Abstract
The worldwide incidence of bone disorders and conditions has trended steeply upward and is expected to double by 2020, especially in populations where aging is coupled with increased obesity and poor physical activity. Engineered bone tissue has been viewed as a potential alternative to the conventional use of bone grafts, due to their limitless supply and no disease transmission. However, bone tissue engineering practices have not proceeded to clinical practice due to several limitations or challenges. Bone tissue engineering aims to induce new functional bone regeneration via the synergistic combination of biomaterials, cells, and factor therapy. In this review, we discuss the fundamentals of bone tissue engineering, highlighting the current state of this field. Further, we review the recent advances of biomaterial and cell-based research, as well as approaches used to enhance bone regeneration. Specifically, we discuss widely investigated biomaterial scaffolds, micro- and nano-structural properties of these scaffolds, and the incorporation of biomimetic properties and/or growth factors. In addition, we examine various cellular approaches, including the use of mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), adult stem cells, induced pluripotent stem cells (iPSCs), and platelet-rich plasma (PRP), and their clinical application strengths and limitations. We conclude by overviewing the challenges that face the bone tissue engineering field, such as the lack of sufficient vascularization at the defect site, and the research aimed at functional bone tissue engineering. These challenges will drive future research in the field.
Keywords: bone tissue engineering stem cells, scaffolds, vascularization, immunomodulation, cell homing, clinical challenges
I. INTRODUCTION
Bone grafts are utilized in a wide array of clinical settings to augment bone repair and regeneration. Bone defect repair using the tissue engineering approach is perceived as a better approach because the repair process may proceed with the patient’s own tissue by the time the regeneration is complete.1–3 Currently, the United States, as well as other countries worldwide, is experiencing an exceedingly high demand for functional bone grafts. Annually in the United States, more than half a million patients receive bone defect repairs, with a cost greater than $2.5 billion. This figure is expected to double by 2020 in the United States and globally due to a variety of factors, including the growing neeeds of the baby-boomer population and increased life expectancy.4
Extensive studies have reported the considerable shortcomings, limitations, and complications of current clinical treatments for bone repair and regeneration; these include autologous and allogeneic transplantations using autografts and allografts.4–10 To date, autografts serve as the gold standard for bone grafts because they are histocompatible and non-immunogenic, and they offer all of the imperative properties required of a bone graft material. Specifically, autografts possess the essential components to achieve osteoinduction (i.e., bone morphogenetic proteins (BMPs) and other growth factors), osteogenesis (i.e., osteoprogenitor cells) and osteoconduction (i.e., three-dimensional and porous matrix). However, autografts involve harvesting bone from the patient’s iliac crest, and thus, requires a second operation at the site of tissue harvest.11 Autologous bone transplants are very expensive procedures, and they may result in significant donor site injury and morbidity, deformity, scarring and they are associated with surgical risks as well: bleeding, inflammation, infection, and chronic pain.12–14 Autografts, further, may be a null treatment option in cases where the defect site requires larger volumes of bone than is feasible or available. Allografts represent the second most common bone-grafting technique; they involve transplanting donor bone tissue, often from a cadaver. Allogeneic bone is also likely histocompatible, and is available in various forms, including demineralized bone matrix (DBM), morcellised and cancellous chips, cortico-cancel-lous and cortical grafts, and osteochondral and whole-bone segments, depending on the host-site requirements. In comparison to autografts, allografts are associated with risks of immunoreactions and transmission of infections. They have reduced osteoinductive properties and no cellular component, because donor grafts are devitalized via irradiation or freeze-drying processing.15–17 Although less than autografts, allogenic grafts come with substantial cost issues. Furthermore, the bone grafting market is experiencing an obvious unmet supply and great demand; there is currently a shortage in allograft bone graft ma-terial.18 Other commonly used bone repair techniques may involve distraction osteogenesis, bone cement fillers, and bone morphogenic proteins. Although the previously mentioned clinical interventions have been shown to improve repair of bone, none possess all of the ideal characteristics: high osteoinductive and angiogenic potentials, biological safety, low patient morbidity, no size restrictions, ready access to surgeons, long shelf life, and reasonable cost.
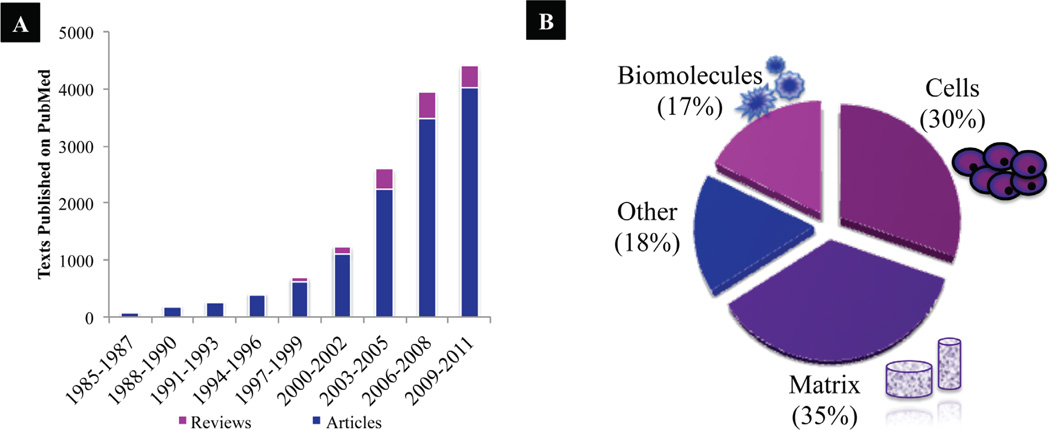
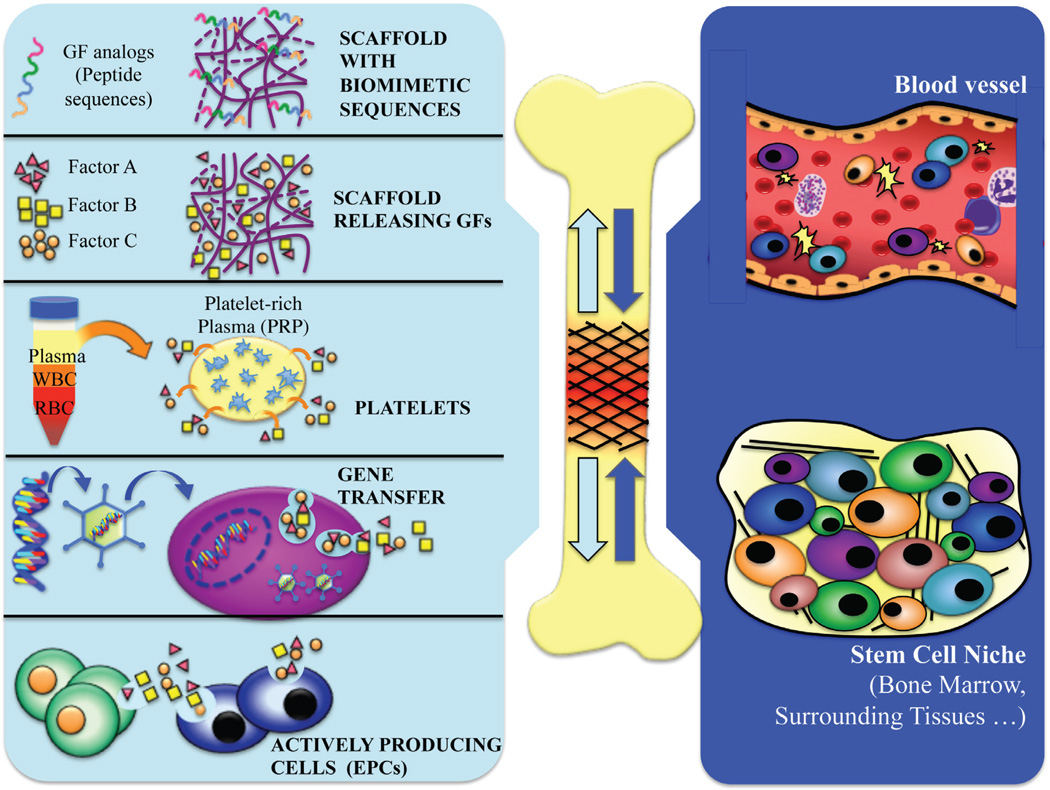
The field of bone tissue engineering (BTE) was initiated nearly three decades ago. Interest and progress in the BTE field has seen tremendous growth over the years, with an exponentially increasing number of studies and reviews published on the PubMed database since the mid-1980s (Fig. 1). The field of BTE focuses on alternative treatment options that will ideally eliminate the previously described issues of current clinically used treatments (i.e., donor site morbidity, limited availability, immune rejection, and pathogen transfer). BTE requires the collaborative efforts of scientists, engineers, and surgeons to achieve this ultimate goal of creating bone grafts that enhance bone repair and regeneration.19 The classic BTE paradigm highlights several key players: (1) a biocompatible scaffold that closely mimics the natural bone extracellular matrix niche, (2) osteo-genic cells to lay down the bone tissue matrix, (3) morphogenic signals that help to direct the cells to the phenotypically desirable type, and (4) sufficient vascularization to meet the growing tissue nutrient supply and clearance needs. Specifically, upon implantation, the construct may influence the host by releasing osteogenic and/or vasculo-genic growth factors (i.e., growth factor-releasing scaffold, scaffold with growth factor analogs, or seeding with platelet-enriched plasma), or by housing cells that are genetically engineered to or naturally release growth factors (Fig. 2). In turn, accelerated cell homing, vascularization, and bone regeneration of the defect site results. Although much progress has been made, many crucial hurdles remain to be cleared on the way to BTE becoming a true clinical reality. The following review critically considers advances and obstacles for functional BTE.
FIGURE 1.
(A) Published articles on BTE since mid-1980s on PubMed.
(B) Break-down of the articles published in 2011 according to bone tissue engineering focus (i.e., biomolecules, cells, matrices, and other, including vascularization approaches and bioreactors).
FIGURE 2.
Schematic illustration of bone tissue engineering paradigm. Factors from the implanted graft at the defect site that influence the host response may include growth factors (or their analogs, or from platelet-enriched plasma), and cells (genetically modified to release factors, or naturally produce factors). In response, cell homing and enhanced vascularization and bone regeneration will occur.
II. FUNDAMENTALS OF BONE AND DEVELOPMENTAL BIOLOGY
Bone tissue engineering (BTE) is based on the understanding of bone structure, bone mechanics, and tissue formation as it aims to induce new functional bone tissues. In other words, to successfully regenerate or repair bone, knowledge of the bone biology and its development is quite essential.
Bone possesses the ability to perform a wide array functions, and bone responds to a variety of metabolic, physical and endocrine stimuli. Bones (1) represent the foundation for our bodily locomotion, (2) provide load-bearing capacity to our skeleton and protection to our internal organs, (3) house the biological elements required for hematopoiesis, (4) trap dangerous metals (i.e., lead), and (5) maintain the homeo-stasis of key electrolytes via calcium and phosphate ion storage. In addition, bone is engaged in a constant cycle of resorption and renewal, undergoing continual chemical exchange and structural remodeling due to both internal mediators and external mechanical demands. Bone has been previously, and most appropriately, referred to as the ultimate smart material for its scar-less regenerative capacity. Functional bone tissue engineering requires the newly restored bone to be fully integrated with the neighboring host bone, and importantly, to perform the previously mentioned functions of native bone.
Bone is a highly dynamic and diverse tissue, both structurally and functionally. Macroscopic structure and mechanical properties of the more than 200 bones in the human skeletal system are largely influenced by distinct loading conditions. Skeletal structures range from long (i.e., tibia, ul-nar, etc.) to short (i.e., phalanges, etc.), flat (i.e., skull, sternum, etc.), and irregular (i.e., pelvic, vertebrae, etc.). Bone functions range from locomotion to vital organ protection. Bone tissue may also either take on a compact (i.e., cortical bone) or trabecular (i.e., cancellous bone) pattern arrangement, ranging in mechanical strength and modulus. Despite these complex features and forms, it has relative simplicity in terms of its microscopic, hierarchical architecture. Specifically, bone extracellular matrix (ECM) is composed of both a non-mineralized organic component (predominantly type-1 collagen) and a mineralized inorganic component (composed of 4-nm-thick plate-like carbonated apatite mineralites). The nano-composite structure (tough and flexible collagen fibers reinforced by hydroxyapatite crystals) is integral to the requisite compressive strength and high fracture toughness of bone.
A. Bone Development
Bone formation occurs via two very distinct pathways, intramembraneous and endochondral. In either case, mesenchymal cellular condensation first occurs and serves as a template for subsequent bone formation. Intramembraneous bone formation involves mesenchymal progenitor cells differentiating directly into osteoblasts and the subsequent development of parts of the mandible, clavicle, and many cranial bones. Most bones in the body (i.e., all long bones and vertebrae), however, are formed through endochondral bone formation. This process involves mesenchymal progenitor cells first differentiating into chondrocytes, which are responsible for depositing a cartilaginous template that is later mineralized and replaced by bone.
Although distinct differences in the bone composition and structure occur via endo-chondral and intramembranous ossification, several molecular regulators are shared.20,21 For instance, several key molecules, including Indian Hedgehog (Ihh), parathyroid hormone related peptide (PTHrP), bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF) and fibroblastic growth factors (FGFs), are critical regulators in both processes.22 In en-dochondral ossification, BMPs are responsible for the initiation of mesenchymal condensations, and Ihh and PTHrP form a critical feedback loop that mediate the balance between chondrocyte proliferation and hypertrophy and regulate the thickness of the growth plate. Likewise, during intramembranous bone formation, these key players are required to induce uncommitted mensenchymal progenitor cells along the osteogenic pathway as pre-osteoblasts, which co-express chondrocytic and osteoblastic markers simultaneously. Furthermore, in both processes, bone remodeling is required for the maintenance of all normal healthy bone, which involves a balance between osteoclastic bone resorption and osteoblastic bone formation.23
1. Bone Defect Repair
Interestingly, upon fracture, bone is repaired by a process that recapitulates many of the events of both intramembraneous and endochondral bone formation, and it uniquely heals without the formation of scar tissue.24,25 Initially, hema-toma formation is accompanied by an inflammatory response, and the recruitment of many of the signaling molecules involved in the regulation of new bone formation (i.e., ILs, TNF-α, FGFs, BMPs, PDGF, VEGF, etc.). At the cortex and periosteum, intramembranous bone formation immediately occurs. The external soft tissues stabilize the fracture by the formation of a callus, which subsequently undergoes chondrogenesis, and then a process highly similar to endochondral ossiflcation. More specifically, after the callus forms, chondrocyte proliferation decreases as the tissues begin to mature (i.e., hypertrophy) and calcify the matrix. In-growing blood vessels carry chondroclasts, which are responsible for resorbing the calcified cartilage and osteoblastic progenitors, which begin the process of new bone formation. The mechanical continuity of the cortex is achieved via subsequent remodeling of the newly formed bone.
The question remains: What is the optimal method for bone regeneration? Should BTE focus more on bone development processes or on bone defect repair? In the opinion of the authors, BTE should not exclusively focus on one or the other, but both. In situations requiring bone regeneration, the initial events always involve he-matoma formation and an early inflammatory response, which is largely responsible for the recruitment of host cells and release of critical signaling molecules. From there, emulation of some aspects of normal bone tissue development and remodeling may hold the key to the future success of BTE. Seminal developmental biology principles that may help the future success of BTE include the following:
The use of pluri- or multipotent stem cells
The identification of critical genes, growth factors, and signal transduction cascades that mediate bone formation
The physical process of bone formation
Complex interactions between epithelium and mesenchyme within the underlying connective tissue
The understanding of mesenchyme encoding tissue-specific patterns
The understanding that normal tissue healing involves progressive remodeling and restructuring of pre-existing tissue structures
The importance of the tissue microenviron-ment’s physical properties (i.e., “mechanother-apy”) (8) Angiogenesis and neo-vascularization of the newly formed bone tissue
Incorporation of developmental biology insights will critically impact future tissue engineering approaches. For instance, future approaches may include appropriate extracellular matrix molecules or adhesive ligands that target stem cells mediating earlier stages of tissue remodeling and regeneration.26 And for the promotion of angiogenesis, BTE will aim to develop scaffolds that incorporate growth factors and possess the necessary porosity for vascular ingrowth.27 Furthermore, engineering featuring micro- and nano- meter surface topography of these scaffolds is critical for directing cellular adhesion, spreading, and proliferation. On a broader scale, for successful bone tissue engineering, it is critical to develop a scaffold that is inspired by the natural processes of developmental biology and promotes tissue remodeling, rather than simply supporting final tissue form and function.
III. RECENT ADVANCES IN BTE
Although bone is a highly vascularized tissue and has the ability to regenerate, beyond a critical point, clinical intervention measures are required. It is the hope that BTE will be the future treatment of choice, as it will likely eliminate many of the pitfalls of current treatments. In this section, we discuss the status and key issues for BTE components (i.e., biomaterials, cells, signaling molecules, and vascularization).
A. Biomaterials
1. Osteoinductive Materials
Osteoinductive or “smart” biomaterials have the ability to induce ectopic bone formation by instructing its surrounding in vivo environment to form bone.28–30 Although the biological mechanisms of this phenomenon have not been fully elucidated, it is well recognized that these materials hold great potential for bone tissue regeneration. An array of biomaterial families have demonstrated having osteoinductive properties, including natural and synthetic ceramics (i.e., hydroxyapatite (HA) and variouscalcium phosphate compositions, and their composites (i.e., HA/ poly(lactic-co-glycolic acid) (PLGA). A number of studies have illustrated osteoinduc-tion by calcium phosphate (CaP)-based bioma-terials in various physical forms.31 Specifically, osteoinductivity has been demonstrated with CaP-based biomaterials in the form of sintered ceramics,32–36 cements,37,38 coatings,39,40 and coral-derived ceramics41–43 in a variety of animal models. Other ceramics, such as alumina ceramic and porous bioglass, have also been recently identi-fied as being osteoconductive.44 In addition, polymer/ceramic composites, such as PLGA/ HA, have been shown to be osteoinductive and to induce bone formation ectopically.45–50 However, it is critical to note that other material properties play a critical role in osteoinduction, aside from the chemical composition of the biomaterial, which may include porosity of the biomaterial implant and its surface properties, such as nano/micro topography. To some extent, the level of osteoinductivity also depends on the species used for the study (i.e., interspecies variation). Two main theories have been proposed to explain the observed osteoinductivity. The first is based on the biomaterial surface features that absorb and present osteoinductive factors to the surrounding cells. The second hypothesis is that the calcium phosphate–based materials release calcium and phosphate ions, which later influence stem cell differentiation into bone cells. No conclusive evidence exists for either of these hypotheses.29
2. Hybrid Materials
A number of synthetic and natural polymers, as well as ceramics have been developed and identified as biomaterials for BTE. Biomaterials for bone-scaffolding applications have to possess certain physical, chemical, and biological properties. Although great strides have been made, it is difficult for any biomaterial to satisfy all of the listed requirements. Recent efforts have been aimed, however, in the direction of developing hybrid biomaterials. These are nothing but the combination of two or more biomaterials, with enhanced functionalities, in the form of either co-polymers, polymer–polymer blends, or polymer–ceramic composites. These are considered an advanced class of biomaterials that are more optimal for bone scaffolding applications.
a. Co-polymers
Co-polymers are defined as being derived from two or more monomeric species. For example, poly (lactide-co-glycolide) (PLGA) co-polymer systems are derived from poly lactide, which displays a glass transition temperature (Tg) above room temperature with an unreasonably long degradation time, and polyglycolide, which displays Tg below room temperature and a shorter degradation time. The development of the PLGA co-polymer system allowed for the tuning of Tg and degradation based on the need. Similarly, other co-polymer systems have been developed, such as PLGA-PCL, PLGA co-polymerized with PLL, and PLA- co-polymerized PCL.54 In addition, DegraPol™ is another example of a co-polymer that was originally synthesized for bone regeneration.55
b. Polymer–polymer blends
Polymer blends involve a mixture of two polymers. By choosing polymers with required intermolecular or Van der Walls interactions, it is possible to design a miscible blend system with enhanced properties. PLGA blends with polyphosphazenes are a prime example. It is known that PLGA biomaterials produce acidic byproducts upon degradation, and this has been a major problem, because the long-term tissue exposure to acidic products may result in tissue necrosis and implant failure. On the other hand, polyphosphazenes release neutral or basic products in degradation.56 Therefore, PLGA has been blended with a wide variety of polyphosphazenes to achieve novel biomaterials with near-neutral degradation products.57–62
c. Polymer-ceramic composites
Composite materials represent attractive candidates for BTE applications because bone is, in fact, a composite material composed of a mix of inorganic hydroxyapatite crystals (HA) and organic collagen fibers.63 Furthermore, polymer-ceramic composites capitalize the advantages of each of its components (i.e., biodegradable polymer and ceramic materials), and have demonstrated success in bone regeneration that exceeds the results when these materials are used separately.64
Composites of HA and various polymers, including poly(lactic acid) (PLA),65 PLGA,66 gelatin,67 chitosan,68,69 and collagen70 have been successfully fabricated and have demonstrated enhanced bone formation in vitro and/or in vivo. These materials are considered to be biomimetic and to stimulate the formation, precipitation, and deposition of calcium phosphate from simulated body fluid (SBF), resulting in enhanced bone-matrix interface strength.62 Furthermore, Ma et al. demonstrated porous poly(L-lactic acid) (PLLA)/HA composite scaffolds to have superior osteoconductivity properties and to promote enhanced osteoblastic cell survival, proliferation, and expression of bone-specific markers (i.e., a bone sialoprotein and osteocalcin) in comparison to pure PLLA scaffolds during 6 weeks of in vitro cultivation.71
Upon implantation, the addition of HA to natural polymer scaffolds has been shown to improve the bioactivity and mechanical properties compared to polymer control scaffolds72 and to potentially reduce adverse effects associated with the degradation of some synthetic polymers.73 For instance, Higashi et al. observed accelerated and increased bone formation with composite PLA/HA scaffolds in a rat femur defect model, in comparison to pure PLA scaffolds.74 Overall, polymer/HA composites demonstrate osteoconductivity superior to their pure polymer counterparts.
3. Advanced Hydrogels
Hydrogels, due to their unique biocompatibility and desirable physical characteristics, have long been used as materials for tissue engineering. Hydrogels not only serve as matrices for tissue engineering and regenerative medicine but also are capable of mimicking extracellular matrix topography and delivering required bioactive agents that promote tissue regeneration.75,76 From the naturally derived collagen and gelatin gels to the synthetic poly(ethylene glycol) materials, poly(vinyl alcohol)-based hydrogel systems have been utilized for bone tissue engineering.76,77
Recently, self-assembling peptides have gained attention for forming scaffolds, as they are completely biological, biocompatible, and biodegradable.78,79 Self-assembling systems aim to mimic the natural extracellular matrix, and peptides, which may be readily synthesized chemically and biologically, conveniently serve as the starting material. For example, self-assembling RAD16-I (i.e., PuraMatrix™, Cambridge, MA) can form an injectable nanofiber network or hydrogel upon implantation. In other words, RAD16-I peptides may be injected, and via interactions with body fluids, they will gel and adopt the physical geometry of the tissue defect. Further, self-assembling RAD16-I, as well as other peptides such as P11-4, have been shown to support osteogenesis both in vitro and in vivo.80–83 For instance, Misawa et al. observed bony bridge formation after the injection of RAD16-I into small (i.e., 3 mm) bone defects of mice calvaria. Lastly, these self-assem-bling nano-featured biomaterials have been sown to be non-immunogenic and biodegradable, safely breaking down into amino acids that may be readily and easily cleared in vivo. Thus, SAPs represent a novel class of biomaterials that offers a promising option for BTE applications.
4. Immuno-modulatory Biomaterials
Immunobioengineering aims to design materials that have the ability to modulate or manipulate the immune system in a favorable manner for enhanced bone repair and regeneration.84 Typically, the host’s immune reaction to an implant begins with the initial acute response to the surgical injury and innate recognition of the foreign material, which is subsequently followed by adaptive immunity mediated chronic inflammation in response specific recognition of antigens. Novel strategies in immunobioengineering are highlighting the importance of incorporating rational control and modulation, and importantly not elimination, of host inflammation into the design of tissue engineering strategies methods. A list of immunomodulating biomate-rial strategies are presented in Table 1.
TABLE 1.
Immunomodulation Strategies for Biomaterials
| Biomaterial choice | Material type |
In vitro: Decreased dentritic cell maturation (decreased levels of CD40,CD80, and CD86, HLA-DQ HLA-DR, CD83); increased secretion of TNF–a (295,296) In vitro: Decreased natural killer cell activity, decreased T- and B-cell proliferation85 |
| Surface property modulation |
Surface treatments | |
| Hydrophilic surface |
In vitro: Increased apoptosis of adherent primary human macrophages; increased levels of anti-inflammatory cytokine IL-10 and decreased levels of inflammation-associated chemokine IL-8297,298 |
|
| Anti-fouling coating |
In vitro: Decreased passive cell attachment and cell activation (i.e., non-specific cell-material interaction)299, 300 |
|
| Surface topography | ||
| Aligned structures |
In vitro: Decreased initial monocyte adhesion In vivo: Increased cell infiltration; decreased fibrous capsule88 |
|
| Micro/Nano structures |
In vitro: Increased pro-inflammatory cytokines IL-1, IL-6, TNF-a301 In vivo: Decreased/ thinner fibrous capsule302 |
|
| Bioactive molecule incorporation |
Providing integrins adhesion sites |
In vitro and in vivo: RGD and PHSRN domains increased formation of FBGC303,304 |
| Coupling of anti- inflammatory drugs to biomaterials |
In vivo: Decreased anti-inflammatory cytokines, prostaglandins, proteolytic enzymes, free oxygen radicals and nitric oxide; Decreased T helper (Th)1-directed immunity305,306 |
|
| Delivery of growth factors/bioactive molecules |
In vivo: Increase macrophage chemotaxis and activation307 In vivo: Decreased capsule formation308 |
|
| Artificial ECM | Hydrogels |
In vivo: host response dependent on species of origin, tissue of origin, processing materials, method of terminal sterilization309 |
| Artificial ECM coatings for synthetic implants |
In vitro and in vivo: Increased cell adhesion and proliferation310,311 | |
Several specific strategies have been proposed in immunobioengineering, namely selection of appropriate material type, biomaterial surface modulate (i.e., surface treatments, surface topography), and incorporation of artificial extracellular matrix and/or bioactive molecules. Although traditionally it has been accepted that the implants should be immune-inert, it is proving to be more beneficial to design materials that allow for enhanced cell-specific responses that encourage accelerated wound healing and bone tissue regeneration (i.e., increased boneforming cell activity, and decreased NK cell activity and T and B cell-mediated immunity). One of these strategies is to design biomateri-als of ECM similar composition and structure. For instance, Smith et al. demonstrated blends of polydioxanon and collagen or elastin to have immunomodulating effects by decreasing the activity of natural killer cells, as well as T- and B-cell proliferation.85 In addition, in immuno-modulating biomaterials, the biomaterial surfaces may be modified to become more immuno-compatible. The biomaterial surface is the first most critical factor for host acute immune response upon implantation, since the surface chemistry is responsible for the type, intensity, and conformation of serum proteins that are absorbed. The biomaterial surface should limit macrophage adhesion and activation as well as their fusion into foreign body giant cells (FBGCs). For instance, hydrophilic surfaces are associated with low-integrin binding sites and, therefore, decreased dendritic cell maturation and macrophage spreading, and increased macrophage apoptosis.86,87 Biomaterial surface topography, and micro/nano-scale architecture play a significant role in modulating and activating the immune system. Cao et al. demonstrated decreased capsule formation and increased tissue regeneration in scaffolds with aligned fiber topography compared to scaffolds with randomly aligned fibers.88 Biomaterial surface treatments may also be employed to shield the biomaterial from protein absorption (i.e., coating with mic-roparticle hydrogels, surfactant polymers, etc.), or to deliver bioactive molecules (i.e., growth factors, anti-inflammatory drugs).88
Specific immunobioengineering studies have investigated the effects of pharmacologic modulation of the inflammatory response on bone regeneration in vivo; they involve cytokine-specific agents, corticosteroids, prostaglandins, non-ste-roidal anti-inflammatory drugs, and selective prostaglandin agonists.89 For instance, pro-inflam-matory synthetic thrombin peptide TP508, which activates the same signaling pathways stimulated by TNF-α, IL-1, and other pro-inflammatory cy-tokines during fracture healing, has been shown to have anabolic effects and to enhance in vivo bone regeneration.90 In a rat model, a single injection of TP508 into a femoral fracture resulted in increased strength of the healed bone, vascularization of fracture site, and accelerated fracture repair and regeneration.91 Furthermore, studies have reported similar effects on bone healing in rabbits, with the controlled release of TP508 from various biodegradable scaffolds (i.e., PLGA microspheres and poly(propylene fumarate) scaffolds).92,93
Selective prostaglandin agonists represent another interesting immunomodulating target for enhanced bone regeneration. Previously, prostaglandins have been avoided as a therapeutic agent for bone repair, due to the risk of well-recognized side effects, including severe systemic inflammation. However, the main effects of prostaglandins on bone have been recently identified to occur selectively via two prosta-glandin receptors (i.e., Prostaglandin E2 type 2 (EP2) and EP4 receptors); thus, the systemic side effects may be avoided. Several studies have demonstrated the positive effects of selective EP2 or EP4 receptor agonists on bone fracture healing in various animal models.94,95 In dogs, healing of critical-size long-bone segmental defects in the radius and tibia was accelerated and significantly enhanced with EP2 agonists encapsulated in a PLGA carrier.95
Although these results are extremely promising thus far, further studies are needed to investigate more immunomodulating targets. Most importantly, strategies to integrate inflammatory modulation into tissue engineering strategies to enhance bone regeneration are needed.
B. Biodegradable Scaffolds
1. Scaffold Mechanical Integrity, Structure, and Mechanotransduction
A key feature of BTE scaffolds is to provide temporary mechanical integrity at the defect site until the bone tissue is repaired or regenerated, and normal biomechanical function is restored. For the bone tissue engineering scaffold to be “functional” immediately upon implantation, its biomechanical properties must match the physical demand of the healthy surrounding bone.96 In addition, the mechanical strength of the scaffold affects the mechanotransduction of the adherent bone cells on the scaffold, which plays a critical role in the bone repair and remodeling processes. It has been proposed that, generally, the structural biomechanics of the BTE scaffold is related to the osteoconductive properties of the scaffold, while mechanotrans-duction is related to its potential osteoinduc-tive properties.97 Biomechanical stimuli of cells due to the scaffold deformation largely influences osteoinduction (i.e., bone ingrowth from the host). Therefore, as suggested by Sikavitsas et al., a mechanotransduction strategy may be used to control the function of bone cells in vivo by designing a scaffold with mechanical properties that allow ‘osteoinductive fluid flow’ in the scaffold. By combining three-dimensional imaging, flow modeling, and numerical simulation of scaffold physical properties, threshold permeability (k = 1/32φr2 where r is the hydraulic radius and φ is equivalent to the required cut-off radius) may be determined. Specifically, it was verified that a threshold permeability of ∼3 × 10−11 m2 of a porous bone graft implant was necessary for inducing vascularization and mineralization in an implant.98,99
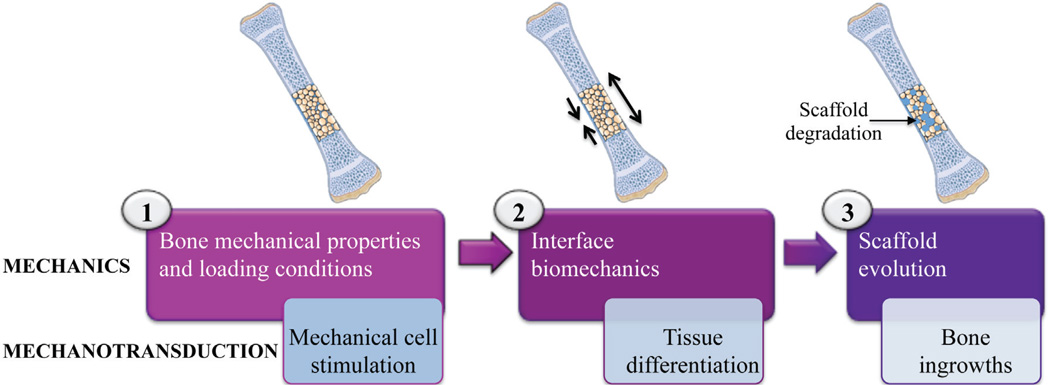
The BTE biomechanical paradigm has been well described in a step-wise fashion, where each step holds the mechanical aspects of the scaffold central to insure the safety of the surgical procedure using a BTE scaffold (Fig. 3).97 The first step, which involves the bone mechanical properties and loading conditions, is analogous to the primary fixation of the scaffold. At this point, the BTE scaffold should not induce a stress-shielding effect, which will result in peri-scaffold bone resorption as seen in metallic joint implants. Also, the elastic property of the BTE scaffold should not exceed that of bone, to maintain a proper mechanical stimulation on the peri-scaffold bone, which depends on the loading conditions. The second step involves interface biomechanics and may be identified as the secondary fixa-tion. Here, the mechanical properties of the BTE scaffold may be adapted to generate interface scaffold-bone mechanotransduction, which has been shown to influence tissue differentiation and osteointegration of the scaffold.100 The third step, which may be termed ‘final fixation,’ involves scaffold evolution, in which the ingrowing bone offers support to the mechanical load as the BTE scaffold degrades. Thus, each step revolves around mechanical aspects, which induces a biological reaction in and around the BTE scaffold via mechanotransduction. It has been suggested that the separation between these steps may be represent an engineering approach in the mechanical design of bone scaffolds. Ideally, if mechanical considerations can be used to confer osteoinductivity to a BTE scaffold, the dependency on osteogenic factors and bioreactors may be reduced. This might eventually lead to the development of an off-the-shelf product.101
FIGURE 3.
Illustration of a three-step biomechanical paradigm in BTE. In the first step, upon implantation, it is critical that the mechanical properties of the BTE scaffold should closely match that of the surrounding host bone tissue and loading conditions to reduce the stress-shielding effect. The second step involves interface biomechanics, and should allow for interface scaffold-bone mechanotransduction for enhanced osteointegration of the scaffold. Lastly, as the scaffold degrades, ingrowing bone tissue will begin to support the mechanical load of BTE scaffold. Adapted from Pioletti (97).
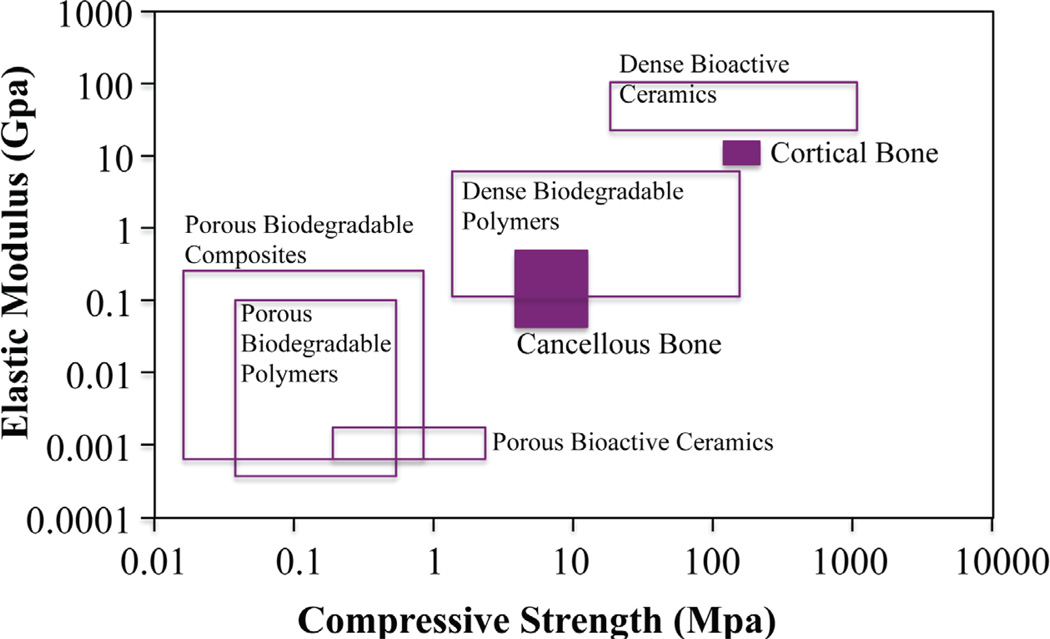
Mechanical properties of human bone vary tremendously according to location and function (i.e., load or non-load bearing). Again, the restorative scaffold’s mechanical properties should be modulated or tailored to match the demands of the defect site, to decrease or avoid complications such as stress shielding, implant-related osteopenia, and subsequent re-fracture.102 The scaffold’s material composition largely influences its mechanical properties. Dense ceramics (HA, calcium triphosphate) possess elastic moduli and compressive strength similar to human cortical bone; however, they are brittle and display slow degradation rates (Fig. 4).103 On the other hand, biodegradable polymer scaffolds display human cancellous bone compatible mechanics with tunable degradation. For this reason, the development of polymer-ceramic composite BTE scaffolds is becoming increasingly attractive: scaffold properties can be tailored to the particular mechanical and physiologic demands of the host tissue by effectively controlling volume fraction, morphology, and arrangement of the inorganic particulate phase in the polymer matrix. For example, widely investigated composites for BTE involve the incorporation of bioceramic and bioglass particles, carbon nanotubes (CNTs), or magnesium metallic or alloy particles.104–107 These inorganic inclusions positively affect the mechanical properties leading to reinforcement of the scaffold structure104 compared with non-composite polymer scaffolds. The enhancement of mechanical properties depends strongly on the inclusion shape and size distribution, as well as on the quality of the inclusion distribution in the matrix and on the strength of the inclusion–matrix interface. Although the composite strategy is promising, the scaffold mechanical properties are nowhere close to demonstrating the human cortical bone mechanical properties. On the other hand, composite scaffolds display enhanced functionality. In a study conducted in our lab on composite CNT/PLGA microsphere scaffolds, we observed increased biomimetic biomineralization of the composite scaffolds after a 14-day incubation in simulated body fluid (SBF) in vitro, in comparison to PLGA polymer scaffolds (Fig. 5). The increased bio-mineralization may be attributed to the CNTs present in the composite scaffold. The increased mechanical strength of the composite scaffolds can be attributed to the increased CNTs at the joining microsphere–microsphere areas. Thus, by forming composites with CNTs, the overall mechanical and biomimetic properties of a polymer scaffold may be effectively enhanced.104
FIGURE 4.
Elastic modulus versus compressive strength values of various BTE biomaterial classes compared to human bone. Adapted from Rezwan et al. (103).
FIGURE 5.
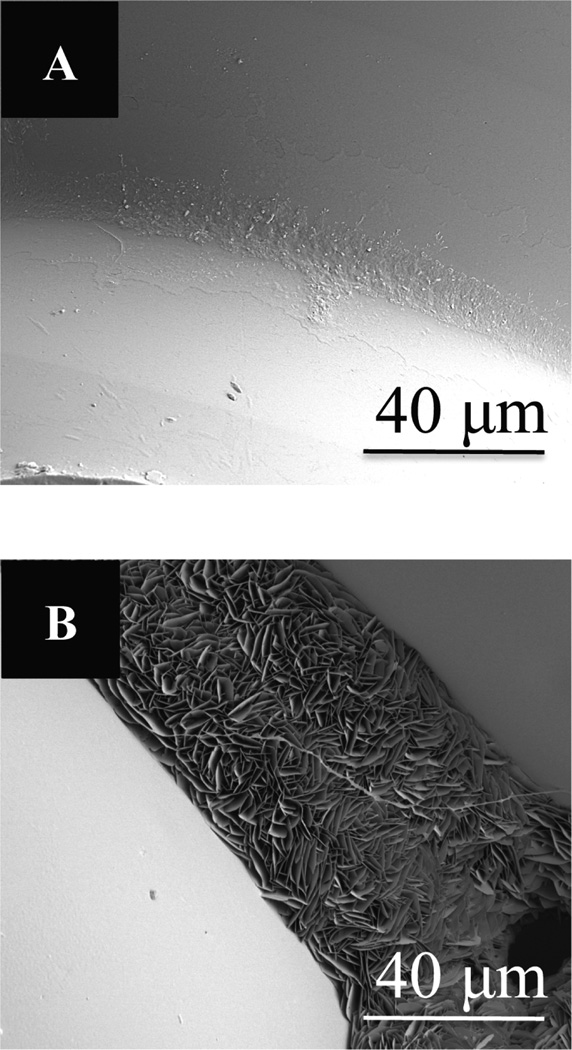
SEM images of (A) PLGA (50/50) microsphere scaffolds, and (B) composite carbon nanotube/PLGA (50:50) microsphere scaffolds after 14 days in simulated body fluid. Crystalization is seen the joining areas of mi-crospheres in only composite CNT/PLGA scaffolds. Scale bar = 40 µm.
Recently, biodegradable metals gained attention as the new generation biomaterials. They offer good mechanical properties, and therefore may be potent biomaterial options to make scaffolds with cortical bone–like mechanical properties. Particularly, magnesium metal has attracted attention because it has density and mechanical strength similar to cortical bone.108–110 Moreover, magnesium is present in small quantities in our bones. One particular disadvantage of magnesium is its rapid and uncontrolled degradation. Although this problem can be partially addressed by alloying magnesium with other metals such as zinc and aluminum, further investigations to develop and characterize magnesium-based scaffold systems for BTE are needed.111
2. Scaffold Porosity
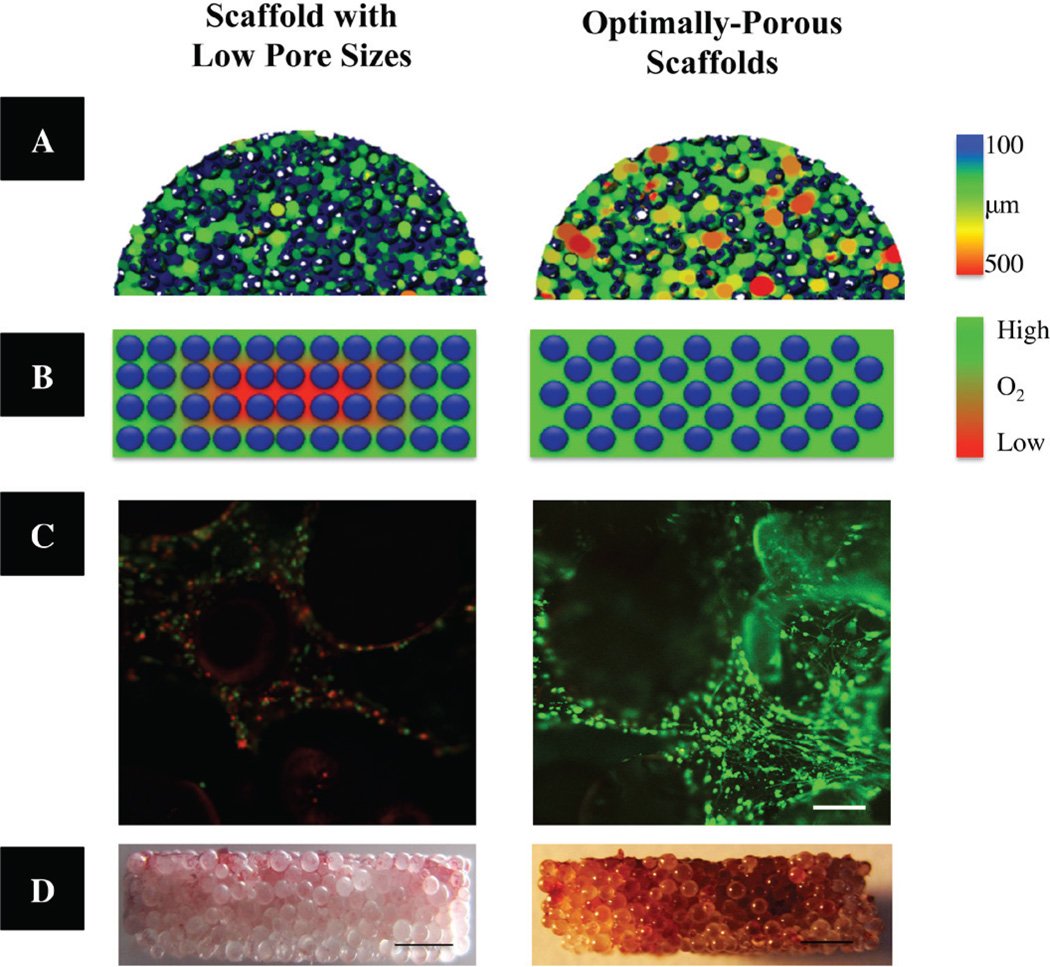
Microporosity is a critical element of the osteoconductive properties of scaffold material and the resultant bone tissue ingrowth and vascularization. Scaffold pore structure (i.e., pore size, volume, and interconnectedness) is an essential consideration for proper cell growth, cell migration, nutrient flow, vascularization, and better spatial organization for cell growth and ECM production.112,113 Although some ambiguity remains surrounding the optimal porosity and pore size for a three-dimensional bone scaffold, studies suggest that scaffolds currently designed with small pore sizes (i.e., < 200 µm) display in vitro and in vivo osteoblast survival and bone formation limited to the periphery, due to decreased oxygen and nutrient diffusion throughout the scaffolds.27 On the other hand, scaffolds with a mean pore size of 300 µm display increased osteoblast proliferation and differentiation throughout the entire scaffold, due to enhanced neo-vascularization and mass transport of oxygen and nutrients.114–117 Porogen leaching was used in combination with several traditional scaffold fabrication techniques, such as gas foaming,118,119 freeze drying,120 and phase separation121 to fabricate highly porous scaffolds. Recently, the authors have combined a microsphere sintering technique with porogen leaching to develop optimally porous and mechanically compatible scaffolds for BTE.122 As seen in Fig. 6, porogen (i.e., NaCl cystrals) leaching combined with thermal sinter of PLGA microspheres allows for the fabrication of consistent and reproducible optimally porous scaffolds with increased porosity and interconnectivity, which consequently allowed for improved oxygen availability (Fig. 6B), pre-osteoblastic cell survival (Fig. 6C) and mineralization (Fig. 6D) in the interior of the constructs. However, as porosity and mean pore sizes increase, mechanical strength is scarified; determination of a balance between mechanical strength and porosity is crucial. This study by the authors demonstrated that, by fabricating scaffolds with optimal pore size, it is possible to maintain oxygen tension and pH levels inside a scaffold that are almost similar to the values measured on the scaffold exterior. Such scaffolds, called oxygen tension controlled matrices, have been proven to support cell proliferation and mineralization throughout the scaffold structure (i.e., fully osteoconductive) in vitro and in static culture, and they may have the potential to repair large-scale or critical-size bone defects in vivo.123,124
FIGURE 6.
Optimally-porous, mechanically strong biodegradable scaffolds for enhanced bone regeneration. (A) Reconstructed MicroCT 3D porosity images demonstrating significantly increased interconnected pore sizes in optimally-porous scaffolds. (B) Schematic illustration of available oxygen levels throughout the scaffolds in vitro. (Scale from red to green demonstrating increasing oxygen levels.) (C) Pre-osteoblast cell viability in the center of the constructs after 14 days in vitro. Scaffolds with increasing porosity resulted significant cell survival in the interior of the macro-porous construct (right) compared to control scaffolds (left) (live cells = green; dead cells = red). Scale bar = 200 µm. (D) After 28 days in osteogenic medium, Alizarin Red staining was performed. Optimally-porous scaffolds displayed mineralization throughout the thickness of the scaffold, where as scaffolds with low pore sizes displayed mineralization to limited to the surface of the scaffolds. Scale bar = 1000 µm. Adapted and modified from Amini et al. (122).” (D) After 28 days in osteogenic medium, Alizarin Red staining was performed. Optimally-porous scaffolds displayed mineralization throughout the thickness of the scaffold, where as scaffolds with low pore sizes displayed mineralization to limited to the surface of the scaffolds. Scale bar = 1000 µm. Adapted and modified from Amini et al. (122).”
3. Nano-Featured Scaffolds
Scaffolds are meant to serve as a synthetic and temporary ECM replica that supports cell attachment and guides three-dimensional bone tissue formation. It has been well established that native bone cells interact with nano-scale proteins and minerals. Specifically, the main constituents of bone ECM (i.e., Type I collagen fibrils and hydroxyapatite (HA) crystals) are nanometers in diameter; all living molecular building blocks (i.e., proteins, lipids, carbohydrates, and nucleic acids) are governed by their nanoscale sizes, patterns, and folding. Thus, bone cells are predisposed to adhere, grow, proliferate, differentiate, and produce ECMs based on these nanoscale interactions, making nano-structural topographical properties of scaffold critical for osteoinductivity.125 Specifically, by decreasing material size to the nanoscale, scientists are able to dramatically increase the scaffold’s surface area, surface roughness, and surface area-to-volume ratios, resulting in superior physiochemical properties.
Nano-topography significantly influences osteoinductivity and osseointegration of the BTE scaffold.126–129 Specifically, studies suggest that osteoblasts demonstrate increased cell adhesion and proliferation, ALP activity, and enhanced expression of osteoblast differentiation markers RunX2, osteocalcin, and bone sialoprotein (BSP) on nano-featured biomaterials.130–134 Results from these studies suggest that nanotopography of bone scaffolds will stimulate bone formation and enhance bone-implant integration, leading to better tissue repair and regeneration at the bone implant–biomaterial interface.
Scaffold fabrication techniques that allow for development of nano-featured scaffolds include electrospinning,135–138 molecular self assembly,139–142 and phase separation.143 Nano-featured scaffolds can also be made from self-assembled into nanotubes/nanofibers that can even more accurately simulate the dimensions of natural entities, such as collagen fibers. For instance, osteogenic helical rosette nanotubes formed through the self-assembly of DNA base pairs (i.e., guanine and cytosine) in aqueous solutions have been reported for use in BTE applications. These osteogenic nanotubes have tailorable amino acid and peptide side chains that enhance osteoblast adhesion and inhibit fi-broblast adhesion (i.e., lysine and RGD); they have served as excellent mineralization templates to assemble a biomimetic nanotube/HA structure.144
4. Scaffold-induced cell homing
Stem-cell homing is a term that describes stem cell recruitment to injured tissues or their ability to navigate to other target niches/locations following mobilization.145,146 For example, a natural healing process in our bodies involves the inherent ability of mesenchymal stem cells (MSCs) to mobilize into circulation and migrate to an injury site, allowing for their participation in the regenerative process.147
For better tissue regeneration, various methods are being investigated to achieve enhanced cell homing to defect sites. These methods involve either cell-based approaches (i.e., stem cells engineered to be more responsive to the cues), or scaffold-based approaches (i.e., defect site, implanted scaffold and/or navigation cues more attractive or obvious to the cells).148 In the cell-based approach, cells are modified or engineered to express markers that are useful in guiding them to the regeneration site. For instance, MSCs modified with a nanometer-scale polymer containing sialyl Lewisx roll toward the inflamed tissue. Sialyl Lewisx is found on the surface of leukocytes and is responsible for cell rolling via the interaction with certain types of selectins present in the inflamed tissue. Sarkar et al. demonstrated surface engineered MSC rolling and homing in an inflamed ear model of the mouse.149
The latter approach or “scaffold-based homing” is based on releasing the chemokines responsible for MSC homing through biodegradable scaffolds placed in the defect site. Although the mechanisms of the mobilization of key cellular players have not yet fully elucidated, several key molecules have been identified as important factors. For instance, specific chemokine receptors (e.g., CCR1, CCR7, CCR9, CXCR4, CXCR5 and CXCR6) are important mediators. CXCR4 is the receptor of CXCR12 [also referred to as stromally derived factor-1 (SDF-1)], which has been identified to have a critical role in the recruitment and cell guidance of MSCs to bone healing sites.150 Furthermore, studies conducted by the authors have shown SDF-1 to be up-regulated in high-density MSC-seeded scaffolds in vitro.151 Also, in a mouse tibia fracture model, Granero-Molto et al. demonstrated that MSCs migrate to the injury site in a CXCR4-dependent manner.152 Another important cell type for bone regeneration and neovascular-ization of the defect site includes endothelial progenitor cells (EPCs). Various cytokines responsible for their mobilization include VEGF, stem cells factor (SCF), monocyte chemoat-tractant protein (MCP)-1/-3, and SDF-1.153 For example, Schantz et al. demonstrated enhanced cell migration and proliferation within polycap-rolactone scaffolds that delivered VEGF, SDF-1, and BMP-6 in a subcutaneous rat model.154 The incorporation of various mimetic peptide sequences (i.e., arginine-glycine-aspartic acid (RGD), glycine-phenlalanine-hydroxyproline-glycine-glutamate-arganine (GFOGER), Tyr-Ile-Gly-Ser-Arg (YIGSR), Arg-Glu-Asp-Val (REDV), and Ile-Lys-Val-Ala-Val (IKVAV)) may be used to mediate cell attachment and spreading of the cells attracted to the defect site, and such scaffolds have ultimately demonstrated enhanced osteoblast functionality and osseointegration in vivo.155 Engineered cell homing, either cell based or scaffold based, is gaining interest and may play an important role for effective bone regeneration in vivo.
5. Engineering Scaffolds for Orthopaedic Tissue Interfaces
Engineering orthopaedic tissue interfaces remains a significant and challenging endeavor in the field of BTE. Tissue–tissue interfaces are ubiquitous in our musculoskeletal system, as is their function in synchronizing joint motion and function. Failure to regenerate these interfaces results in the compromise of graft stability and long-term clinical outcome.156–158
The complexity of regenerating hard tissue– soft tissue orthopaedic interfaces (i.e., bone to soft tissues, such as ligament, tendon or cartilage) is a result of a number of factors. Orthopaedic tissue interfaces are structurally heterogeneous and intricate. The involved tissues are composed of very distinct cell populations that must operate in unison to maintain physiologic function and homeostasis. Furthermore, within these tissue interfaces is a distinct gradient of mechanical properties that allows for load transfer between the tissue types. Engineering the mechanical properties progressing from soft tissue to bone should also account for the native controlled distribution of non-mineralized and mineralized interface regions, as well as collagen fiber organization. Furthermore, the spatial distribution and interactions between interface-relevant cells are critical for the formation, maintenance, and repair of orthopaedic interfacial tissue. Thus, interface tissue engineering should strategically incorporate these biomimetic parameters into stratified scaffolds that enable both distinct and continuous multi-tissue regeneration and seamless graft integration.
A variety of multi-phased scaffolds have been designed to structurally and functionally mimic native soft tissue-to-bone to support the formation of integrated multi-tissue systems. For example, Lu et al. developed a biomimetic stratified scaffold for ligament-to-bone interface tissue engineering. Briefly, a tri-phasic stratified scaffold was designed to mimic the three interface regions (ligament, fibrocartilage, and bone).159 Phase 1 is designed with PLGA (10:90) mesh intended for soft tissue (i.e., ligament) formation, phase 2 consists of PLGA (85:15) microspheres and is the interface fibrocartilage region, and phase 3 is composed of sintered PLGA (85:15) and 45S5 bioactive glass composite microspheres for bone formation. This innovative scaffold design allowed for three distinct yet continuous phases to support the formation of the multi-tissue regions observed across a ligament-bone junction after 2 months in vivo.160
For a more comprehensive review of the interfacial tissue engineering strategies, the reader is encouraged to refer to recent reviews by Lu et al. and Yang et al.157,161 Although much progress has been achieved in regenerating orthopaedic tissue interfaces, significant challenges remain. More insight into the basic developmental biology of these interfaces is required to better understand the mechanisms by which these unique transition tissues are created. Also, more information on the spatial distribution of matrix molecules and the relation of tissue structure and mechanical properties is needed. With this, biomaterials that are able to temporally and spatially control the application of cells and soluble factors, as well as bioreactors that better mimic the native stresses at these interfaces may be created and improved upon.
6. New Scaffold Fabrication Techniques
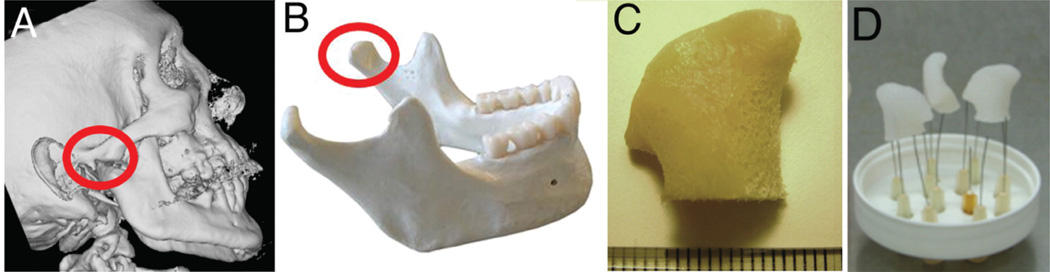
The development of personalized and anatomically shaped bone grafts has opened new doors for clinically relevant BTE. By combining computer assisted design (CAD) with computer assisted manufacturing (CAM), tissue engineers are able to produce custom-made and individualized complex scaffold architectures for the repair of complex bone defects that are often encountered in craniomaxillofacial surgeries. Grayson et al. successfully utilized the CAD/CAM systems to engineer personalized, clinically sized anatomically shaped bone grafts for the repair of human temporomandibular joint (TMJ) (Fig. 7).162
FIGURE 7.
Tissue engineering of anatomically shaped bone grafts. (A–C) Scaffold preparation. (A, B) Clinical CT images were used to obtain high-resolution digital data for the reconstruction of exact geometry of human TMJ condyles. (C) These data were incorporated into MasterCAM software to machine TMJ-shaped scaffolds from fully decellularized trabecular bone. (D) A photograph illustrating the complex geometry of the final scaffolds that appear markedly different in each projection. Adapted from Grayson et al.162
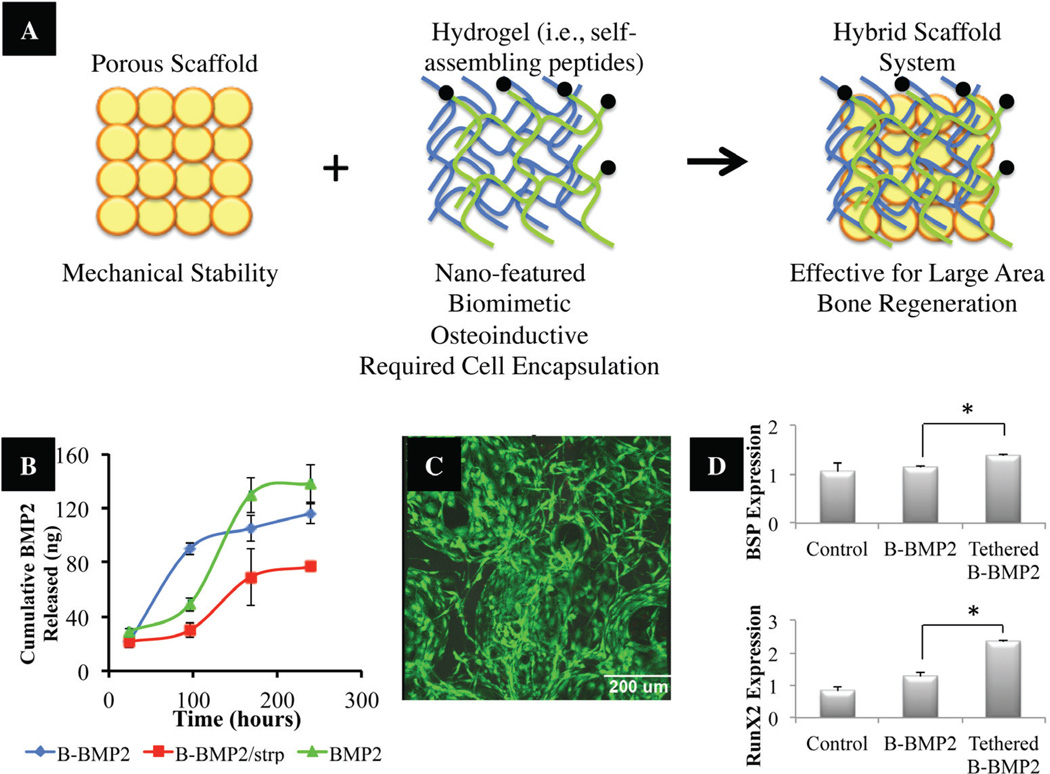
In addition, as previously described, micro-and nano-scale architectures play a critical role in native bone physiology, so scientists are investigating new fabrication techniques that would allow for multi-scale hierarchical manipulation. Several techniques, including electrospinning combined with fiber bonding,137,163,164 templating,165–167 and modified rapid prototyping,168,169 have demonstrated the ability to construct multi-scale synthetic scaffolds. Moreover, a novel hybrid approach involving the combination of mechanically strong, porous scaffolds and nano-featured self-assembling peptide hydrogels as an osteoinductive scaffold system is being investigated.79,80,170 In this approach, the mechanically strong scaffold component would allow for mechanical stability of the load-bearing defect site; whereas, the hydrogel phase will allow for efficient cell delivery into the defect implantation site, cell niche establishment and promotion of mineralization (Fig. 8). Growth factors for the promotion of accelerated bone and vascularization (i.e., BMP, VEGF) may also be covalently tethered to the hydrogel phase to allow for enhanced effects upon implantation. In a recent study, the authors have incorporated the features described above and developed a hybrid system comprised of a mechanically load-bearing scaffold infused with a self-assembling peptide hy-drogel with tethered BMP-2 (Fig. 8). The newly developed “polymer-hydrogel” hybrid system is robust: it not only satisfies mechanical needs but also has the ability to load the cells and factors required for osteogenesis and vasculogenesis. The hybrid system supported the encapsulation of rat bone-marrow–derived stromal cells and pre-os-teoblastic MC3T3-E1 cells, and it llowed for the later cell expression of key bone markers (i.e., BSP and RunX2) in vitro. Although BTE methods are currently not the gold standard in clinical practice, due to related high costs and insufficient universal manufacturing methods, recent studies have revealed methods for accelerated bone regeneration that are proving effective and are paving exciting roads for the use of BTE methods in the clinic.
FIGURE 8.
(A) Illustration of hybrid scaffolds composed of a mechanically strong component, and a hydrogel phase for enhanced bone regeneration abilities.170 (B) In vitro release kinetics of biotinylated BMP2. Amount of BMP2 released over time was measured by ELISA. Results show cumulative release of rhBMP2 for untethered groups (BMP2-biotin, BMP2) versus tethered group. (C) Survival of pre-osteoblastic MC3T3-E1 cells in hybrid scaffold. Images show live and dead cells cultured on hybrid scaffolds; green represents live cells. (D) Bone Sialoprotein (BSP) and RunX2 gene expression profile of pre-osteoblastic MC3T3-E1 grown in BMP2 untethered versus tethered SAP gel PLGA/nHA hybrid scaffolds (p < 0.05). Adapted and modified from Igwe et al.170
C. Cellular Approaches
An unresolved debate on the most effective cell type for clinical bone regeneration continues, but it has been established that cellular-based bone regeneration approach is indeed effective. Cellular-based approaches in BTE primarily target the early stages of bone repair when the recruitment of skeletal progenitors may be impaired. Proposed mechanisms by which implanted cells enhance bone regeneration in BTE involve (1) early release of key osteogenic and vasculogenic molecules and growth factors, (2) formation of a template to recruit host osteogenic and vascu-logenic cells, and (3) actively laying down bone matrix and vascularizing the bone construct.
The major challenge in making these cellular therapies more efficient is the identification of the cell sources that can be implanted to the bone defect site and will differentiate into os-teoblasts and form neo-vasculature.171,172 Thus far, studies have investigated several cell types for their abilities to promote bone repair and regeneration: mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), adipose derived stem cells (ADSCs) and stem cells from human exfoliated deciduous teeth (SHED). This variety of possible candidates for cell transplantation can be explained by the finding that cells involved in the reconstruction of osseous tissue undergo a progression from undifferentiated progenitors to biosynthetically mature cells; therefore, therapeutic strategies can approach supporting the healing process at different stages of bone tissue development.173 For successful clinical application in the regeneration of bone, the properties of choice include isolation and expansion efficiency, expression and stability of osteogenic markers, “bona fide” bone tissue formation, and long-term safety (i.e., immunorejection, graft-versus-host disease, tumorigenicity). Table 2 summarizes the cell types that have been utilized for clinical bone defect repair thus far.
TABLE 2.
Cell Choices for Bone Tissue Engineering
| Cell type | Source | Clinical use |
|---|---|---|
| Embryonic stem cells (ESCs) |
Embryonic bodies (EBs) | N/D |
| Induced Pluoripotent stem cells (iPSCs) |
Any cell type that could be induced to become osteoblasts |
N/D |
| Adult stem cells | Bone marrow | Segmental defects of long bones312 Large bone diaphysis defects313 Maxillary sinus augmentation314 Posterior spinal fusion251 Bone tumor resection315 |
| Adipose tissue | Large calvarial defect316 Osteonecrotic femoral heads317 Hip osteonecrosis318 |
|
| Peripheral blood | N/D | |
| Teeth (pulp, exfoliated teeth) | N/D | |
| Cord blood | N/D | |
| Amniotic fluid | N/D | |
| Stem cells derived from ESCs and iPSCs | N/D | |
| Genetically modified cells | Any cell type that could be genetically modified |
N/D |
| Autologous cells and growth factor cocktail |
Platelet-rich plasma bone marrow aspirate |
Necrosis of femur head, avascular necroses, Non-unions319 Sinus graft320 |
1. Embryonic stem cells (ESCs)
Embryonic stem cells (ESCs) are, by definition, obtained from ‘embryos,’ which are formed following the fertilization up until the ninth week of gestation. ESCs are frequently obtained from extra embryos developed by in vitro fertilization techniques, to bypass ethical debates regarding their usage. Because ESCs are pluripotent, with high proliferative activity, they can potentially be used as a single source for the derivation of multiple lineages present in adult bone, including osteogenic cells, vascular cells, osteoclasts, nerve cells for bone regeneration.174
Since their first isolation more than 30 years ago, ESCs have been intensely investigated. Specifically, it has been established that undif-ferentiated ESCs express the following surface antigens: stage-specific embryonic antigen-4 (SSEA-4), tumor rejection antigens TRA-1–60 and TRA-1–81. However, they lack the expression of SSEA-1.78 ESCs are not highly im-munogenic; they express very few major histo-compatibility complex (MHC) class I molecules. ESCs possess high alkaline phosphatase and telomerase activities, and expression of transcription factors Oct4, Sox2, and Nanog, which are crucial for the maintenance of pluripotency. Self-renewal of ESCs is regulated by cytokines of the IL-6 family. Human ESCs are confirmed in vitro by their ability to induce differentiation in embryoid bodies, which are defined as aggregates of cells cultured in suspension. Also, ESC confirmation tests involve observing their differentiation potential in vivo and the formation of tissues from all three germ layers.
ESCs require complex proliferative culture conditions, including various growth factors, feeder cell layers, specific media and/or coated culture plates.175 For instance, murine ESCs require presence of leukaemia inhibitory factor (LIF) or a feeder layer of murine embryonic fibro-blasts (MEF) to remain in the undifferentiated and proliferative state. On the other hand, human ESCs should be cultured on Matrigel or laminin in the presence of MEF-conditioned medium.176 In the absence of these conditions, ESCs differentiate spontaneously into embryoid bodies. ESCs have the potential to differentiate into numerous cell types including cardiomyo-cytes, haematopoietic cells, endothelial cells, neurons, chondrocytes, adypocytes, hepatocytes pancreatic islets, and importantly, osteoblasts.177
The potential use of ESCs for BTE has gained considerable attention among tissue engineers.178,179 Osteogenic differentiation of ESCs may be achieved by either first forming or not forming embryoid bodies. For the first method, embryoid body formation is followed by its dissociation, re-plating of dissociated single cells, and then administration of osteogenic supplements (i.e., β-glycerophosphate, ascorbic acid, dexamethasone, retinoic acid, and 1,25-hydroxy vitamin D3).180,181 However, due to the limited control of lineage-specific differentiation of ESCs within EBs, this method may result in a limited number of the cell type of interest. Thus, scientists have searched for more efficient, simple, and convenient culture strategies by directly differentiating ESCs into the osteogenic lineage, bypassing EB formation. In this method, ESCs are directly plated as a single-cell suspension and cultured in the presence of β-glycerophosphate, ascorbic acid and dexamethasone.182,183 These findings suggest that this may be a good culture strategy for applying functional ESC-derived osteogenic cells effectively to BTE.
Many studies have demonstrated the effectiveness and potential use of ESCs for BTE purposes when combined with various three-dimensional scaffolds. For instance, the expression of alkaline phosphatase and osteocalcin were significantly enhanced in human ESC culture on three-dimensional PLGA scaffolds in comparison with the same cells cultured on a two-dimensional culture plate.184 Another approach for ESC-based three-dimensional bone tissue generation involved the development of BMP-inoculated three-dimensional scaffolds, composed of PLGA and hydroxyapatite, as an ESC-derived osteoblast delivery vehicle for generating bone-like tissue in vivo. Studies have demonstrated successful bone tissue formation using ESC-derived osteoblasts subcutaneously implanted into immunodeficient mice.185
Despite their enormous potential, concerns about ESCs must be addressed prior to their potential use for tissue engineering applications. It is critical to confirm the stability of the donor ESCs and that they are not tumorogenic; prolonged culture of undifferentiated ESCs may result in spontaneous development of abnormal karyotypes, and their implantation resulting in the formation of teratomas in vivo. Also, the immunological incompatibility between donor ESCs and host cells must be addressed.
2. Induced Pluripotent Stem Cells
Induced pluripotent stem cells (iPSCs) are pluripotent stem cells that are artificially derived from a non-pluripotent cell via the induction of a “forced” expression of specific genes. iPSCs were first produced from mouse fibroblasts by retroviral delivery of four transcription factors (i.e., Oct4, Sox2, Klf4, and Myc) in 2006.186 In the following year, terminally differentiated human somatic cells were also converted into iP-SCs using a similar method.187,188 Studies have shown human iPSCs to possess properties similar to those of human ESCs, not only in respect to their morphology, gene expression, surface antigens but also their in vitro differentiation potential and pluripotency. However, the inherent epigenetic memory of the starting non-pluripo-tent cell may influence the differentiation potential and in vivo functionality of tissues derived from such iPSCs.189 Additional research in this area is needed to determine the best starting somatic cell for iPSC generation for human clinical applications. Furthermore, possible resultant tumor formation due to integrated oncogenes requires special attention and investigation. In addition, it is paramount to develop non-viral induction methods to produce clinically safe iPS cells for BTE.
3. Adult Stem Cells
A. Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) have long been recognized for their potential in engineering bone grafts because they differentiate and form bone during the natural bone development process. Their great potential in BTE has led to their characterization and the identification of a plethora of sources for their isolation. MSCs have been defined through the expression of various CD markers (i.e., negative for CD34, CD45, CD14, CD11a, CD19, and HLA-DR and positive for STRO-1, CD29, CD73, CD90, CD105, CD106, CD166, CD146, and CD44).190 Also, MSCs have been isolated from a number of adult sources including bone marrow,191 peripheral blood,192 umbilical cord blood,193 sy-novial membrane,194 deciduous teeth,195 dental pulp,196 amniotic fluid,197 adipose tissue,198 brain, skin, heart, kidneys and liver199 through a relatively simple protocol that primarily relies on their ability to adhere to plastic in tissue culture. 200 In addition, their high proliferative potential combined with their ability to withstand freezing conditions allows for their in vitro expansion to obtain clinically relevant cell numbers.191
In addition to adult sources, MSCs have recently been derived from embryonic stem cells, as well as iPS cells.201 These embryonic- and iPSC-derived MSCs have the same in vitro and in vivo multi-potent characteristics as MSCs derived from other adult sources (i.e., bone marrow). However, unlike MSCs derived from adult sources, iPSC-derived MSCs can be expanded with a lower rate of senescence. Their enhanced survival potential, both in vitro and in vivo, may be attributed to higher telomerase activity.201 In any case, MSCs of embryonic and iPSC origin must be further tested to rule out the possibility of teratoma formation before they are considered for clinical application.
The incorporation of MSCs into BTE bio-materials is a widely studied strategy for accelerated bone formation and osteointegration during bone defect repair and regeneration. Mechanisms by which enhanced bone regeneration occurs involves directly providing MSCs for osteogenic differentiation and bone formation, as well as enhanced osteoinductivity of the biomaterial via the release of osteogenic growth factors and stimulation of the migration and differentiation of host osteoprogeni-tors. In addition, pre-differentiating MSCs into the osteogenic lineage before implantation has been shown to further accelerate defect repair and osteointegration of the construct in vivo by delivering a more mature osteogenic population capable of immediate bone formation. Pre-clinical trials with MSC-seeded constructs have proven effective in accelerating bone repair in various scenarios, including critical-size femoral defects, cranio-maxillofacial deformities, and spinal fusions.202
Although MSCs may seem to represent a great cellular option for enhanced BTE, several issues with their use have been identified. First, several studies have shown that a maximum of 24–40 population doublings are reached before MSCs reach senescence-associated growth arrest. Also, osteogenic differentiation potential in vitro and bone forming efficiency in vivo signifi-cantly decreases with increasing donor age and systemic disease. Additionally, the lack of knowledge about common markers for MSCs isolated from different sources makes it difficult to define MSCs.190,203 These factors significantly limit the actual amount and the quality of MSCs obtainable for clinical application. Approximately 4–6 weeks is required for cell expansion before possible patient treatment. Furthermore, long-term culture may lead to forced selection under artificial culture conditions, which increases the possibility of abnormal karyotype development and malignant cell transformation. Lastly, the use of fetal bovine serum (FBS) during in vitro expansion poses a risk of transmitting zoonotic or prion-related diseases, which may induce an immune response triggered by xenogenic proteins. The option of using synthetic serum with range of recombinant growth factors or serum-free media are being explored as alternatives.173
At present, a number of strategies have been reported that are capable of augmenting the loss of both proliferative capacity and osteogenic differentiation potential of MSCs after extensive population doublings ex vivo. These methods include cultivation of MSCs in the presence of basic fibroblastic growth factor (FGF-2) and maintenance of MSCs on several extracellular matrices (i.e., basement membrane-like extracellular matrix produced by bovine corneal endo-thelial cells, denatured collagen type I matrix) instead of conventional tissue-culture plastic during progressive passages.202 The mechanism controlling how various ECMs may influence the retention of MSC osteogenic differentiation potential after ex vivo expansion remains to be elucidated; however, it has been suggested that the physical interactions between MSCs and certain ECM motifs (i.e., integrins and their li-gands) may play a significant role.
The variability of colony formation and culture conditions necessary to sustain prolifera-tive capacity have led to an interesting proposal regarding the creation of a universal allogenic human MSC cell line providing “off the shelf” or “ready to use” cells.112 Though it may not seem possible without requiring the use of im-munosuppressive drugs to reduce associated risks of rejection, it has recently been shown that cultured MSCs exhibit a poorly immuno-genic phenotype (i.e., evidenced by MHC class I+, MHC Class II-, and low levels of expression of co-stimulatory molecules, CD40, CD80, and CD86). Also, MSCs have been shown to be immune suppressive (i.e., immune privileged). Specifically, MSCs do not induce the proliferation of lymphocytes, and they suppress the proliferation of T cells and cytokine production in response to alloantigens or insignificant mito-gens, as well as inhibiting the function of B cells, dendritic cells, and the natural killer cells. These data greatly enhance the therapeutical appeal of MSCs in BTE.
B. Adipose-Derived Stem Cells
Adipose-derived stem cells (ADSCs) represent an easily accessible, widely available, and abundant source of autologous osteogenic cells. ADSCs have multi-lineage differentiation potential (i.e., osteogenic, chondrogenic, adipo-genic, neural, cardiomyocyte, and endothelial lineages).203–206 Isolation protocols of ADSCs include density gradient centrifugation of the col-lagenase-digested lipoaspirates (ranging from 100 ml to several liters), and culture expansion of the adherent cell population. Lipoaspirates house a relatively high frequency of ADSCs (1 to 5% of isolated cells), in comparison to MSCs in bone marrow (0.001% to 0.1% of isolated cells). Similar to MSC isolation, the successful numbers of cell isolated are influenced by the tissue harvesting procedure, as well as the site of tissue harvesting (e.g., arm, thigh, abdomen, breast). ADSCs also share a common surface-antigen expression pattern, including CD44, CD90, CD13, CD29, CD73, CD166 and CD105.205,207 However, the expression of STRO-1 and CD34 antigens remains controversial. Some researchers have reported that ADSCs do not express STRO-1,208 and others have reported the absence or extremely low levels of expression of STRO-1 or CD34 antigens on the surface of ADSCs.198 Expression of surface markers on ADSCs may vary according to passage number and isolation techniques;209 hence, the characterization of ADSCs requires further clarification.
The osteogenic differentiation potential of ADSCs has been demonstrated not only in vitro but also in vivo. Osteogenic differentiation in vitro may be achieved through the addition of supplements, including β-glycerophosphate, ascorbic acid, and dexamethasone, which are similar to those used for osteogenic differentiation of bone-marrow-derived MSCs.205,210,211 It has also been shown that other exogenic factors may be applied to direct osteogenic differentiation of ADSCs, such as brief treatment of BMP-2.212 Furthermore, pre-differentiated ADSCs have demonstrated good adhesion, proliferation activity, and homogenous bonelike tissue formation on various biocompatible three-dimensional scaffolds in vitro and on ec-topic bone formation in vivo.213–215 In addition, osteogenic performance of ADSCs has been assessed in orthotopic in vivo environments, which provide a more inductive, and physiological/ clinically relevant environment. Although enhanced bone regeneration has been demonstrated with implanted ADSCs,216,217 the mechanisms by which ADSCs promote bone healing in these orthotopic models has not been investigated.
ADSCs may further serve as an attractive cell population for the implementation of a one-step, intra-operative bone grafting approach, avoiding the cost and time of cell expansion. In this approach, tissue harvest, cell isolation, cell seeding onto a scaffold, and subsequent implantation could occur within a few hours, with no ex vivo cell culture. As previously mentioned, because ADSCs frequency in human lipoaspirates is relatively high (i.e., 500-fold larger numbers of colony forming units than human bone-marrow aspirates), these cells may represent a suitable cell source for such a one-step surgical procedure.218,219 Muller et al. offered proof-of-principle for this intra-operative approach by demonstrating vascularized tissue formation with positive staining of bone sialoprotein and osteocalcin 8 weeks post-implantation of ADSCs, which were implanted ectopically in nude mice 3 hours after harvest.220 These findings have significant implications for BTE applications in which ADSCs could be used for the fabrication of tissue-engineered bone.
C. Peripheral Blood–Derived Stem Cells
The isolation of stem cells from the patient’s peripheral blood (PB) represents a minimally invasive (i.e., no donor site morbidity) and convenient method for obtaining two effective cell populations for bone and vascular regeneration, namely MSCs and endothelial progenitor cells (EPCs). Traditionally, MSCs have been isolated from bone marrow; however, recent studies have identified peripheral blood as a site for MSC isolation (PB-MSCs). In fact, Chong et al. suggested that from 2 mL of peripheral blood, approximately 0.5–1 million cells were obtainable after 2 weeks from cell seeding (passage 0) and approximately 5 million PB-MSCs were obtainable at the end of passage 2. PB-MSCs display the same differentiation potential profile as MSCs isolated from bone marrow (i.e., tri-lineage; chondrogenic, osteogenic, and adipogenic).221 Furthermore, EPCs, especially those isolated from peripheral blood, have demonstrated high angiogenic potential and an effective cell population for promoting neo-vascu-larization of bone defect sites.222 Although the effectiveness of these two cell types, both isolated from peripheral blood, has not been thoroughly investigated in bone regeneration, other isolation sources (i.e., bone marrow) have been sought that have demonstrated superior bone regeneration and vascularization of bone defect sites.223,224 Thus, the effectiveness of PB-EPCs and PB-MSCs in vivo should be further investigated; they represent a potent alternative to cell transplantation procedures.
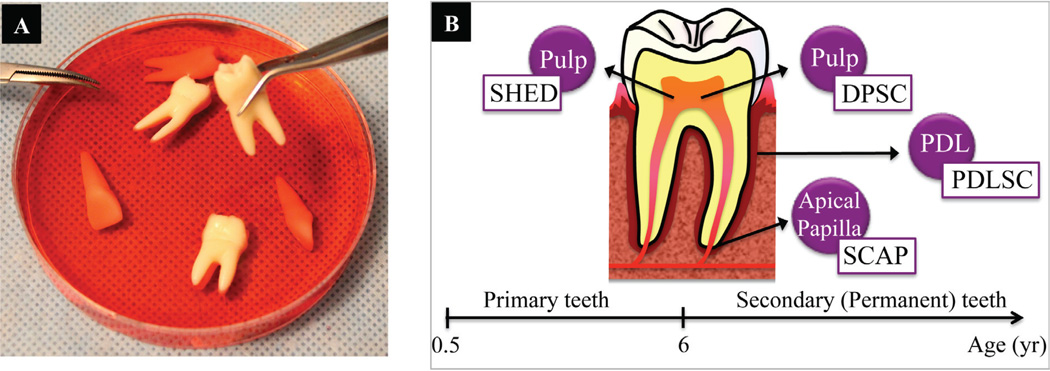
D. Tooth-Derived Stem Cells
a. Dental Pulp–Derived Stem Cells
Interesting findings within the last decade have pointed to stem cells residing in dental pulp tissue as an attractive cell source for BTE.225,226 Dental pulp derived stem cells (DPDSCs) can differentiate into a number of cell types, including odontoblasts, chon-drocytes, osteoblasts, endothelial cells, adi-pocytes and neural cells.227,228 DPSCs have been isolated from digested pulp tissue by either a single-colony selection or an immunomagnetic isolation method using anti-STRO-1 antibody and magnetically activated cell sorting (MACS). DPDSCs are highly proliferative, and they display the typical immunoreactivity profile of MSCs.230
Successful osteogenic differentiation of DPDSCs has been demonstrated and involves the administration of osteogenic medium (i.e., supplements of ascorbic acid, dexamethasone, β-glycerophosphate). Furthermore, in vivo transplantation of such DPSCs into nude rats generated living fibrous lamellar bone tissues containing osteocytes.231 Recent BTE studies have also shown the potential for use of DPSCs in combination with a three-dimensional scaffolds. For example, highly mineralized tissue formation was reported in an autologous implantation study with rabbit DPSCs and a poly(lactide-co-glycolide) (PLGA) scaffold construct implanted subcutaneously.232
b. Stem cells from Human Exfoliated Deciduous Teeth (SHEDs)
With the discovery of DPDSCs in 2000, that of stem cells in deciduous teeth came soon after, in 2003. Stem cells from human exfoliated deciduous teeth (SHED) have a similar differentiation potential as DPDSCs; however, they also have several key advantages. SHEDs have a higher proliferation rate, and they may also be readily accessible in young patients that have disposable primary teeth. Also, SHEDs have a distinctive osteoinductive ability and high plasticity. Lastly, upon transplantation, SHEDs are capable of differentiating into blood vessels that anastomose with the host vasculature.233
Numerous studies have proven that tooth-derived DPSCs and SHEDs might be an ideal resource of stem cells to induce bone regeneration. Further, tooth-derived stem cells are readily accessible, and provide an easy and minimally invasive way to obtain and store stem cells for future use (Fig. 9). Due to an increase in interest and demand for tooth-derived stem cell storage, many dental professionals are now undergoing training for proper recovery and transport of the teeth to optimize stem cell recovery. Also, many cell banking companies, including BioEDEN and Provia Laboratories Inc. (Store-A-Tooth) have been created with the hope of offering a reasonable and simple option to preserving tooth-derived stem cells. BioEDEN, founded in 2006, is the world’s first international private stem cell storage bank that collects, assesses, and cryogenically stores viable deciduous teeth-derived stem cells.
FIGURE 9.
(A) Image of extracted teeth that may be used to tooth-derived stem cell isolation. (B) Stem cells that may be isolated from primary teeth [i.e., stem cells from human exfoliated deciduous teeth (SHED)], and secondary teeth [i.e., dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSC), and stem cells of apical papilla (SCAP)].
At this stage, it is not clear how to obtain large amounts of tooth-derived cells. Also, questions remain about the mineral matrix that will be laid out by these cells and how different or similar its composition compared to bone.169 Despite these limitations or lack of understanding about the mineralization, tooth-derived stem cells (DPDSCs and SHEDs) certainly present an interesting and alternative adult stem cell source for BTE.
4. Genetically Modified Cells
Approaches involving cell and gene therapy offer great potential for enhanced bone regeneration. Proposed mechanisms for enhanced bone regeneration include the obvious growth factor release, as well as the recruitment of host cells to the site of implantation by genetically modified cells, resulting in a reduced number of exogenous cells that need to be implanted.234 The creation of genetically modified cell populations expressing growth or transcription factors of interest requires a gene-delivery vector, which is often vi-rally based. Gene therapy in many BTE studies has involved the osteoinductive bone morpho-genetic protein (BMP) family for bone repair. Several investigations have involved the use of BMP-2–transfected bone marrow cells or MSCs and their ability to enhance the repair of calvarial and critical-size bone defects in mice and rats.235– 238 In addition, Breitbart et al. cultured perios-teal cells retrovirally transduced with BMP-7 in a PGA scaffold in a critical-size calvarial defect model in rabbits for enhanced bone formation.239 Other genes of interest include transcription factors essential for osteoblast differentiation (i.e., core binding factor α1 (Cbfa1), and Osterix), factors enhancing angiogenesis (i.e., VEGF), as well as combinations of several factors. For example, Jabbarzadeh et al. transfected ADSCs with VEGF and cultured them on PLGA micro-sphere scaffolds for enhanced vascularization at a bone defect site (Fig. 10A, B).240 Sefcik et al. has demonstrated enhanced vascularization and bone formation via sustained delivery of sphin-gosine 1-phosphate (S1P) (Fig. 10C, D).241 Also, 70% L-lactide and 30% DL-lactide co-polymer (PLDL) scaffolds loaded with recombinant human growth factors (combinations of BMP-2, TGF-β3, and VEGF) were utiltized to promote enhanced vascular and bone regeneration at a segmental bone defect (Fig. 10E, F).242
FIGURE 10.
(A) Immunofluorescent staining of VEGF production by transfected adipose stem cells (ADSCs) cultured for 10 days on PLAG sintered microsphere scaffolds in vitro. ADSCs were stained with antibody directed against VEGF (green) and nuclei counterstained with DAPI (blue). Scale bar = 100 mm. Adapted from Jabbarzadeh et al.240 (B) Representative histological cross sections of transfected ADSCs with VEGF implanted with sintered microsphere scaffolds 21 days after subcutaneous implantation in SCID mice. Scale bar = 10 mm. Adapted from Jabbarzadeh et al.240 (C) Intravital microscopy images of control PLAG films (left) or S1P loaded films (right) in a dorsal skinfold window chamber at 7 days post-implantation. Significant lume-nal expansion of both arterioles (black) and venules (white) is induced by S1P over the course of 7 days (arrows). Scale bar = 500 µm. (241) (D) New bone volume formed within defect area following 6 weeks of healing. (*p<0.05) (238) (E, F) Micro-CT images of vascular (E) and bone (F) ingrowth several weeks after implantation of 70% L-lactide and 30% DL-lactide co-polymer (PLDL) scaffold loaded with recombinant human growth factors (combinations of BMP-2, TGF-β3, and VEGF). Adapted from Guldberg et al.242
Another highly attractive gene therapy approach includes the extension of the in vitro lifes-pan of cells for tissue engineering applications. As previously described, the proliferative ability of human MSCs decreases after prolonged culture periods, resulting in senescence. This observation is largely due to telomere shortening of chromosomes occurring with each cell division. However, by ectopically expressing human telomerase reverse transcriptase (hTERT), the life expectancy of cells can be significantly extended.
Clearly, gene therapy holds great promise for future clinical applications. However, to fulfill this clinical goal, gene therapy must be proven safe for human use.243 Currently, the majority of gene therapy BTE studies involve the use of viral gene therapy, which has unresolved issues involving immunogenicity, stimulation of acute immune-modulatory responses, and uncontrollable insertional mutagenesis leading to the risk of malignant transformation. Consequently, a hold by the FDA has recently been imposed on all retroviral gene therapy clinical trials, and the enthusiasm for the safety and success of viral gene therapy has decreased.244 Thus, the challenge now lays in developing nonviral gene therapy strategies. These are mostly based on the direct gene transfer of naked plasmid DNA (i.e., nucleofec-tion) via physical and chemical methods. In this approach, the plasmid DNA is transferred by forming lipoflexes (cationic lipid/DNA complex), polyflex (cationic polymer/DNA complex), complexation with cationic nanoparticles, and via chimeric RNA/DNA oligonucleotides.234,245
5. Autologous Cells and Factor Cocktail
Tissue regeneration is the result of interplay between cells and growth factors. In fact, growth factors/singling molecules regulate cell expression, migration, and tissue formation and its morphogenesis. Multiple cell types and a number of signaling molecules are involved during bone development, as well as bone repair processes. To recapitulate some of these events, orthopaedic researchers and clinicians have proposed the use of bone-marrow aspirate concentrate (BMAC) and platelet rich plasma (PRP) as two natural sources for bone regeneration.
A. Bone Marrow Aspirate Concentrate (BMAC)
Bone marrow aspirate concentrates (BMACs) are commonly harvested from the patient’s iliac crest to yield a rich source of osteogenic stem cells and osteoconductive growth factors, ultimately to repair and regeneration of bone defect. Specifically, BMACs contain a loaded mix of regenerative cells, including MSCs, endothe-lial progenitor cells (EPCs), hematopoietic stem cells (HSCs), platelets, lymphocytes, and granu-locytes. Each one of these components plays a critical role in the bone regeneration process. Namely, EPCs stimulate angiogesis; HSCs differentiate into growth-factor-releasing platelets; MSCs form osteo- and chondro- progenitor cells; platelets mediate cell-to-cell adhesion and growth factor release; lymphocytes support the migration and proliferation of EPCs; and granu-locytes release angiogenic factor VEGF. In numerous clinical cases beginning in 1978, BMACs have been shown to be effective in the treatment of bone defects, including tibial non-unions and osteonecrosis of the femoral head.247–253 Over the years, BMACs have proven to be a safe alternative method to promoting bone unions, with effectiveness similar to autologous bone grafting.254
B. Platelet-Rich Plasma (PRP)
Though not a cell source, platelet-rich plasma (PRP) may be used as an autologous source of growth factors to be added to BTE constructs for enhanced bone regeneration. PRP is a concentration of platelets in a small volume of plasma, and it may be easily isolated from freshly drawn whole blood activated with a mixture of thrombin and calcium. Autologous PRP may be used to facilitate bone regeneration, as it contains high levels of growth factors that are involved in chemotaxis, cell proliferation, differentiation and extracellular matrix synthesis (i.e., TGF-β1, PDGF-BB, VEGF-A and IGF-1). The use of PRP for enhanced bone regeneration has proven to be advantageous in several aspects. For instance, PRP is more potent than a single recombinant growth factor, due the synergis-tic effect of the combination of growth factors present in PRP.255 In relation to vascularization, PRP not only has pro-angiogenic properties, it also induces the endothelial cells to express a pro-osteogenic phenotype. However, in vivo animal experiments involving PRP offer conflicting results, and perhaps to variations in the animal species used, protocol designs, types of bone defects, outcome parameters measured, the presence of MSCs, and types scaffolds or bone substitutes. Furthermore, PRP may have high variability in GF concentrations, due both to host health factors and to different preparation methods.256 Although PRP has been investigated clinically for the repair of soft tissues, including nerve, tendon, cartilage, ligament, its usefulness for bone defect repair and regeneration has yet to be clinically established.257,258
C. Bioreactors
For functional BTE, the use of bioreactor cultivating systems for three-dimensional cell-scaffold constructs has been proposed to achieve homogeneous bone tissue development at clinically relevant sizes (i.e., millimeter to centimeter sizes and beyond).259 The fundamental mechanisms by which this occurs involves (1) improved cell seeding efficiency, (2) increased mechanical stimulation of osteogenic cells, and (3) overcoming the limited diffusional exchange of nutrients and oxygen that is typically observed in static culture. By enabling reproducible and controlled changes of specific environmental factors (e.g., pH, temperature, pressure, nutrient supply and waste removal), bioreactors allow for automated and standardized tissue manufacturing with reduced production costs, and thus, they facilitate large-scale use of BTE strategies.
Several different types of bioreactors with unique flow patterns have been investigated for BTE purposes, including stirred flasks, rotating bioreactors, and perfusion bioreactors (Fig. 11). Stirred (i.e., spinner) flask bioreactors are the most simple and inexpensive systems. In this system, convective forces are generated by a stir-rer, which allows for medium flow around the cell-seeded constructs that are positioned in the center of the flask. In comparison to static controls, there are increased levels of osteogenic cell proliferation, expression of osteogenic marker genes, and mineralization in stirred-flask bio-reactors.260 However, the use of a spinner flask system often results in the formation of a dense superficial cell layer, which may hamper oxygen and nutrient supply to the cells residing in the scaffold interior.261 In contrast, rotating bioreactor systems produce a laminar flow as its concentric cylinders rotate horizontally. In particular, studies using a NASA-designed rotating wall bioreactor showed increased osteo-blast performance and mineralization.262,263 The limitations are that the scaffolds have to have water equivalent density and certain dimensions to be able to continuously rotate without falling to the bioreactor center.264 In addition, rotating bioreactors have been negatively associated with the possible collision of scaffolds with the bio-reactor wall, which may damage scaffolds and disrupt attached cells.265 Unlike the previously described bioreactor systems, perfusion-based bioreactors enable the mass transport of nutrients and oxygen throughout the entire scaffold. Perfusion systems generally consist of a chamber that houses the cell-seeded constructs and the peristaltic roller pump that delivers the culture medium. The mode of the fluid flow (i.e., steady, oscillating, pulsed) can significantly influence the stimulation of the osteogenic cells. Perfusion-stimulated constructs not only show enhanced osteogenic cell distribution, density, proliferation, differentiation, and deposition of mineralized extracellular matrix throughout the entire scaffold in vitro,266 but also have shown enhanced bone in vivo compared to statically cultivated controls.267 Finally, because bone is a mechanically sensitive and active tissue, some bioreactors have been designed to generate direct mechanical stimulation on the culture constructs. The cultivation of constructs in a bioreactor capable of exerting direct mechanical strain has been associated with increased levels of ALP activity, mineralized matrix production, and osteogenic gene expressions.268
FIGURE 11.
Schematic illustration of bioreactors utilized in BTE. Specifically, (A) spinner (red arrows show movement of the stir bar), (B) rotating wall (red arrows show movement of the vessel), and (C) perfusion bioreactors (red arrows show movement of the medium) are the most commonly used. (D) Comparison of bioreactor culture of bone constructs (right) versus static culture (middle). Bioreactors allow for increased nutrient perfusion throughout construct, and enhanced bone formation in vitro. Adapted from Martin et al. and Olivier et al.321,322
Highly efficient and uniform cell seeding is critical for the success of engineered bone grafts in clinical application. First, efficient cell seeding not only aids in limiting the biopsy size but also the extent of cell expansions. Second, the rate of bone mineralization and formation is directly associated with the density of the seeded cells. Specifically, constructs with increased osteo-genic cell density are associated with enhanced osteogenic gene expression, differentiation, and bone mineralization.269 Manual, static loading of cells onto a scaffold, although most commonly used in BTE studies, has low seeding efficiencies and non-uniform cell distributions within scaffolds. Significantly higher efficiencies and uniformities may be obtained using biore-actors for dynamic seeding. Perfusion bioreac-tors provide the highest cell seeding efficiency and uniformity; dilute cell suspension is allowed to flow directly through the pores of the scaffold, allowing for cell deposition throughout the entire construct.270,271
Although bioreactors have proven to be an effective tool for improved bone formation results in BTE, compliance with “Good Manufacturing Practice’’ (GMP, national quality assurance guidelines) is necessary before it is considered to be clinically applicable. Additionally, bioreactor designs that accommodate cell seeding, in vitro expansion, and perfusion culture have been proposed for even more efficiency. These designs also aim to reduce the safety risks associated with the handling and transferring of constructs between separate bioreactors for the generation of clinically relevant BTE constructs.
D. Vascularization Techniques
The importance of vascularization to the development and repair of bone tissue has been extensively documented in BTE investigations.272 The greatest amount of new bone formation occurs in the most vascularized areas, whereas inadequate vascularization at bone defect sites is associated with decreased bone tissue repair and regeneration, and has been identified as the major pitfall to successful BTE. Specifically, until the timely onset of construct vasculariza-tion, which is typically on the order of hours to days (i.e., less than 1 mm/day), seeded cells in an implanted BTE construct rely on diffusion for the uptake of nutrients (i.e., oxygen, glucose, etc.) and the clearing of metabolic byproducts (i.e., carbon dioxde, lactic acid, etc.), a transport mechanism that is only efficient over short distances (i.e., less than 200 µm).273 These diffusional constraints result in viable cells located only superficially (i.e., at the periphery of the constructs) and thus limit the success of the engineering bone tissue throughout the entire thickness of the defect. Although in vitro delivery of nutrients to engineered constructs may be alleviated via bioreactor systems, this only delays the diffusional constraint problem when it is implanted in the host defect site. There is a critical obstacle in maintaining the survival of large masses of cells upon transfer from the in vitro culture conditions into the host defect site in vivo.274 To remedy this obstacle, scientists have proposed several methods to accelerate the onset of neo-vascualization for survival and integration of BTE grafts with host tissue including (1) scaffold design, (2) inclusion of angiogenic growth factors, (3) in vitro pre-vascularization (i.e., co-culture of endothelial and osteogen-ic cells), and (4) in vivo pre-vascularization. Although it is still unclear which method is the best for successful in vivo application, a combination of these methods may prove to be most effective. The following is a brief review of each method and its challenges.
1. Scaffold Design
Scaffold design has a profound effect on the rate of vascularization after implantation. Specifically, mean pore size of the scaffold is a critical determinant of blood vessel ingrowth. BTE studies that suggest pore sizes greater than 300 µm are required for vascular ingrowth.27 Interconnectivity of pores is also critical, as it significantly affects cell migration, and in turn, vascularization. Scaffold fabrication techniques including gas foaming, phase separation, and freeze drying are employed in association with porogen leaching for the generation of increasingly porous scaffolds. Recently, the authors developed thermal sintering and porogen leaching method and fabricated scaffolds with the desired pore size and volume. These scaffolds have proven to be superior because they not only support vascular in growth but also meet the mechanical requirements for bone regeneration.122,275 On the other hand, methods such as layer-by-layer deposition (i.e., solid free-form fabrication) are now commonly used to actively design scaffold porosity and interconnectivity. With these fabrication systems, production of complex scaffolds with well-defined architecture and optimized pore in-terconnectivity is possible.276
2. Inclusion of Angiogenic Growth Factors
The local delivery of angiogenic growth factors certainly accelerates vascularization of an implanted graft. Angiogenic growth factors may be incorporated into the BTE construct design either by way of the scaffold or seeded cells. In the first scenario, the growth factor may be (1) incorporated onto the scaffold by simple soaking for resultant fast release, (2) encapsulated in scaffolds, or (3) covalently immobilized for controlled and extended release. Otherwise, growth factors may be incorporated into the seeded cells via genetic modification.
Several critical considerations determine the success of this method. First, the choice of growth factors is crucial. Several commonly studied angiogenic growth factors in BTE include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF). Secondly, the proper dosage of the growth factor has been shown to affect the quality of the neo-vascu-lature. For instance, excess amounts of VEGF have been shown to cause severe vascular leakage and hypotension.277 Finally, multiple growth factors should be considered along spatial and temporal gradients may allow for enhanced results, as bone tissue development is controlled by the interaction of multiple growth factors. Studies have shown that the incorporation of VEGF and bFGF results in accelerated vascu-larization of engineered tissues via the mobilization and recruitment of endothelial progenitor cells (EPCs), though the resulting vessels are often disorganized, leaky and hemorrhagic.278 For this reason, the addition of growth factors that stimulate the recruitment of smooth muscle cells or pericytes for the stabilization and maturation of the vessels may be considered, including PDGF, transforming growth factor-β (TGF-β) and angiopoietin 1 (Ang1).279,280
The addition of growth factors to scaffolds is a relatively easy and widely studied approach. Because this type of growth factor delivery is either driven by passive diffusion or coupled to the rate of biomaterial degradation, growth factor release may be altered only to some extent by the amount of growth factor added or by varying the degradation rate of the material. The release profile for this method is, therefore, often not in tune with the actual healing process and cellular demands.281 On the other hand, growth factors covalently linked to the scaffolds may be released according to the cellular demands. It has been demonstrated that the vasculature formed in this manner via controlled release of VEGF formed organized vasculature, in comparison to the vasculature that arose from an uncontrolled VEGF release.282
The incorporation of growth factors into the scaffolds is not an efficient process. Adding the high price associated with the human recombi-nant growth factors makes the growth-factor-loaded scaffold approach unattractive. On the other hand, the incorporation of genetically modified cells, such as VEGF releasing ADSCs, has demonstrated enhanced vascular forma-tion.240 In addition, cells releasing the combination of osteogenic and angiogenic factors (i.e., BMP-4 and VEGF, respectively) together have been shown to increase not only vascular formation but also the quantity of regenerated bone, compared to the each factor alternatively delivered alone.283 However, gene therapy in general has safety concerns, and it is not yet approved for clinical use.
3. In Vitro Pre-Vascularization
Current in vitro pre-vascularization strategies of BTE involve the prior seeding and co-culture of endothelial cells and osteogenic cells in BTE constructs in vitro.284 The mechanism underlying this strategy depends on the direct and indirect communication of these two cell types, and the formation of premature vessels by the endothe-lial cells in vitro, which may later mature, and anastomose with the host vasculature upon implantation. This approach has not only demonstrated accelerated vascularization in vivo but also has enhanced osteogenic differentiation in vitro and bone formation in vivo.222,223 With this method, anastomoses occurs more quickly in comparison to non-prevascularized constructs, as host vessels only need to grow into the outer region of the constructs to meet the pre-vascular structures. This method may decrease the time needed for vascularization from weeks to days.
An important consideration for in vitro pre-vascularization is the type of utilized cells, and identifying an abundant source of effective au-tologous endothelial cells. Although mature en-dothelial cells, isolated from biopsies of skin or saphenous veins, may be used, they present major drawbacks, including insufficient numbers with limited proliferative abilities (i.e., limited in vitro expansion).284 In contrast, various stem cells have recently been the topic of discussion. Endothelial progenitor cells (EPCs) have been recognized as an attractive autologous endo-thelial cell population that may be easily isolated from peripheral blood or bone marrow in clinical practice. EPCs have very high and long-term proliferative potential (more than 1,000 population doublings), and may be quickly expanded for clinical use. EPCs display the typical cobblestone morphology of endothelial cells when culture in vitro, and they have good angio-genic ability, as demonstrated by complex and intricate network when cultured on and within Matrigel (i.e., angiogenic assay) (Fig. 12).275 Our recent work established peripheral blood derived EPCs as a more potent cell population than the bone marrow-derived EPCs. We co-cultured peripheral blood–derived EPCs with bone marrow–derived MSCs to demonstrate their synergy (i.e., increased expression of vasculogenic and osteogenic factor expression) in vitro, and other groups implanted at defect sites. Our results showed enhanced levels of vascularization and bone formation.285 Yu et al. also noted that central necrosis is avoided when scaffolds are seeded with EPCs and MSC-derived osteoblasts, which is not the case when only osteoblasts are seeded alone and implanted.286
FIGURE 12.
(A) Isolation of rabbit peripheral blood-derived endothelial progenitor cells (EPCs) via terminal exsan-guination. (B) Phase contrast image of PB-EPCs cultured in endothelial growth media. (C) Two-dimensional angio-genesis assay showing network formation by PB-EPCs (cells cultured on Matrigel for 8 hours in vitro). Scale bar = 500 mm. (B) Hematoxylin/eosin staining demonstrating capillary network and branch point formation of PB-EPCs cultured in Matrigel after 7 days in vitro. Scale bar = 250 mm. Adapted and modified from Amini et al.222
Perhaps the most desirable cell source is one that contains both osteogenic and vasculogenic progenitor cells. For instance, MSCs, which may be isolated from bone marrow for osteoprogenitor cells, also have been shown to have the potential to differentiate toward an endothelial lineage.288 Another attractive autologous source that may be used to isolate both osteo- and endothelial- progenitors is the stromal vascular fraction (SVF) of adipose tissue, which is abundantly available, easy to harvest, and associated with minimal donor site morbidity. In addition, in comparison to bone marrow, it has a much higher frequency of clono-genic mesenchymal progenitors compared.289
Several issues regarding in vitro pre-vascu-larization remain uncertain. For instance, it is unclear whether it is better to maintain the pre-vascularization in vitro long term to establish a premature vascular network formation, or to implant the construct shortly after seeding the cells to allow the in vivo environment help establish a functional vasculature. Also, even though endothelial cells have the potential to form new vessels within the scaffolds that may anastomose with host vasculature when implanted in vivo, it is important to consider the presence of other cell types (i.e., smooth muscle cells, pericytes) to ensure the formation of functional vasculature, so a tri-culture approach should be further in-vestigated.290 Finally, the potential benefits of this approach have been treated with sskepticism because it involves cell-containing constructs, which, like other approaches, requires immediate supply with nutrients and oxygen after implantation. One approach to solve this problem may involve the engineering of a vascular axis within the in vitro construct, which can be surgically connected to the host vasculature as it is when vessels are surgically implanted.
4. In Vivo (Surgically-Induced) Pre-Vascularization
In vivo pre-vascularization may be performed to allow for vascularization of bone constructs. The “flap pre-fabrication” approach utilizes an “extrinsic” mode of vascularization and involves two main stages. First, the BTE construct is implanted in axially vascularized tissue (i.e., in subcutaneous, intramuscular, or intraperitoneal sites), where microvascular network formation within the constructs occurs within several weeks. The construct is then harvested and transferred as free bone flap to the bone defect site, where the vascular axis is connected via microsurgical vascular anastomosis techniques, resulting in instantaneous perfusion of the entire construct. Several drawbacks of this technique include the obvious requirement of two required surgeries, cost, the formation of a random vascularization pattern, degree of vascularization based on host tissue vascularity, as well as donor site morbidity.291 In another method, an “intrinsic” mode of vascularization is used where vessels that are suitable for microsurgical transfer (i.e., carotid artery, jugular vein saphenous bundle, or arteriovenous (AV) loop) are incorporated into the construct.292 Though this procedure has clear advantages over the “flap pre-fabrication” approach, including that it does not require two separate operations like the “flap pre-fabrication” approach, is not dependent upon local vascular conditions and the included vasculature is not randomly oriented, this method is still very challenging because most load-bearing osteogenic constructs are not able to be molded or shaped around the AV loops.
E. Functional Bone Tissue Engineering
The term functional bone tissue engineering was coined more than a decade ago by Dr. Pioletti at an American Society of Biomechanics meeting. It was defined as an approach that allows for full functional ability of the graft immediately following surgical implantation. In this approach, the bone graft to be implanted is required to have carefully defined biomechani-cal properties to allow its immediate use upon completion surgery. However, functional BTE entails more than just the ability for immediate mechanical usage. Instead, functional BTE approaches allow for the newly restored bone to be fully integrated with the neighboring host bone, and importantly, possess the ability to perform all the functions of native bone. The quality of the newly regenerated tissue should seamlessly match that of the host bone. In the future, effective quality assessment tests should be developed to ensure truly functional engineered bone for patients.
IV. REMAINING LIMITATIONS & CHALLENGES FACING BTE
The field of tissue engineering and in particular, BTE, is rapidly growing. BTE-based products are beginning to be used in clinical practice. Based on the current success, even more BTE technologies are expected to become available to patients in the next few years. Current efforts are focused on developing effective strategies for BTE, but we predict that the future discussion will turn toward the identification of the most cost-effective BTE strategies. Although the race to make BTE a clinical reality is well warranted, significant challenges and limitations in this field still exist (Table 3).
TABLE 3.
Current limitations and challenges facing the field of bone tissue engineering.
| Fundamental Challenges | Selecting most effective:
|
| Achieving proper vascularization | |
| Achieving seamless host integration | |
| Knowledge Limitation | Donor versus host cell contribution |
| Appropriate immunomodulatory biomaterials/agents | |
| Possible side effects/ complications of donor cells/growth factors | |
| Most appropriate animal models | |
| Evaluation Challenges | Regenerated Bone Quality |
| Regenerated Bone Functionality | |
| Long –term tracking of the regenerated bone | |
| Clinical Challenges | FDA approval |
| Multi- vs. single- component | |
| Expensive | |
| Patient specific |
Generally, the field of tissue engineering has undergone tremdous advances in the last several decades, especially with simple tissues (i.e., skin). Engineering bone tissue, however, is not only based on principles of cellular and molecular developmental biology and morphogenesis, it is very much guided by bioengineering and biome-chanics. Bone tissue structure and mechanical strength varies by distinct and dynamic loading conditions, as well as location in the body. Perhaps one of the largest challenges facing bone tissue engineering is developing mechanically strong porous scaffolds that retain proper vascularization and host integration properties. Currently, the vast majority of reported mechanically strong BTE scaffolds experience bone tissue regeneration that is limited to the periphery of the scaffold upon implantation, due to lack of sufficient and timely vascularization of the construct. In addition, the incorporation of immunomodulatory strategies is becoming increasingly popular for modulating the host’s foreign-body response (i.e., fibrous tissue encapsulation), an event that is often observed to be an inhibitory factor for optimal tissue regeneration and integration. Scientists are attempting to tackle both enhanced vascularization and inhibition of fibrous tissue formation by incorporating growth factors via the scaffold or genetically modified cells that release increased levels of angiogenic VEGF, or even by coating the scaffold with anti-inflammatory molecules, such as dexamethasone. Animal models pose another critical challenge to testing various BTE approaches pre-clinically. In pre-clinical studies, load-bearing large animal models should generally be used to assess graft functionality because research on small animals (i.e., mice) does not yield relevant results due to major differences in graft size and healing properties.
Although many BTE strategies have been investigated, so far only a few have been approved for clinical use.293 These are mostly single-component strategies involving cells, factors, or defect-filling materials. For BTE to become a widespread clinical reality, it must incorporate the recent technologies that utilize all the necessary components (i.e., scaffolds, cells, and growth factors) for successful bone repair and regeneration. One concern is that the technologies that include more components may have difficulty obtaining regulatory approval. Furthermore, BTE may even pose as a health care burden in its current form, as it comes with high manufacturing costs and is patient specific. To increase efficiency, patient-independent methods need to be considered. In addition, more effective cell isolation, seeding, and cul-turing methods need to be developed to streamline the engineering process and to decrease the safety risks associated with the handling the constructs during the pre-implantation period. Bioreactors that can combine all three steps have been proposed for this purpose and may drive the way for safer and more effective bone tissue engineering. Ultimately, however, the best bioreactor for BTE scaffolds is bone itself with the idea that a scaffold could indeed mature into a normal bone tissue if an adequate environment is provided in vivo. Perhaps the quickest route to clinical success will avoid utilizing the in vitro bioreactor approach.294 Therefore, further efforts must be made to establish efficient intraoperative cell seeding methods to minimize in vitro culture of the BTE constructs, and allow for maximized bone tissue regeneration in vivo.
ACKNOWLEDGEMENTS
Drs. Nukavarapu and Laurencin acknowledge funding from the Department of Defence under Grant W81XWH-11-1-0262. Dr. Amini would like to thank support from NIH through an F-30 Fellowship (NIH-1F30-DE22477010). The authors also acknowledge support from the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences Center, and Institute for Regenerative Engineering, the University of Connecticut.
REFERENCES
- 1.Laurencin CT, Ambrosio AM, Borden MD, Cooper JA. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Laurencin CT, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3(1):49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Nukavarapu SP, Wallace J, Elgendy H, Lieberman J, Laurencin CT. Bone and biomaterials. In: group TF, editor. An introduction to biomaterials and their applications. 2 ed. CRC Press; 2011. pp. 571–593. [Google Scholar]
- 4.Baroli B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci. 2009;98(4):1317–1375. doi: 10.1002/jps.21528. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17(2):175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 7.Soucacos PN, Johnson EO, Babis G. An update on recent advances in bone regeneration. Injury. 2008;39:S1–S4. doi: 10.1016/S0020-1383(08)70009-3. [DOI] [PubMed] [Google Scholar]
- 8.Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3(1):1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Arrington ED, Smith WJ, Chambers HG, Buck-nell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;(329):300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 10.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 1995;20(9):1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg. 2001;9(3):210–218. doi: 10.5435/00124635-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 14.St John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, Albert T, et al. Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop (Belle Mead NJ) 2003;32(1):18–23. [PubMed] [Google Scholar]
- 15.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: What they can offer and what they cannot. J Bone Joint Surg Br. 2007;89(5):574–579. doi: 10.1302/0301-620X.89B5.19039. [DOI] [PubMed] [Google Scholar]
- 16.Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70(3):369–376. [PubMed] [Google Scholar]
- 17.Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63(2):244–248. [PubMed] [Google Scholar]
- 18.Greenwald ASB, Scott D, Goldberg VM, Yaszemski M, Hem CS. Bone-graft substitutes: facts, fictions, & applications. American Academy of Orthopaedic Surgeons: 75th Annual Meeting. J Bone Joint Surg. 2001:98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 19.O’Keefe RJ, Mao J. Bone tissue engineering and regeneration: from discovery to the clinic--an overview. Tissue Eng Part B Rev. 2011;17(6):389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y. Skeletal morphogenesis during embryonic development. Crit Rev Eukaryot Gene Expr. 2009;19(3):197–218. doi: 10.1615/critreveukargeneexpr.v19.i3.30. [DOI] [PubMed] [Google Scholar]
- 21.Fröhlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther. 2008;3(4):254–264. doi: 10.2174/157488808786733962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 23.Zuo C, Huang Y, Bajis R, Sahih M, Li YP, Dai K, et al. Osteoblastogenesis regulation signals in bone remodeling. Osteoporos Int. 2012 doi: 10.1007/s00198-012-1909-x. [DOI] [PubMed] [Google Scholar]
- 24.Fazzalari NL. Bone fracture and bone fracture repair. Osteoporos Int. 2011;22(6):2003–2006. doi: 10.1007/s00198-011-1611-4. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53–76. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- 26.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 27.Karageorgiou V, Kaplan D. Porosity of 3D bio-material scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Blokhuis TJ, Arts JJ. Bioactive and osteoinductive bone graft substitutes: definitions, facts and myths. Injury. 2011;42(Suppl 2):S26–S29. doi: 10.1016/j.injury.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Barradas AM, Yuan H, van Blitterswijk CA, Habibo-vic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 2011;21:407–429. doi: 10.22203/ecm.v021a31. discussion 29. [DOI] [PubMed] [Google Scholar]
- 30.Habibovic P, de Groot K. Osteoinductive biomaterials—properties and relevance in bone repair. J Tissue Eng Regen Med. 2007;1(1):25–32. doi: 10.1002/term.5. [DOI] [PubMed] [Google Scholar]
- 31.LeGeros RZ. Properties of osteoconductive bioma-terials: calcium phosphates. Clin Orthop Relat Res. 2002;(395):81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Ripamonti U. The induction of bone in osteogenic composites of bone matrix and porous hydroxyapatite replicas: an experimental study on the baboon (Papio ursinus) J Oral Maxillofac Surg. 1991;49(8):817–830. doi: 10.1016/0278-2391(91)90010-j. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki H, Sakai H. Osteogenic response to porous hydroxyapatite ceramics under the skin of dogs. Bio-materials. 1992;13(5):308–312. doi: 10.1016/0142-9612(92)90054-r. [DOI] [PubMed] [Google Scholar]
- 34.Klein C, de Groot K, Chen W, Li Y, Zhang X. Osseous substance formation induced in porous calcium phosphate ceramics in soft tissues. Biomaterials. 1994;15(1):31–34. doi: 10.1016/0142-9612(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 35.Pollick S, Shors EC, Holmes RE, Kraut RA. Bone formation and implant degradation of coralline porous ceramics placed in bone and ectopic sites. J Oral Maxillofac Surg. 1995;53(8):915–922. doi: 10.1016/0278-2391(95)90281-3. discussion 22-3. [DOI] [PubMed] [Google Scholar]
- 36.Ripamonti U. Osteoinduction in porous hydroxyapa-tite implanted in heterotopic sites of different animal models. Biomaterials. 1996;17(1):31–35. doi: 10.1016/0142-9612(96)80752-6. [DOI] [PubMed] [Google Scholar]
- 37.Gosain AK, Song L, Riordan P, Amarante MT, Nagy PG, Wilson CR, et al. A 1-year study of os-teoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: part I. Plast Reconstr Surg. 2002;109(2):619–630. doi: 10.1097/00006534-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 38.Habibovic P, Gbureck U, Doillon CJ, Bassett DC, van Blitterswijk CA, Barralet JE. Osteoconduction and osteoinduction of low-temperature 3D printed bioc-eramic implants. Biomaterials. 2008;29(7):944–953. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Barrère F, van der Valk CM, Dalmeijer RA, Meijer G, van Blitterswijk CA, de Groot K, et al. Osteo-genecity of octacalcium phosphate coatings applied on porous metal implants. J Biomed Mater Res A. 2003;66(4):779–788. doi: 10.1002/jbm.a.10454. [DOI] [PubMed] [Google Scholar]
- 40.Habibovic P, van der Valk CM, van Blitterswijk CA, De Groot K, Meijer G. Influence of octacalcium phosphate coating on osteoinductive properties of bioma-terials. J Mater Sci Mater Med. 2004;15(4):373–380. doi: 10.1023/b:jmsm.0000021104.42685.9f. [DOI] [PubMed] [Google Scholar]
- 41.Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am. 1991;73(5):692–703. [PubMed] [Google Scholar]
- 42.Ripamonti U, Crooks J, Khoali L, Roden L. The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials. 2009;30(7):1428–1439. doi: 10.1016/j.biomaterials.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 43.Ripamonti U, Klar RM, Renton LF, Ferretti C. Synergistic induction of bone formation by hOP-1, hTGF-beta3 and inhibition by zoledronate in macro-porous coral-derived hydroxyapatites. Biomaterials. 2010;31(25):6400–6410. doi: 10.1016/j.biomaterials.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 44.Yuan H, de Bruijn JD, Zhang X, van Blitterswijk CA, de Groot K. Bone induction by porous glass ceramic made from Bioglass (45S5) J Biomed Mater Res. 2001;58(3):270–276. doi: 10.1002/1097-4636(2001)58:3<270::aid-jbm1016>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Laurencin CT, Devin J, Attawia M. Polymeric-hydroxyapatite bone composite. United States; 1998. inventors; Massachusetts Institute of Technology, assignee. [Google Scholar]
- 46.Devin JE, Attawia MA, Laurencin CT. Three-dimensional degradable porous polymer-ceramic matrices for use in bone repair. J Biomater Sci Polym Ed. 1996;7(8):661–669. doi: 10.1163/156856296x00435. [DOI] [PubMed] [Google Scholar]
- 47.Khan YM, Katti DS, Laurencin CT. Novel polymer-synthesized ceramic composite-based system for bone repair: an in vitro evaluation. J Biomed Mater Res A. 2004;69(4):728–737. doi: 10.1002/jbm.a.30051. [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa S, Neo M, Tamura J, Fujibayashi S, Take-moto M, Shikinami Y, et al. In vivo evaluation of a porous hydroxyapatite/poly-DL-lactide composite for bone tissue engineering. J Biomed Mater Res A. 2007;81(4):930–938. doi: 10.1002/jbm.a.31109. [DOI] [PubMed] [Google Scholar]
- 49.Barbieri D, Renard AJ, de Bruijn JD, Yuan H. Het-erotopic bone formation by nano-apatite containing poly(D,L-lactide) composites. Eur Cell Mater. 2010;19:252–261. doi: 10.22203/ecm.v019a24. [DOI] [PubMed] [Google Scholar]
- 50.Khan Y, El-Amin SF, Laurencin CT. In vitro and in vivo evaluation of a novel polymer-ceramic composite scaffold for bone tissue engineering. Conf Proc IEEE Eng Med Biol Soc. 2006;1:529–530. doi: 10.1109/IEMBS.2006.259656. [DOI] [PubMed] [Google Scholar]
- 51.El-Ghannam A. Bone reconstruction: from bioceram-ics to tissue engineering. Expert Rev Med Devices. 2005;2(1):87–101. doi: 10.1586/17434440.2.1.87. [DOI] [PubMed] [Google Scholar]
- 52.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Yuan H, Yang Z, Li Y, Zhang X, De Bruijn JD, De Groot K. Osteoinduction by calcium phosphate bio-materials. J Mater Sci Mater Med. 1998;9(12):723–726. doi: 10.1023/a:1008950902047. [DOI] [PubMed] [Google Scholar]
- 54.Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys. 2011;49(12):832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saad B, Kuboki Y, Welti M, Uhlschmid GK, Neuen-schwander P, Suter UW. DegraPol-foam: a degradable and highly porous polyesterurethane foam as a new substrate for bone formation. Artif Organs. 2000 Dec;24(12):939–945. doi: 10.1046/j.1525-1594.2000.06664.x. PubMed PMID: 11121973. eng. [DOI] [PubMed] [Google Scholar]
- 56.Nukavarapu SP, Kumbar S, Laurencin C. Biodegradable polyphosphazene scaffolds for tissue engineering. Polyphosphazenes for Biomedical Applications Taylor & Francis group. 2009:117–138. [Google Scholar]
- 57.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Krogman NR, et al. Miscibility and in vitro osteocompatibility of biodegradable blends of poly[(ethyl alanato) (p-phenyl phenoxy) phospha-zene] and poly(lactic acid-glycolic acid) Biomaterials. 2008;29(3):337–349. doi: 10.1016/j.biomaterials.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ibim SE, Ambrosio AM, Kwon MS, El-Amin SF, Allcock HR, Laurencin CT. Novel polyphosphazene/ poly(lactide-co-glycolide) blends: miscibility and degradation studies. Biomaterials. 1997;18(23):1565–1569. doi: 10.1016/s0142-9612(97)80009-9. [DOI] [PubMed] [Google Scholar]
- 59.Ambrosio AM, Allcock HR, Katti DS, Laurencin CT. Degradable polyphosphazene/poly(alpha-hy-droxyester) blends: degradation studies. Biomaterials. 2002;23(7):1667–1672. doi: 10.1016/s0142-9612(01)00293-9. [DOI] [PubMed] [Google Scholar]
- 60.Lakshmi S, Katti DS, Laurencin CT. Biodegradable polyphosphazenes for drug delivery applications. Adv Drug Deliv Rev. 2003;55(4):467–482. doi: 10.1016/s0169-409x(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 61.Krogman NR, Singh A, Nair LS, Laurencin CT, All-cock HR. Miscibility of bioerodible polyphospha-zene/poly(lactide-co-glycolide) blends. Biomacromolecules. 2007;8(4):1306–1312. doi: 10.1021/bm061064q. [DOI] [PubMed] [Google Scholar]
- 62.Krogman NR, Weikel AL, Kristhart KA, Nukavar-apu SP, Deng M, Nair LS, et al. The influence of side group modification in polyphosphazenes on hydrolysis and cell adhesion of blends with PLGA. Biomaterials. 2009;30(17):3035–3041. doi: 10.1016/j.biomaterials.2009.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Athanasiou KA, Zhu C, Lanctot DR, Agrawal CM, Wang X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000;6(4):361–381. doi: 10.1089/107632700418083. [DOI] [PubMed] [Google Scholar]
- 64.Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43–56. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 65.Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Kim SS, Park MS, Gwak SJ, Choi CY, Kim BS. Accelerated bonelike apatite growth on porous polymer/ ceramic composite scaffolds in vitro. Tissue Eng. 2006;12(10):2997–3006. doi: 10.1089/ten.2006.12.2997. [DOI] [PubMed] [Google Scholar]
- 67.Kim HW, Kim HE, Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Bio-materials. 2005;26(25):5221–5230. doi: 10.1016/j.biomaterials.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Ni M, Zhang M, Ratner B. Calcium phos-phate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng. 2003;9(2):337–345. doi: 10.1089/107632703764664800. [DOI] [PubMed] [Google Scholar]
- 69.Chesnutt BM, Yuan Y, Buddington K, Haggard WO, Bumgardner JD. Composite chitosan/nano-hydroxy-apatite scaffolds induce osteocalcin production by os-teoblasts in vitro and support bone formation in vivo. Tissue Eng Part A. 2009;15(9):2571–2579. doi: 10.1089/ten.tea.2008.0054. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues CV, Serricella P, Linhares AB, Guerdes RM, Borojevic R, Rossi MA, et al. Characterization of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials. 2003;24(27):4987–4997. doi: 10.1016/s0142-9612(03)00410-1. [DOI] [PubMed] [Google Scholar]
- 71.Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54(2):284–293. doi: 10.1002/1097-4636(200102)54:2<284::aid-jbm16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 72.Ho E, Lowman A, Marcolongo M. Synthesis and characterization of an injectable hydrogel with tunable mechanical properties for soft tissue repair. Biomacromolecules. 2006;7(11):3223–3228. doi: 10.1021/bm0602536. [DOI] [PubMed] [Google Scholar]
- 73.Santin M, Motta A, Borzachiello A, Nicolais L, Am-brosio L. Effect of PMMA cement radical polymerisation on the inflammatory response. J Mater Sci Mater Med. 2004;15(11):1175–1180. doi: 10.1007/s10856-004-5668-x. [DOI] [PubMed] [Google Scholar]
- 74.Higashi S, Yamamuro T, Nakamura T, Ikada Y, Hyon SH, Jamshidi K. Polymer-hydroxyapatite composites for biodegradable bone fillers. Biomaterials. 1986;7(3):183–187. doi: 10.1016/0142-9612(86)90099-2. [DOI] [PubMed] [Google Scholar]
- 75.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 76.Slaughter BV, Khurshid SS, Fisher OZ, Khadem-hosseini A, Peppas NA. Hydrogels in regenerative medicine. Advanced materials (Deerfield Beach, Fla) 2009;21(32–33):3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park JB. The use of hydrogels in bone-tissue engineering. Med Oral Patol Oral Cir Bucal. 2011;16(1):e115–e118. doi: 10.4317/medoral.16.e115. [DOI] [PubMed] [Google Scholar]
- 78.Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25(7):803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 79.Semino CE. Self-assembling peptides: from bio-inspired materials to bone regeneration. J Dent Res. 2008;87(7):606–616. doi: 10.1177/154405910808700710. [DOI] [PubMed] [Google Scholar]
- 80.Bokhari MA, Akay G, Zhang S, Birch MA. The enhancement of osteoblast growth and differentiation in vitro on a peptide hydrogel-polyHIPE polymer hybrid material. Biomaterials. 2005;26(25):5198–5208. doi: 10.1016/j.biomaterials.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 81.Misawa H, Kobayashi N, Soto-Gutierrez A, Chen Y, Yoshida A, Rivas-Carrillo JD, et al. PuraMatrix facilitates bone regeneration in bone defects of calvaria in mice. Cell Transplant. 2006;15(10):903–910. doi: 10.3727/000000006783981369. [DOI] [PubMed] [Google Scholar]
- 82.Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, et al. Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res. 2007;86(5):426–430. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- 83.Kyle S, Aggeli A, Ingham E, McPherson MJ. Recom-binant self-assembling peptides as biomaterials for tissue engineering. Biomaterials. 2010;31(36):9395–9405. doi: 10.1016/j.biomaterials.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17(6):393–402. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith MJ, Smith DC, Bowlin GL, White KL. Modulation of murine innate and acquired immune responses following in vitro exposure to electrospun blends of collagen and polydioxanone. J Biomed Mater Res A. 2010;93(2):793–806. doi: 10.1002/jbm.a.32579. [DOI] [PubMed] [Google Scholar]
- 86.Kou PM, Babensee JE. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater Res A. 2011;96(1):239–260. doi: 10.1002/jbm.a.32971. [DOI] [PubMed] [Google Scholar]
- 87.Anderson J, Rodriguez A, Chang D. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93(3):1151–1159. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amini AR, Wallace JS, Nukavarapu SP. Short-term and long-term effects of orthopedic biodegradable implants. J Long Term Eff Med Implants. 2011;21(2):93–122. doi: 10.1615/jlongtermeffmedimplants.v21.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X, Wang H, Touma E, Qi Y, Rousseau E, Quigg RJ, et al. TP508 accelerates fracture repair by promoting cell growth over cell death. Biochem Biophys Res Commun. 2007;364(1):187–193. doi: 10.1016/j.bbrc.2007.07.202. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Li X, Tomin E, Doty SB, Lane JM, Carney DH, et al. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J Orthop Res. 2005;23(3):671–679. doi: 10.1016/j.orthres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Sheller MR, Crowther RS, Kinney JH, Yang J, Di Jorio S, Breunig T, et al. Repair of rabbit segmental defects with the thrombin peptide, TP508. J Orthop Res. 2004;22(5):1094–1099. doi: 10.1016/j.orthres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 93.Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, et al. Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005;72(4):343–353. doi: 10.1002/jbm.a.30265. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka M, Sakai A, Uchida S, Tanaka S, Nagashi-ma M, Katayama T, et al. Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819.CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone. 2004;34(6):940–948. doi: 10.1016/j.bone.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A. 2003;100(11):6736–6740. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122(6):570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 97.Pioletti DP. Biomechanics in bone tissue engineering. Comput Methods Biomech Biomed Engin. 2010;13(6):837–846. doi: 10.1080/10255841003630660. [DOI] [PubMed] [Google Scholar]
- 98.Jones AC, Arns CH, Hutmacher DW, Milthorpe BK, Sheppard AP, Knackstedt MA. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials. 2009;30(7):1440–1451. doi: 10.1016/j.biomaterials.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 99.Sikavitsas VI, Temenoff JS, Mikos AG. Biomateri-als and bone mechanotransduction. Biomaterials. 2001;22(19):2581–2593. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 100.Blecha LD, Zambelli PY, Ramaniraka NA, Bourban PE, Månson JA, Pioletti DP. How plate positioning impacts the biomechanics of the open wedge tibial osteotomy; a finite element analysis. Comput Methods Biomech Biomed Engin. 2005;8(5):307–313. doi: 10.1080/10255840500322433. [DOI] [PubMed] [Google Scholar]
- 101.Martin I, Smith T, Wendt D. Bioreactor-based roadmap for the translation of tissue engineering strategies into clinical products. Trends Biotechnol. 2009;27(9):495–502. doi: 10.1016/j.tibtech.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Byrne DP, Lacroix D, Planell JA, Kelly DJ, Pren-dergast PJ. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: application of mechano-biological models in tissue engineering. Biomaterials. 2007;28(36):5544–5554. doi: 10.1016/j.biomaterials.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 104.Mikael PE, Nukavarapu SP. Functionalized carbon nanotube composite scaffolds for bone tissue engineering: prospects and progress. J Biomater Tissue Engin. 2011;1(1):76–85. [Google Scholar]
- 105.Wallace JS, Mikael PE, Nukavarapu SP, editors. Society for Biomaterials meeting. 2010. Biodegradable polymer-magnesium composite scaffolds for bone tissue engineering. [Google Scholar]
- 106.Nukavarapu SP, Kumbar S, Brown J, Krogman N, Weikel A, Hindenlang M, et al. Polyphosphazene/ nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules. 2008;9(7):1818–1825. doi: 10.1021/bm800031t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong HM, Yeung KW, Lam KO, Tam V, Chu PK, Luk KD, et al. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials. 2010 Mar;31(8):2084–2096. doi: 10.1016/j.biomaterials.2009.11.111. PubMed PMID: 20031201. eng. [DOI] [PubMed] [Google Scholar]
- 108.Zhang E, Xu L, Yu G, Pan F, Yang K. In vivo evaluation of biodegradable magnesium alloy bone implant in the frst 6 months implantation. J Biomed Mater Res A. 2009;90(3):882–893. doi: 10.1002/jbm.a.32132. [DOI] [PubMed] [Google Scholar]
- 109.Kraus T, Fischerauer SF, Hänzi AC, Uggowitzer PJ, Löffler JF, Weinberg AM. Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. Acta Biomater. 2012;8(3):1230–1238. doi: 10.1016/j.actbio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 110.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 111.Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 112.Logeart-Avramoglou D, Anagnostou F, Bizios R, Petite H. Engineering bone: challenges and obstacles. J Cell Mol Med. 2005;9(1):72–84. doi: 10.1111/j.1582-4934.2005.tb00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeltinger J, Sherwood JK, Graham DA, Müeller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7(5):557–572. doi: 10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 114.Holtorf H, Datta N, Jansen J, Mikos A. Scaffold mesh size affects the osteoblastic differentiation of seeded marrow stromal cells cultured in a flow perfusion bio-reactor. J Biomed Mater Res A. 2005;74(2):171–180. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 115.Kühne J, Bartl R, Frisch B, Hammer C, Jansson V, Zimmer M. Bone formation in coralline hydroxyapatite. Effects of pore size studied in rabbits. Acta Or-thop Scand. 1994;65(3):246–252. doi: 10.3109/17453679408995448. [DOI] [PubMed] [Google Scholar]
- 116.Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997;121(2):317–324. doi: 10.1093/oxfordjournals.jbchem.a021589. [DOI] [PubMed] [Google Scholar]
- 117.Volkmer E, Drosse I, Otto S, Stangelmayer A, Stengele M, Kallukalam B, Mutschler W, Schieker M. Hypoxia in static and dynam- ic 3D culture systems for tissue engineering of bone. Tissue Eng Part A. 2008;14(8):1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- 118.Salerno A, Guarnieri D, Iannone M, Zeppetelli S, Netti PA. Effect of micro- and macroporosity of bone tissue three-dimensional-poly(epsilon-caprolactone) scaffold on human mesenchymal stem cells invasion, proliferation, and differentiation in vitro. Tissue Eng Part A. 2010;16(8):2661–2673. doi: 10.1089/ten.tea.2009.0494. [DOI] [PubMed] [Google Scholar]
- 119.Kim SS, Ahn KM, Park MS, Lee JH, Choi CY, Kim BS. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J Biomed Mater Res A. 2007;80(1):206–215. doi: 10.1002/jbm.a.30836. [DOI] [PubMed] [Google Scholar]
- 120.Hou Q, Grijpma DW, Feijen J. Preparation of interconnected highly porous polymeric structures by a replication and freeze-drying process. J Biomed Mater Res B Appl Biomater. 2003;67(2):732–740. doi: 10.1002/jbm.b.10066. [DOI] [PubMed] [Google Scholar]
- 121.Schugens C, Maquet V, Grandfils C, Jerome R, Te-yssie P. Polylactide macroporous biodegradable implants for cell transplantation. II. Preparation of polylactide foams by liquid–liquid phase separation. J Biomed Mater Res. 1996;30(4):449–461. doi: 10.1002/(SICI)1097-4636(199604)30:4<449::AID-JBM3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 122.Amini AR, Adams D, Laurencin CT, Nukavarapu SP. Optimally porous and biomechanically compatible scaffolds for large area bone regeneration. Tissue Eng. 2012;18:1376–1388. doi: 10.1089/ten.tea.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amini AR, Mikael P, Adams D, Laurencin CT, Nu-kavarapu SP. Design and Characterization of Fully Osteoconductive Scaffolds for Homogeneous and Enhanced Bone Regeneration. Society for Biomaterials. 2011 [Google Scholar]
- 124.Amini AR, Mikael P, Adams D, Laurencin CT, Nu-kavarapu SP. Fully osteoconductive and mechanically compatible scaffolds for effective bone regeneration. OrthoRes Soc. 2011 [Google Scholar]
- 125.Harvey EJ, Henderson JE, Vengallatore ST. Nano-technology and bone healing. J Orthop Trauma. 2010;24(Suppl 1):S25–S30. doi: 10.1097/BOT.0b013e3181ca3b58. [DOI] [PubMed] [Google Scholar]
- 126.Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotech-nology and Orthopedics: A personal perspective. Nano-medicine: Wiley Interdisciplinary Reviews. 2009:6–10. doi: 10.1002/wnan.25. [DOI] [PubMed] [Google Scholar]
- 127.Christenson EM, Anseth KS, van den Beucken JJ, Chan CK, Ercan B, Jansen JA, et al. Nanobioma-terial applications in orthopedics. J Orthop Res. 2007;25(1):11–22. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 128.Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25(6):1539–1560. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 129.Ward BC, Webster TJ. The effect of nanotopography on calcium and phosphorus deposition on metallic materials in vitro. Biomaterials. 2006;27(16):3064–3074. doi: 10.1016/j.biomaterials.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 130.Igwe J, Amini AR, Mickael P, Laurencin CT, Nuka-varapu SP. Nanostructured scaffolds for Bone Tissue Engineering. In: Zilberman M, editor. Studies in mechanobiology, tissue engineering and Biomaterials. Verlag Berlin Heidelberg: Springer; 2011. [Google Scholar]
- 131.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteo-blast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 132.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31(6):1133–1139. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith LA, Liu X, Hu J, Ma PX. The enhancement of human embryonic stem cell osteogenic differentiation with nano-fibrous scaffolding. Biomaterials. 2010;31(21):5526–5535. doi: 10.1016/j.biomaterials.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ashammakhi N, Ndreu A, Yang Y, Ylikauppila H, Nikkola L, Hasirci V. Tissue engineering: a new takeoff using nanofiber-based scaffolds. J Craniofac Surg. 2007;18(1):3–17. doi: 10.1097/01.scs.0000236444.05345.53. [DOI] [PubMed] [Google Scholar]
- 135.Lee JH, Rim NG, Jung HS, Shin H. Control of os-teogenic differentiation and mineralization of human mesenchymal stem cells on composite nanofibers containing poly[lactic-co-(glycolic acid)] and hydroxy-apatite. Macromol Biosci. 2010;10(2):173–182. doi: 10.1002/mabi.200900169. [DOI] [PubMed] [Google Scholar]
- 136.Lao L, Wang Y, Zhu Y, Zhang Y, Gao C. Poly(lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J Mater Sci Mater Med. 2011;22(8):1873–1884. doi: 10.1007/s10856-011-4374-8. [DOI] [PubMed] [Google Scholar]
- 137.Tuzlakoglu K, Santos MI, Neves N, Reis RL. Design of nano- and microfiber combined scaffolds by electrospinning of collagen onto starch-based fiber meshes: a man-made equivalent of natural extracellular matrix. Tissue Eng Part A. 2011;17(3–4):463–473. doi: 10.1089/ten.TEA.2010.0178. [DOI] [PubMed] [Google Scholar]
- 138.Jose MV, Thomas V, Johnson KT, Dean DR, Nyairo E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009;5(1):305–315. doi: 10.1016/j.actbio.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 139.Matson JB, Stupp SI. Self-assembling peptide scaffolds for regenerative medicine. Chem Commun (Camb) 2012;48(1):26–33. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yokoi H, Kinoshita T, Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci U S A. 2005;102(24):8414–8419. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brun P, Ghezzo F, Roso M, Danesin R, Palù G, Ba-gno A, et al. Electrospun scaffolds of self-assembling peptides with poly(ethylene oxide) for bone tissue engineering. Acta Biomater. 2011;7(6):2526–2532. doi: 10.1016/j.actbio.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 142.Mata A, Geng Y, Henrikson KJ, Aparicio C, Stock SR, Satcher RL, et al. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Bio-materials. 2010;31(23):6004–6012. doi: 10.1016/j.biomaterials.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30(25):4094–4103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang L, Webster T. Nanotechnology and nanoma-terials: Promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 145.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132(4):612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 146.Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and trans-lational perspectives. Biomaterials. 2011;32(12):3189–3209. doi: 10.1016/j.biomaterials.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 147.Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32(2):395–409. doi: 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24(24):4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 149.Sarkar D, Spencer JA, Phillips JA, Zhao W, Schafer S, Spelke DP, et al. Engineered cell homing. Blood. 2011;118(25):e184–e191. doi: 10.1182/blood-2010-10-311464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Igwe J, Amini AR, Nukavarapu SP. Orthopaedic Research Society. San Francisco, CA: USA; 2012. Fabrication and evaluation of a novel scaffold system with high-density cell seeding for bone regeneration: an investigation of cell density enhanced osteogenic expression. [Google Scholar]
- 152.Granero-Moltó F, Weis JA, Miga MI, Landis B, Myers TJ, O’Rear L, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee DY, Cho TJ, Kim JA, Lee HR, Yoo WJ, Chung CY, et al. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 2008;42(5):932–941. doi: 10.1016/j.bone.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 154.Schantz JT, Chim H, Whiteman M. Cell guidance in tissue engineering: SDF-1 mediates site-directed homing of mesenchymal stem cells within three-dimensional polycaprolactone scaffolds. Tissue Eng. 2007;13(11):2615–2624. doi: 10.1089/ten.2006.0438. [DOI] [PubMed] [Google Scholar]
- 155.García AJ, Reyes CD. Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. J Dent Res. 2005;84(5):407–413. doi: 10.1177/154405910508400502. [DOI] [PubMed] [Google Scholar]
- 156.Lu HH, Jiang J. Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91–111. [PubMed] [Google Scholar]
- 157.Lu HH, Subramony SD, Boushell MK, Zhang X. Tissue engineering strategies for the regeneration of orthopedic interfaces. Ann Biomed Eng. 2010;38(6):2142–2154. doi: 10.1007/s10439-010-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Friedman MJ, Sherman OH, Fox JM, Del Pizzo W, Snyder SJ, Ferkel RJ. Autogeneic anterior cruciate ligament (ACL) anterior reconstruction of the knee. A review. Clin Orthop Relat Res. 1985;(196):9–14. [PubMed] [Google Scholar]
- 159.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12(12):3497–3508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 160.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A. 2008;86(1):1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 161.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15(2):127–141. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107(8):3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 164.Srouji S, Kizhner T, Suss-Tobi E, Livne E, Zussman E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J Mater Sci Mater Med. 2008;19(3):1249–1255. doi: 10.1007/s10856-007-3218-z. [DOI] [PubMed] [Google Scholar]
- 165.Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007;28(21):3188–3197. doi: 10.1016/j.biomaterials.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 166.Porter JR, Henson A, Popat KC. Biodegradable poly(epsilon-caprolactone) nanowires for bone tissue engineering applications. Biomaterials. 2009;30(5):780–788. doi: 10.1016/j.biomaterials.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 167.Tao SL, Desai TA. Aligned arrays of biodegradable poly(epsilon-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007;7(6):1463–1468. doi: 10.1021/nl0700346. [DOI] [PubMed] [Google Scholar]
- 168.Yeong WY, Chua CK, Leong KF, Chandrasekaran M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004;22(12):643–652. doi: 10.1016/j.tibtech.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 169.Heo SJ, Kim SE, Wei J, Hyun YT, Yun HS, Kim DH, et al. Fabrication and characterization of novel nano-and micro-HA/PCL composite scaffolds using a mod-ified rapid prototyping process. J Biomed Mater Res A. 2009;89(1):108–116. doi: 10.1002/jbm.a.31726. [DOI] [PubMed] [Google Scholar]
- 170.Igwe J, Mikael PE, Nukavarapu SP. Design, fabrication and in vitro evaluation of novel polymer-hy-drogel hybrid scaffold for bone tissue engineering. J Regen Med Tissue Engin. 2012 Jun 11; doi: 10.1002/term.1506. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 171.Robey PG. Cell sources for bone regeneration: the good, the bad, and the ugly (but promising) Tissue Eng Part B Rev. 2011;17(6):423–430. doi: 10.1089/ten.teb.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Colnot C. Cell sources for bone tissue engineering: insights from basic science. Tissue Eng Part B Rev. 2011;17(6):449–457. doi: 10.1089/ten.teb.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Drosse I, Volkmer E, Capanna R, De Biase P, Mutschler W, Schieker M. Tissue engineering for bone defect healing: an update on a multi-component approach. Injury. 2008;39(Suppl 2):S9–S20. doi: 10.1016/S0020-1383(08)70011-1. [DOI] [PubMed] [Google Scholar]
- 174.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23(6):699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 175.Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21(5):546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- 176.Bishop AE, Buttery LD, Polak JM. Embryonic stem cells. J Pathol. 2002;197(4):424–429. doi: 10.1002/path.1154. [DOI] [PubMed] [Google Scholar]
- 177.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 178.Hwang YS, Cho J, Tay F, Heng JY, Ho R, Kazarian SG, et al. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bio-reactor in bone tissue engineering. Biomaterials. 2009;30(4):499–507. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 179.Warotayanont R, Frenkel B, Snead ML, Zhou Y. Leu-cine-rich amelogenin peptide induces osteogenesis by activation of the Wnt pathway. Biochem Biophys Res Commun. 2009;387(3):558–563. doi: 10.1016/j.bbrc.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7(1):89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 181.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36(5):758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 182.Karp JM, Ferreira LS, Khademhosseini A, Kwon AH, Yeh J, Langer RS. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006;24(4):835–843. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- 183.Hwang YS, Polak JM, Mantalaris A. In vitro direct osteogenesis of murine embryonic stem cells without embryoid body formation. Stem Cells Dev. 2008;17(5):963–970. doi: 10.1089/scd.2007.0228. [DOI] [PubMed] [Google Scholar]
- 184.Tian XF, Heng BC, Ge Z, Lu K, Rufaihah AJ, Fan VT, et al. Comparison of osteogenesis of human embryonic stem cells within 2D and 3D culture systems. Scand J Clin Lab Invest. 2008;68(1):58–67. doi: 10.1080/00365510701466416. [DOI] [PubMed] [Google Scholar]
- 185.Kim S, Kim SS, Lee SH, Eun Ahn S, Gwak SJ, Song JH, et al. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials. 2008;29(8):1043–1053. doi: 10.1016/j.biomaterials.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 186.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 187.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichi-saka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 188.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 189.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, et al. Cell type of origin infuences the molecular and functional properties of mouse induced plu-ripotent stem cells. Nat Biotechnol. 2010;28(8):848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, et al. Bone regeneration and stem cells. J Cell Mol Med. 2011;15(4):718–746. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 192.Kuznetsov SA, Mankani MH, Gronthos S, Satomu-ra K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153(5):1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rosada C, Justesen J, Melsvik D, Ebbesen P, Kas-sem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72(2):135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- 194.De Bari C, Dell’Accio F, Tylzanowski P, Luy-ten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 195.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 197.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 198.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 200.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 201.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 202.Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11(5–6):787–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- 203.Kagami H, Agata H, Tojo A. Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for bone tissue engineering: basic science to clinical translation. Int J Biochem Cell Biol. 2011;43(3):286–289. doi: 10.1016/j.biocel.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 204.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332(3):415–426. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fraser JK, Schreiber R, Strem B, Zhu M, Alfonso Z, Wulur I, et al. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S33–S37. doi: 10.1038/ncpcardio0444. [DOI] [PubMed] [Google Scholar]
- 207.Tapp H, Hanley EN, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood) 2009;234(1):1–9. doi: 10.3181/0805/MR-170. [DOI] [PubMed] [Google Scholar]
- 208.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 209.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-as-sociated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 210.Al-Salleeh F, Beatty MW, Reinhardt RA, Petro TM, Crouch L. Human osteogenic protein-1 induces osteo-genic differentiation of adipose-derived stem cells harvested from mice. Arch Oral Biol. 2008;53(10):928–936. doi: 10.1016/j.archoralbio.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 211.Ahn HH, Kim KS, Lee JH, Lee JY, Kim BS, Lee IW, et al. In vivo osteogenic differentiation of human adi-pose-derived stem cells in an injectable in situ-forming gel scaffold. Tissue Eng Part A. 2009;15(7):1821–1832. doi: 10.1089/ten.tea.2008.0386. [DOI] [PubMed] [Google Scholar]
- 212.Knippenberg M, Helder MN, Zandieh Doulabi B, Wuisman PI, Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342(3):902–908. doi: 10.1016/j.bbrc.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 213.Hattori H, Masuoka K, Sato M, Ishihara M, Asa-zuma T, Takase B, et al. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J Biomed Mater Res B Appl Biomater. 2006;76(1):230–239. doi: 10.1002/jbm.b.30357. [DOI] [PubMed] [Google Scholar]
- 214.Müller AM, Davenport M, Verrier S, Droeser R, Alini M, Bocelli-Tyndall C, et al. Platelet lysate as a serum substitute for 2D static and 3D perfusion culture of stromal vascular fraction cells from human adipose tissue. Tissue Eng Part A. 2009;15(4):869–875. doi: 10.1089/ten.tea.2008.0498. [DOI] [PubMed] [Google Scholar]
- 215.Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS, Lee SH. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A. 2008;14(8):1285–1294. doi: 10.1089/ten.tea.2007.0253. [DOI] [PubMed] [Google Scholar]
- 216.Di Bella C, Farlie P, Penington AJ. Bone regeneration in a rabbit critical-sized skull defect using au-tologous adipose-derived cells. Tissue Eng Part A. 2008;14(4):483–490. doi: 10.1089/tea.2007.0137. [DOI] [PubMed] [Google Scholar]
- 217.Hao W, Pang L, Jiang M, Lv R, Xiong Z, Hu YY. Skeletal repair in rabbits using a novel biomimetic composite based on adipose-derived stem cells encapsulated in collagen I gel with PLGA-beta-TCP scaffold. J Orthop Res. 2010;28(2):252–257. doi: 10.1002/jor.20969. [DOI] [PubMed] [Google Scholar]
- 218.Helder MN, Knippenberg M, Klein-Nulend J, Wuis-man PI. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13(8):1799–1808. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 219.Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascular-ization capacity. Stem Cells. 2007;25(7):1823–1829. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 220.Müller AM, Mehrkens A, Schäfer DJ, Jaquiery C, Güven S, Lehmicke M, et al. Towards an intraopera-tive engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127–135. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 221.Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30(4):634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 222.Amini AR, Laurencin CT, Nukavarapu SP. Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J Orthop Res. 2012;30(9):1507–1515. doi: 10.1002/jor.22097. [DOI] [PubMed] [Google Scholar]
- 223.Fedorovich NE, Haverslag RT, Dhert WJ, Alblas J. The role of endothelial progenitor cells in pre-vascularized bone tissue engineering: development of heterogeneous constructs. Tissue Eng Part A. 2010;16(7):2355–2367. doi: 10.1089/ten.TEA.2009.0603. [DOI] [PubMed] [Google Scholar]
- 224.Seebach C, Henrich D, Kähling C, Wilhelm K, Tami AE, Alini M, et al. Endothelial progenitor cells and mesenchymal stem cells seeded onto beta-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng Part A. 2010;16(6):1961–1970. doi: 10.1089/ten.TEA.2009.0715. [DOI] [PubMed] [Google Scholar]
- 225.Mangano C, Paino F, d’Aquino R, De Rosa A, Iezzi G, Piattelli A, et al. Human dental pulp stem cells hook into biocoral scaffold forming an engineered biocom-plex. PLoS One. 2011;6(4):e18721. doi: 10.1371/journal.pone.0018721. PubMed PMID: 21494568. Pubmed Central PMCID: PMC3073992. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tirino V, Paino F, d’Aquino R, Desiderio V, De Rosa A, Papaccio G. Methods for the identification, characterization and banking of human DPSCs: current strategies and perspectives. Stem Cell Rev. 2011 Sep;7(3):608–615. doi: 10.1007/s12015-011-9235-9. PubMed PMID: 21318597. eng. [DOI] [PubMed] [Google Scholar]
- 227.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12(10):2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 228.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 229.d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endo-theliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 230.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DP-SCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: a useful source of living autol-ogous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20(8):1394–1402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 232.El-Backly RM, Massoud AG, El-Badry AM, Sherif RA, Marei MK. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust Endod J. 2008;34(2):52–67. doi: 10.1111/j.1747-4477.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- 233.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyaza-wa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34(8):962–699. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 234.Marolt D, Knezevic M, Novakovic GV. Bone tissue engineering with human stem cells. Stem Cell Res Ther. 2010;1(2):10. doi: 10.1186/scrt10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chew SA, Kretlow JD, Spicer PP, Edwards AW, Baggett LS, Tabata Y, et al. Delivery of plasmid DNA encoding bone morphogenetic protein-2 with a biodegradable branched polycationic polymer in a critical-size rat cranial defect model. Tissue Eng Part A. 2011;17(5–6):751–763. doi: 10.1089/ten.tea.2010.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Park SY, Kim KH, Koo KT, Lee KW, Lee YM, Chung CP, et al. The evaluation of the correlation between histomorphometric analysis and micro-computed tomography analysis in AdBMP-2 induced bone regeneration in rat calvarial defects. J Periodontal Implant Sci. 2011;41(5):218–226. doi: 10.5051/jpis.2011.41.5.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces hetero-topic and orthotopic bone formation in rodents. J Or-thop Res. 1998;16(3):330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 238.Blum JS, Barry MA, Mikos AG, Jansen JA. In vivo evaluation of gene therapy vectors in ex vivo-derived marrow stromal cells for bone regeneration in a rat critical-size calvarial defect model. Hum Gene Ther. 2003;14(18):1689–1701. doi: 10.1089/104303403322611719. [DOI] [PubMed] [Google Scholar]
- 239.Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured peri-osteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999;42(5):488–495. [PubMed] [Google Scholar]
- 240.Jabbarzadeh E, Starnes T, Khan Y, Jiang T, Wirtel A, Deng M, et al. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc Natl Acad Sci U S A. 2008;105(32):11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchw-ey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29(19):2869–2877. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Guldberg RE, Duvall CL, Peister A, Oest ME, Lin AS, Palmer AW, et al. 3D imaging of tissue integration with porous biomaterials. Biomaterials. 2008;29(28):3757–3761. doi: 10.1016/j.biomaterials.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hollinger JO, Winn S, Bonadio J. Options for tissue engineering to address challenges of the aging skeleton. Tissue Eng. 2000;6(4):341–350. doi: 10.1089/107632700418065. [DOI] [PubMed] [Google Scholar]
- 244.Jadlowiec JA, Celil AB, Hollinger JO. Bone tissue engineering: recent advances and promising therapeutic agents. Expert Opin Biol Ther. 2003;3(3):409–423. doi: 10.1517/14712598.3.3.409. [DOI] [PubMed] [Google Scholar]
- 245.Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7(1):31–34. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 246.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87(7):896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 247.Connolly JF, Guse R, Tiedeman J, Dehne R. Autolo-gous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;(266):259–270. [PubMed] [Google Scholar]
- 248.Kettunen J, Mäkelä EA, Turunen V, Suomalainen O, Partanen K. Percutaneous bone grafting in the treatment of the delayed union and non-union of tibial fractures. Injury. 2002;33(3):239–245. doi: 10.1016/s0020-1383(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 249.Goel A, Sangwan SS, Siwach RC, Ali AM. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury. 2005;36(1):203–206. doi: 10.1016/j.injury.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 250.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteo-necrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A(6):1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 251.Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973–3982. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 252.Jäger M, Jelinek EM, Wess KM, Scharfstädt A, Ja-cobson M, Kevy SV, et al. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4(1):34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 253.Salama R, Weissman SL. The clinical use of combined xenografts of bone and autologous red marrow. A preliminary report. J Bone Joint Surg Br. 1978;60(1):111–115. doi: 10.1302/0301-620X.60B1.342531. [DOI] [PubMed] [Google Scholar]
- 254.Neen D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine (Phila Pa 1976) 2006;31(18):E636–E640. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 255.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414(6859):118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 256.Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97(2):110–118. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 257.Del Buono A, Papalia R, Denaro V, Maccauro G, Maffulli N. Platelet rich plasma and tendinopathy: state of the art. Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):79–83. doi: 10.1177/03946320110241S215. [DOI] [PubMed] [Google Scholar]
- 258.Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc. 2011;19(3):244–250. doi: 10.1097/JSA.0b013e318227b2dc. [DOI] [PubMed] [Google Scholar]
- 259.Salter E, Goh B, Hung B, Hutton D, Ghone N, Gray-son WL. Bone tissue engineering bioreactors: a role in the clinic? Tissue Eng Part B Rev. 2012;18(1):62–75. doi: 10.1089/ten.TEB.2011.0209. [DOI] [PubMed] [Google Scholar]
- 260.Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32(1):112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 261.Stiehler M, Bünger C, Baatrup A, Lind M, Kassem M, Mygind T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2009;89(1):96–107. doi: 10.1002/jbm.a.31967. [DOI] [PubMed] [Google Scholar]
- 262.Yu X, Botchwey EA, Levine EM, Pollack SR, Lau-rencin CT. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast pheno-typic expression and matrix mineralization. Proc Natl Acad Sci U S A. 2004;101(31):11203–11208. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Botchwey EA, Pollack SR, El-Amin S, Levine EM, Tuan RS, Laurencin CT. Human osteoblast-like cells in three-dimensional culture with fluid flow. Biorheology. 2003;40(1–3):299–306. [PubMed] [Google Scholar]
- 264.Botchwey EA, Pollack SR, Levine EM, Johnston ED, Laurencin CT. Quantitative analysis of three-dimensional fluid flow in rotating bioreactors for tissue engineering. J Biomed Mater Res A. 2004;69(2):205–215. doi: 10.1002/jbm.a.10163. [DOI] [PubMed] [Google Scholar]
- 265.Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62(1):136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 266.Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22(11):1279–1288. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 267.Wang Y, Uemura T, Dong J, Kojima H, Tanaka J, Tateishi T. Application of perfusion culture system improves in vitro and in vivo osteogenesis of bone marrow-derived osteoblastic cells in porous ceramic materials. Tissue Eng. 2003;9(6):1205–1214. doi: 10.1089/10763270360728116. [DOI] [PubMed] [Google Scholar]
- 268.Mauney JR, Sjostorm S, Blumberg J, Horan R, O’Leary JP, Vunjak-Novakovic G, et al. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74(5):458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 269.Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. J Biomed Mater Res. 2000;51(3):376–382. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 270.Bancroft GN, Sikavitsas VI, van den Dolder J, Shef-field TL, Ambrose CG, Jansen JA, et al. Fluid flow increases mineralized matrix deposition in 3D per-fusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99(20):12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Vunjak-Novakovic G, Obradovic B, Martin I, Bur-sac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14(2):193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 272.Das A, Botchwey E. Evaluation of angiogenesis and osteogenesis. Tissue Eng Part B Rev. 2011;17(6):403–414. doi: 10.1089/ten.TEB.2011.0190. [DOI] [PubMed] [Google Scholar]
- 273.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A(7):1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 274.Mooney DJ, Mikos AG. Growing new organs. Sci Am. 1999;280(4):60–65. doi: 10.1038/scientificamerican0499-60. [DOI] [PubMed] [Google Scholar]
- 275.Nukavarapu SP, Amini AR. Optimal scaffold design and effective progenitor cell identification for the regeneration of vascularized bone. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2464–2467. doi: 10.1109/IEMBS.2011.6090684. [DOI] [PubMed] [Google Scholar]
- 276.Moroni L, de Wijn JR, van Blitterswijk CA. 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials. 2006;27(7):974–985. doi: 10.1016/j.biomaterials.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 277.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardio-vasc Pathol. 2003;12(6):295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 278.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 279.Rouwkema J, Rivron NC, van Blitterswijk CA. Vas-cularization in tissue engineering. Trends Biotechnol. 2008;26(8):434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 280.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 281.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(15):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 282.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res. 2004;94(8):1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 283.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogen-ic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20(5):848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 284.Buschmann J, Welti M, Hemmi S, Neuenschwander P, Baltes C, Giovanoli P, et al. Three-dimensional co-cultures of osteoblasts and endothelial cells in DegraPol foam: histological and high-field magnetic resonance imaging analyses of pre-engineered capillary networks in bone grafts. Tissue Eng Part A. 2011 Feb;17(3–4):291–299. doi: 10.1089/ten.TEA.2010.0278. PubMed PMID: 20799888. eng. [DOI] [PubMed] [Google Scholar]
- 285.Kim S, von Recum H. Endothelial stem cells and precursors for tissue engineering: cell source, differentiation, selection, and application. Tissue Eng Part B Rev. 2008;14(1):133–147. doi: 10.1089/teb.2007.0304. [DOI] [PubMed] [Google Scholar]
- 286.Amini AR, Laurencin CT, Nukavarapu SP. Development and evaluation of optimized scaffolds pre-seed-ed with effective progenitor combination for vascular-ized bone regeneration. Ortho Res Soc. 2012 [Google Scholar]
- 287.Yu H, Vandevord P, Gong W, Wu B, Song Z, Matthew H, et al. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res. 2008;26(8):1147–1152. doi: 10.1002/jor.20609. [DOI] [PubMed] [Google Scholar]
- 288.Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A. 2010;16(9):2809–2820. doi: 10.1089/ten.tea.2010.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 290.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23(7):879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 291.Cassell OC, Hofer SO, Morrison WA, Knight KR. Vascularisation of tissue-engineered grafts: the regulation of angiogenesis in reconstructive surgery and in disease states. Br J Plast Surg. 2002;55(8):603–610. doi: 10.1054/bjps.2002.3950. [DOI] [PubMed] [Google Scholar]
- 292.Tanaka Y, Sung KC, Tsutsumi A, Ohba S, Ueda K, Morrison WA. Tissue engineering skin flaps: which vascular carrier, arteriovenous shunt loop or ar-teriovenous bundle, has more potential for angio-genesis and tissue generation? Plast Reconstr Surg. 2003;112(6):1636–1644. doi: 10.1097/01.PRS.0000086140.49022.AB. [DOI] [PubMed] [Google Scholar]
- 293.Hollister SJ, Murphy WL. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev. 2011;17(6):459–474. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Alman BA, Kelley SP, Nam D. Heal thyself: using endogenous regeneration to repair bone. Tissue Eng Part B Rev. 2011;17(6):431–436. doi: 10.1089/ten.TEB.2011.0189. [DOI] [PubMed] [Google Scholar]
- 295.Yoshida M, Babensee JE. Differential effects of agarose and poly(lactic-co-glycolic acid) on dendritic cell maturation. J Biomed Mater Res A. 2006;79(2):393–408. doi: 10.1002/jbm.a.30798. [DOI] [PubMed] [Google Scholar]
- 296.Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J Biomed Mater Res A. 2005;74(4):503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- 297.Brodbeck W, Shive M, Colton E, Nakayama Y, Mat-suda T, Anderson J. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J Biomed Mater Res. 2001;55(4):661–668. doi: 10.1002/1097-4636(20010615)55:4<661::aid-jbm1061>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 298.Brodbeck W, Nakayama Y, Matsuda T, Colton E, Ziats N, Anderson J. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18(6):311–319. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 299.Schmidt DR, Kao WJ. Monocyte activation in response to polyethylene glycol hydrogels grafted with RGD and PHSRN separated by interpositional spacers of various lengths. J Biomed Mater Res A. 2007;83(3):617–625. doi: 10.1002/jbm.a.31270. [DOI] [PubMed] [Google Scholar]
- 300.Zhu J, Tang C, Kottke-Marchant K, Marchant RE. Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug Chem. 2009;20(2):333–339. doi: 10.1021/bc800441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Collie AM, Bota PC, Johns RE, Maier RV, Stayton PS. Differential monocyte/macrophage interleukin-1 beta production due to biomaterial topography requires the beta 2 integrin signaling pathway. J Biomed Mater Res A. 2010 doi: 10.1002/jbm.a.32963. [DOI] [PubMed] [Google Scholar]
- 302.Bota PC, Collie AM, Puolakkainen P, Vernon RB, Sage EH, Ratner BD, et al. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A. 2010;95(2):649–657. doi: 10.1002/jbm.a.32893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kao W, Lee D. In vivo modulation of host response and macrophage behavior by polymer networks grafted with fibronectin-derived biomimetic oligopeptides: the role of RGD and PHSRN domains. Biomaterials. 2001;22(21):2901–2909. doi: 10.1016/s0142-9612(01)00037-0. [DOI] [PubMed] [Google Scholar]
- 304.Kao WJ, Lee D, Schense JC, Hubbell JA. Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J Biomed Mater Res. 2001;55(1):79–88. doi: 10.1002/1097-4636(200104)55:1<79::aid-jbm110>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 305.Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12(2):188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6(6):887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 307.Hosgood GWound healing. The role of platelet-derived growth factor and transforming growth factor beta. Vet Surg. 1993;22(6):490–495. doi: 10.1111/j.1532-950x.1993.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 308.Liu L, Chen G, Chao T, Ratner BD, Sage EH, Jiang S. Reduced foreign body reaction to implanted biomaterials by surface treatment with oriented osteopontin. J Biomater Sci Polym Ed. 2008;19(6):821–835. doi: 10.1163/156856208784522083. [DOI] [PubMed] [Google Scholar]
- 309.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88(12):2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 310.Nagai M, Hayakawa T, Fukatsu A, Yamamoto M, Fukumoto M, Nagahama F, et al. In vitro study of collagen coating of titanium implants for initial cell attachment. Dent Mater J. 2002;21(3):250–260. doi: 10.4012/dmj.21.250. [DOI] [PubMed] [Google Scholar]
- 311.Morra M. Biochemical modification of titanium surfaces: peptides and ECM proteins. Eur Cell Mater. 2006;12:1–15. doi: 10.22203/ecm.v012a01. [DOI] [PubMed] [Google Scholar]
- 312.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 313.Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13(5):947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 314.Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, Ahmadbeigi N. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(2):203–209. doi: 10.1016/j.tripleo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 315.Morishita T, Honoki K, Ohgushi H, Kotobuki N, Matsushima A, Takakura Y. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients’ mesen-chymal stem cells. Artif Organs. 2006;30(2):115–118. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 316.Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Cranio-maxillofac Surg. 2004;32(6):370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 317.Pak J. Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician. 2012;15(1):75–85. [PubMed] [Google Scholar]
- 318.Pak J. Regeneration of human bones in hip osteone-crosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Reports. 2011;5(1):296. doi: 10.1186/1752-1947-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hendrich C, Franz E, Waertel G, Krebs R, Jäger M. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev (Pavia) 2009;1(2):e32. doi: 10.4081/or.2009.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lozada JL, Caplanis N, Proussaefs P, Willardsen J, Kammeyer G. Platelet-rich plasma application in sinus graft surgery: Part I—Background and processing techniques. J Oral Implantol. 2001;27(1):38–42. doi: 10.1563/1548-1336(2001)027<0038:PPAISG>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 321.Martin I, Wendt D, Heberer M. The role of bio-reactors in tissue engineering. Trends Biotechnol. 2004;22(2):80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 322.Olivier V, Faucheux N, Hardouin P. Biomaterial challenges and approaches to stem cell use in bone recon-structivesurgery. Drug discov Today. 2004;9(18):803–811. doi: 10.1016/S1359-6446(04)03222-2. [DOI] [PubMed] [Google Scholar]