Abstract
Background
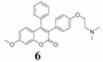
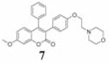
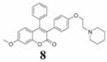
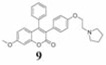
Coumarins belong to an important group of useful drugs with diverse pharmacological properties. In the present study, the in vitro cytotoxicity of new coumarin-based benzopyranone derivatives containing diethylaminoethoxy (5), dimethylaminoethoxy (6), morpholinoethoxy (7), piperidinylethoxy (8) and pyrrolidinylethoxyl (9) amino side chain against human carcinoma (A549) and normal (LL47) lung cell lines was evaluated.
Materials and Methods
The cytotoxicity was evaluated by crystal violet dye binding assay. The effect of compound 9 on different phases of the cell cycle was determined using flow cytometry.
Results
In A549 cells, the 50% lethal dose (LD50) for compounds 5–9 were found to be 7.08, 5.0, 34.2, 8.33 and 5.83 µM, respectively, while in LL47 cells, the LD50 values were found to be 16.7, 20.4, 34.6, 15.4 and 8.75 µM, respectively after 48 h treatment. Cell cycle data indicated that A549 cells were arrested at different phases depending on the concentration.
Conclusion
Compounds 5–9 showed anticancer activity against lung cancer cell lines, while compound 6 showed highly selective anticancer activity.
Keywords: Coumarin, basic amino side chain, cell viability, cell cycle, benzopyranone derivatives
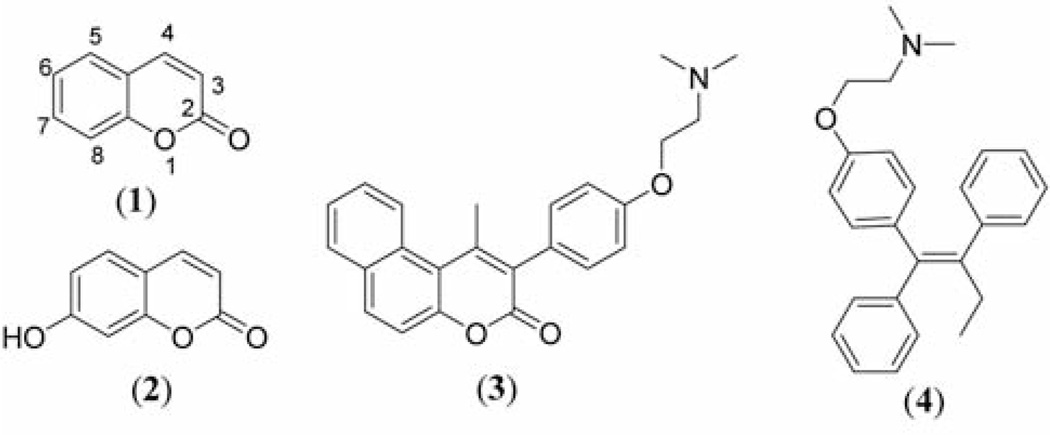
Coumarin (2H-l-benzopyran-2-one) and many of its derivatives exhibit useful and diverse biological activities including antitumor and antiproliferative activities (1–6). For example, coumarin (compound 1) and its metabolite, 7-hydroxycoumarin (compound 2) (Figure 1) have demonstrated antitumor activity in human cancer cell lines, such as A549 (lung), ACHN (renal), H727 (lung), MCF-7 (breast) and HL-60 (leukemia) (7–9). They display both cytostatic and cytotoxic activities (10, 11). The cytostatic and apoptotic actions of compounds 1 and 2 in non-small cell lung carcinoma (NSCLC) cell lines depend on both the histological type of lung cancer and the coumarin concentration used (12, 13). Furthermore, they inhibit cell growth by inducing cell cycle arrest in the G1 phase in human lung carcinoma cell lines (12, 14).
Figure 1.
Structures of coumarin (compound 1), 7-hydroxycoumarin (compound 2), coumarin-based benzopyranone derivatives containing basic sides chain (compound 3) and tamoxifen (TAM, compound 4).
Most recently, the current authors have been interested in benzopyranone derivatives containing basic amino side chain (compound 3, Figure 1) as potential anticancer agents. The basic amino side chain (tertiary aminoethoxy group), responsible for tissue-selective activity, is one of the chemical features of estrogen receptor (ER) ligands often referred to as selective estrogen receptor modulators (SERMs), e.g. tamoxifen (TAM, compound 4, Figure 1) (15–18). Although TAM is widely used for breast cancer treatment, it inhibits growth and exerts dose-dependent inhibitory activities in ER-negative lung cancer cell lines (19–20).
Preliminary in vitro results obtained from the National Cancer Institute Developmental Therapeutics program revealed that compound 3, with a dimethylaminoethoxy side chain, showed antiproliferative activity against the following NCI-9 NSCLC cancer cell lines: A549/ATCC, EKVX, HOP-62, NCI-H226, NCI-H23, NCI-H322M, NCI-H460 and NCI-H522. As part of an on-going investigation, this study evaluated the in vitro cytotoxicity activity of new coumarin-based benzopyranone analogs containing basic amino side chain group at the C-3 substituted position of the benzopyranone core structure, compounds 5–9, against A549 (cancer) and LL47 (normal) human lung cell lines. In the present study, the effect of compound 9 on cell cycle progression was also studied in A549 cell line using flow cytometry.
Materials and Methods
Chemicals
F12 K medium, penicillin-streptomycin anti-biotic solution (100×), fetal bovine serum (FBS), Trypsin-EDTA solution (1×), phosphate bufferred saline (PBS), 50% glutaraldehyde, crystal violet, propidium iodide and RNase were obtained from Sigma-Aldrich Company (St. Louis, MO, USA). The potassium phosphate, EDTA, D-glucose, ethanol were obtained from Thomas Scientific Company (Swedesboro, NJ, USA).
Cell line maintenance
A549 lung (cancer) and LL47 lung (normal) cell lines were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA) and cultured as per the guidelines supplied. The cells were maintained in F12K medium containing 100 units of penicillin/ml, 100 µg of streptomycin/ml, 2 mM L-glutamine and 10% FBS in T-75 cm2 flasks at 37°C in a 5% CO2 incubator.
Treatment of cells
The cells (LL47 or A549) were plated at a density of 10×104 cells per well in polystyrene, flat bottom 24-well microtiter plates (Corning Costar, Rochester, NY, USA) in F12K medium containing 10% FBS and allowed to stabilize overnight in a CO2 incubator at 37°C. Following this, the cells were treated with compounds 5–9 at different concentrations (0, 10, 25, 50, 75 and 100 µM) in a final volume of 1 ml per well in triplicate wells for each treatment for 24 h or 48 h at 37°C in a 5% CO2 incubator. All studies were repeated at least twice.
Evaluation of cell viability
At the end of the incubation period, the viability was evaluated by dye uptake assay according to Badisa et al. (21). Glutaraldehyde (400 µl of 0.25%) was added to each well and incubated for 30 min at room temperature to fix the cells. The glutaraldehyde (0.07% final concentration in the well) in the crystal violet dye staining assay procedure fixed the viable cells after the treatment with compound. The plates were rinsed with water to wash off the dead cells and dried under airflow inside a laminar hood for 5–10 min. Crystal violet (400 µl of 0.1%) was added to each well, incubated for 15 min, washed and dried. To solubilize the dye, 1 ml of 0.05 M sodium phosphate solution (monobasic) in 50% ethyl alcohol was added to each well and the plates were read at 540 nm in a Bio-Tek EL800 plate reader (Bio-Tek, Winooski, VT, USA). The mean absorbance value of control was considered as 100% and the treated sample percentages were calculated by comparing the absorbance of treated samples with the mean absorbance of the control.
Cell cycle analysis by flow cytometry
The effect of compound 9 on cell cycle phases in A549 lung cancer cell line was studied by using a C6 Accuri flow cytometer (Accuri Cytometers, Ann Arbor, MI, USA) according to Badisa et al. (22). Cells at a density of 1.3 × 106 cells per T-25 cm2 flask were plated overnight. The following day, the cells were treated with 0, 25, 50 and 75 µM of compound in triplicate flasks for 24 h in a 5% CO2 incubator at 37°C. At the end of incubation, the cells were trypsinized and centrifuged at 2,500 rpm for 10 min at room temperature. Each pellet was re-suspended in 100 µl PBS and singlet cells were made by vortexing. The cells were fixed in pre-cooled 95% ethanol (5 ml added in a drop-wise manner to each tube while vortexing) and incubated at 4°C at least for 24 h. The cells were then harvested and re-suspended in ethanol (100 µl of 95%). The cell suspensions were transferred into Eppendorf tubes. Staining solution (1 ml) containing final concentrations of 1.25 mg/ml ribonuclease A, 1 mg/ml D-glucose and 50 µg/ml propidium iodide was added to each tube in the dark. The tubes were incubated at room temperature for 1 h in the dark with occasional stirring. The distribution of cells in each phase was analyzed within 2 h with the C6 Accuri flow cytometer. In each sample, a total of 10,000 events from the gated subpopulation were analyzed separately. C6 Accuri flow cytometer software was used for the acquisition and analysis of the data, and the percentage of cells in each phase was determined in the gated population of singlet cells.
Selectivity index (SI)
The degree of the selectivity of compounds 5–9 were calculated according to the previous reported method (23): SI=LD50 of pure compound in the normal cell line/LD50 of the same pure compound in cancer cell line, where LD50 is the lethal concentration that required to kill 50% of the cells.
Statistical analysis
The viability and cell cycle analysis results were presented as mean±standard deviation (n=3). All treated cells data were presented as percentage values in comparison to the untreated control (100%). The data were analyzed for significance by one-way ANOVA, and then compared by Dunnett’s multiple comparison tests, using GraphPad Prism Software, version 3.00 (GraphPad Software, Inc., San Diego, CA, USA). Differences with the respective untreated control were considered statistically significant when p<0.05.
Results
Cytotoxicity
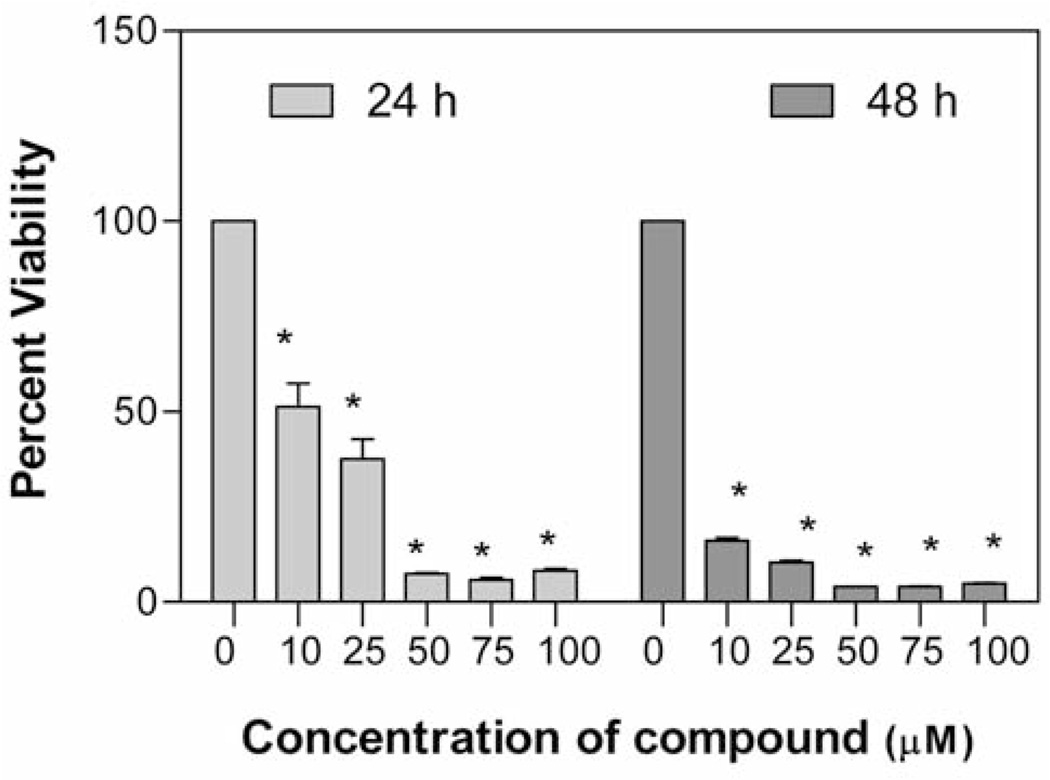
The in vitro anticancer activity of compounds 5–9 was evaluated at different concentrations (0, 25, 50, 75 and 100 µM) against A549 human lung carcinoma cell line after 24 and 48 h of treatment. The value, lethal dose 50 (LD50, the dose (concentration) of tested drug where a 50% growth reduction is observed in cell growth compared to the untreated control) after 24 h or 48 h treatment of compound was calculated according to the Ipsen method (24) and is given in Table I. It was observed that all synthesized compounds caused significant dose-dependent cell death in comparison to the untreated control. At 24 h treatment, compounds 5 and 9 (LD50=35.6 and 38.8 µM, respectively) and 6 and 8 (LD50=46.5 and 51.05 µM, respectively) showed comparable toxicity to a similar extent, while compound 7 (LD50=82.3 µM) showed markedly lower toxicity. At 48 h treatment, compounds 6 and 9 (LD50=5.0 µM and 5.83 µM, respectively) and 5 and 8 (LD50=7.08 µM and 8.33 µM, respectively) also showed comparable toxicity to a similar extent, while compound 7 (LD50=34.2 µM) showed markedly lower toxicity. These results revealed that compounds 6 and 9 show the highest cytotoxicity and the most significant decrease in cell viability against A549 lung cancer cell line at 48 h drug treatment. Figure 2 shows dose- and time-dependent cytotoxicity of potent compound 9 at 24 and 48 h treatment on A549 cells.
Table I.
In vitro cytotoxicity (lethal dose LD50, µM) of benzopyranone derivatives 5–9 containing basic amino side chain.
| LD50, µM (µg/ml) |
|||||
|---|---|---|---|---|---|
| LL47 Lung normal cells |
A549 Lung cancer cells |
||||
| Product | 48 h | 24 h | 48 h | Selectivity Index | |
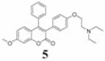 |
16.7 (37.7) | 35.6 (80.3) | 7.08 (15.9) | 2.36 | |
 |
20.4 (49.1) | 46.5 (111.9) | 5.0 (12.0) | 4.08 | |
 |
34.6 (75.6) | 82.3 (179.9) | 34.2 (74.8) | 1.01 | |
 |
15.4 (33.8) | 51.1 (112.2) | 8.33 (18.3) | 1.85 | |
 |
8.75 (19.8) | 38.8 (87.9) | 5.83 (13.2) | 1.50 | |
Figure 2.
Effect of compound 9 on A549 viability. The cells at a initial density of 1.0×104 per well were treated with 0, 10, 25, 50, 75 or 100 µM of the compound in a final volume of 1 ml F12K complete medium containing 10% FCS for 24 h or 48 h. Data are represented as the mean (boxes) and SEM (errorbars) for n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
After 48 h treatment against LL47 human (normal) lung cell line, compounds 5–9 showed slightly less toxicity in comparison to the A549 cancer cell line with LD50 values of 16.7, 20.4, 34.6, 15.4 and 8.75 µM, respectively. The selectivity index (SI) values of compounds 5–9 were: 2.36, 4.08, 1.01, 1.85 and 1.5, respectively (Table I). These observations revealed that compound 6, containing the pyrodinyl ethoxy chain group, exhibited the most tumor selective cytotoxicity based on its SI, which was the highest among all tested compounds.
Concentration-dependent inhibition at different phases of the A 549 cell cycle
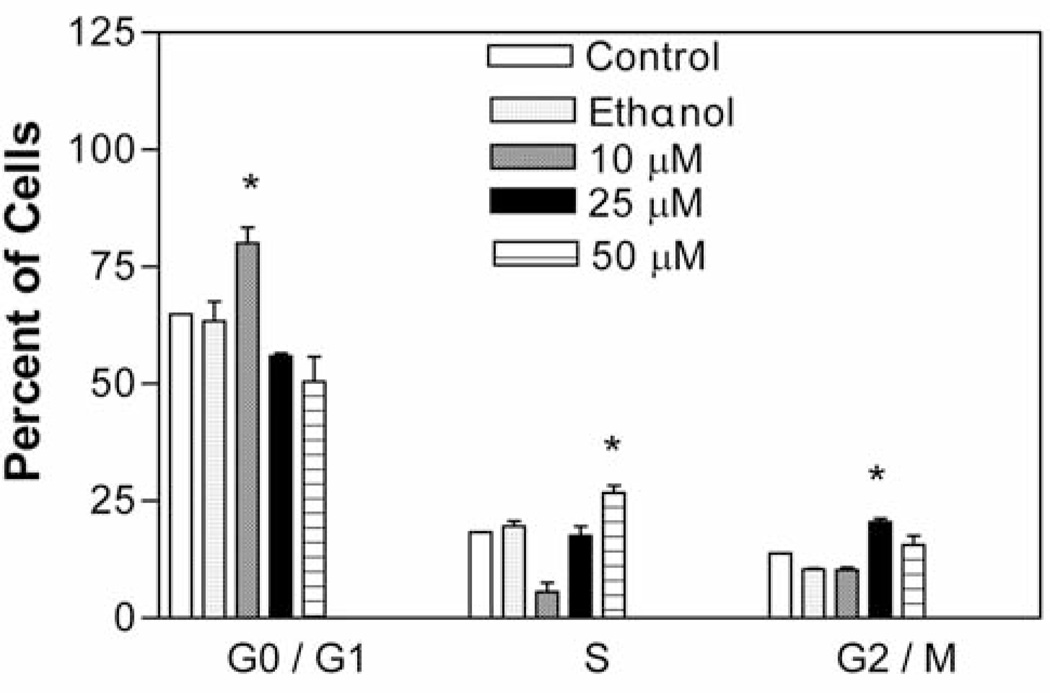
Figure 3 shows the effect of increasing concentrations of compound 9 on A549 cell progression through G0/G1–, S– and G2/M– phases. It was observed that treatment with compound 9 at 10, 25 and 50 µM concentrations for 24 h resulted in significant cell cycle arrest (p<0.05) in G0/G1–, G2/M– and S–phases, respectively, depending on the concentration. There was a marked increase in accumulation of treated cells in comparison to untreated control cells in the G0/G1 (15.25%) phase at 10 µM concentration and in the G2/M–(8.45%) and S– (6.7%) phases at 25 and 50 µM concentrations respectively. The cell cycle analysis data showed that the compound 9 exhibits a concentration dependent cell arrest
Figure 3.
Effect of compound 9 on A549 cell cycle. The cells at an initial density of 1.3×106/ml per T-25 flask in F12K complete medium containing 10% FCS were treated with 0, 10, 25 or 50 µM of the compound for 24 h. Cells were pelleted and stained by propidium iodide staining solution for 1 h in the dark and analyzed by flow cytometry. Data are represented as mean (boxes) and SEM (error bars) for n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
Discussion
The cytotoxicity of new benzopyranone derivatives with basic amino side chain (compounds 5–9) against human lung normal and cancer cell lines was evaluated by a simple and reproducible crystal violet dye-staining assay (21). Compounds 5–9 bear a similar structural resemblance to TAM in that they possess triphenylethylene core pharmacophore with a basic amino side chain functional group. TAM substantially mediates ER-independent growth-inhibitory effect in NSCLC cell line via type II estrogen binding sites (type II EBSs) (20). Although it may be assumed that compounds 5–9 act at the same target sites as TAM and other related triphenylethylene compounds in lung cancer cell lines, further studies are needed to confirm this claim. The in vitro cytotoxicity results indicated that the tested compounds display higher cytotoxicity against A549 cell line at 48 h treatment. The significant difference in the LD50 value of compound 6 in normal and cancerous lung cell lines indicates an interesting difference in its mode of action in cancer and normal cells. The SI indicates the differential cytotoxicity of a pure compound (the greater the index, the more selective the anticancer drug). The differential toxicity exhibited by compound 6 was highly significant (SI=4.08) and may be considered as a future candidate for the development of potent chemo- therapeutic agents.
The elicited cytotoxic effect of compound 9 at 10 µM may be due to the selective increase of cells in the G0/G1 phase, which suggests apoptotic cell death (Figure 3). This result is consistent with previous studies where it was shown that coumarin and derivatives inhibit cell growth by inducing cell cycle arrest in the G1 phase in lung carcinoma cell lines (13, 14). It was also evident that the cells arrest at different phases was concentration dependent. Future work would involve the establishment of the mechanism for cytotoxicity of compound 9 and its target site in the A549 cancer cell line.
Conclusion
The in vitro cytotoxicity of a new group of benzopyranone derivatives containing a basic amino side chain was studied. It was shown that coumarin compounds possessing basic amino side chains display higher cytotoxicity both in A549 and LL49 lung cell lines. However, the higher toxicity of these compounds against normal lung cell lines is of the utmost concern, since one of the important criteria in the development of therapeutic drugs for cancer treatment is to have few or no side-effects on normal cells of patients undergoing chemotherapy. Future studies would involve the testing of compound 6, which had the highest SI, in animal models as a chemotherapeutic agent.
Acknowledgements
Faculty Research Development Funds (NIH/NCRR/RCMI grant G12RR03020 is gratefully acknowledged for financial support.
References
- 1.Nofal ZM, El-Zahar MI, AbdEl-Karim SS. Novel Coumarin Derivatives with expected biological activity. Molecules. 2000;5:99–113. [Google Scholar]
- 2.Takeuchi Y, Xie L, Cosentino LM, Lee KH. Anti-AIDS agents-XXVIII. synthesis and anti-HIV activity of methoxy substituted 3’, 4’-di-O-(–)-camphanoyl-(+)-cis khellactone (DCK) analogues. Bioorg Med Chem Lett. 1997;7:2573–2578. doi: 10.1016/s0960-894x(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 3.Madari H, Panda D, Wilson L, Jacobs RS. Dicoumarol: A unique microtubule-stabilizing natural product that is synergistic with Taxol. Cancer Res. 2003;63(6):1214–1220. [PubMed] [Google Scholar]
- 4.Musiciki B, Periers AM, Laurin P, Ferroud D, Benedetti Y, Lachaud S, Chatreaux F, Haesslein JL, LLtis A, Pierre C, Khider J, Tessol N, Airault M, Demassey J, Dupuis-Hamelin C, Lassaigne P, Bonnefoy A, Vicat P, Klich M. Improved antibacterial activities of coumarin antibiotics bearing 5’,5’-dialkylnoviose: biological activity of RU 79115. Bioorg Med Chem Lett. 2000;10:1695–1699. doi: 10.1016/s0960-894x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- 5.Abd Allah OA. Synthesis and biological studies of some benzopyran. Farmaco. 2000;55(9–10):641–649. doi: 10.1016/s0014-827x(00)00090-2. [DOI] [PubMed] [Google Scholar]
- 6.Lacy A, O’Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 2004;10(30):3797–3811. doi: 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- 7.Stanchev S, Momekov G, Jensen F, Manolov I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur J Med Chem. 2008;43(4):694–706. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Thornes RD, Daly L, Lynch G, Breslin B, Browne H, Browne HY, Corrigan T, Daly P, Edwards G, Gaffney E, Henley J, Healy F, Keane F, Lennon F, McMurray N, O’Loughlin S, Shine M, Tanner A. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J Cancer Res Clin Oncol. 1994;120(Suppl):S32–S34. doi: 10.1007/BF01377122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohler JL, Gomella LG, Crawford ED, Glode LM, Zippe CD, Fair WR, Marshall ME. Phase II evaluation of coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma. Prostate. 1992;20(2):123–131. doi: 10.1002/pros.2990200208. [DOI] [PubMed] [Google Scholar]
- 10.Stanchev S, Momekov G, Jensen F, Manolov I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur J Med Chem. 2008;43(4):694–706. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Egan D, James P, Cooke D, O’Kennedy R. Studies on the cytostatic and cytotoxic effects and mode of action of 8-nitro-7-hydroxycoumarin. Cancer Lett. 1997;118(2):201–211. doi: 10.1016/s0304-3835(97)00331-5. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Gonzalez JS, Prado-Garcia H, Aguilar-Cazares D, Molina-Guarneros JA, Morales-Fuentes J, Mandoki JJ. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer. 2004;43(3):275–283. doi: 10.1016/j.lungcan.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Goel A, Prasad AK, Parmar VS, Ghosh B, Saini N. 7,8-Dihydroxy-4-methylcoumarin induces apoptosis of human lung adenocarcinoma cells by ROS-independent mitochondrial pathway through partial inhibition of ERK/MAPK signaling. FEBS Lett. 2007;581(13):2447–2454. doi: 10.1016/j.febslet.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Kahn J, Preis P, Waldman F, Tseng A., Jr Coumarin modulates the cell-cycle progression of an MTV-EJras cell line. J Cancer Res Clin Oncol. 1994;120(Suppl):S19–S22. doi: 10.1007/BF01377118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YW, Mobley JA, Brueggemeier RW. Synthesis and estrogen receptor binding affinities of 7-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-ones containing a basic side chain. Bioorg Med Chem Lett. 2003;13(8):1475–1478. doi: 10.1016/s0960-894x(03)00132-x. [DOI] [PubMed] [Google Scholar]
- 16.Sharma AP, Saeed A, Durani S, Kapil RS. Structure–activity relationship of antiestrogens. Effect of the side chain and its position on the activity of 2,3-diaryl-2-H-1-benzopyrans. J Med Chem. 1990;33(12):3216–3222. doi: 10.1021/jm00174a019. [DOI] [PubMed] [Google Scholar]
- 17.Jain N, Xu J, Kanojia RM, Du F, Jian-Zhong G, Pacia E, Lai MT, Musto A, Allan G, Reuman M, Li X, Hahn DW, Cousineau M, Peng S, Ritchie D, Russell R, Lundeen S, Sui Z. Identification and structure–activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symptoms. J Med Chem. 2009;52(23):7544–7569. doi: 10.1021/jm900146e. [DOI] [PubMed] [Google Scholar]
- 18.Wallace OB, Bryant HU, Shetler PK, Adrian MD, Geiser AG. Benzothiophene and naphthalene derived constrained SERMs. Bioorg Med Chem Lett. 2004;14(20):5103–5106. doi: 10.1016/j.bmcl.2004.07.072. [DOI] [PubMed] [Google Scholar]
- 19.Croxtall JD, Emmas C, White JO, Choudhary Q, Flower RJ. Tamoxifen inhibits growth of oestrogen receptor-negative A549 cells. Biochem Pharmacol. 1994;47(2):197–202. doi: 10.1016/0006-2952(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 20.Caltagirone S, Ranelletti FO, Rinelli A, Maggiano N, Colasante A, Musiani P, Aiello FB, Piantelli M. Interaction with type II estrogen binding sites and antiproliferative activity of tamoxifen and quercetin in human non-small cell lung cancer. Am J Respir Cell Mol Biol. 1997;17(1):51–59. doi: 10.1165/ajrcmb.17.1.2728. [DOI] [PubMed] [Google Scholar]
- 21.Badisa RB, Tzakou O, Couladis M, Pilarinou E. Cytotoxic activities of some Greek Labiatae herbs. Phytother Res. 2003;17:472–476. doi: 10.1002/ptr.1175. [DOI] [PubMed] [Google Scholar]
- 22.Badisa RB, Darling-Reed SF, Joseph P, Cooperwood S, Latinwo LM, Goodman CB. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009;29:2993–2996. [PMC free article] [PubMed] [Google Scholar]
- 23.Koch A, Tamez P, Pezzuto J, Soejarto D. Evaluation of plants used for antimalarial treatment by the Massai of Kenya. J Ethnopharmacol. 2005;101:95–99. doi: 10.1016/j.jep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Ipsen J, Feigl P. Bancroft's Introduction to Biostatistics. 2nd edition. New York: Harper and Row; 1970. p. 164. [Google Scholar]