (71%), eyes (67%), skin (59%), and lungs (54%). Overall, these studies have demonstrated consistently high (≥50%) response rates in the treatment of cutaneous and oral chronic GVHD, while reported responses in hepatic and lung forms have been much more variable. For example, reports of less than optimal responses in patients with hepatic chronic GVHD [Foss et al. 2005; Seaton et al. 2003; Sniecinski et al. 1998] are contrasted with groups who have shown good responses across all affected organ systems [Couriel et al. 2006; Greinix et al. 1998; Rubegni et al. 2005]. Studies have demonstrated a benefit in initiating ECP earlier in the course of chronic GVHD therapy [Apisarnthanarax et al. 2003; Couriel et al. 2006]. However, responses have also been seen in heavily pretreated patients [Apisarnthanarax et al. 2003; Foss et al. 2005]. Of these uncontrolled, nonrandomized studies assessing long-term outcomes, mortality was significantly better in patients who responded to ECP therapy compared with those who did not [Couriel et al. 2006; Foss et al. 2005].
Flowers and colleagues published the first prospective randomized controlled trial evaluating ECP for the management of patients with chronic GVHD in 2008 [Flowers et al. 2008]. This single-blind, multicenter study randomized 100 patients in a 1:1 ratio to ECP therapy in addition to conventional immunosuppression versus conventional immunosuppression alone. All patients had received at least 2 weeks of corticosteroid treatment prior to enrollment and were considered steroid-refractory, dependent or intolerant. ECP was performed using the UVAR system (Therakos) and administered on 3 days for the first week, and then twice weekly (on consecutive days) through 12 weeks. Patients in the ECP arm who responded were allowed to continue with 2 ECP sessions every 4 weeks until week 24 with the study analysis only including the first 12 weeks of treatment. In the control arm, 29 of 41 patients crossed over to receive open-label ECP after the 12-week assessment. A total of 95 patients were included in the final analysis with no significant differences between the two arms in baseline demographics, transplant or chronic GVHD characteristics. In an effort to provide an objective measure, the primary endpoint for this study was the median change from baseline in total skin score (TSS) of 10 body regions at 12 weeks. The results for this unvalidated measure favored the ECP arm after 12 weeks of therapy (–14.5% versus –8.5%, p = 0.48), although it did not reach statistical significance. A composition of a ≥50% reduction in daily steroid dose and ≥25% improvement in TSS occurred in a significantly higher number of patients in the ECP arm (4 versus 0 patients; p = 0.04); however, a difference in the ability to reduce steroids (≥50%) was not seen. An unblinded assessment was performed at 12 weeks showing a significant difference in overall skin response favoring those receiving ECP therapy (17 versus 4 patients; p = 0.002). Some of the limitations of this study include the assessment of cutaneous disease only as the primary endpoint (compared with chronic GVHD involving all organ systems), the use of an unvalidated endpoint (TSS), the short duration of ECP treatment at the time of the primary endpoint (12 weeks) and the absence of a protocol-mandated steroid tapering schedule. These limitations reflect the difficulties of designing prospective trials in patients with chronic GVHD and are not unique to this one study.
More recently, outcomes were reported for the 29 patients who were originally assigned to the control arm but subsequently received treatment with ECP (crossed over) due to GVHD progression or inadequate response to steroids alone [Greinix et al. 2011]. Roughly one-third of these patients achieved a partial or complete skin response following 24 weeks of therapy, with the greatest improvement detected between weeks 12 to 24. In addition, one-third of the patients achieved a 50% or greater reduction in steroids. Responses were also seen in oral mucosa (65%), liver (50%), joint (36%), and ocular (27%) manifestations. These results demonstrate the efficacy of ECP and suggest the 12 week time-point chosen in the original study was too early to fully determine efficacy.
Acute GVHD
There is less experience with ECP for the treatment of acute GVHD with no published randomized controlled studies to date. Similar to chronic GVHD reports, acute GVHD studies consist of patients who failed or were resistant to front-line therapy with steroids. Table 5 summarizes responses rates of selected ECP trials in acute GVHD. Greinix and colleagues have the largest number of publications detailing the use of ECP for the treatment of acute GVHD. Recently, a prospective phase II trial by this group assessed the effects of an intensified ECP regimen in 59 patients with steroid-refractory acute GVHD. ECP was administered on 2 consecutive days weekly until response and thereafter every 2–4 weeks until maximal response was achieved. Complete responses were achieved in 82% of patients with cutaneous, 61% with liver, and 61% with gut involvement [Greinix et al. 2006]. Only 25% of patients with all three organ systems affected by acute GHVD achieved a complete response. The intensified ECP schedule used in this phase II trial produced higher complete response rates in patients with GI or grade IV acute GVHD when compared with an earlier study conducted by this group using a less-intensive ECP schedule (12% versus 60% for grade IV disease and 25% versus 73% for GI disease) [Greinix et al. 2000, 2006]. Kaplan–Meier estimates for overall survival at 4 years were significantly different between patients achieving a complete response (59%) and those who did not (11%) [Greinix et al. 2006].
Table 5.
Clinical trials with ECP in acute GVHD.
| Study | ECP regimen | Number of patients | Skin | GI | Liver | OS (%) |
|---|---|---|---|---|---|---|
| Greinix et al. [1998] | Twice every 2 weeks for 3 months, then twice monthly | 6 | OR=100% | OR=100% | 100 | |
| CR=67% | CR=100% | |||||
| Greinix et al. [2000] | Twice every 1–2 weeks to response then twice every 2–4 weeks | 21 | OR=81% | OR=None | OR=67% | 57 |
| CR=62% | CR=67% | |||||
| Salvaneschi et al. [2001]* | Thrice weekly to response then twice every 2 weeks for 3 months | 9 | OR=89% | OR=60% | OR=20% | 67 |
| CR=67% | CR=60% | CR=20% | ||||
| Messina et al. [2003]* | Twice weekly for 1 month, then twice every 2 weeks for 2 months, then twice monthly for 3 months | 33 | OR=82% | OR=75% | OR=60% | 58 |
| CR=76% | CR=75% | CR=60% | ||||
| Perotti et al. [2010]* | Twice–thrice weekly to response then twice weekly for 1 week then twice every 2 weeks then twice monthly | 50 | OR=83% | OR=73% | OR=67% | 44 |
| Greinix et al. [2006] | Twice weekly to response then twice every 2–4 weeks | 59 | CR=82% | CR=60% | CR=61% | 47 |
| Perfetti et al. [2008] | Twice weekly for 1 months, then twice every 2 weeks for 2 months, then twice monthly until CR or stabilization | 23 | CR=66% | CR=40% | CR=27% | 48 |
CR, complete response; ECP, extracorporeal photopheresis; GI, gastrointestinal; GVHD, graft-versus-host disease; OR, overall response (complete + partial response); OS, overall survival.
Study involving pediatric patients.
The use of ECP for the treatment of steroid-refractory acute GVHD has also been studied in the pediatric population with results highlighted in Table 5 [Messina et al. 2003; Perotti et al. 2010; Salvaneschi et al. 2001].
Conclusion
From a historical perspective, ECP was the first FDA-approved selective immunotherapy for any malignancy. Determining the mechanism by which this therapy exerts its immunomodulatory affect has been hampered by the limited understanding of the pathophysiology for the disorders in which this therapy is indicated. Recent research identifying potential cellular and inflammatory targets of ECP have paralleled similar research implicating these same factors in the pathogenesis of the target disorders. However, despite these recent findings, our understanding of ECP remains limited.
Nearly two decades have passed since the original case report detailed the use of ECP for the management of GVHD. Over this period there has been a wealth of retrospective case series establishing the safety and to lesser degree efficacy of this treatment in patients with GVHD. This data provided support for a decision by the Centers for Medicare and Medicaid Services (CMS) allowing expanded coverage for ECP in patients with extensive chronic GVHD that is resistant to standard immunosuppressive therapy, or who require a steroid-sparing strategy. Current studies are in progress to answer the most fundamental of questions: when and for whom should this therapy be provided.
There are currently two prospective, randomized trials underway evaluating ECP in patients with GVHD, which hope to provide further guidance for treating clinicians. The first of these trials is a single-center, unblinded, randomized trial of ECP in patients with newly diagnosed acute GVHD being conducted by researchers at MD Anderson Cancer Center [ClinicalTrials.gov identifier: NCT00609609]. Eligible patients must have clinically diagnosed acute GVHD and have not had more than 72 hours of treatment with systemic steroids. Patients are adaptively randomized to either an intensive ECP arm combined with steroids versus steroids alone. The primary endpoint consists of a composite of being alive, in remission, with a GVHD response and on a tapered dose of steroids on day 60 of study. Extensive correlative studies are also being conducted exploring the mechanism of action for ECP in this patient population.
The second ongoing trial is a randomized trial of ECP in patients with new onset moderate or severe (NIH Consensus Graded) Chronic GVHD [ClinicalTrials.gov identifier: NCT01380535]. In December 2011, the first patient was enrolled onto this international, multicenter, single-blinded trial that randomizes patients to ECP with corticosteroids and cyclosporine versus corticosteroids and cyclosporine alone. Unlike the study performed by Flowers and colleagues the primary endpoint is overall response according to NIH Response Criteria and includes the assessment of chronic GVHD involving all organ systems by week 28 of study. Several secondary measures are also being assessed including individual organ response, underlying malignancy relapse, adverse events and patient-assessed quality of life. While the trial was specifically designed to capture response based on the NIH criteria, recent results from the multicenter chronic GVHD consortium suggest that these response criteria may not correlate with long-term survival outcomes or patient assessed quality of life [Inamoto et al. 2012]. Despite this limitation, it is hoped that this study will answer many questions left unanswered from the Flowers and colleagues trial.
Patients who suffer from acute and chronic GVHD have limited treatment options. ECP remains an important therapeutic option. Future basic, translational, and clinical research studies will provide a better understanding of its mechanism of action and optimize its therapeutic potential.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: None of the authors have any conflicts of interest to declare.
Contributor Information
James W. Hart, Department of Pharmacy Clinical Programs, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Lisa H. Shiue, Department of Dermatology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Elizabeth J. Shpall, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Amin M. Alousi, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 423, Houston, TX 77030, USA
References
- Abreu M., von Tirpitz C., Hardi R., Kaatz M., Van Assche G., Rutgeerts P., et al. (2009) Extracorporeal photopheresis for the treatment of refractory Crohn’s disease: results of an open-label pilot study. Inflamm Bowel Dis 15: 829–836 [DOI] [PubMed] [Google Scholar]
- Apisarnthanarax N., Donato M., Korbling M., Couriel D., Gajewski J., Giralt S., et al. (2003) Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant 31: 459–465 [DOI] [PubMed] [Google Scholar]
- Barr M., Meiser B., Eisen H., Roberts R., Livi U., Dall’Amico R, et al. (1998) Photopheresis for the prevention of rejection in cardiac transplantation. Photopheresis Transplantation Study Group. N Engl J Med 339: 1744–1751 [DOI] [PubMed] [Google Scholar]
- Berger C., Hanlon D., Kanada D., Girardi M., Edelson R. (2002) Transimmunization, a novel approach for tumor immunotherapy. Transfus Apher Sci 26: 205–216 [DOI] [PubMed] [Google Scholar]
- Biagi E., Di Biaso I., Leoni V., Gaipa G., Rossi V., Bugarin C., et al. (2007) Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation 84: 31–39 [DOI] [PubMed] [Google Scholar]
- Bladon J., Taylor P. (2006) Extracorporeal photopheresis: a focus on apoptosis and cytokines.J Dermatol Sci 43(2): 85–94 [DOI] [PubMed] [Google Scholar]
- Brunstein C., Miller J., Cao Q., McKenna D., Hippen K., Curtsinger J., et al. (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlens S., Ringden O., Remberger M., Lonnqvist B., Hagglund H., Klaesson S., et al. (1998) Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant 22: 755–761 [DOI] [PubMed] [Google Scholar]
- Chen X., Vodanovic-Jankovic S., Johnson B., Keller M., Komorowski R., Drobyski W. (2007) Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood 110: 3804–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child F., Ratnavel R., Watkins P., Samson D., Apperley J., Ball J., et al. (1999) Extracorporeal photopheresis (ECP) in the treatment of chronic graft-versus-host disease (GVHD). Bone Marrow Transplant 23: 881–887 [DOI] [PubMed] [Google Scholar]
- Chu Y., Gress R. (2008) Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant 14: 365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G., Gamper H., Isaacs S., Hearst J. (1985) Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem 54: 1151–1193 [DOI] [PubMed] [Google Scholar]
- Clark F., Gregg R., Piper K., Dunnion D., Freeman L., Griffiths M., et al. (2004) Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood 103: 2410–2416 [DOI] [PubMed] [Google Scholar]
- Cloud T., Hakim R., Griffin A. (1961) Photosensitization of the eye with methoxsalen. Arch Ophthalmol 66: 689–694 [DOI] [PubMed] [Google Scholar]
- Couriel D., Hosing C., Saliba R., Shpall E., Anderlini P., Rhodes B., et al. (2006) Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood 107: 3074–3080 [DOI] [PubMed] [Google Scholar]
- Di Ianni M., Falzetti F., Carotti A., Terenzi A., Castellino F., Bonifacio E., et al. (2011) Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117: 3921–3928 [DOI] [PubMed] [Google Scholar]
- Dignan F., Greenblatt D., Cox M., Cavenagh J., Oakervee H., Apperley J., et al. (2012) Efficacy of bimonthly extracorporeal photopheresis in refractory chronic mucocutaneous GVHD. Bone Marrow Transplant 47: 824–830 [DOI] [PubMed] [Google Scholar]
- Edelson R., Berger C., Gasparro F., Jegasothy B., Heald P., Wintroub B., et al. (1987) Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med 316: 297–303 [DOI] [PubMed] [Google Scholar]
- Ehrenstein M., Evans J., Singh A., Moore S., Warnes G., Isenberg D., et al. (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 200: 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara J., Reddy P. (2006) Pathophysiology of graft-versus-host disease. Semin Hematol 43: 3–10 [DOI] [PubMed] [Google Scholar]
- Filipovich A., Weisdorf D., Pavletic S., Socie G., Wingard J., Lee S., et al. (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11: 945–956 [DOI] [PubMed] [Google Scholar]
- Fimiani M., Di Renzo M., Rubegni P. (2004) Mechanism of action of extracorporeal photochemotherapy in chronic graft-versus-host disease. Br J Dermatol 150: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Flowers M., Apperley J., van Besien K., Elmaagacli A., Grigg A., Reddy V., et al. (2008) A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood 112: 2667–2674 [DOI] [PubMed] [Google Scholar]
- Flowers M., Inamoto Y., Carpenter P., Lee S., Kiem H., Petersdorf E., et al. (2011) Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117: 3214–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss F., DiVenuti G., Chin K., Sprague K., Grodman H., Klein A., et al. (2005) Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant 35: 1187–1193 [DOI] [PubMed] [Google Scholar]
- Foss F., Gorgun G., Miller K. (2002) Extracorporeal photopheresis in chronic graft-versus-host disease. Bone Marrow Transplant 29: 719–725 [DOI] [PubMed] [Google Scholar]
- Fukushi N., Arase H., Wang B., Ogasawara K., Gotohda T., Good R., et al. (1990) Thymus: a direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci U S A 87: 6301–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparro F., Dall’Amico R., Goldminz D., Simmons E., Weingold D. (1989) Molecular aspects of extracorporeal photochemotherapy. Yale J Biol Med 62: 579–593 [PMC free article] [PubMed] [Google Scholar]
- Gatza E., Rogers C., Clouthier S., Lowler K., Tawara I., Liu C., et al. (2008) Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood 112: 1515–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Gooden C., Guo L., Kirklin J. (2008) Role for CD4(+)CD25(+) T cells in inhibition of graft rejection by extracorporeal photopheresis.J Heart Lung Transplant 27: 616–622 [DOI] [PubMed] [Google Scholar]
- Giorgini A., Noble A. (2007) Blockade of chronic graft-versus-host disease by alloantigen-induced CD4+CD25+Foxp3+ regulatory T cells in nonlymphopenic hosts. J Leukoc Biol 82: 1053–1061 [DOI] [PubMed] [Google Scholar]
- Greinix H., Knobler R., Worel N., Schneider B., Schneeberger A., Hoecker P., et al. (2006) The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica 91: 405–408 [PubMed] [Google Scholar]
- Greinix H., van Besien K., Elmaagacli A., Hillen U., Grigg A., Knobler R., et al. (2011) Progressive improvement in cutaneous and extracutaneous chronic graft-versus-host disease after a 24-week course of extracorporeal photopheresis - results of a crossover randomized study. Biol Blood Marrow Transplant 17: 1775–1782 [DOI] [PubMed] [Google Scholar]
- Greinix H., Volc-Platzer B., Kalhs P., Fischer G., Rosenmayr A., Keil F., et al. (2000) Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood 96: 2426–2431 [PubMed] [Google Scholar]
- Greinix H., Volc-Platzer B., Rabitsch W., Gmeinhart B., Guevara-Pineda C., Kalhs P., et al. (1998) Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood 92: 3098–3104 [PubMed] [Google Scholar]
- Hahn T., McCarthy P., Jr, Zhang M., Wang D., Arora M., Frangoul H., et al. (2008) Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol 26: 5728–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A., Horwitz L., Beschorner W., Santos G. (1985) Development of graft-versus-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-Ia specificity, including autoreactivity. J Exp Med 161: 718–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A., Thoburn C., Bright E., Horwitz L. (1997) Specificity of effector mechanisms in syngeneic graft-vs-host disease: recognition of the MHC class II invariant chain peptide (CLIP). Transplant Proc 29: 725–727 [DOI] [PubMed] [Google Scholar]
- Hings I., Severson R., Filipovich A., Blazar B., Kersey J., Ramsay N., et al. (1994) Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation 58: 437–442 [DOI] [PubMed] [Google Scholar]
- Hoffmann P., Ermann J., Edinger M., Fathman C., Strober S. (2002) Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med 196: 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander G., Widmer B., Burakoff S. (1994) Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease. J Immunol 152: 1609–1617 [PubMed] [Google Scholar]
- Huang F., Platt N., Wykes M., Major J., Powell T., Jenkins C., et al. (2000) A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med 191: 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamoto Y., Martin P., Chai X., Jagasia M., Palmer J., Pidala J., et al. (2012) Clinical benefit of response in chronic graft-versus-host disease. Biol Blood Marrow Transplant 18: 1517–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C., Wilen G., Ylitalo P., Malmiharju T. (1983) Inter- and intraindividual variations in serum methoxsalen levels during repeated oral exposure. Curr Ther Res 33: 258–264 [Google Scholar]
- Knobler R., Barr M., Couriel D., Ferrara J., French L., Jaksch P., et al. (2009) Extracorporeal photopheresis: past, present, and future. J Am Acad Dermatol 61: 652–665 [DOI] [PubMed] [Google Scholar]
- Knobler R., French L., Kim Y., Bisaccia E., Graninger W., Nahavandi H., et al. (2006) A randomized, double-blind, placebo-controlled trial of photopheresis in systemic sclerosis. J Am Acad Dermatol 54: 793–799 [DOI] [PubMed] [Google Scholar]
- Knobler R., Graninger W., Lindmaier A., Trautinger F., Smolen J. (1992) Extracorporeal photochemotherapy for the treatment of systemic lupus erythematosus. A pilot study. Arthritis Rheum 35: 319–324 [DOI] [PubMed] [Google Scholar]
- Lee S., Kim H., Ho V., Cutler C., Alyea E., Soiffer R., et al. (2006) Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant 38: 305–310 [DOI] [PubMed] [Google Scholar]
- Lee S., Vogelsang G., Flowers M. (2003) Chronic graft-versus-host disease. Biol Blood Marrow Transplant 9: 215–233 [DOI] [PubMed] [Google Scholar]
- Lim H., Edelson R. (1995) Photopheresis for the treatment of cutaneous T-cell lymphoma. Hematol Oncol Clin North Am 9: 1117–1126 [PubMed] [Google Scholar]
- Ludvigsson J., Samuelsson U., Ernerudh J., Johansson C., Stenhammar L., Berlin G. (2001) Photopheresis at onset of type 1 diabetes: a randomised, double blind, placebo controlled trial. Arch Dis Child 85: 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan M., Weisdorf D., Wagner J., DeFor T., Burns L., Ramsay N., et al. (2002) Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant 8: 387–394 [DOI] [PubMed] [Google Scholar]
- Malawista S., Trock D., Edelson R. (1991) Treatment of rheumatoid arthritis by extracorporeal photochemotherapy. A pilot study. Arthritis Rheum 34: 646–654 [DOI] [PubMed] [Google Scholar]
- Marks D., Rockman S., Oziemski M., Fox R. (1990) Mechanisms of lymphocytotoxicity induced by extracorporeal photochemotherapy for cutaneous T cell lymphoma. J Clin Invest 86: 2080–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Weisdorf D., Przepiorka D., Hirschfeld S., Farrell A., Rizzo J., et al. (2006) National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant 12: 491–505 [DOI] [PubMed] [Google Scholar]
- Mathur K., Morris S., Deighan C., Green R., Douglas K. (2008) Extracorporeal photopheresis improves nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: three case reports and review of literature. J Clin Apher 23: 144–150 [DOI] [PubMed] [Google Scholar]
- McLeod B., Sniecinski I., Ciavarella D., Owen H., Price T., Randels M., et al. (1999) Frequency of immediate adverse effects associated with therapeutic apheresis. Transfusion 39: 282–288 [DOI] [PubMed] [Google Scholar]
- Messina C., Locatelli F., Lanino E., Uderzo C., Zacchello G., Cesaro S., et al. (2003) Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol 122: 118–127 [DOI] [PubMed] [Google Scholar]
- Miyara M., Amoura Z., Parizot C., Badoual C., Dorgham K., Trad S., et al. (2005) Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol 175: 8392–8400 [DOI] [PubMed] [Google Scholar]
- Morelli A., Larregina A., Shufesky W., Zahorchak A., Logar A., Papworth G., et al. (2003) Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood 101: 611–620 [DOI] [PubMed] [Google Scholar]
- Mutis T., van Rijn R., Simonetti E., Aarts-Riemens T., Emmelot M., van Bloois L., et al. (2006) Human regulatory T cells control xenogeneic graft-versus-host disease induced by autologous T cells in RAG2-/-gammac-/- immunodeficient mice. Clin Cancer Res 12: 5520–5525 [DOI] [PubMed] [Google Scholar]
- Owsianowski M., Gollnick H., Siegert W., Schwerdtfeger R., Orfanos C. (1994) Successful treatment of chronic graft-versus-host disease with extracorporeal photopheresis. Bone Marrow Transplant 14: 845–848 [PubMed] [Google Scholar]
- Pavletic S., Martin P., Lee S., Mitchell S., Jacobsohn D., Cowen E., et al. (2006) Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 12: 252–266 [DOI] [PubMed] [Google Scholar]
- Perfetti P., Carlier P., Strada P., Gualandi F., Occhini D., Van Lint M., et al. (2008) Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant 42: 609–617 [DOI] [PubMed] [Google Scholar]
- Perotti C., Del Fante C., Tinelli C., Viarengo G., Scudeller L., Zecca M., et al. (2010) Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion 50: 1359–1369 [DOI] [PubMed] [Google Scholar]
- Przepiorka D., Weisdorf D., Martin P., Klingemann H., Beatty P., Hows J., et al. (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15: 825–828 [PubMed] [Google Scholar]
- Quaranta S., Shulman H., Ahmed A., Shoenfeld Y., Peter J., McDonald G., et al. (1999) Autoantibodies in human chronic graft-versus-host disease after hematopoietic cell transplantation. Clin Immunol 91: 106–116 [DOI] [PubMed] [Google Scholar]
- Radenhausen M., Michelsen S., Plewig G., Bechara F., Altmeyer P., Hoffmann K. (2004) Bicentre experience in the treatment of severe generalised atopic dermatitis with extracorporeal photochemotherapy. J Dermatol 31: 961–970 [DOI] [PubMed] [Google Scholar]
- Rieger K., Loddenkemper C., Maul J., Fietz T., Wolff D., Terpe H., et al. (2006) Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood 107: 1717–1723 [DOI] [PubMed] [Google Scholar]
- Rook A., Heald P., Nahass G., Macey W., Witmer W., Lazarus G., et al. (1989) Treatment of autoimmune disease with extracorporeal photochemotherapy: pemphigus vulgaris - preliminary report. Yale J Biol Med 62: 647–652 [PMC free article] [PubMed] [Google Scholar]
- Rubegni P., Cuccia A., Sbano P., Cevenini G., Carcagni M., D’Ascenzo G., et al. (2005) Role of extracorporeal photochemotherapy in patients with refractory chronic graft-versus-host disease. Br J Haematol 130: 271–275 [DOI] [PubMed] [Google Scholar]
- Sakoda Y., Hashimoto D., Asakura S., Takeuchi K., Harada M., Tanimoto M., et al. (2007) Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood 109: 1756–1764 [DOI] [PubMed] [Google Scholar]
- Salvaneschi L., Perotti C., Zecca M., Bernuzzi S., Viarengo G., Giorgiani G., et al. (2001) Extracorporeal photochemotherapy for treatment of acute and chronic GVHD in childhood. Transfusion 41: 1299–1305 [DOI] [PubMed] [Google Scholar]
- Seaton E., Szydlo R., Kanfer E., Apperley J., Russell-Jones R. (2003) Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood 102: 1217–1223 [DOI] [PubMed] [Google Scholar]
- Shimada M., Onizuka M., Machida S., Suzuki R., Kojima M., Miyamura K., et al. (2007) Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol 139: 458–463 [DOI] [PubMed] [Google Scholar]
- Shulman H., Kleiner D., Lee S., Morton T., Pavletic S., Farmer E., et al. (2006) Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 12: 31–47 [DOI] [PubMed] [Google Scholar]
- Smith E., Sniecinski I., Dagis A., Parker P., Snyder D., Stein A., et al. (1998) Extracorporeal photochemotherapy for treatment of drug-resistant graft-versus-host disease. Biol Blood Marrow Transplant 4: 27–37 [DOI] [PubMed] [Google Scholar]
- Sniecinski I., Parker P., Dagis A., Collier T., Wang S., Richard K, et al. (1998) Extracorporeal photopheresis is effective treatment for chronic refractory graft-versus-host disease. In: American Society of Hematology, 40th Annual Meeting, Miami Beach, FL, December 4–8, 1998 [Google Scholar]
- Steinman R., Turley S., Mellman I., Inaba K. (2000) The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med 191: 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima T., Reddy P., Liu C., Williams D., Cooke K., Ferrara J. (2003) Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood 102: 429–435 [DOI] [PubMed] [Google Scholar]
- Therakos (2001) Photopheresis. Available from: http://www.therakos.com/healthcare-professionals (accessed 15 May 2012).
- Toubai T., Sun Y., Reddy P. (2008) GVHD pathophysiology: is acute different from chronic? Best Pract Res Clin Haematol 21: 101–117 [DOI] [PubMed] [Google Scholar]
- Tsirigotis P., Kaloyannidis P., Papalexandri A., Baltadakis I., Karakasis D., Batsis I., et al. (2012) Extracorporeal photopheresis in the treatment of chronic graft-versus-host disease. The Hellenic experience: a study by the Hellenic association of hematology. Transfus Apher Sci 46: 173–180 [DOI] [PubMed] [Google Scholar]
- Vowels B., Cassin M., Boufal M., Walsh L., Rook A. (1992) Extracorporeal photochemotherapy induces the production of tumor necrosis factor-alpha by monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol 98: 686–692 [DOI] [PubMed] [Google Scholar]
- Weisdorf D., Haake R., Blazar B., Miller W., McGlave P., Ramsay N., et al. (1990) Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 75: 1024–1030 [PubMed] [Google Scholar]
- Zhao D., Zhang C., Yi T., Lin C., Todorov I., Kandeel F., et al. (2008) In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood 112: 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zic J. (2003) The treatment of cutaneous T-cell lymphoma with photopheresis. Dermatol Ther 16: 337–346 [DOI] [PubMed] [Google Scholar]
- Zic J., Miller J., Stricklin G., King L., Jr (1999) The North American experience with photopheresis. Ther Apher 3: 50–62 [DOI] [PubMed] [Google Scholar]
- Zorn E., Kim H., Lee S., Floyd B., Litsa D., Arumugarajah S., et al. (2005) Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 106: 2903–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]