Abstract
AD is a public health epidemic, which seriously impacts cognition, mood and daily activities; however, one type of activity, exercise, has been shown to alter these states. Accordingly, we sought to investigate the relationship between exercise and mood, in early-stage AD patients (N = 104) from California, over a 1-year period. Patients completed the Mini-Mental State Examination (MMSE), Geriatric Depression Scale (GDS), and Blessed-Roth Dementia Rating Scale (BRDRS), while their caregivers completed the Yale Physical Activity Survey (YALE), Profile of Mood States (POMS), the Neuropsychiatric Inventory (NPI) and Functional Abilities Questionnaire (FAQ). Approximately half of the participants were female, from a variety of ethnic groups (Caucasian = 69.8%; Latino/Hispanic Americans = 20.1%). Our results demonstrated that the patients spent little time engaged in physical activity in general, their overall activity levels decreased over time, and this was paired with a change in global cognition (e.g., MMSE total score) and affect/mood (e.g., POMS score). Patients were parsed into Active and Sedentary groups based on their Yale profiles, with Active participants engaged in walking activities, weekly, over 1 year. Here, Sedentary patients had a significant decline in MMSE scores, while the Active patients had an attenuation in global cognitive decline. Importantly, among the Active AD patients, those individuals who engaged in walking for more than 2 h/week had a significant improvement in MMSE scores. Structured clinical trials which seek to increase the amount of time AD patients were engaged in walking activities and evaluate the nature and scope of beneficial effects in the brain are warranted.
Keywords: Exercise, Physical activity, Alzheimer's, Mild impairment, MMSE, Cognitive decline
1. Introduction
AD is a public health issue affecting every 1 in 8 Americans who are 65 years and older and nearly half of all Americans aged 85 years and older. In California alone, roughly 480,000 individuals were affected in 2010 and this number is expected to increase by 50% in the next 15 years (Thies, Bleiler, & Alzheimer's Association, 2011). AD has devestating consequences for both the diagnosed individual and the entire family, because it impairs cognitive-behavioral functioning and disrupts activities of daily living (ADL; Melrose et al., 2011; Rolland et al., 2007). Given the significant emotional and financial toll AD has on the individual, family, and society, identifying promising treatment strategies is imperative, in order to reduce the risk, slow the decline and alleviate symptoms in AD.
Exercise is one such promising strategy. Several epidemiological studies involving older adults have suggested that physical activity may slow the progression of cognitive decline (Larson, 2010; Yu, Kolanowski, Strumpf, & Eslinger, 2006), improve performance on tests of cognition and mood (Angevaren, Aufdemkampe, Verhaar, Aleman, & Vanhees, 2008; Antunes, Stella, Santos, Bueno, & de Mello, 2005; Vogt, Schneider, Bruumer, & Struder, 2010; Weuve et al., 2004; Williams & Tappen, 2007), and enhance sleep quality (Benloucif et al., 2004). Consistent with these epidemiological studies, a limited number of clinical trials have linked increased physical activity in healthy elderly adults to improved cognition, particularly executive functioning (Colcombe et al., 2003; Colcombe & Kramer, 2003; Colcombe, Kramer, McAuley, Erickson, & Scalf, 2004; Kramer et al., 2003; van Gelder et al., 2004). Human epidemiological, clinical and neuroimaging studies are supported by animal research which has showed enhanced cerebral function through the upregulation of neurotrophic factors (Cotman, Berchtold, & Christie, 2007), increased neurogenesis (Pereira et al., 2007; van Praag, Kempermann, & Gage, 1999; van Praag, Shubert, Zhao, & Gage, 2005), increased blood flow (Pereira et al., 2007), and reduced oxidative stress (Kiraly & Kiraly, 2005), as well as reduced β-amyloid (Adlard, Perreau, Pop, & Cotman, 2005) in response to exercise. Taken together, these findings support the premise that exercise has beneficial effects on cognition and brain function.
Exercise benefits on cognition are also supported by the results of controlled trials in normal aging. A recent meta-analysis showed an overall benefit of aerobic exercise on episodic memory, attention, processing speed and executive function in non-demented older adults (Smith et al., 2010). To date, the results of 2 randomized controlled trials provide preliminary support for a potential cognition-enhancing effect of aerobic exercise in adults with Mild Cognitive Impairment (MCI). Lautenschlager et al. (2008) showed that 6 months of home-based exercise vs. usual care improved performance on well-established measures of global cognition, e.g., the AD Assessment Scale for Cognition. In the second study, Baker et al. (2010) observed that 6 months of structured and supervised aerobic exercise vs. stretching exercise improved executive function in sedentary adults with MCI. In people with dementia, several studies support a possible role of physical activity on improved cognitive function, slowed disease course and improved ADL (Heyn, Abreu, & Ottenbacher, 2004; Rolland et al., 2007; Scarmeas et al., 2010; Venturelli, Scarsini, & Schena, 2011). However, the field is still in the developmental stage and there is no clear consensus. Since physical activity may slow the onset of age-related cognitive decline and improve cognition in MCI, the possibility exists that exercise could also improve cognition and/or reduce the rate of decline in mild-to-moderate AD. If this is the case, then those individuals who are physically active might show a slower rate of decline than those who are sedentary.
In order to evaluate whether physical activity may affect cognitive decline in AD, we surveyed a sample of AD patients evaluated at nine outpatient clinics across the state of California. This study had two objectives: (1) to quantify the prevalence, type, and amount of physical activity (measured by the YALE in total hours per week; e.g., the dependent variable of interest) in mild-to moderate-AD patients seen at the Alzheimer's Research Centers of California (ARCC), now called the California Alzheimer's Disease Centers (CADC), and (2) to determine whether those participants who engaged in physical activities showed altered global cognition, functional capacity and/or mood states compared to those who were more sedentary. To this end, patients and their informants, were surveyed at baseline and after 1 year (1 year) to determine the prevalence and type of exercise activity participants engaged in. Here, the total number of hours engaged in physical activity was examined, and then that amount of activity (as measured by the YALE) was evaluated with respect to performance on a set of global cognitive (MMSE), affective (POMS), behavioral (GDS, NPI), and functional (BRDRS, FAQ) evaluations, in order to elucidate whether any correlations existed among these variables.
2. Materials and methods
2.1. Setting: The Alzheimer's Disease Research Centers of California
Statewide, California has 10 ARCCs, 9 of which were involved in recruiting the cognitively impaired participants and their informants for this study. Located in outpatient clinics at large university medical centers, the ARCCs receive funding through the California Department of Public Health and collect a common set of demographic, diagnostic, and medical data (i.e., Minimum Uniform Data Set (MUDS)) on each participant. Key clinical personnel at each of the ARCC sites participate in educational conferences and training exercises to insure high inter-site reliability and accuracy of the MUDS dataset. In addition to collecting the MUDS, investigators at the ARCCs have collaborated on multiple research projects (e.g., Tinklenberg et al., 2007; Weinstein, Barton, Ross, Kramer, & Yaffe, 2009) over the past decade investigating topics of mutual interest. The nine ARCC sites involved in the current study included the University of California at Davis-Sacramento, University of California at Davis-Martinez, University of California at Irvine, University of California at San Diego, University of California in San Francisco, University of California in San Francisco – Fresno, University of Southern California in Los Angles, University of Southern California – Rancho, and Stanford University. Once collected, data from each of the participating ARCC sites was electronically submitted to a central facility located at the Institute for Health and Aging at the University of California at San Francisco for additional accuracy checking and storage. Written consent, approved by the Institutional Review Board of each ARCC site, was obtained from each patient or the designated surrogate prior to data collection.
2.2. Participants
Patients diagnosed with mild-to-moderate AD, who completed both baseline and 1-year assessments, were included in this study's analyses. All patients fulfilled the National Institute for Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorder Association (NINDS-ADRDA) criteria (McKhann et al., 1984) for probable/possible AD. Diagnoses were determined by multidisciplinary conferences at each of the ARCC sites, which typically consisted of a team of neurologists, neuropsychologists, psychiatrists, geriatric specialists, and nurses. At baseline, all patients were required to be age 60 or older, be either English- or Spanish-speaking, and have a score of 18 out of 30 points or higher on the MMSE and a score of less than 8 out of 15 points on the GDS. Patients who were visually impaired (i.e., legally blind), with uncorrected hearing impairments, or restricted mobility (e.g., used a wheelchair or walker) were excluded, because these physical limitations would restrict their ability to engage in activities. Patients had to have an informant with whom they had frequent face-to-face contact (i.e., a minimum of 3 days/week); this person provided key information about the patient's physical activity as well as affective and functional abilities. All informant responses were verified by trained clinical personnel.
2.3. Measures
2.3.1. Physical activities
A modified version of the Yale Physical Activities Survey for Older Adults (YALE) was administered to each patient and/or their informant at baseline and at the 1-year re-assessment, either over the telephone or in-person at the clinic. Then, either the patients or their informants answered questions from this commonly used, validated instrument (Bonnefoy et al., 2001; Di Pietro, Caspersen, Ostfeld, & Nadel, 1993) pertaining to the number of hours and minutes per week that the patient engaged in a variety of mental, physical, and social activities and is available in English and Spanish (De Abajo, Larriba, & Marquez, 2001). Physical activities included housework (e.g., dishwashing, shopping, laundry), yard-work (e.g., gardening, raking, sweeping), exercise (e.g., walking, swimming, aerobics), and recreational tasks (e.g., dancing, bowling, needlework). In the current study, physical activity (e.g., scores from the YALE) is considered a measure of the overall “fitness” of the individual, because it takes into account their lifestyle choices which contribute to their overall health status.
2.3.2. Affect/mood
Changes in the participant's affective status and mood were assessed with the GDS (Yesavage et al., 1982) and POMS (McNair, Lorr, & Droppleman, 1971). The GDS is a common measure of depression utilizes in geriatric populations (Sheikh et al., 1991), where the participant responds ‘yes’ or ‘no’ to a set of 15 questions that assess mood during the past week. In the POMS, the informant rates the extent to which 65 adjectives describe the participant's mood during the past week. The POMS included both positive and (e.g., lively, energetic, relaxed) and negative (e.g., angry, listless, grouchy) adjectives that were rated along the following 5-point Likert scale: (1) very slightly or not at all, (2) a little, (3) moderately, (4) quite a bit, and (5) extremely or all the time. For data analysis, the 65 adjectives were broken down into 6 factors, each of which had been empirically validated, previously, in geriatric populations (Albani et al., 2005; Nyenhuis, Yamamoto, Luchetta, Terrien, & Paramentier, 1999). Presumably, individuals who are physically active would be rated higher on the “positive” descriptors (e.g., lively, active, relaxed, alert) than their sedentary peers. In comparison to the GDS, which evaluates normal to severely depressed mood, the POMS assesses a range of both positive and negative emotional states.
2.3.3. Functional abilities
Functional abilities were assessed with two informant-based instruments, the BRDRS (Blessed, Tomlinson, & Roth, 1988) and FAQ (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982). The BRDRS examines aspects of personal, domestic and social ADL that have been correlated with AD brain-related changes (Bondareff, Raval, Colletti, & Hauser, 1998). The shortened BRDRS used in this study asked the informant to rate from normal to totally dependent the participant's ability to perform basic as well as instrumental activities of daily living (IADLs), with total scores ranging from 0 to 17. In the FAQ, the informant rates the participant's performance over the preceding 4 weeks on each of 10 IADLs, such as shopping, preparing meals, handling finances, keeping track of current events, and traveling out of the neighborhood. Performance on each item is rated on a scale of 0 – normal, 1 – has difficulty but does by self, 2 – requires assistance, and 3 – dependent on others. Scores on the FAQ range from a low of 0 to high of 30, with >6 points suggestive of a dementia (Teng et al., 2008).
2.3.4. Behavioral symptoms
The presence of behavioral symptoms, such as delusions, depression, anxiety, apathy, and agitation, were assessed using the 12-item Neuropsychiatric Inventory (NPI-Q; Cummings, Mega, Gray, Rosemberg-Thompson, & Gornbein, 1994). Here, informants answered questions pertaining to the AD patients’ behavior in recent weeks.
2.3.5. Global cognition
Global cognitive functioning was assessed using the MMSE (Folstein, Folstein, & McHugh, 1975), wherein patients were asked a series of questions pertaining to working memory, executive function, processing speed and attention. The MMSE has been previously used to categorize the expected rate of decline in AD populations (Doody, Massman, & Dunn, 2001), to track the rate of conversion from MCI to AD (Maioli et al., 2007; Marra, Ferraccioli, Vita, Quaranta, & Gainotti, 2011), to establish a correlation between cognitive scores and neuroimaging in AD conversion, progression and decline (Barrio, Kepe, Satyamurthy, Huang, & Small, 2008; Davatzikos, Bhatt, Shaw, Batmanghelich, & Trojanowski, 2011; Leow et al., 2009), and as an outcome measure for a few behavioral intervention studies in AD (see Table 4 in Baum, Jarjoura, Polen, Faur, & Rutecki, 2003; Rolland et al., 2007; Van de Winckel, Feys, De Weerdt, & Dom, 2004).
2.4. Procedures
Participants and/or their informants completed the same set of instruments at baseline and the 1-year follow-up assessment, in order to identify any changes in activity patterns, mood and/or behavior during the year. Any informant-based instruments used in this study could be mailed to the informant prior to the clinic visit for completion, or administered directly to the informant during the clinic visit. If the instruments were completed at home, a trained staff person would verify the responses with the informant during the clinic visit. Every effort was made to ensure that the same informant completed the measures at baseline and the 1-year re-evaluation. Additionally, the 1-year re-evaluation was scheduled as close as possible to 12 months after the baseline assessment. Ensuring a constant 1-year interval was necessary in order to conduct analyses on the rate of change over a specified period.
Given the multiple sites and personnel involved in this longitudinal project, the investigators took a number of steps to ensure uniformity of data collection. First, all staff members involved in the project received training in how to administer and score the modified YALE along with the POMS, GDS, NPI, MMSE, FAQ, and BRDRS. Secondly, research personnel at the lead site (i.e., UCI) were available to address any questions of staff collecting data at the other ARCCs. Finally, personnel at the Institute for Health and Aging, which houses the ARCC central database, screened the data for any inconsistencies or unusual values (e.g., outliers) that could represent an error. In case of an error, the site that submitted the data would be contacted to verify its accuracy.
2.5. Analytical methods
The current study employed a mixed design ANCOVA for all of its primary outcome analytical measures. Repeated measures designs, like the one utilized in the present study, reduce the error that might be due to individual variability within a population; a problem more commonly found in cross-sectional designs. This type of repeated measures design is utilized most frequently in longitudinal studies, and for our purposes, the change in MMSE over the 1-year interval was the dependent variable of interest (e.g., MMSE at baseline vs. MMSE at 1 year). Age, years of education and sex were utilized as the covariates. The changes to mood/affect (GDS and POMS), behavior/psychiatric symptoms (NPI), functional abilities (BRDRS and FAQ) and physical activities (YALE; e.g., baseline hours vs. 1 year hours) were evaluated only with respect to their effects on change in the MMSE, and comprised the independent variables. That is, separate ANCOVAs were performed in which each of these tests (YALE, GDS, BRDRS, POMS, FAQ and NPI) served as the independent variables while the MMSE was utilized as the dependent variable. For the YALE, the YALE total score was not utilized in the current study. Instead, this study focused on the total amount of time the adults engaged in any activity, in order to determine if any change on cognition, mood, neuropsychiatric behavior and/or ADL performance was activity-specific. In order to evaluate this, the patient's informants responded in hours (e.g., 1 h, 1.25 h, 1.5 h, 2 h, etc.) the amount of time spent engaged in any activity, and those reported values were included in the subsequent analyses. All activities from the YALE were evaluated, and the reported time spent engaged in those activities are detailed below. Factors such as age, years of education and sex were utilized as the covariates in order to account for any variance in the model due to these factors. All significant covariates, main effects and interactions are reported in the subsequent section along with their supporting F, probability (p), degrees of freedom (df) and confidence interval (CI) values. All significant within-subjects’ main effects were evaluated with paired t-tests, with supporting T, p, df and CI values reported.
3. Results
3.1. Characteristics of the population and results at baseline
The California AD study sample (N = 104) included older adults aged 63–98 (Mean: 81 ± 6.54) with an average of 16.67 years of education (±3.25 years). Approximately half the study participants were female (44.2%) and the sample included primarily Caucasian American (69.8%) and Latino/Hispanic Americans (20.1%; see Table 1).
Table 1.
Demographics and baseline cognitive and activity scores for AD patients.
| Mean | SD | |
|---|---|---|
| Sample size | 104 | NA |
| Age | 81 | 6.54 |
| Years of education | 16 | 2.73 |
| % female | 44.2 | NA |
| % Caucasian American | 69.8 | NA |
| % Latino/Hispanic American | 20.1 | NA |
| Baseline GDS | 0.86 | 1.99 |
| Baseline MMSE for all AD | 24.8 | 13.57 |
| Baseline BRDRS | 0.58 | 1.01 |
| Baseline NPI | 0.3 | 0.84 |
| % leisurely walking at baseline | 67.3 | NA |
| MMSE for leisurely all walking AD at baseline | 25.55 | 3.24 |
| MMSE for all sedentary AD at baseline | 23.07 | 3.65 |
3.1.1. Physical activities vs. cognition at baseline
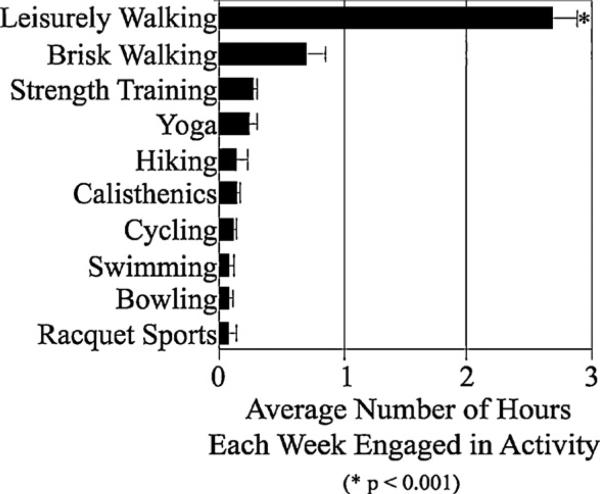
As previously described in Sections 2.3.1 and 2.4, the number of hours per week engaged in any exercise activity over the course of the 1-year period was evaluated (e.g., YALE). According to AD patient informants, at baseline, the primary exercise activity participants engaged in was walking (see Fig. 1; Table 1). Based on the YALE profile, 70 out of 104 AD patients (67.3%) engaged in leisurely walking for an average of 2.76 h/week, and an additional 14 engaged in both leisurely and brisk walking for an average of 0.68 h/week. ANCOVA analyses indicated that the population spent more time leisurely walking than in any other type of exercise activity reported by the patients’ informants at baseline (F8,784 = 5.12, p < 0.001, Ts103 = 3.23–6.14, ps < 0.001, CI = 0.39–1.21 and 0.59–2.37; see Fig. 1). Age, years of education and sex were not found to be significant covariates in the model (ps > 0.5) and were not utilized as factors in any follow-up analyses. Paired samples t-tests, Bonferoni corrected for multiple comparisons, revealed that those participants who leisurely walked had significantly higher MMSE scores (25.55 ± 3.24) than those who did not (23.07 ± 3.65; T103 = 2.97, p = 0.01, CI = 2.63–0.82). As reported in Section 2.3.2, Informants provided information to assess Affect/Mood across 6 sub-dimensions of the POMS. After reviewing the participants’ affect/mood profiles, and independent of all other factors, baseline ANCOVA and t-tests indicated that AD patients experienced feelings of vigor-activity at higher levels than any other mood state (F1,103 = 98.98, p < 0.001); while feelings of fatigue-inertia and confusion-bewilderment (Ts103 = 7.82–14.89, ps < 0.001, CIs = 0.41–1.09 and 0.34–1.11 respectively) were experienced at the next highest levels in the population.
Fig. 1.
Mean Hours Engaged in Activity (SEM) as measured by the Yale Physical Activity Questionnaire, across the entire AD population at baseline. Results showed that the AD population engaged in Leisurely Walking activities for more hours each week than any other type of physical activity (N = 104; *p < 0.001).
3.1.2. Affect/mood vs. global cognition at baseline
No participants were considered clinically depressed at baseline as measured by the GDS (0.86 ± 1.99) and there were no significant correlations between affect/mood and cognition
3.1.3. Functional abilities vs. global cognition or physical activity at baseline
Separate ANCOVA models were fitted to the data, with each sub-dimension of the BRDRS at baseline comprising the independent variables, and baseline MMSE score as the dependent variable. This process was repeated for the FAQ vs. MMSE analysis. At baseline, participants were classified as mildly impaired (Mean MMSE = 24.8 ± 13.57) and functionally capable of performing daily activity (Mean BRDRS: 0.58 ± 1.01). Functional limitations were noted as AD patients had difficulty with navigating their local area, eating, dressing, managing financial obligations, keeping track of current events, and comprehending news/entertainment media (BRDRS: F6,432 = 9.53, p < 0.001, Paired-Ts103 = 2.64–4.13, p < 0.005, CIs = 0.21–1.38; FAQ: F9,603 = 33.38, p < 0.001, Paired Ts103 = 0.18–10.23, ps < 0.001; CIs 0.11–1.24 and 0.67–1.63, respectively). The relationship between global cognitive performance and IADLs, without accounting for physical activity, has been thoroughly evaluated in the literature, and will not be evaluated here (see Section 4 for a review of the evidence). These, as well as all results reported in the subsequent sections, were independent of age, years of education and sex (i.e., age, years of education and sex were not found to be significant covariates for the model). No relationship between functional abilities and physical activity were found at baseline.
3.1.4. Behavioral/neuropsychiatric symptoms vs. cognition at baseline
There was no evidence of behavioral/psychiatric disturbances at baseline as measured by total scores on the NPI (0.30 ± 0.84), nor any significant correlations between behavioral/psychiatric disturbances and cognition.
3.2. Characteristics of the population and results at the 1-year follow-up
The same participants as at baseline were evaluated at the 1-year follow-up. As previously described in Section 2.5, a series of separate repeated measures ANCOVAs were performed, evaluating the relationships between the changes (Δ) in cognitive and neuropsychiatric performance (MMSE, POMS, GDS, BRDRS, FAQ and NPI) over the 1-year time period and measures of exercise activity (YALE) over that same interval. Again, age, years of education and sex were utilized as the covariates, however, they were not shown to be significant covariates (ps > 0.5) in the model, and were not utilized in any follow-up analyses. While no significant relationships between depression, functional ability or behavioral/psychiatric disturbances were noted (ΔGDS, ΔBRDRS, ΔFAQ and ΔNPI, respectively), the results showed a difference in global cognitive function (e.g., ΔMMSE) and mood state (e.g., ΔPOMS F5,395 = 19.91, p < 0.01) that was dependent on the amount of time spent engaged in walking activities, on average, over the 1-year period (ΔYALE). This relationship was further parsed and evaluated in the subsequent analyses.
3.2.1. Physical activities and cognition at 1 year
In order to evaluate whether a change in the amount of time engaged in walking activities (ΔYALE) affected the change in global cognitive function in AD patients (ΔMMSE), participants were divided into two groups: those who did not engage in any activity at all over the course of the 1 year (Sedentary, N = 20) and those who walked over that same interval (Active, N = 84). Here, repeated measures ANCOVA were conducted, where MMSE (baseline vs. 1 year) and Walking Group (Sedentary vs. Active) were compared, with age, years of education and sex as the covariates. Age, years of education and sex were not shown to be significant covariates (ps > 0.5) and were not utilized in any follow-up analyses. The results revealed a significant interaction between the change in global cognition and the time engaged in walking activities (F6,96 = 142.11, p < 0.001), with Active participants having higher MMSE (ΔMMSE = + 2.22 + 0.82; N = 84) than Sedentary participants (ΔMMSE = – 1.97 + 0.98; Paired T73 = 6.08, p < 0.005, CI = 0.06–2.67; N = 20).
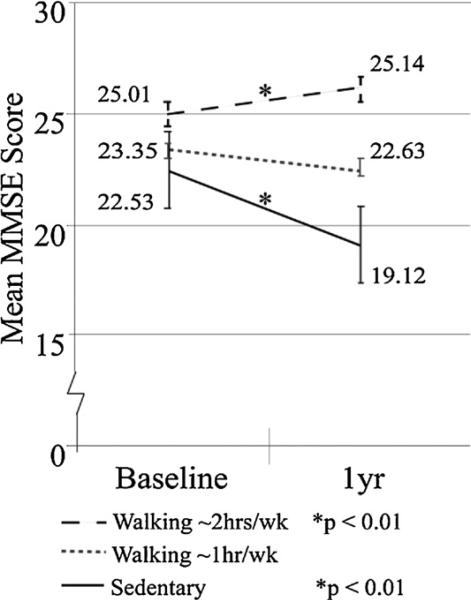
A follow-up analysis was conducted to determine if the effects of walking on cognition were dose-dependent. Here, the walking group was subdivided into those who walked for approximately 1 h/week (i.e., 1 h/week; N = 50) and those who walked more than 2 h/week (i.e., 2+ h/week; N = 34). A repeated measures ANOVA was conducted comparing MMSE scores (baseline vs. 1 year) with Walking Group (Sedentary, 1 h/week, or 2+ h/week) and the results demonstrated that the effects of walking on cognition were dose-dependent. That is, AD patients who were Sedentary experienced a significant drop in MMSE scores (T29 = 4.61, p = 0.001, CI = 2.69–5.09, N = 20) over the 1-year interval (see Fig. 2, black solid). There was an attenuation in the decline of MMSE scores in those participants who walked for 1 h/week (see Fig. 2, gray dashed; N), and a significant improvement in MMSE scores in those individuals who walked for 2+ h/week (see Fig. 2, black dashed; T23 = 4.19, p = 0.001, CI = 3.14–0.28).
Fig. 2.
Baseline and 1-year Mean total MMSE score and Mean total hours engaged in Leisurely Walking activities per week for the AD population. Standard Error of the Mean for MMSE scores and Data Values for all groups are shown. Black dashed: Results for active AD patients who engaged in walking for ~2 h/week (e.g., walking ~2 h/week; N = 34; *p < 1.0e–7). Gray dashed: Results for AD patients engaged in walking for ~1 h/week (e.g., Walking ~1 h/week; N = 50; p > 0.1). Black solid: Results for AD patients who were not engaged in leisurely walking activities at either time point (e.g., Sedentary; N = 20; *p < 0.01).
Due to the statistically significant difference in baseline MMSE scores between those who were engaged in walking activities and those who were not, a variable was created to account for baseline global cognition (i.e., “Baseline MMSE”). First, the change in global cognition over the 1-year interval was utilized (i.e., “1-year MMSE” “Baseline MMSE” = “ΔMMSE”) and compared with “Baseline MMSE.” A bivariate correlation analysis was conducted to determine if there was a correlation between the baseline MMSE score and the overall change to MMSE scores over the 1-year interval (i.e., “Baseline MMSE” vs. “ΔMMSE”). The results indicated that “Baseline MMSE” was not significantly correlated with the change to cognitive function over the 1-year period our study (p = 0.70). Next, this study sought to determine if baseline global cognition affected how much time the AD population engaged in walking activities over the 1-year interval. To this end, a second bivariate correlation analysis was conducted wherein baseline global cognition (“Baseline MMSE”) was compared with the change in time spent engaged in walking activity over the 1-year interval (i.e., “1-year Walking hours” “Baseline Walking hours” = “ΔWalking hours”). The results revealed that “Baseline MMSE” was not significantly correlated with the change in the amount of time spent engaged in walking activities over the 1-year period (p = 0.52).
3.2.2. Affect/mood and cognition at 1 year
POMS scores were collected over 1 year. Separate repeated measures ANCOVAs were conducted, with each ANCOVA evaluating one of the six clusters derived from the POMS (i.e., anger-hostility, confusion-bewilderment, depression-dejection, fatigue-inertia, tension-anxiety and vigor-activity; the dependent variables), and these dimensions were evaluated over the 1-year interval (e.g., “time” = independent variable). Age, education and sex were not found to be significant covariates in the model. However, mood was shown to change over time in this population (F5,515 = 4.74, p < 0.001), as AD patients reported higher levels of vigor-activity than any other type of affect/mood state, followed by fatigue-inertia and then confusion-bewilderment; however, the level of vigor-activity at 1 year was significantly less than observed at baseline (Ts103 = 7.82–14.89, ps < 0.001). Feelings of anger-hostility, fatigue-inertia, depression-dejection and confusion-bewilderment increased over the same interval (Ts103 = 2.21–12.91, ps < 0.001). Since age, education and sex were not shown to be significant covariates, the subsequent analysis utilized 6 separate ANOVAs, which compared each sub-dimension of the POMS vs. global cognitive performance (total MMSE) and walking activities (Walking Gp) over the 1-year interval. Results from these separate ANOVAs indicate that lower MMSE scores and a Sedentary lifestyle correlated with a loss in vigor-activity (F5,515 = 96.84, p < 0.001) along with an increase in anger-hostility, confusion-bewilderment, depression-dejection and fatigue-inertia (Ts103 = 7.82–14.89, ps < 0.001) over the 1-year interval.
4. Discussion
The objective of the current study was to quantify the prevalence of physical activity in AD patients, and to investigate whether those participants who engaged in physical activities over a 1-year interval showed altered affect/mood states, IADL performance or cognitive function as compared to those who were more sedentary. The sample consisted of males and female AD patients, who were well educated (16.67 ± 3.25 years) and were primarily Caucasian and Latino-Hispanic Americans. Analyses indicated that these AD patients were mildly impaired at baseline and did not show evidence of clinical depression or behavioral/psychiatric disturbances (see Section 3.1; Table 1).
According to AD patient informants at baseline, the primary physical activity AD patients were engaged in was walking, with 68% of the population walking for one or more hours per week, and 32% remaining largely sedentary. MMSE scores were significantly higher among the active patients, relative to the sedentary AD patients. Regardless of physical activity level, AD patients reported a higher level of vigor-activity than any other mood state (see Section 3.1.1). A previous investigation by Nyenhuis et al. (1999) found that “age” itself was positively correlated with higher than average feelings of vigor-activity among healthy adult samples, and individuals who volunteer to participate in structured clinical trials could possibly be more vigorous and active than their peers in the general population (Nyenhuis et al., 1999). In our study, it is possible that AD patients reported higher than average feelings of vigor-activity, even among the “Sedentary” AD patients, because our sample includes individuals who were evaluated in the dynamic environment of the CADC/ARCC Centers, and may be more vigorous and active than found among other AD patients in the overall residential population.
We examined the relationship between the amount of time spent walking and MMSE scores at baseline and 1 year, in order to determine if there was any relationship between physical activity and global cognition. Our study was able to demonstrate that AD patients who were sedentary declined significantly and this is consistent with the overall literature (Davatzikos et al., 2011; Barrio et al., 2008; Yáguez, Shaw, Morris, & Matthews, 2011). In contrast, those who walked for one or more hours, weekly, during the year, experienced a stabilization in cognitive functioning and those who walked for two or more hours, weekly, showed a significant improvement on the MMSE (see Section 3.2). Correlation analyses indicated that baseline MMSE was not a significant factor in predicting walking behavior over the 1 year, suggesting that other factor(s) besides baseline cognitive function were affecting the decision to engage in physical activity in these AD patients.
Thus, our results support the premise that some level of physical activity, especially walking, is beneficial to cognitive function in those with mild-to-moderate AD. To our knowledge, this is the first USA-based clinical study to show an improvement in AD cognitive scores due to an association with physical activity. Our results, however, are consistent with a recent Australian-based study, which showed that 4 months of a walking-exercise intervention increased MMSE scores in AD patients (Vreugdenhil, Cannell, Davies, & Razay, 2011). Additionally, our findings are consistent with research showing that exercise activity in healthy older adults is associated with both a slower rate of cognitive decline and improved cognitive functioning (Colcombe & Kramer, 2003; Colcombe et al., 2003, 2004; Kramer et al., 2003; Larson, 2010; van Gelder et al., 2004; Vogt et al., 2010; Weuve et al., 2004; Yu et al., 2006), and that exercise-based investigations in dementia populations are feasible (Baker et al., 2010; Lautenschlager et al., 2008; Rolland et al., 2007; Yáguez et al., 2011; Yu & Kolanowski, 2009; Yu et al., 2006).
Recent research may be able to explain the lack of decline and, in some cases, improvement in MMSE scores for the walking group. Specifically, both animal and human research have shown that aerobic exercise, defined as activity that improves oxygen consumption by the body through purposeful, rhythmic use of large muscle groups for an extended period of time, resulted in beneficial changes to brain structure and function that are paired with improvements in cognition (Cotman & Berchtold, 2002; Nichol, Parachikova, & Cotman, 2007; Nichol, Deeny, Seif, Camaclang, & Cotman, 2009; Parachikova, Nichol, & Cotman, 2008) even in AD patients (Yu & Kolanowski, 2009; Yu et al., 2006). We suggest that a lifestyle which includes regular walking may result in the beneficial biological changes to the brain's structure/function and overall cognition that have been associated with exercise. Therefore, walking, even in low amounts (i.e., ~1 h/week on average) may help to stabilize and improve cognitive function of AD patients. This statement is supported by further evidence from our study, in which lower MMSE scores significantly correlated with a Sedentary lifestyle (e.g., individuals did not report regularly engaging in walking activities over the 1 year), a loss vigor and an increase in anger, confusion, depression and fatigue (see Section 3.2.2). For those AD patients with low MMSE scores, increased walking activities could possibly alleviate many of the deleterious cognitive, affect/mood, neuropsychiatric symptoms associated with AD. Further, walking activities may be the most beneficial for those AD patients who walk for longer periods of time (i.e., 2+ h/week on average), as indicated by the dose-dependent effect of exercise evident in the present study. Thus, we suggest that walking activities may be one intervening strategy that could be useful in AD populations. Clearly, our results indicate that future structured AD clinical trials investigating the dose-dependent effects of exercise on cognition are warranted.
Our conclusions are further supported by previous clinical research, which has suggested that approximately 2–4 h/week of walking in healthy elderly can improve cognitive function (Colcombe & Kramer, 2003; Colcombe et al., 2003, 2004; Kramer et al., 2003; van Gelder et al., 2004) and an epidemiological study (Weuve et al., 2004) which quantified the relationship between total physical activity and cognition showed benefits in as little as 1.5 h/week. It may be that the threshold of the AD brain to benefit from exercise is relatively low, a supposition that is supported by evidence from some rodent animal studies (Nichol et al., 2009). Additional research is needed to establish the thresholds for brain-related benefits of physical activity in AD patients.
We also sought to determine if walking behavior was related to the affect/mood states of AD patients over the course of a year (see Section 3.2.2). First, the analysis was conducted across the entire population regardless of activity level and evaluated the relationship between the change in MMSE scores during the 1-year period and any change in mood. Subsequently, the analysis was repeated, taking into account both walking behavior and MMSE scores. In this case, most of the AD patients demonstrated a loss in their feelings of vigor-activity over the 1-year period. This was true even in those individuals who were active at baseline, or who increased the amount of time they spent walking over the 1 year. Similarly, AD patients evidenced an increase in feelings of anger-hostility, fatigue-inertia, confusion-bewilderment and depression-dejection. The increase in depression-dejection, though, was not significant enough to warrant a diagnosis of “clinical depression” at the follow-up assessment, as noted by the low GDS scores in the population at this time point.
It may be that the threshold for the benefit of physical activity on mood in AD patients is different than that for cognition. There may be extraneous factors that are disproportionately affecting the affect/mood states of AD patients that are unrelated to walking behavior and/or AD pathology. It is possible that our POMS results reflect the fact that the exercise this population engaged in was, at best, mildly aerobic and, therefore, did not have the same impact on mood as noted in previous reports (see Heyn et al., 2004; Rolland et al., 2007). Higher levels of aerobic exercise, in a form other than walking, may be necessary to improve affect/mood in individuals with AD. Future research is warranted.
Finally, this study sought to determine if there were any relationships between measures of functional abilities and cognitive function over the 1-year interval, however, none such occurred (see Sections 3.1.2 and 3.1.3). Similar to the findings reported for affect/mood, it is possible that the threshold for the beneficial impact of exercise on functional capacity is different than that for cognitive function. Alternatives to the scales used here may also be suitable to detect such changes. This also warrants future study.
Limitations of the current investigation include a relatively small sample size compared with other epidemiological studies, the use of correlation analyses as the indication of relatedness, and the use of informant-based questionnaires as the main measure of physical activity, functional abilities, and mood. Rigorous screening processes, meticulous training of the research personnel administering the neuropsychological testing, and the wide range of persons recruited in the current study, which is common among multi-site clinical investigations like this one, suggests that there is a low probability of any caregiver bias present in the current data. This study was not able to determine if unknown, unmeasured or confounding factors, unrelated to walking activity, contributed to the stabilization of cognitive functioning in AD patients. Nevertheless, while the current study is descriptive in nature, it indicates that a controlled clinical trial investigating the effects of increased walking on cognitive functioning and disease progression in individuals with mild-to-moderate AD would be warranted.
5. Conclusions
Our study concludes that a sedentary lifestyle correlates with a decline in cognitive function, a loss of vigor, and an increase in feelings of anger, confusion, depression and fatigue. We also conclude that some level of physical activity, especially walking, is beneficial to cognitive function in those with mild-to-moderate AD. This study supports the premise that walking activities may be one intervening strategy that could be useful in AD populations, and that future structure AD clinical trials investigating the dose-dependent effect of exercise on cognition are warranted.
Acknowledgements
We wish to acknowledge the efforts of the Clinical Staff from the various CADC, ADRC and ARCC sites across the state of California. This study was funded by the California Alzheimer's Disease Centers and the following grants: 5P50AG016573, 3P50AG016573, and 5P01AG000538-32.
Footnotes
Conflict of interest statement
The current study has no conflicts of interest to report.
References
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in ransgenic model of Alzheimer's disease. Journal of Neuroscience. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani C, Gunzelmann T, Schmutzer G, Grulke N, Bailer H, Blaser G, et al. The emotional sensitivity for elderly people – Validation of the profile of mood states for people over 60 years. Zeitschrift fur Gerontologie und Geriatrie. 2005;38:431–440. doi: 10.1007/s00391-005-0314-x. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systemic Reviews. 2008;2:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- Antunes HK, Stella SG, Santos RF, Bueno OF, de Mello MT. Depression, anxiety and quality of life scores in seniors after endurance exercise program. Revista Brasileira de Psiquiatria. 2005;27:266–271. doi: 10.1590/s1516-44462005000400003. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Archives of Neurology. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio JR, Kepe V, Satyamurthy N, Huang SC, Small G. Amyloid and tau imaging, neuronal losses and function in mild cognitive impairment. Journal of Nutrition, Health and Aging. 2008;12:61S–65S. doi: 10.1007/BF02982589. [DOI] [PubMed] [Google Scholar]
- Baum EE, Jarjoura D, Polen AE, Faur D, Rutecki G. Effectiveness of group exercise program in a long-term care facility: A randomized pilot trial. Journal of the American Medical Directors Association. 2003;4:74–80. doi: 10.1097/01.JAM.0000053513.24044.6C. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Orbeta L, Ortiz R, Janssen I, Finkel SI, Bleiberg J, et al. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;15:1542–1551. doi: 10.1093/sleep/27.8.1542. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. Blessed-Roth Dementia Scale (DS). Psychopharmacology Bulletin. 1988;24:705–708. [PubMed] [Google Scholar]
- Bondareff W, Raval J, Colletti PM, Hauser DL. Quantitative magnetic resonance imaging and the severity of dementia in Alzheimer's disease. American Journal of Psychiatry. 1998;145:853–856. doi: 10.1176/ajp.145.7.853. [DOI] [PubMed] [Google Scholar]
- Bonnefoy M, Normand S, Pachiaudi C, Lacour JR, Laville M, Kostka T. Simultaneous validation of ten physical activity questionnaires in older men: A doubly labeled water study. Journal of the American Geriatrics Society. 2001;49:28–35. doi: 10.1046/j.1532-5415.2001.49006.x. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2003;11:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: Recent findings and future directions. Journal of Molecular Neuroscience. 2004;24:9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain healthy and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega MS, Gray K, Rosemberg-Thompson S, Gornbein T. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Bhatt P, Shaw LM, Batmanghelich KN, Trojanowski JQ. Prediction of MCI to AD conversion, via MRI, CSF biomarkers and pattern classification. Neurobiology of Aging. 2011;32:2322, e19–27. doi: 10.1016/j.neurobiolaging.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Abajo S, Larriba R, Marquez S. Validity and reliability of the Yale Physical Activity survey in Spanish elderly. Journal of Sports Medicine and Physical Fitness. 2001;41:479–485. [PubMed] [Google Scholar]
- Di Pietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Medicine and Science in Sports and Exercise. 1993;25:628–642. [PubMed] [Google Scholar]
- Doody RS, Massman P, Dunn JK. A method for estimating progression rates in Alzheimer disease. Archives of Neurology. 2001;58:449–454. doi: 10.1001/archneur.58.3.449. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Archives of Physical Medicine and Rehabilitation. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Kiraly MA, Kiraly SJ. The effect of exercise on hippocampal integrity: Review of recent research. International Journal of Psychiatry in Medicine. 2005;35:75–89. doi: 10.2190/HX7L-4B40-PQNY-2A4P. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Colcombe SJ, McAuley E, Eriksen KI, Scalf P, Jerome GJ, et al. Enhancing brain and cognitive function of older adults through fitness training. Journal of Molecular Neuroscience. 2003;20:213–221. doi: 10.1385/JMN:20:3:213. [DOI] [PubMed] [Google Scholar]
- Larson EB. Prospects for delaying the rising tide of worldwide, late-life dementias. International Psychogeriatrics. 2010;22:1196–1202. doi: 10.1017/S1041610210001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Cod KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. Journal of the American Medical Association. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Leow A, Yanovzky I, Parikshak N, Lee S, Toga AW, Jack CR, Jr., et al. The Alzheimer's Disease Neuroimaging Initiative Alzheimer's disease neuro-imaging initiative: A one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. NeuroImage. 2009;45:645–655. doi: 10.1016/j.neuroimage.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maioli F, Coveri M, Pagni P, Chiandetti C, Marchetti C, Ciarrocchi R, et al. Conversion of mild cognitive impairment to dementia in elderly subjects: A preliminary study in a memory and cognitive disorder unit. Archives of Gerontology and Geriatrics. 2007;44(Suppl. 1):233–241. doi: 10.1016/j.archger.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Marra C, Ferraccioli M, Vita MG, Quaranta D, Gainotti G. Patterns of cognitive decline and rates of conversion to dementia in patients with degenerative and vascular forms of MCI. Current Alzheimer Research. 2011;8:24–31. doi: 10.2174/156720511794604552. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. Educational and Industrial Testing Service; San Diego, CA: 1971. p. 27. [Google Scholar]
- Melrose RJ, Ettenhofer ML, Harwood D, Achamallah N, Campa O, Mandelkern M, et al. Cerebral metabolism, cognition and functional abilities in Alzheimer disease. Journal of Geriatric Psychiatry and Neurology. 2011;24:127–134. doi: 10.1177/0891988711405333. [DOI] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE ε4 mice. Alzheimer's & Dementia. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behavioural Brain Research. 2007;184:124–132. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Paramentier A. Adult and geriatric normative data and validation of the profile of mood states. Journal of Clinical Psychology. 1999;55:79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiology of Disease. 2008;30:121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer's disease: A 1-year randomized, controlled trial. Journal of the American Geriatrics Society. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Brickman AM, Cosentino S, Schupf N, Xin-Tang M, et al. Physical activity and Alzheimer disease course. American Journal of Geriatric Psychiatry. 2010;19:471–481. doi: 10.1097/JGP.0b013e3181eb00a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA, Brooks JO, III, Friedman LF, Gratzinger P, Hill RD, et al. Proposed factor structure of the Geriatric Depression Scale. International Psychogeriatrics. 1991;3:23–28. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Struman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;79:232–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Ringman J, Ross LK, Mulnard RA, Dick MB, Bartzokis G, et al. Diagnosis depression in Alzheimer's disease with the National Institute of Mental Health Provisional Criteria. American Journal of Geriatric Psychiatry. 2008;16:469–477. doi: 10.1097/JGP.0b013e318165dbae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W, Bleiler L, Alzheimer's Association Alzheimer's disease facts and figures. Alzheimer's Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Tinklenberg JR, Kraemer HC, Yaffe K, Ross K, Sheikh J, Ashford JW, et al. Donepezil treatment and Alzhiemer disease: Can the results of randomized clinical trials be applied to Alzheimer disease patients in clinical practice? American Journal of Geriatric Psychiatry. 2007;15:953–960. doi: 10.1097/JGP.0b013e3180986138. [DOI] [PubMed] [Google Scholar]
- Van de Winckel A, Feys H, De Weerdt W, Dom R. Cognitive and behavioral effects of music-based exercise in patients with dementia. Clinical Rehabilitation. 2004;18:253–260. doi: 10.1191/0269215504cr750oa. [DOI] [PubMed] [Google Scholar]
- van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: The FINE study. Neurology. 2004;28:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. American Journal of Alzheimer's Disease and Other Dementias. 2011;26:381–388. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T, Schneider S, Bruumer V, Struder HK. Frontal EEG asymmetry: The effects of sustained walking in the elderly. Neuroscience Letters. 2010;485:134–137. doi: 10.1016/j.neulet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer's disease: A randomized controlled trial. Scandinavian Journal of Caring Sciences. 2011 doi: 10.1111/j.1471-6712.2011.00895.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Barton C, Ross L, Kramer JH, Yaffe K. Treatment practices of mild cognitive impairment in California Alzheimer's Disease Centers. Journal of the American Geriatrics Society. 2009;57:686–690. doi: 10.1111/j.1532-5415.2009.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Journal of the American Medical Association. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Williams CL, Tappen RM. Effects of exercise on mood in nursing home residents with Alzheimer's disease. American Journal of Alzheimer's Disease and Other Dementias. 2007;22:389–397. doi: 10.1177/1533317507305588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáguez L, Shaw KN, Morris R, Matthews D. The effects on cognitive functions of a movement-based intervention in patients with Alzheimer's type dementia: a pilot study. International Journal of Geriatric Psychiatry. 2011;2:173–181. doi: 10.1002/gps.2510. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey MB, et al. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yu F, Kolanowski AM. Facilitating aerobic exercise training in older adults with Alzheimer's disease. Geriatric Nursing. 2009;30:250–259. doi: 10.1016/j.gerinurse.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Yu F, Kolanowski AM, Strumpf NE, Eslinger PJ. Improving cognition and function through exercise intervention in Alzheimer's disease. Journal of Nursing Scholarship. 2006;38:358–365. doi: 10.1111/j.1547-5069.2006.00127.x. [DOI] [PubMed] [Google Scholar]