Abstract
Purpose
Bone marrow–derived endothelial progenitor cells (EPCs) contribute to vascular repair although it is uncertain how local endothelial cell apoptosis influences their reparative function. This study was conducted to determine how the presence of apoptotic bodies at sites of endothelial damage may influence participation of EPCs in retinal microvascular repair.
Methods
Microlesions of apoptotic cell death were created in monolayers of retinal microvascular endothelial cells (RMECs) by using the photodynamic drug verteporfin. The adhesion of early-EPCs to these lesions was studied before detachment of the apoptotic cells or after their removal from the wound site. Apoptotic bodies were fed to normal RMECs and mRNA levels for adhesion molecules were analyzed.
Results
Endothelial lesions where apoptotic bodies were left attached at the wound site showed a fivefold enhancement in EPC recruitment (P < 0.05) compared with lesions where the apoptotic cells had been removed. In intact RMEC monolayers exposed to apoptotic bodies, expression of ICAM, VCAM, and E-selectin was upregulated by 5- to 15-fold (P < 0.05– 0.001). EPCs showed a characteristic chemotactic response (P < 0.05) to conditioned medium obtained from apoptotic bodies, whereas analysis of the medium showed significantly increased levels of VEGF, IL-8, IL-6, and TNF-α when compared to control medium; SDF-1 remained unchanged.
Conclusions
The data indicate that apoptotic bodies derived from retinal capillary endothelium mediate release of proangiogenic cytokines and chemokines and induce adhesion molecule expression in a manner that facilitates EPC recruitment.
The vascular endothelium is a dynamic cell layer that sends and receives complex chemical signals to and from other cells in the vessel wall. These signals contribute to monolayer integrity and vascular function.1 The balance is seriously disrupted in the diabetic retinal microvasculature because of accelerated apoptosis of pericytes and endothelial cells2 resulting in progressive vasodegeneration3 and progression to sight-threatening diabetic retinopathy (DR).4 Elevated glucose levels in the diabetic milieu can disrupt normal cell substrate communication and vascular function,5,6 thereby contributing to endothelial cell loss.7,8 Damage to the endothelium is accompanied by breakdown of the inner blood–retinal barrier9 and the local release of cytokines and chemokines such as VEGF, IGF-1 SDF-110 by the adjacent endothelium.11
Dead or dying endothelial cells in capillary lumens are replaced through mitosis of neighboring cells in the monolayer12 or by the recruitment of circulating endothelial progenitor cells (EPCs).13 It is becoming widely recognized that EPCs make a significant contribution to normal vascular repair by homing to the site of injury, differentiating into endothelium, and thereby maintaining monolayer integrity.14 EPCs are recruited to the damaged vasculature via cytokine gradients and local expression of adhesion molecules (e.g., ICAM-1 and VCAM-1).15,16 Subsequent differentiation and incorporation of these progenitors into vessels is mediated by complex interactions with exposed basement membrane (BM) proteins.17 In addition, injury and/or ischemia-induced release of cytokines such as VEGF, IL, and GM-CSF appear to play a key role in mobilization of EPCs from the bone marrow and attracting EPCs to sites of vascular injury.11
Emerging evidence suggests a potential link between endothelial cell apoptosis and initiation of vascular repair by EPCs. Uptake of apoptotic bodies by neighboring cells is known to provoke EPC recruitment and differentiation in macrovascular endothelium,18 although the existence of this phenomenon in microvascular endothelium and particularly that of the retinal microvasculature has not been established. Therefore, the present study was undertaken to investigate EPC incorporation into the highly specialized retinal microvascular endothelium after apoptotic cell death in the monolayer (akin to what happens in DR).
Materials and Methods
Isolation of Retinal Microvascular Endothelial Cells
Retinal microvascular endothelial cells (RMECs) were isolated from bovine retina by established, published protocols.19 Briefly, isolated bovine retinal homogenate was subjected to enzyme digestion consisting of pronase, DNase, and collagenase. This mixture was agitated at 37°C and filtered through 53-µm gauze followed by spinning at 1100 rpm for 10 minutes. Finally, the pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM) containing heparin and insulin. The isolated cells were then transferred onto 1% gelatin-coated flasks and incubated at 37°C. During culturing, retinal microvascular endothelial cells migrated from the vessel fragments and proliferated to form a monolayer over the course of 7 to 10 days. RMECs from passages 2 to 4 were used in all experiments.
Isolation and Characterization of EPCs
EPCs were isolated and characterized as described previously.20 Briefly, they were cultured on fibronectin-coated (2 µg/cm2) culture plates supplemented with endothelial cell basal medium (EBM-2; containing EGM-2 MV, SingleQuots; Cambrex Bio Science Wokingham, Ltd., Berkshire, UK) containing 5% FBS, human VEGF, FGF-2, EGF, IGF-1, and ascorbic acid. Spindle-shaped adherent EPCs at the end of a 7-day culture period were assayed for CD133, CD34, VEGFR2, CD31, CD14, and uptake of acLDL DiI, UEA-1 lectin.20
Verteporfin In Vitro Model of Endothelial Loss and Repair by EPCs
We used an in vitro model to assess the repair of damaged retinal endothelial cells by EPCs, as described previously20 with slight modification. Briefly, confluent RMEC monolayers were treated with the photodynamic drug verteporfin 0.5 to 1 µg/mL (Novartis Inc., Basel, Switzerland). Discrete regions of the monolayer were illuminated by a collimated beam of red light (600 nm) from a monochromator (Cairn Research, Faversham, UK) to create wounds of 1-mm diameter. This model produces microlesions in the monolayer whereby the red light–exposed endothelial cells undergo synchronous apoptosis while maintaining the integrity of the subendothelial matrix.20
In some preparations, apoptotic cells were left attached at the lesion site, whereas others were thoroughly washed with PBS to remove the characteristically rounded and fragmented cells. DiI-labeled (Biomedical Technologies, Stoughton, MA) EPCs were added to these wounded RMECs and the adherent cells counted in a 1-mm2 area of a verteporfin-induced wound after staining of the cultures with biotinylated BS1-B4 isolectin (Sigma-Aldrich, St. Louis, MO) and streptavidin-conjugated AlexaFluor 488 (Molecular Probes, Inc., Eugene, OR).
Adhesion Molecule Expression at the Sites of RMEC Denudation
Adhesion molecule expression at the denuded regions on RMEC mono-layers was studied by immunohistochemistry for ICAM-1, VCAM-1, and E-Selectin. Wounded and unwounded RMECs were treated with FITC-conjugated mouse monoclonal anti-human ICAM-1, VCAM-1, or E-selectin antibodies (Dako Cytomation, Glostrup, Denmark or Chemicon international, Temecula, CA) and the slides were viewed by confocal scanning laser microscopy. The regions of the monolayer in the immediate vicinity of lesions were evaluated for the expression of adhesion molecules and compared to regions distant from wound sites (unharmed). The intensity of adhesion molecule immune staining was compared by keeping confocal microscope settings (e.g., laser power, brightness-contrast, and gain) constant during the recording of images from the penumbra of lesions and undamaged periphery of the monolayer.
Preparation of Apoptotic Bodies and Conditioned Medium
RMECs were grown to confluence on 20-mm Petri dishes that had been coated with fibronectin (2 µg/cm2; Sigma-Aldrich). RMEC monolayers were treated with the photodynamic drug verteporfin (Visudyne; Novartis Inc.) at cytotoxic concentration of 0.5 to 1 µg/mL in dark conditions. After incubation for 30 minutes, these monolayers were exposed to red light for 5 minutes and maintained at 37°C to generate a highly synchronous apoptotic response in the monolayer. After 3 hours of red light exposure, apoptotic bodies were harvested by gentle detachment with trypsin-EDTA and separated from conditioned medium by centrifuging at 15000g for 20 minutes and quantified with an epifluorescence microscope (Nikon, Tokyo, Japan) and software (Lucia Software, Exeter, UK). Some apoptotic RMEC cultures were maintained for 24 hours after initial light exposure, and the conditioned medium analyzed by ELISA as described later.
Quantification of Apoptosis by JC-1 Flow Cytometry
Apoptosis in RMECs was assessed with a JC-1 assay (MitoProbe; Molecular Probes, Eugene, OR) as per the manufacturer’s instructions. Briefly, 1 × 106 verteporfin-treated RMECs were suspended in 1 mL warm PBS to which 10 µL of 200 µM JC-1 was added. Analysis of JC-1 fluorescence was quantified with the 488 nm green filter and PE-filter on a flow cytometer (FACScalibur; BD Biosciences, Oxford, UK). Data are expressed in terms of the reduced red/green fluorescence ratio in comparison to control (nonapoptotic) RMECs.
Quantitative Real-Time RT-PCR (qRT-PCR) to Study the Ability of Apoptotic Bodies to Induce Adhesion Molecule Expression in Monolayers of RMECs
Apoptotic bodies were added to healthy RMEC monolayers at a concentration of 2 × 105. After 6- and 24-hour exposures at 37°C to apoptotic bodies, RMECs were harvested and subjected to total RNA extraction (RNeasy mini kit; Qiagen, West Sussex, UK) as per the manufacturer’s instructions. The amount of RNA was quantified with a spectrophotometer (model ND-1000; NanodropTechnologies, Wilmington, DE) by measuring absorbance at 260/280 nm. cDNA was prepared from 1 µg equivalent of total RNA (Sensiscript Reverse Transcription [RT] kit; Qiagen), as per the manufacturer’s instructions. qRT-PCR reaction was conducted (QunatiTectSYBR green qPCR kit; Qiagen) and ICAM-1 (forward GCAACTTCTCCTGCTCTGCT, reverse TTCCGGCTACAGTTCAGCTT), VCAM-1 (forward AAGTGTAGCTACT-GCGTGAAAG, reverse CACAGTAAGGGCCATAGGCA), E-selectin (forward AGTCATCCTGCCACTTCACC, reverse TCTTTCAGGACATG-CAAACG) and 28S (forward TTGAAAATCCGGGGGAGAG, reverse ACATTGTTCCAACATGCCAG) primers. A thermocycler (Lightcycler; Roche Diagnostics, UK) was programmed for initial activation at 95°C for 15 minutes, amplification involving denaturation at 94°C for 15 seconds, annealing at 50°C to 55°C (variable) for 30 seconds and extension at 72°C for 15 seconds followed by cooling at 40°C. A total of 45 to 50 cycles were conducted, and PCR efficiency was confirmed with melting curve analysis. A quantification report from the thermocycler was used to obtain average crossover values and data were expressed in terms of ΔΔCT (relative gene expression crossover value in comparison to 28S housekeeping mRNA) values in comparison with the control group results.
EPC Chemotaxis in Apoptotic Endothelial Cell–Conditioned Medium
Conditioned medium obtained from panapoptotic cultures were tested for chemotaxis with Dunn’s chemotaxis assay, as described previously.20 Briefly, the outer well of the Dunn’s chamber was filled with conditioned medium obtained from apoptotic endothelial cells and the assembled Dunn chamber slide was maintained at 37°C. With a shutter control device, the images were digitally recorded over the span of 10 hours, at a time-lapse of 5 minutes. Data were analyzed with commercial software (AQM 2001 software; Kinetic Imaging Ltd. Manchester, UK) and expressed as circular histograms.
Cytokine and Chemokine ELISA of Conditioned Medium from Apoptotic RVECs
Conditioned medium obtained from apoptotic RVECs was analyzed for VEGF (Biosource International-Invitrogen Inc., Carlsbad, CA), SDF-1α (R&D Systems, Abingdon, UK), IL-8 (R&D Systems), IL-6, and TNF-α (Diaclon, Boldon, UK), as per the manufacturers’ instructions. For each cytokine ELISA, the optical density for both standards and samples was obtained with a spectrophotometer (Tecan Systems, Männedorf, Switzerland) and concentrations were expressed in picograms per milliliter.
Statistical Analysis
Data were expressed as the mean ± SEM. Statistical differences in the mean were assessed by using one way analysis of variance (ANOVA) followed by the Tukey-Kramer post hoc test for multiple comparisons, unless stated specifically. Chemotaxis data were analyzed with the Raleigh test for statistical significance. All statistical analyses were performed with commercial software (SPSS 14.0; SPSS Inc, Chicago, IL, or GraphPad InStat 3.0; GraphPad Software, San Diego, CA).
Results
In Vitro Model for the Study of Repair by EPCs in the Presence and Absence of Apoptotic Bodies at the Wound Site
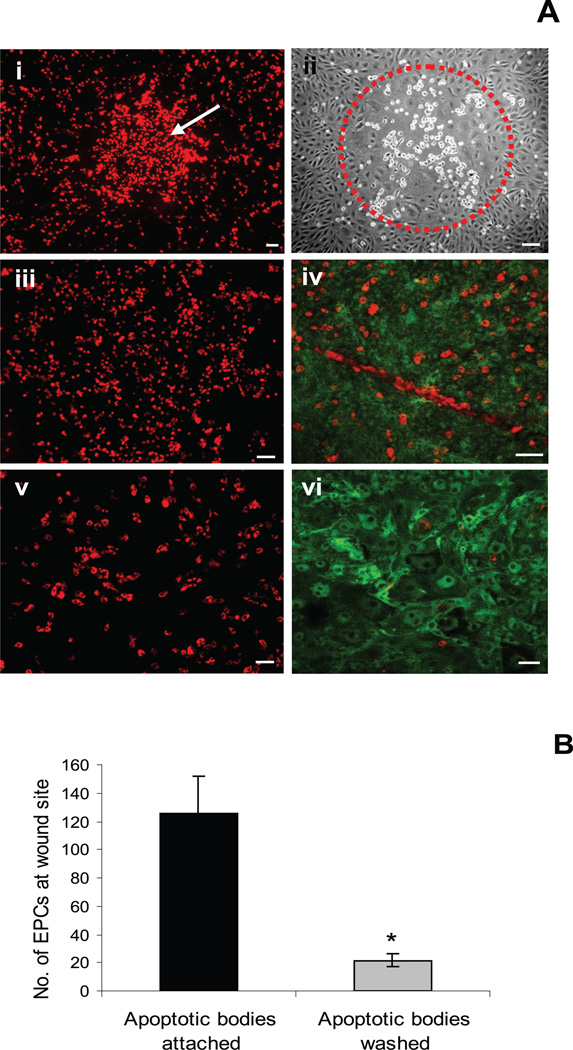
To explore the anecdotal observation from a previous study that EPC recruitment could be influenced by the presence of apoptotic cells at the site of an endothelial wound, we introduced EPCs to wounded RMEC monolayers in the presence or absence of apoptotic cells at the site of the lesion in the monolayer. The verteporfin model created highly circumscribed regions of endothelial apoptosis, and to test the reparative capacity of EPCs, we prelabeled these cells with DiI and added them to the verteporfin-treated RMEC monolayers at 3 hours after wounding. EPCs preferentially targeted the denuded regions (Fig. 1Ai) and subsequently adhered to the wound sites (Fig. 1Aii). The presence of apoptotic bodies at the wound site attracted EPCs (Fig. 1Aiii) and these cultures showed a complete healing of the wound (Fig. 1Aiv), which contrasted with the monolayers from which apoptotic bodies had been washed off (Fig. 1Av). These cultures were observed until restoration of endothelial monolayer in wounded regions. Complete healing of wounds was observed with enhanced contribution by EPCs among apoptotic body attached wounds (Figs. 1Aiv, 1Avi). Quantification showed that the presence of apoptotic bodies provoked a sixfold increase in EPC homing (P < 0.05; Fig. 1B).
Figure 1.
Apoptotic bodies attract EPCs to the wound site. (A) Highly circumscribed delineated regions of apoptosis were created with a highly collimated beam of red light in endothelial monolayers that had been pretreated with the photodynamic agent verteporfin. (Ai) Addition of DiI-labeled EPCs showing site-specific targeting as indicated by the cluster of red fluorescent cells (white arrow). (Aii) Phase-contrast micrograph of a similar wound showing clustering of EPCs at 24 hours (red circle: area of wound and adhesion of EPCs). (Aiii) Presence of apoptotic bodies attracted more EPCs compared with wounds in which they had been removed from the wound site (Av). Large proportion of EPCs (red) showed incorporation among RMECs (green) in the presence of apoptotic bodies (Aiv), whereas the number was reduced in their absence (Avi). Scale bar, 100 µm. (B) Quantification of EPCs at the wound site showed a significantly large proportion of EPCs due to the presence of apoptotic bodies, P < 0.05.
Effect of Verteporfin Wounding on Adhesion Molecule Expression at Wound Site
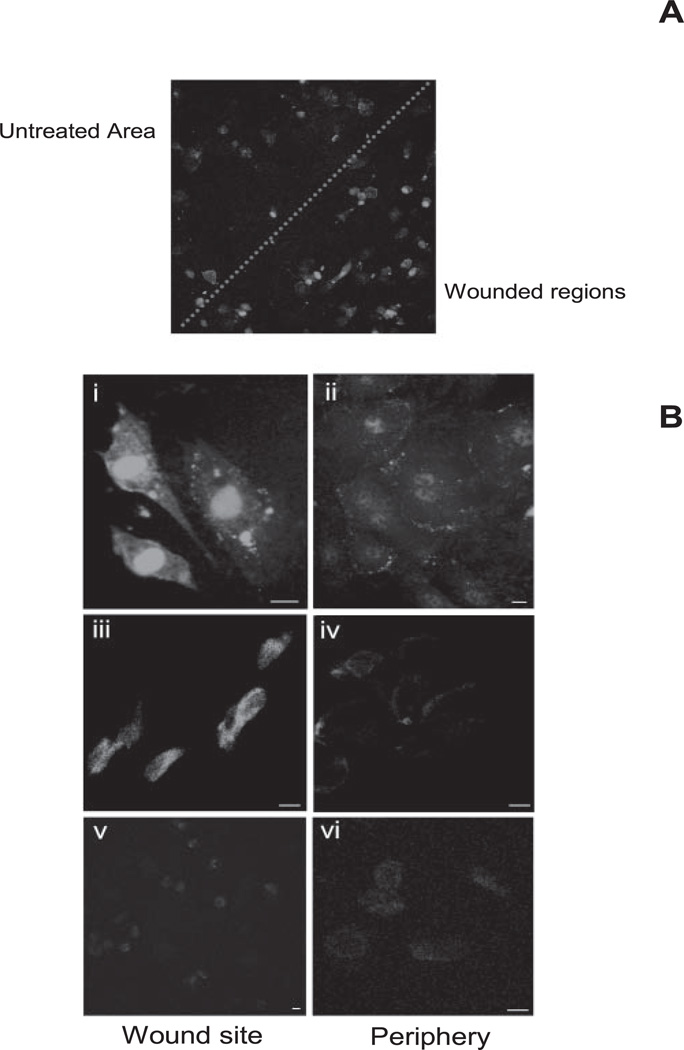
We observed that exposure of apoptotic bodies to healthy endothelium upregulates adhesion molecule expression. To confirm whether such mechanisms are involved in the recruitment of EPCs, we studied expression of adhesion molecules at the wound site in the above model. By maintaining contrast, brightness staining of confocal microscope consistent images were taken for wounded and unwounded regions (Fig. 2A. It was observed that induction of endothelial apoptosis with verteporfin caused a marked increase in ICAM-1 expression around the edge of the wound (Fig. 2Bi) compared with distant or untreated regions on the same plate (Fig. 2Bii). VCAM-1 surface expression at the site of wounds (Fig. 2Biii) was also upregulated when compared to unharmed regions on same plate (Fig. 2Biv). There was no apparent change in E-selectin expression between wounded and nonwounded regions (Fig. 2Bv, 2Bvi).
Figure 2.
Adhesion molecule expression after verteporfin induction of wound. (A) A typical verteporfin-induced wound showing enhanced expression of ICAM at the wound site compared with the periphery (dotted line: peripheral and central wound areas). (B) Strong ICAM-1 expression at the wound site (Bi) compared with the periphery (Bii). Similarly intense VCAM-1 expression was observed at the wound (Biii) site compared with the distant regions on same image (Biv). There was no significant change in E-selectin expression at the wound site and at the periphery (Bv, Bvi). Scale bar, 10 µm.
Preparation and Quantification of Apoptotic Bodies
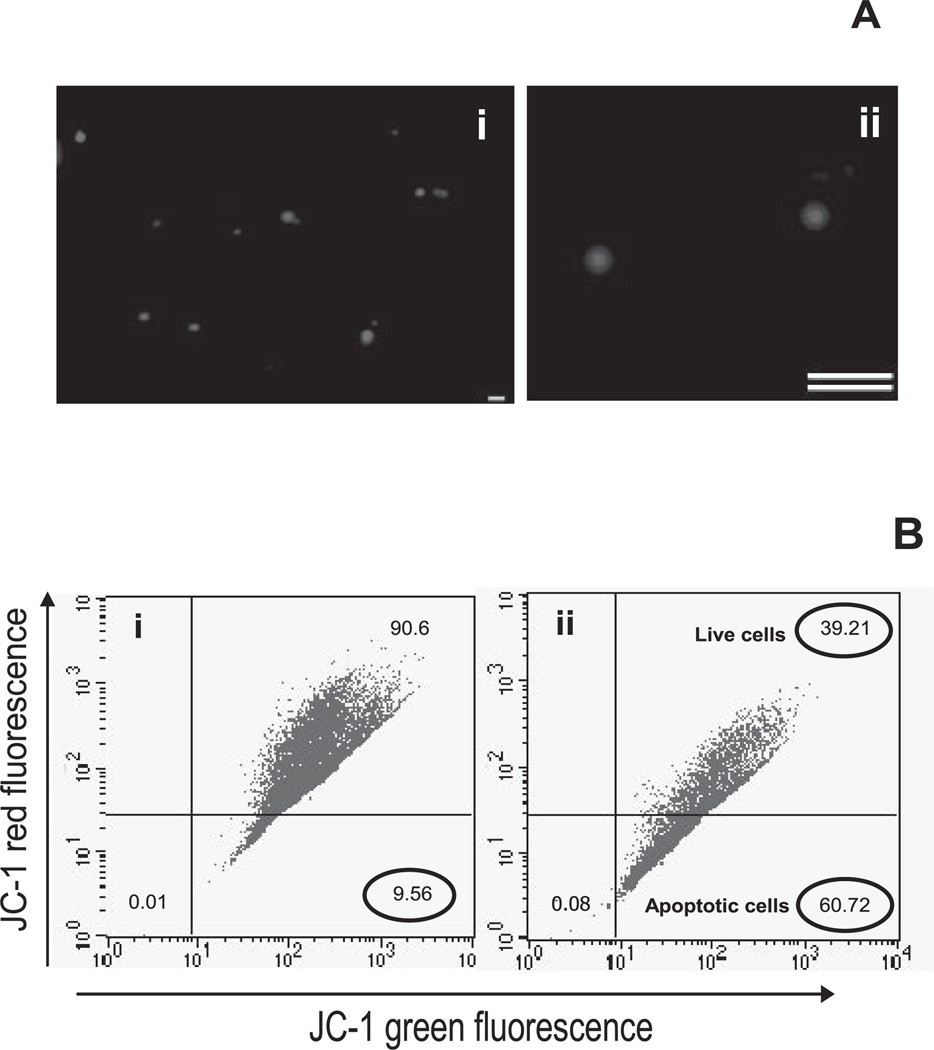
A large number of apoptotic bodies was generated from RMECs by treatment with verteporfin rather than serum starvation, which produces a highly asynchronous apoptotic response, even in cells at the same stage of the cycle.21,22 The size of the apoptotic bodies was assessed by epifluorescence microscopy with PI staining and was shown to range from 1 to 5 µm in diameter (Fig. 3A). Studies have shown that activation of verteporfin by red light generates the singlet oxygen radical,23 resulting in endothelial cell apoptosis via generation of multiple caspase-2, -3, -6, -7, -8, and -9.24 To confirm the apoptotic nature of the cells and to establish the model system, we conducted JC-1 flow cytometry, wherein a decrease in red to green fluorescence was associated with the formation of J-1 aggregates as indicative of apoptosis. JC-1 flow cytometry confirmed the apoptotic nature of these bodies, as indicated by the decrease in red to green fluorescence. Verteporfin treatment and light exposure rendered more than 60% of cells apoptotic at the end of 2 to 3 hours (Fig. 3B).
Figure 3.
Quantification of apoptotic bodies. (A) PI-stained apoptotic bodies were observed to be present in the range of 1 to 5 µm. (B) Apoptosis was confirmed by using JC-1 flow cytometry. The initial gate (bottom left) was selected by excluding debris and using healthy endothelial cells. Verteporfin induced robust apoptosis and thus a marked decrease in the red to green fluorescence, with 60% apoptotic cells, whereas untreated healthy counterparts showed the presence of 9% apoptotic cells. Flow cytometry dot plots are representative of three independent experiments. Scale bar, 10 µm.
Upregulation of Adhesion Molecule Expression in Endothelial Monolayer after Exposure to Apoptotic Bodies
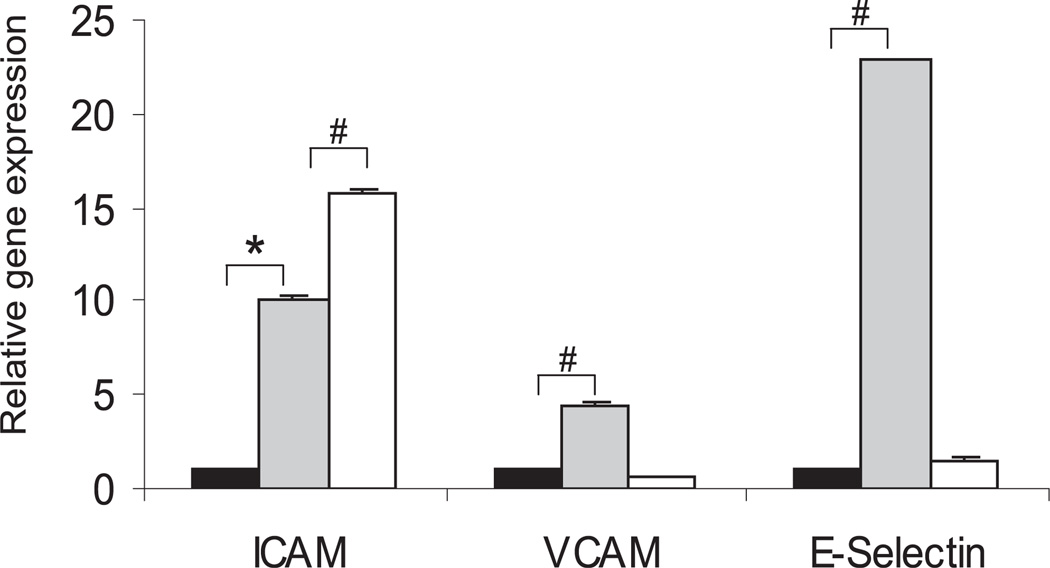
Adhesion molecule mRNA expression by healthy RMECs exposed to apoptotic bodies was studied at 6 and 24 hours. Before qRT-PCR was performed, all the primers were assessed for any primer dimer contamination by conventional RT-PCR (data not shown). Treatment of healthy RMECs with apoptotic bodies upregulated expression of ICAM-1 and E-selectin by 10-and 23-fold (P < 0.001), respectively, whereas VCAM-1 was upregulated by fivefold (P < 0.05) after a 6-hour exposure to apoptotic bodies. ICAM-1 mRNA showed a time-dependent increase with upregulation to >15-fold (P < 0.001), whereas there was decline in VCAM-1 and E-selectin expression at 24 hours after exposure to apoptotic bodies (Fig. 4).
Figure 4.
Adhesion molecule expression after exposure of healthy endothelium to apoptotic bodies. Quantitative real-time PCR mean data showing that exposure of apoptotic bodies to the healthy endothelium upregulated ICAM-1, VCAM-1, and E-selectin expression at 6 and 24 hours. n = 3; *P < 0.05, #P < 0.001.
Chemotaxis of Conditioned Medium from Apoptotic Endothelial Cells
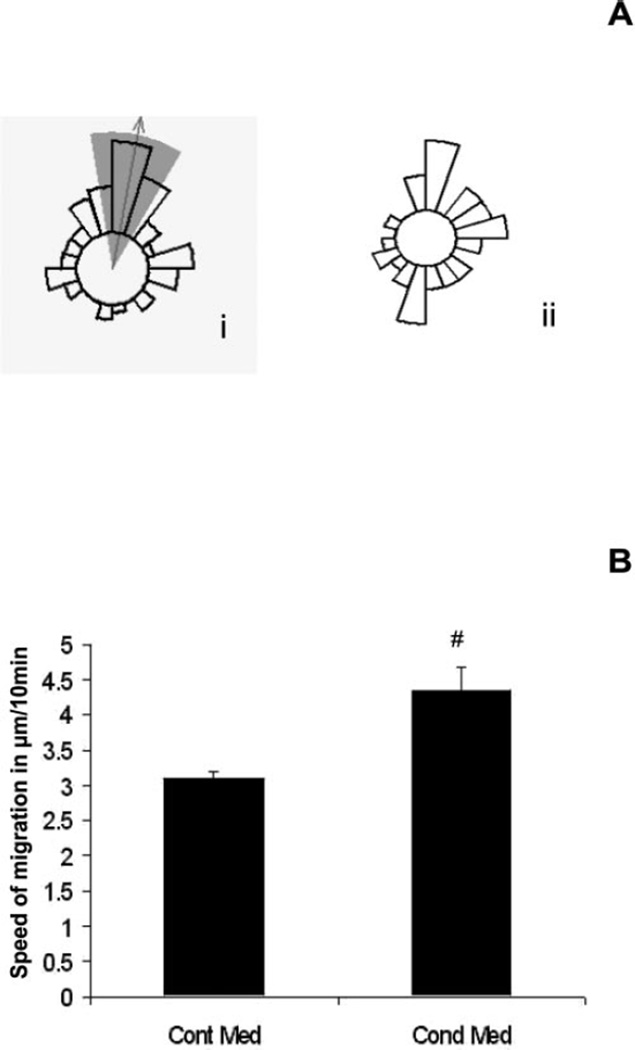
Conditioned medium obtained from apoptotic endothelial cells was tested for its effects on EPC migration by using Dunn’s chemotaxis assay. The presence of conditioned medium in the outer well of the Dunn’s chamber induced a robust chemotactic response by EPCs (Fig. 5Ai). EPCs exposed to control medium showed no chemotaxis but demonstrated random movement in all directions (Fig. 5Aii). This response indicated the release of chemokines from apoptotic RMEC cultures that may be involved in homing and recruitment of EPCs at the site of the endothelial injury. The mean speed of migration was determined for group of cells at each 10-minute interval, and analysis indicated a significant increase in EPC migration for conditioned medium compared with control medium (P < 0.001; Fig. 5B).
Figure 5.
EPC migration to the conditioned medium from apoptotic RMECs. (A) Chemotaxis was represented in terms of the circular histogram; (Ai, arrow) direction of migration; shaded portion: area of the confidence interval for the set of a particular experiment. Characteristic migratory response by EPCs with conditioned medium while with control medium EPCs moved (Aii) randomly in all directions (P < 0.05). Graphs are representative of three individual experiments. (B) Speed of migration for EPCs significantly increased in conditioned medium compared with control medium; #P < 0.001.
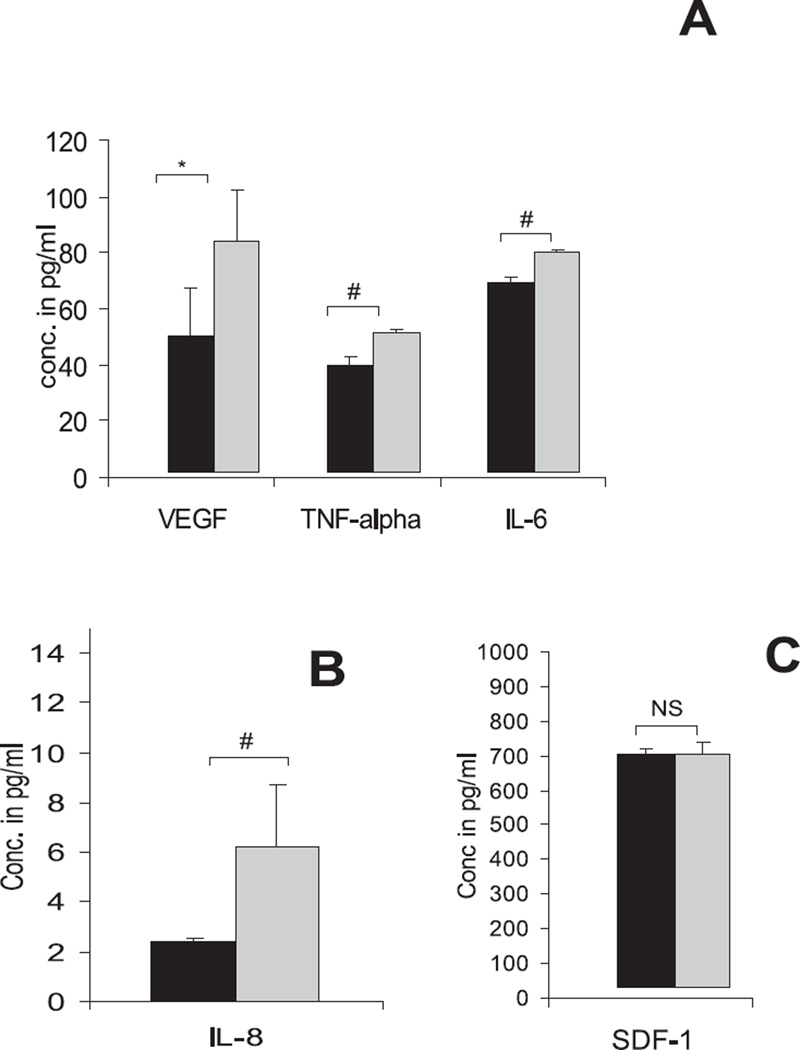
ELISAs were conducted on the control and conditioned medium to analyze possible candidate cytokines and chemokines that could have been responsible for the chemotaxis induced by the conditioned medium from apoptotic cells. VEGF was observed to be the predominant cytokine in conditioned medium, which exhibited a twofold increase compared with control medium (P < 0.05; Fig. 6A). The levels of the proinflammatory cytokines IL-8, IL-6, and TNF-α were also significantly increased (P < 0.001) in conditioned medium when compared with that in control medium; however, SDF-1 concentrations were not significantly different (Figs. 6A, 6B).
Figure 6.
Analysis of conditioned medium for cytokines. (A) Conditioned medium analyzed by ELISA showing increase in the levels of VEGF, IL-6, and TNF-α compared with the control medium. A similar increase was observed in the expression of IL-8 (B); however, SDF-1 (C) levels remained unchanged, *P < 0.05, #P< 0.001.
Discussion
Accumulating evidence suggests that bone marrow–derived EPCs possess reparative potential in the healing of injured endothelium, thus maintaining vascular integrity during vasodegeneration.14,25 Although the current investigation has been in vitro, the findings implicate apoptotic bodies at sites of endothelial injury as being crucial to the homing responses by EPCs. We have shown that the presence of apoptotic bodies at the site of microlesions in the monolayer enhances recruitment of EPCs and reduces the time needed to re-establish the integrity of the endothelium. One study showed that the expression of adhesion molecules is crucial to EPC homing to sites of endothelial injury.26 At sites of tissue hypoxia or inflammation, the stimuli for induction of endothelial adhesion molecule expression are well characterized.27 However, in the absence of factors derived from the local parenchyma, such as can be envisaged at discrete sites of endothelial cell death, the stimulus for local adhesion molecule expression is less clear. Addition of apoptotic bodies to uninjured endothelial monolayers upregulates adhesion molecule expression, suggesting that the increased immunostaining for adhesion molecules in the penumbra of the endothelial lesions could be stimulated by local exposure to apoptotic cells and not solely to disruption of the monolayer, thus promoting EPC adhesion at the wound site. In addition, cytokines released by apoptotic bodies induce strong chemokine stimuli for homing of EPCs and improves monolayer integrity in vitro.
Apoptotic bodies (1–5 µm) differ from microparticles (<1 µm), which transport cell-derived compounds like DNA, phospholipids, and/or peptides and subsequently induce variety of signals.28 Efficient removal of apoptotic bodies is crucial for homeostasis to avoid acute inflammatory responses induced by dying cells.12 It has been reported previously that apoptotic bodies from human umbilical vein endothelial cells induce EPC maturation and differentiation.18 The present study has demonstrated that exposure of apoptotic bodies to healthy endothelium induces a significant upregulation of adhesion molecules signals which, in turn, promotes recruitment of reparative EPCs. How these apoptotic bodies induce such signals is intriguing and has important implications for microvascular pathophysiology. It is particularly important for the retinal microvasculature in which capillary blood flow (especially the deep plexus) can be relatively sluggish,29 and it may mean that apoptotic capillary endothelium is not immediately removed and can influence EPC migration and their interactions with the resident vessels. Patients with acute myocardial infarction (MI) show increased levels of desquamated endothelial cells, microparticles,30 and apoptosis of vascular cells after local injury.31 In addition, vascular trauma is associated with rapid mobilization of EPCs.32 Although we did not study these two processes in vivo, our study highlights a possible link between endothelial cell apoptosis and increased mobilization of EPCs.
Apoptotic cells display a variety of the so-called eat-me signals such as externalization of phosphatidylserine and surface expression of oxidized LDL particles, thrombospondin-1 binding sites, and ICAM-1.33 These signals are recognized by the tethering receptors on phagocytes and culminates in an endosome-phagosome interaction that induces digestion of the apoptotic bodies.12 In the present study, it was interesting that apoptotic bodies induced upregulation of adhesion molecules on healthy endothelium at 6 hours, which subsequently decreased after clearance at 24 hours. This initial window of adhesion molecule expression is likely to be crucial for the homing and recruitment of EPCs to sites of injury.32 Exposure to apoptotic bodies consistently increased ICAM-1 expression, although this was not apparent for VCAM-1 or E-selectin. Harrington et al.34 have demonstrated that E-selectin expression on the surface of endothelial cell is dependent on specific caspase-3 activation, and serum deprivation of endothelial cells can induce apoptosis without upregulation of the protein on the cell surface. E-selectin surface expression on endothelial cells may persist for only short time frames and disappear after endocytosis and lysosomal degradation of the protein.35 Verteporfin, a spontaneous inducer of endothelial apoptosis,36 can cause a rapid clearance of surface E-selectin expression that may not always be shown by immunofluorescence, whereas clearance of apoptotic bodies by endothelial cells has upregulated this expression. By contrast, ICAM-1 is known to be crucial for recruitment of EPCs,26 and its blockade by neutralizing antibodies significantly inhibits ischemic wound healing.26,37,38 ICAM-1 expression accompanied EPC homing to the site of injury, and this may also account for migration of all monocytic cells in the context of the retinal vasculature. In DR, capillary leukostasis has been demonstrated to be ICAM-1 dependent, and the pathologic result is speculated to induce vessel occlusion.39
VEGF is a key regulator of angiogenesis that represents an important target for therapeutic intervention in proliferative retinopathies.40 Conditioned medium obtained from apoptotic RMECs showed a significant increase in VEGF, indicating that this factor may be a stimulus for EPC homing in the current model system. Indeed, VEGF165 gene transfer is a promising strategy for the improvement of EPC function whereby it improves adhesion, proliferation, and incorporation of progenitors to the vascular endothelium.41,42 ELISA analysis of conditioned medium failed to show a significant increase in the level of SDF-1 compared with that in control medium. Although this could be viewed as surprising, a prior report has shown similar observations, indicating very low levels of SDF-1 with concomitant upregulation of CXCR4 by endothelial cells.43 SDF-1 is a hypoxia-responsive molecule44,45 that is released after platelet accumulation.46 Therefore, this discrepancy may be a function of the in vitro conditions that may not precisely mimic the complex milieu of ischemic tissues.
This study has shown that the proinflammatory cytokines IL-6, IL-8, and TNF-α occur at high concentrations in medium conditioned by apoptotic RMECs and are probably released in response to cell damage.47,48 This finding is important because EPCs are known to express high levels of the IL-6 receptor (gp-80), and administration of IL-6 stimulates proliferation, migration, and tubule formation via activation of gp80/gp130 signaling pathways.49,50 Furthermore, IL-8 can act as a proangiogenic cytokine with wound-healing properties,51 and it also induces homing of EPCs. Patients with acute myocardial infarction show an increase in the levels of IL-8 and VEGF compared with patients with stable angina.52 The increase in these cytokines suggests important role of IL-8 and VEGF in EPC mobilization. Significantly elevated levels of IL-8 were also observed in conditioned medium in our study, which may be a strong stimulus for EPCs in our model system (in addition to VEGF and IL-6). Likewise, the TNFR2/p75 receptor system must be activated in EPCs for EPC-mediated neovascularization to occur,53 although whether the modest increases in TNF-α observed in the current model system play a major role in EPC activity is unknown. There have been conflicting observations that suggest that blockage of TNF-α could improve EPC-mediated repair in inflammatory conditions such as rheumatoid arthritis.54 It is difficult to ascertain the precise role of TNF- α in the homing of EPCs; however, further studies with neutralizing antibodies would help to establish the modulatory role of this cytokine.
It is known that EPCs are crucial for vascular repair and regeneration. This study points toward a crucial role played by apoptotic endothelial cells in homing and recruitment of EPCs, a response modulated by monolayer expression of cytokines and adhesion molecules. These findings form an important foundation for ongoing in vitro and in vivo investigations of vasodegeneration in the context of diabetic retinopathy.
Acknowledgments
Supported by Guide Dogs for the Blind Association, Action Medical Research, and the Juvenile Diabetes Research Foundation of the Medical Research Council.
Footnotes
Disclosure: A.D. Bhatwadekar, None; J.V. Glenn, None; T.M. Curtis, None; M.B. Grant, None; A.W. Stitt, None; T.A. Gardiner, None
References
- 1.Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, vom Hagen F, Lin J, Hammes HP. Incipient diabetic retinopathy: insights from an experimental model. Ophthalmologica. 2007;221:269–274. doi: 10.1159/000101930. [DOI] [PubMed] [Google Scholar]
- 3.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermans MP. Diabetes and the endothelium. Acta Clin Belg. 2007;62:97–101. doi: 10.1179/acb.2007.017. [DOI] [PubMed] [Google Scholar]
- 5.Boyd-White J, Williams JC., Jr Effect of cross-linking on matrix permeability: a model for AGE-modified basement membranes. Diabetes. 1996;45:348–353. doi: 10.2337/diab.45.3.348. [DOI] [PubMed] [Google Scholar]
- 6.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- 7.Dobler D, Ahmed N, Song L, Eboigbodin KE, Thornalley PJ. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes. 2006;55:1961–1969. doi: 10.2337/db05-1634. [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–2299. doi: 10.2174/1381612054367300. [DOI] [PubMed] [Google Scholar]
- 9.Yamashiro K, Tsujikawa A, Ishida S, et al. Platelets accumulate in the diabetic retinal vasculature following endothelial death and suppress blood-retinal barrier breakdown. Am J Pathol. 2003;163:253–259. doi: 10.1016/S0002-9440(10)63648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 12.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–R805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 13.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 14.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 15.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 16.Hibbert B, Olsen S, O’Brien E. Involvement of progenitor cells in vascular repair. Trends Cardiovasc Med. 2003;13:322–326. doi: 10.1016/j.tcm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Wijelath ES, Rahman S, Murray J, Patel Y, Savidge G, Sobel M. Fibronectin promotes VEGF-induced CD34 cell differentiation into endothelial cells. J Vasc Surg. 2004;39:655–660. doi: 10.1016/j.jvs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 19.Stitt AW, Chakravarthy U, Archer DB, Gardiner TA. Increased endocytosis in retinal vascular endothelial cells grown in high glucose medium is modulated by inhibitors of nonenzymatic glycosylation. Diabetologia. 1995;38:1271–1275. doi: 10.1007/BF00401758. [DOI] [PubMed] [Google Scholar]
- 20.Bhatwadekar AD, Glenn JV, Li G, Curtis TM, Gardiner TA, Stitt AW. Advanced glycation of fibronectin impairs vascular repair by endothelial progenitor cells: implications for vasodegeneration in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:1232–1241. doi: 10.1167/iovs.07-1015. [DOI] [PubMed] [Google Scholar]
- 21.Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messam CA, Pittman RN. Asynchrony and commitment to die during apoptosis. Exp Cell Res. 1998;238:389–398. doi: 10.1006/excr.1997.3845. [DOI] [PubMed] [Google Scholar]
- 23.Levy JG. Photosensitizers in photodynamic therapy. Semin Oncol. 1994;21:4–10. [PubMed] [Google Scholar]
- 24.Granville DJ, Shaw JR, Leong S, et al. Release of cytochrome c, Bax migration, Bid cleavage, and activation of caspases 2, 3, 6, 7, 8, during endothelial cell apoptosis. Am J Pathol. 1999;155:1021–1025. doi: 10.1016/S0002-9440(10)65202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caballero S, Sengupta N, Afzal A, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–967. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon CH, Hur J, Oh IY, et al. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26:1066–1072. doi: 10.1161/01.ATV.0000215001.92941.6c. [DOI] [PubMed] [Google Scholar]
- 27.Shreeniwas R, Koga S, Karakurum M, et al. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha: an autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992;90:2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 29.Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- 30.Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin R, Kelly CJ, Kay E, Bouchier-Hayes D. The role of apoptotic cell death in cardiovascular disease. Ir J Med Sci. 2001;170:132–140. doi: 10.1007/BF03168827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 33.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 34.Harrington EO, Stefanec T, Newton J, Rounds S. Release of soluble E-selectin from activated endothelial cells upon apoptosis. Lung. 2006;184:259–266. doi: 10.1007/s00408-005-2589-5. [DOI] [PubMed] [Google Scholar]
- 35.Subramaniam M, Koedam JA, Wagner DD. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol Biol Cell. 1993;4:791–801. doi: 10.1091/mbc.4.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houle JM, Strong A. Clinical pharmacokinetics of verteporfin. J Clin Pharmacol. 2002;42:547–557. doi: 10.1177/00912700222011607. [DOI] [PubMed] [Google Scholar]
- 37.Zen K, Okigaki M, Hosokawa Y, et al. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated micro-bubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40:799–809. doi: 10.1016/j.yjmcc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 38.van Buul JD, Voermans C, van den Berg V, et al. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 39.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 40.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 41.Kalka C, Masuda H, Takahashi T, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 42.Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 43.Yun HJ, Jo DY. Production of stromal cell-derived factor-1 (SDF-1) and expression of CXCR4 in human bone marrow endothelial cells. J Korean Med Sci. 2003;18:679–685. doi: 10.3346/jkms.2003.18.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima e Silva R, Shen J, Hackett SF, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21(12):3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 45.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massberg S, Konrad I, Schurzinger K, et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaugler MH, Squiban C, Mouthon MA, Gourmelon P, van der Meeren A. Irradiation enhances the support of haemopoietic cell transmigration, proliferation and differentiation by endothelial cells. Br J Haematol. 2001;113:940–950. doi: 10.1046/j.1365-2141.2001.02852.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee PC, Ho IC, Lee TC. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol Sci. 2005;85:541–550. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- 49.Fan Y, Ye J, Shen F, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28(1):90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li N, Eljaafari A, Bensoussan D, et al. Human umbilical vein endothelial cells increase ex vivo expansion of human CD34(+) PBPC through IL-6 secretion. Cytotherapy. 2006;8:335–342. doi: 10.1080/14653240600845062. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22:719–722. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 52.Schomig K, Busch G, Steppich B, et al. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006;27:1032–1037. doi: 10.1093/eurheartj/ehi761. [DOI] [PubMed] [Google Scholar]
- 53.Goukassian DA, Qin G, Dolan C, et al. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 54.Ablin JN, Boguslavski V, Aloush V, et al. Effect of anti-TNFalpha treatment on circulating endothelial progenitor cells (EPCs) in rheumatoid arthritis. Life Sci. 2006;79:2364–2369. doi: 10.1016/j.lfs.2006.07.035. [DOI] [PubMed] [Google Scholar]