Summary
Oxidative damage affects protein structure and function. Progressive accumulation of oxidized proteins is considered a putative mechanism of aging; however, empirical evidence supporting their role in aging is inconsistent. This inconsistency may reflect a failure to distinguish damage to particular cellular compartments. We found significant reduction of protein carbonyl in the insoluble, but not the soluble, fraction of liver tissues of long-lived compared to short-lived animals. Of cellular components analyzed, only nuclear protein carbonyl level was uniformly reduced in long-lived compared with short-lived animals. This observation suggests that attenuated accumulation of protein carbonyls in the nucleus, where they can affect multiple aspects of gene expression and DNA repair, might contribute to the longevity in mammalian species.
Keywords: naked-mole rat, marmoset, bats, protein carbonylation, dietary restriction, oxidative stress
The oxidative stress theory of aging and age-related disease (Harman 1956; Bokov et al. 2004) asserts that compromised tissue function with age is due to progressively increasing oxidative damage, particularly to proteins. If the hypothesis is valid, oxidative damage should be lower in long-lived compared with short-lived animals at similar ages. However, empirical data have been conflicting and inconsistent (Van Remmen et al. 2003; Andziak et al. 2006; Perez et al. 2009). Oxidative damage may be critical only for certain molecules or classes of molecules and/or only in some cellular compartments; assays which do not distinguish types of damage or damage to specific cellular compartments may mask informative, mechanistically important patterns.
Oxidative damage to proteins represents a reasonable candidate for a mechanism of aging because of proteins’ ubiquitous roles in cellular processes, the requirement that proteins remain precisely folded, and that protein homeostasis is significantly compromised with age (Morimoto & Cuervo 2009; Koga et al. 2010). The most common method of assessing protein oxidative damage is to measure the level of protein carbonyls (Sohal et al. 1993; Olivares-Corichi et al. 2005). Carbonylated proteins that escape degradation by the proteasome and the mitochondrial proteolytic machinery can accumulate over time to form high molecular weight insoluble aggregates (Shringarpure & Davies 2002; Nystrom 2005). However, few attempts have been made to measure protein carbonyls specifically in the insoluble fraction of cellular lysates, which contains cellular organelles. Thus, assessing the insoluble cellular fraction may be critical to understanding the impact of oxidative damage to proteins within organelles. Separating insoluble from soluble carbonyls may reveal functional patterns not evident when total carbonyls are assayed.
Even studies that focus on soluble carbonyls are frequently inconsistent in the range of cellular compartments they assay. Some studies combine all cellular compartments, others employ centrifugation strategies that omit the nucleus (i.e. 600-1000g), and still others (11,000-30,000g) include the cytosol mitochondria, lysosomes and other small cellular organelles (Levine et al. 1990; Youngman et al. 1992; Cao & Cutler 1995; Dubey et al. 1996; Alam et al. 1997; Goto et al. 1999; Levine 2002; Dalle-Donne et al. 2003; Breusing et al. 2009; Ahmed et al. 2010). To reduce potential confounds, we have quantified soluble protein carbonyls strictly in the cytosol with minimal or no interference from free probe or organelles using a sensitive fluorescence-based gel assay (Chaudhuri et al. 2006). Using the same methodology with minor modification in buffer composition, we can assess total protein carbonyls in the insoluble fraction. These techniques, in combination with specific centrifugation strategies, allow us to assay insoluble protein carbonyls in specific cellular compartments as well.
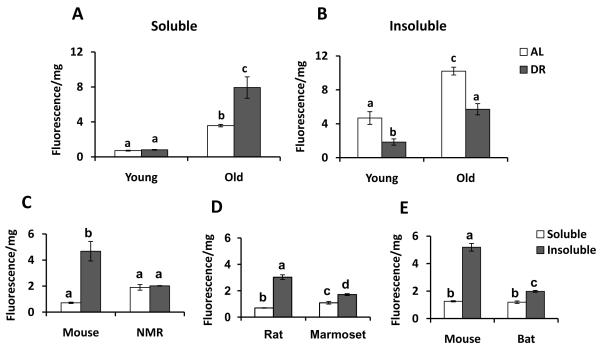
Previous reports indicate that protein carbonylation increases with age and dietary restriction (DR) reduces the rate of accumulation of carbonylation compared to ad lib-fed animals (Sohal et al. 1994; Dubey et al. 1996; Nagai et al. 2000; Radak et al. 2002). Examining carbonylation in liver, older (24-30 mo) mice indeed show increased carbonylation relative to young (8-10 mo) mice in both soluble and insoluble fractions (Fig. 1A,B), but carbonyl attenuation by DR is entirely due to reduced carbonylation in the insoluble fraction (Fig. 1B). These results suggest that 1) attenuation of insoluble protein carbonyls is correlated with longevity, and 2) soluble protein carbonyls may not be reduced in long-lived animals.
Figure 1. Assessment of Protein Carbonyl in the soluble and insoluble fractions of various short- and long-lived species.
Carbonylation in (A) soluble and (B) insoluble fractions from livers of young (4-6 mo; n = 3-6) and old (24-30 mo; n = 3-12) C57BL/6 mice fed ad libitum (AL, white bar) or with their caloric intake restricted by 40% (DR, grey bar). DR of this mouse genotype increases longevity by roughly 25% (Turturro et al. 1999). Note the significant reduction in carbonylation of the DR mice in the insoluble fraction only of young (p < 0.01) and old (p < 0.001) animals. Similarly, significantly (p < 0.01 to 0.0001) lower carbonylation was seen in the insoluble (grey bar) fraction only of the longer-lived species in comparisons of (C) young adult wild-derived ID mice (6 mo) vs. young adult naked mole-rats (NMR; 2 yr), (D) young adult rats (4-6 mo) vs. young adult marmosets (4 yr) and (E) young adult wild-derived mice (6 mo) vs. young adult Brazilian free-tailed bats (young wild caught). Samples were homogenized in 50mM sodium phosphate buffer pH 6.0 followed by centrifugation at 100,000×g to obtain soluble and insoluble fractions. Supernatant and pellet proteins were labeled with the fluorescent probe FTC and were run on 12% SDS-PAGE. Protein carbonyls were quantified using typhoon with excitation and emission wavelengths 488 nm and 520 nm, respectively. Coomassie stain was performed in the same samples for correction of unequal protein loading. Results are mean ± SEM of n = 3-12 animals per group, analyzed by one-way ANOVA with Newman-Keuls multiple comparison test. Bars with different superscripts are significantly different at p < 0.01.
To investigate the relationship between longevity and protein carbonylation in specific cellular fractions, we performed three paired comparisons using mammal species of approximately the same body mass but different longevities. We controlled for body size because the general correlation between size and longevity can confound interspecific patterns (Lorenzini et al. 2005; Gorbunova & Seluanov 2009). We compared mice (maximum longevity 4 years) with naked mole-rats (NMRs, maximum longevity 30 years) and separately with Brazilian free-tailed bats (longevity record in the wild 12 years). We also compared Norway rats (maximum longevity 4 years) with the common marmoset (maximum longevity 16 years). In all comparisons, the shorter lived species exhibited greater carbonylation in the insoluble fraction of these liver extracts (Fig. 1C-E).
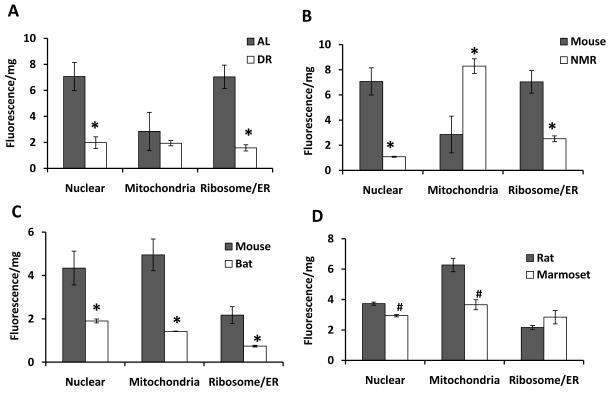
To assess whether protein oxidation in some cellular compartments was more strongly correlated with longevity than others, we employed the same four comparisons – one dietary, three interspecific - and fractionated the insoluble pellet into nuclear, mitochondrial and microsomal [ER/Ribosome] subfractions using differential centrifugation and assessed protein carbonyls in each subfraction. Carbonylation of proteins in the nuclear fraction was reduced in all longer-lived groups (Fig. 2). Interestingly, in the mitochondrial fraction we observed significantly increased carbonyl content in NMR compared to all of the other species, which may explain why total carbonylation has been reported to be increased in naked mole-rats relative to mice (Andziak et al. 2006).
Figure 2. Distribution of Protein Carbonyl in cellular subfractions of short- versus long-lived species.
The insoluble cellular organelles were fractionated from liver samples into nuclear, microsomal (ribosomal/ER) and mitochondrial fractions by differential centrifugation from young adult animals of various groups as in Figure 1. Note that the only uniform result was reduced protein carbonylation in the insoluble nuclear fraction of all longer-lived groups. (A) AL vs. DR C57BL/6 mice, (B) wild-derived mice vs. NMR, (C) wild-derived mice vs. Brazilian free-tailed bats, (D) rats vs. marmosets. Cellular organelles were separated from the insoluble fraction by differential centrifugation: nuclear (1,000g), mitochondrial (16,000g) and microsomal (ribosomal/ER, 100,000g) followed by labeling the protein carbonyls with FTC. The labeled proteins were then subjected to 12% SDS-gel electrophoresis. Protein carbonyls were quantified using typhoon at excitation and emission wavelengths 488 nm and 520 nm, respectively. Coomassie stain was performed with the same samples for correction of unequal protein loading. Results were analyzed by unpaired t-test (#p < 0.05,*p < 0.01). Results are mean ± SEM of n = 3 animals per group.
In summary, reduced protein carbonylation in the nucleus is correlated with increased longevity. This distinct pattern is observed at a young age, consistent with the hypotheses that the biological qualities that regulate lifespan should be evident in young adults, and age-specific mortality should correlate with maximum lifespan. Protein oxidation is likely to interfere with transcriptional regulation on several levels; from damage to transcription factors and polymerases to inhibition of proteasomal degradation of regulatory proteins to modification of the interactions between DNA and histone (Breusing et al. 2009; Nakamura et al. 2010; Kwak et al. 2011; Pashikanti et al. 2011). Future studies to identify the nuclear target proteins that show elevated level of carbonyls in the short-lived species and decreased level in the long-lived species would help to understand their role in modulating chromatin structure and transcriptional regulation associated with longevity. Moreover, determining the underlined mechanisms such as activation of nuclear proteosomal function in long-lived species and consequent decreased accumulation of protein carbonylation would also highlight the importance of nuclear protein homeostasis in mammalian longevity.
Acknowledgments
This work was supported by K07 AG025063 04 (A.R.C., S.N.A.); and NIH grant AG022873 (S.N.A.).
References
- Ahmed EK, Rogowska-Wrzesinska A, Roepstorff P, Bulteau AL, Friguet B. Protein modification and replicative senescence of WI-38 human embryonic fibroblasts. Aging Cell. 2010;9:252–272. doi: 10.1111/j.1474-9726.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- Andziak B, O’Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Breusing N, Arndt J, Voss P, Bresgen N, Wiswedel I, Gardemann A, Siems W, Grune T. Inverse correlation of protein oxidation and proteasome activity in liver and lung. Mech Ageing Dev. 2009;130:748–753. doi: 10.1016/j.mad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Cao G, Cutler RG. Protein oxidation and aging. I. Difficulties in measuring reactive protein carbonyls in tissues using 2,4-dinitrophenylhydrazine. Arch Biochem Biophys. 1995;320:106–114. doi: 10.1006/abbi.1995.1347. [DOI] [PubMed] [Google Scholar]
- Chaudhuri AR, de Waal EM, Pierce A, Van Remmen H, Ward WF, Richardson A. Detection of protein carbonyls in aging liver tissue: A fluorescence-based proteomic approach. Mech Ageing Dev. 2006;127:849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A. Coevolution of telomerase activity and body mass in mammals: from mice to beavers. Mech Ageing Dev. 2009;130:3–9. doi: 10.1016/j.mad.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Nakamura A, Radak Z, Nakamoto H, Takahashi R, Yasuda K, Sakurai Y, Ishii N. Carbonylated proteins in aging and exercise: immunoblot approaches. Mech Ageing Dev. 1999;107:245–253. doi: 10.1016/s0047-6374(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev. 2010 doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J, Workman JL, Lee D. The proteasome and its regulatory roles in gene expression. Biochim Biophys Acta. 2011;1809:88–96. doi: 10.1016/j.bbagrm.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Lorenzini A, Tresini M, Austad SN, Cristofalo VJ. Cellular replicative capacity correlates primarily with species body mass not longevity. Mech Ageing Dev. 2005;126:1130–1133. doi: 10.1016/j.mad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Takahashi R, Goto S. Dietary restriction initiated late in life can reduce mitochondrial protein carbonyls in rat livers: western blot studies. Biogerontology. 2000;1:321–328. doi: 10.1023/a:1026590819033. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Kawakami K, Kametani F, Nakamoto H, Goto S. Biological significance of protein modifications in aging and calorie restriction. Ann N Y Acad Sci. 2010;1197:33–39. doi: 10.1111/j.1749-6632.2009.05374.x. [DOI] [PubMed] [Google Scholar]
- Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Corichi IM, Ceballos G, Medina-Santillan R, Medina-Navarro R, Guzman-Grenfell AM, Hicks JJ. Oxidation by reactive oxygen species (ROS) alters the structure of human insulin and decreases the insulin-dependent D-glucose-C14 utilization by human adipose tissue. Front Biosci. 2005;10:3127–3131. doi: 10.2741/1769. [DOI] [PubMed] [Google Scholar]
- Pashikanti S, Boissonneault GA, Cervantes-Laurean D. Ex vivo detection of histone H1 modified with advanced glycation end products. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Takahashi R, Kumiyama A, Nakamoto H, Ohno H, Ookawara T, Goto S. Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities, and protein carbonylation of rat skeletal muscle and tendon. Exp Gerontol. 2002;37:1423–1430. doi: 10.1016/s0531-5565(02)00116-x. [DOI] [PubMed] [Google Scholar]
- Shringarpure R, Davies KJ. Protein turnover by the proteasome in aging and disease. Free Radic Biol Med. 2002;32:1084–1089. doi: 10.1016/s0891-5849(02)00824-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem Biophys Res Commun. 1993;196:7–11. doi: 10.1006/bbrc.1993.2208. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Youngman LD, Park JY, Ames BN. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci U S A. 1992;89:9112–9116. doi: 10.1073/pnas.89.19.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]