Abstract
The GABAA receptor (GABAAR) is a major target of antiseizure drugs (ASDs). A variety of agents that act at GABAARs s are used to terminate or prevent seizures. Many act at distinct receptor sites determined by the subunit composition of the holoreceptor. For the benzodiazepines, barbiturates, and loreclezole, actions at the GABAAR are the primary or only known mechanism of antiseizure action. For topiramate, felbamate, retigabine, losigamone and stiripentol, GABAAR modulation is one of several possible antiseizure mechanisms. Allopregnanolone, a progesterone metabolite that enhances GABAAR function, led to the development of ganaxolone. Other agents modulate GABAergic “tone” by regulating the synthesis, transport or breakdown of GABA. GABAAR efficacy is also affected by the transmembrane chloride gradient, which changes during development and in chronic epilepsy. This may provide an additional target for “GABAergic” ASDs. GABAAR subunit changes occur both acutely during status epilepticus and in chronic epilepsy, which alter both intrinsic GABAAR function and the response to GABAAR-acting ASDs. Manipulation of subunit expression patterns or novel ASDs targeting the altered receptors may provide a novel approach for seizure prevention.
Keywords: inhibition, epilepsy, antiepileptic drugs, GABA receptor, seizures, chloride channel
Seizures frequently result from an imbalance of excitation and inhibition due to a failure of inhibitory neurotransmission. Most agents that enhance GABAA receptor (GABAAR) function have antiseizure properties due to their ability to increase inhibitory neurotransmitter tone. The evidence linking epilepsy with dysfunction of GABAergic inhibition is substantial, and has been extensively reviewed1-4.
GABAARs and Epilepsy
GABAARs are pharmacologically complex, with binding sites for benzodiazepines (BZs), barbiturates, neurosteroids, general anesthetics, loreclezole, and the convulsant toxins, picrotoxin and bicuculline. Protein subunits from seven different subunit families5 assemble to form pentameric6 transmembrane chloride channels (Fig. 1). In mammals, 16 subunit subtypes have been cloned, including 6 α, 3 β and 3 γ subtypes, as well as δ, π7, ε8 and θ9 and alternatively spliced variants of the β2 and γ2 subtypes. Subunit expression is regulated by region, cell type10 and developmental stage11, reducing the number of isoforms expressed in specific brain regions and individual neurons. The most common composition includes two α1, two β2 and a single γ2 subunit; the δ subunit is found instead of γ in receptors expressed extrasynaptically. The subunits are arranged around a central water-filled pore, which gates to conduct Cl− ions when GABA is bound (Fig. 1). Individual subunit subtypes confer different sensitivities to GABAAR modulators including BZs12, loreclezole13 and zinc ions14.
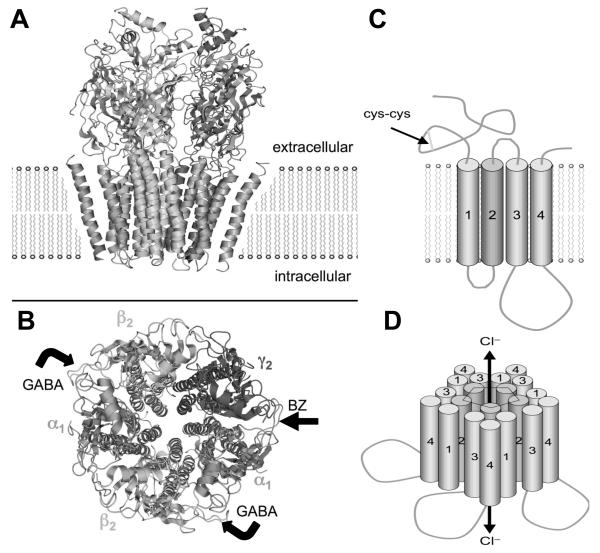
Figure 1.
Model of a GABAA receptor in the plasma membrane. A. A space-filling model of the pentomer in side view (A) and top view (B) based on the high sequence homology with the nicotinic acetylcholine receptor. There are with two binding sites for GABA, between α and β subunits (bent arrows), and one for BZs between the alpha and gamma subunits (arrow). C. A schematic view shows the topology of each subunit with a large extracellular loop containing a cysteine loop and 4 transmembrane domains (M1-M4), the second of which forms the chloride ion channel. Binding of GABA allows the channel to open and conduct Cl− ions, resulting in the fast inhibitory post-synaptic potential (IPSP). D. Putative arrangement of 5 subunits to form a pentamer with central chloride channel lined by the M2 subunit. [Derived from the published structure: RCSB PDB Database·PDB ID: 2BG9 from Unwin, N. (2005)198. Images modified from en.wikipedia.org/wiki/GABA_A_receptor, used with permission (public domain)].
GABAARs are the target not only of the BZs, but other ASDs including barbiturates and agents like tiagabine and vigabatrin1 that increase GABA concentration at the synapse. Bicuculline and picrotoxin, which are GABAAR antagonists, induce seizures in animals and epileptiform activity in brain slice preparations. Several animal models of epilepsy have altered GABAAR number or function1,2,15. GABAAR subunit expression is altered in the hippocampi of experimental animals with recurrent seizures16 and in patients with temporal lobe epilepsy17,18. . Angelman’s syndrome is a human neurodevelopmental disorder associated with severe mental retardation and epilepsy, which is linked to a deletion mutation on chromosome 15q11-1320 in a region encoding the GABAAR β3 subunit21. Two mutations in the γ2 subunit that impair GABAAR function22, K289M23 and R43Q24, have been linked to a human syndrome of childhood absence epilepsy and febrile seizures, and a loss-of-function mutation in the α1 subunit causes autosomal dominant Juvenile Myoclonic Epilepsy25. The R43Q mutation in the γ2 subunit reduces BZ sensitivity26 by altering GABAAR assembly27-29 and trapping the receptor in the endoplasmic reticulum30. Other point mutations in GABAAR subunits have also been associated with generalized epilepsies (see Fig. 2). Hence, GABAAR modulation by GABAergic ASDs is likely critical to their antiseizure activity.
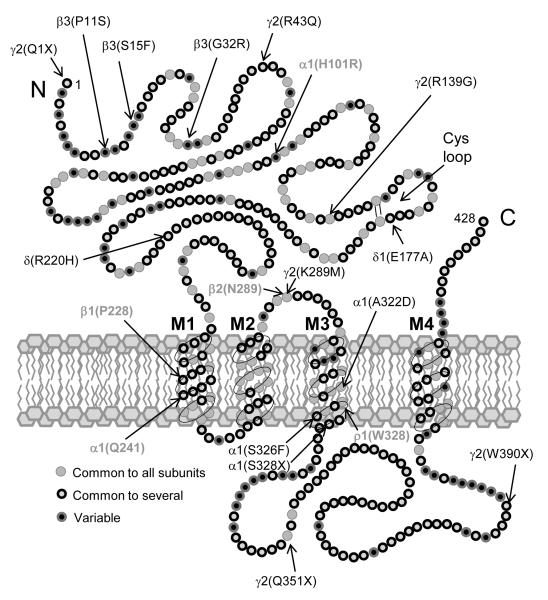
Figure 2.
Model of a prototype GABAAR subunit (based on α1 subunit diagram from Olsen & Tobin, 1990)199 showing approximate locations of point mutations associated with generalized epilepsies (see also Macdonald et al., 2012 200) in black, and locations of point mutations associated with ASD sites of action. See text for details.
Benzodiazepines
BZs were initially developed as anxiolytic agents in the 1950’s. Chlordiazepoxide was introduced in 1960, followed by diazepam31 and nitrazepam.32 In 1965, diazepam was first used to treat status epilepticus in humans.33 Clonazepam was introduced in the 1970’s primarily as an ASD,34 and clobazam, a 1,5 benzodiazepine, was later developed as an ASD with less sedative effect.35 However, the induction of tolerance limits their use as ASDs. The 1,5 BZs may produce less tolerance than the 1,4 BZs, but tolerance to 1,5 BZs does occur.36
BZ activity at GABAARs is a function of the drug’s affinity for the BZ binding site and its intrinsic allosteric effect on the GABAAR. The efficacy of individual compounds varies widely. Most BZs in clinical use are full agonists that maximally enhance GABAAR activity. Flumazenil, a competitive antagonist used to reverse BZ-induced sedation,37 binds to the BZ site without affecting GABA R function. Abecarnil,39 imidazenil,40 and bretazenil41 are “partial agonists” at the BZ site which have antiseizure efficacy in animal models and appear less prone to tolerance.42 Several β-carbolines are “inverse BZ agonists” that inhibit GABA binding43 and can induce seizures or anxiety.44
Antiseizure Activity
BZs are effective against most experimental seizure types, but individual drugs vary in their potency/efficacy in specific seizure models and their other clinical effects.45 BZs are particularly effective against convulsive seizures induced by pentylenetetrazol46 and less effective against tonic seizures induced by maximal electroshock.47 BZs also slow the development of kindling.48
GABAAR Subunits and BZ Pharmacology
BZ augmentation of GABAAR currents requires a γ subunit, and the selectivity of BZ responsiveness is determined by which α subunits are present.5,49 The BZ binding site is located in a cleft between the extracellular amino termini of the α and γ subunits.51 The α1 subunit results in a receptor with high affinity for the hypnotic, zolpidem, defining the “BZ-1” (or Ω-1) receptor type.50 The α2 and α3 subunits result in receptors with moderate zolpidem affinity, termed BZ-2 receptors. GABAARs containing the α5 subunit and/or the γ3 subunit are sensitive to diazepam but not to zolpidem and are termed BZ-3 receptors. GABAARs with the α4 or α6 subunits are insensitive to most BZs.
The subunit composition not only determines the affinity for particular BZs, but also the clinical/behavioral effect of the BZ at that receptor. The role of the α subunits in BZ pharmacology was revealed by the discovery of a single histidine (H) residue found in all BZ-sensitive α subunits (H101 in the rat α1 subunit), but not in the BZ-insensitive α4 or α6 subunits, which instead have a charged arginine (R) residue. This H residue was discovered in a strain of “alcohol-non-tolerant” rats, which were found to have a spontaneous point mutation in the α6 subunit (R100Q) that made their α6-containing GABAARs (found mostly in the cerebellum) diazepam-sensitive, accounting for their ethanol and BZ intolerance.53 Mutation of R100 to H in α6 dramatically increased BZ binding in this normally insensitive subunit, while mutation of H101 to R in α1 reduced BZ sensitivity.52 BZ-insensitive α subunit mutations were subsequently “knocked-in” to identify BZ actions at receptors containing that subunit. In homozygous α1(H101R) knock-in mice, the anxiolytic effect was intact, but BZs were not protective against pentylenetetrazol-induced convulsions and did not produce sedation or amnesia, suggesting that binding to the (wild type) α1 subunit is responsible for sedative, amnestic and antiseizure actions.54 Moreover, the sedative-hypnotic, zolpidem, showed no sedative effect in α1(H101R) mice.55 Unfortunately, these findings underscore the association between sedative and antiseizure efficacy at α1-containing GABAARs. Similarly, the anxiolytic56 and myorelaxant57 properties of BZs appear to derive from α2- and α3-containing GABAARs, while the α5 subunit was critical for amnestic effects.58 BZs may also have a true analgesic effect independent of their sedative and anxiolytic actions, associated with the α2 and α3 more than α5 subunits.59 Since there is no evidence of biophysically distinct effects of BZs on receptors composed of different α subunits, the different behavioral effects are likely due to the brain regions and neuronal populations expressing these specific GABAAR isoforms. New α2/α3 subunit-selective BZs appear to be anxiolytic but not sedating,60 and non-sedating antiseizure BZs that do not induce tolerance61 may also be possible.
BZ Actions at GABAARs
BZs increase the amplitude62 or decay time63 of GABA-mediated inhibitory post-synaptic potentials (IPSPs). Current noise fluctuation analysis64 and single channel studies65 demonstrated that the BZs increased the opening frequency of the GABAAR chloride channel. In patch-clamp recordings of CNS neurons, BZs produce a leftward shift of the concentration-response curve for GABA,66 due to an increase in the affinity for GABA at its binding site, with no change in the kinetics of channel gating65 or single channel conductance. In contrast, an inverse agonist at the BZ site reduced the channel opening frequency for a given GABA concentration. These findings are consistent with binding studies showing the allosteric interaction between the BZ and GABA binding sites. By increasing the affinity of the receptor for GABA through slowing the unbinding rate, the BZs increase the current produced by low GABA concentrations, but not by high GABA concentrations at which receptor binding is saturated, as observed in the synaptic cleft67. Thus, BZs generally do not increase the amplitude of miniature inhibitory postsynaptic currents (mIPSCs) from individual synapses, but instead prolong the mIPSC decay phase68 by slowing the dissociation of GABA from the receptor.69 Prolongation of the mIPSC increases temporal and spatial summation of multiple synaptic inputs, which in turn increases the amplitude of stimulus-evoked polysynaptic IPSCs. The BZs thus increase the inhibitory “tone” of GABAergic synapses, which reduces the hypersynchronous firing of neuron populations that underlies seizures.1
An alternative mechanism for BZ enhancement of GABAAR currents has been proposed based on the controversial finding that BZs progressively increase GABAAR “single channel” conductance.70 Unlike prior reports of a 27 pS main conductance level and a 19 pS subconductance level,65 Eghbali et al.70 found conductance levels ranging from 8 to 53 pS in response to GABA alone, and 70-80 pS in the presence of diazepam, with up to 7-fold increase in conductance observed when diazepam was added. The increases in conductance were seen predominantly in cell-attached patches onto neurons expressing native receptors, but were also observed in outside-out patches. They found similar increases in channel conductance induced by pentobarbital,71 neuroactive steroids,72 propofol,73 and GABA itself.74 The same group75 subsequently found that high conductance (>40 pS) GABAAR channels were not observed in recombinant α1-β1-γ2 GABAARs expressed in L929 cells, nor was conductance increased by diazepam unless the GABAAR-associated protein (GABARAP) was also co-expressed, apparently facilitating clustering of GABAAR proteins through its interaction with the cytoplasmic loop of the γ2 subunit as occurs at synapses with native receptors. Since peptides mimicking the intracellular γ2 loop (γ2 381-403) self-associate, and application of this peptide to the cytoplasmic surface of inside-out patches prevented the diazepam-induced increase in conductance, they hypothesized that the apparent single channel conductance changes induced by BZs are due to synchronized openings of multichannel clustered GABAARs via concerted action through the interacting cytoplasmic loops of conjoined receptors. Such interactions might occur through shared transmembrane domains between interacting receptors, as observed in G-protein-coupled receptors.76 This hypothesis could be addressed using concatenated subunits, chimeras and other strategies. However, synchronization of multiple identical channels by such a mechanism should still require a discrete 9 pS unitary conductance of which the larger conductance states are integer multiples, which has not been reported.
Barbiturates
Phenobarbital was synthesized by Emil Fischer at Bayer in 1911 and introduced as an ASD by Alfred Hauptmann in 1912. Its tendency to produce sedation and cognitive slowing or confusion, as well as paradoxical hyperactivity in children, has curtailed its use in favor of more modern alternatives.77 In addition to their enhancement of GABAAR currents, barbiturates also inhibit repetitive action potential firing at neuronal sodium channels78 by reducing fractional open time and shifting the potential of half-maximal opening towards hyperpolarized potentials.79 These actions contribute to both their antiseizure action and their adverse effect profile.
When co-applied with low concentrations of GABA, barbiturates increased the mean open time, but had no effect on the lengths of 3 distinct open state durations.80 The increase in mean open time resulted from fewer openings in the shorter two durations (O1 and O2) and more long duration (O3) openings. There was also an increase in long burst durations, but no change in the single channel opening frequency or in the closed frequency duration histogram. The increase in channel open time results in greater chloride current flux and increased likelihood that channel openings will summate, producing larger inhibitory currents. In the absence of GABA, high concentrations of pentobarbital (EC50 0.33 mM) and phenobarbital (EC50 3 mM) directly activated GABAAR chloride currents with lower efficacy (smaller maximal currents) than GABA82. Barbiturate-activated GABAAR currents were blocked by bicuculline and picrotoxin, and at high concentrations both phenobarbital and pentobarbital produced open channel block that rapidly terminated the induced currents. The concentrations involved in both direct activation and open channel block are far higher than usual therapeutic levels, thus the main antiseizure mechanism is likely the GABAAR allosteric effect in concert with synaptic and extrasynaptic GABA.
Unlike the BZs, barbiturate sensitivity does not require a specific subunit composition. Homomeric β1 receptors expressed in Xenopus oocytes were directly activated by pentobarbital even though they were not responsive to GABA; these currents were blocked by picrotoxin or penicillin but not bicuculline.83 Pentobarbital also induced current in both α1-β3 and β3 homomeric GABA Rs.84 A At saturating GABA concentrations, pentobarbital markedly potentiated α1-β3-δ currents, increasing desensitization and single channel open duration,85 suggesting that barbiturates may be particularly effective at enhancing tonic GABAAR currents at extrasynaptic sites activated by low GABA concentrations. The ε subunit, which increases spontaneous GABAAR channel openings, significantly reduced86 but did not completely eliminate87 barbiturate responsiveness when co-expressed with α and β subunits. These findings suggest that barbiturate sensitivity does not require either an α or a γ subunit as it is present in β homomeric receptors; however, presence of the δ or ε subunits that substitute for γ can dramatically modify barbiturate responsiveness by altering the efficacy of GABA for activating the channel.
Chimera and mutagenesis studies have identified domains and residues that contribute to barbiturate action, though in less detail than for the BZs. Mutation of the proline (P228A) in the first transmembrane domain of the β1 subunit reduced barbiturate enhancement of GABA-evoked currents in α1-β1-γ2L GABAARs while increasing apparent GABA affinity, without otherwise altering single channel kinetics.88 A chimera study using constructs created from the amino terminal end of the β3 subunit and the carboxyl terminal end of the ρ1 subunit, which forms homomeric GABAC receptors that are insensitive to barbiturates, found that residues of the β3 subunit involved in pentobarbital binding to GABAARs are located downstream from the middle of the M2 region.89 Similarly, mutation of a ρ1 tryptophan (W328) in the third transmembrane domain to a hydrophobic residue produced both allosteric and direct channel activation by pentobarbital.90
Loreclezole
Loreclezole has antiseizure activity in a variety of seizure models, acting more like a barbiturate than a BZ in that the increase in seizure threshold produced by loreclezole was potentiated rather than blocked by the BZ antagonist, flumazenil.91 In the hippocampal slice, loreclezole, potentiated paired pulse inhibition92 and inhibited epileptiform discharges induced by low Ca2+ or low Mg2+. Loreclezole strongly potentiated recombinant GABAARs containing a β2 or β3 subunit but did not enhance currents from β1-containing receptors.93 A single asparagine residue (β2(N289) or β3(N290)) at the cytoplasmic end of the 2nd transmembrane domain confers sensitivity to loreclezole; this amino acid is a serine in the β1 subunit.94 Mutation of β1S290 to N conferred loreclezole sensitivity to β1-containing GABAARs, while mutation of β2N289 or β3N290 to S eliminated loreclezole enhancement. When both β1 and β3 were co-expressed in the same receptor, loreclezole sensitivity was abolished, suggesting a dominant effect of the β1 subunit.95 Coexpression with different alpha subunits altered the degree of loreclezole potentiation (α1=α2=α3>α5>α4) ;96 expression of α5 and β3 with the π subunit reduced loreclezole potentiation,97 while α1-β3-ε receptors showed normal loreclezole enhancement.87
At higher concentrations (> 6 μM) loreclezole caused concentration-dependent inhibition of GABAAR currents by enhancing the rate of apparent desensitization.98 The effect was inconsistent with open channel block as it was voltage independent, non-competitive, and increased with increasing GABA concentration. The BZ site was not involved as the inhibition was not antagonized by flumazenil and did not require a γ subunit. This finding has important clinical implications, since inadvertent high levels of drug could inhibit rather than enhance GABAAR function and potentially trigger seizure activity. Loreclezole inhibited α1-β1-γ2L GABAAR currents that were not potentiated by low concentrations of loreclezole, suggesting separate sites for enhancement and inhibition. At the single channel level, high loreclezole concentrations decreased α1-β1-γ2L mean open time by decreasing the average durations of the open states, and also increased the occurrence of a 20 ms closed state. Loreclezole inhibition was equally effective when applied to the intracellular side of the receptor, suggesting that its inhibitory binding site was accessible from both sides of the membrane, and pre-application of loreclezole prior to GABA inhibited the subsequent GABAAR current, indicating that binding did not require an open channel. Loreclezole was also found to inhibit ρ1 homomeric GABAC receptors with an IC50 of 0.5 μM, which was proposed as a rapid means of pharmacological identification of these receptors.99
Ganaxolone
Ganaxolone (3α-OH-3β-methyl-5α-pregnan-20-one) is the 3β-methylated synthetic analog of the neurosteroid, allopregnanolone, a natural metabolite of progesterone that allosterically enhances GABAAR current. It has a broad range of antiseizure activity in animal epilepsy models100 including seizures induced by bicuculline, t-butylbicyclophosphorothionate (TBPS, a high affinity ligand for the GABAAR picrotoxin site), aminophylline and corneal kindling, and is well tolerated and effective against seizures in humans. Ganaxolone is currently under evaluation for partial onset seizures. It may have special utility in women with catamenial epilepsy who have increased seizures during periods of low progesterone in the menstrual cycle, as well as in children with infantile spasms.101
The antiseizure effect of neurosteroids is not due to action at the progesterone receptor (PR), as exogenous allopregnanolone prevented seizures in PR knockout mice.102 Moreover, progesterone’s antiseizure effect requires metabolism to allopregnanolone, as it was blocked by finasteride, a 5α-reductase inhibitor that blocks allopregnanolone synthesis from progesterone. Hence, the antiseizure effect of allopregnanolone (and ganaxolone) is mediated by GABAARs.
Specific neurosteroids can allosterically modulate GABAAR activity, either positively or negatively, via distinct binding sites on GABAARs.104 Since a variety of GABAAR-interacting steroid hormones are synthesized in the brain,105 these agents represent endogenous modulators of GABAAR function. Allopregnanolone is synthesized in two steps from progesterone: 5α-reductase converts progesterone to 5α-dihydroprogesterone, then 3-α-hydroxysteroid oxidoreductase reversibly converts 5α-dihydroprogesterone to 3α-OH-5α-pregnan-20-one (allopregnanolone). Addition of the 3β-methyl group on ganaxolone does not alter binding to GABAARs, but prevents further metabolism of the 3α-OH group and thus prolongs its GABAAR-modulating actions.100 Ganaxolone enhanced GABA and BZ binding via positive allosteric modulation of GABAAR activity, and at nanomolar concentrations potentiated GABA-evoked currents at α1-β1-γ2L, α2-β1-γ2L or α3-β1-γ2L GABAARs expressed in Xenopus oocytes, while direct activation of chloride flux occurred to a limited extent only at micromolar concentrations.100
The effects of GABAAR-enhancing neurosteroids on single channel GABAAR currents were studied using androsterone (5α-androstan-3α-ol-17-one) and pregnanolone (5β-pregnan-3α-ol-20-one) but likely apply to other positive steroid GABAAR modulators including ganaxolone. Like the barbiturates and loreclezole, these agents increased the proportion of openings to the two longer open states (O2 and O3) without altering the intrinsic durations of those states, and produced longer burst durations.106 There was no change in single channel conductance. Unlike the barbiturates, however, the neurosteroids also increased single channel opening frequency and reduced closed time durations in all but the shortest closed time distributions (thought to represent intraburst closures). At high concentrations (10 μM), both agents reduced open channel durations consistent with open channel (“flickering”) block.
The subunit selectivity of GABAAR-enhancing neurosteroids was initially controversial. An early study107 of recombinant receptors expressed from combinations of α1, α6, β3, γ2 and δ showed loss of neurosteroid responsiveness with combinations containing the delta subunit. There was also reduced sensitivity of cerebellar granule neurons to neurosteroids later in in vitro development, when δ subunit expression increases. These findings were interpreted as showing inhibition of neurosteroid responsiveness by inclusion of the δ subunit. However, mice in which the δ subunit was knocked out showed decreased behavioral sensitivity to neurosteroids.108 Moreover, in receptors composed of α1 or α6, β3 and either γ2L or δ, the greatest potentiation by tetrahydrodeoxycorticosterone (THDOC) was seen in α1-β3-δ GABAARs.109 At high concentrations (1 μM), THDOC inhibited this isoform. There is currently consensus that δ subunit-containing receptors are more sensitive to neurosteroid enhancement, though other subunits are also involved in mediating neurosteroid actions. Presence of the α6 subunit reduced neurosteroid sensitivity.110 THDOC (1 μM) enhanced α1-β3-δ more than α6-β3-δ currents, but increased the extent of desensitization and prolonged deactivation for both receptor isoforms; α1-α6 and α6-α1 chimeras (spliced in transmembrane domain M1) suggested that differences in deactivation rate and its voltage-dependence correlated with N-terminal domains, while the extent of desensitization and its voltage-dependence correlated with C-terminal domains.111 In dentate granule cells from epileptic animals, an increase in α4 subunit expression was associated with decreased neurosteroid enhancement.112 In receptors composed of one α subunit (from α2 through α5), β2 and γ2S, neurosteroid potentiation was dependent on the conserved glutamine residue (α1(Q241)) in the first transmembrane domain of the respective α subunit.113 The δ subunit did not affect neurosteroid binding but likely influenced the efficacy of neurosteroid potentiation. Mutation of α1Q241 to L abolished neurosteroid potentiation, while mutation to W mimicked the effect of steroids and prevented further augmentation; the neighboring S240 residue also participated in steroid binding.114 A subsequent study using concatenated subunits demonstrated that a single functional binding site (not disrupted by the Q241 mutation) was sufficient for neurosteroid modulation.115
GABAAR effects of other ASDs
Topiramate
Topiramate is a heterotricyclic sulfamate with several mechanisms of action including inhibition of sodium and calcium channels, inhibition of carbonic anhydrase, and augmentation of GABA-evoked currents. Topiramate’s effects on GABAARs may contribute both to its antiseizure efficacy and its side effect profile including memory problems, fatigue and psychomotor slowing. It is approved for partial and generalized seizures, the Lennox Gastaut syndrome, and migraine.
Topiramate inhibited voltage-gated sodium channels, with a left shift of the steady state inactivation curve116 resulting in intermittent blockade of sustained repetitive action potential firing during prolonged depolarization.117 It also blocked repetitive firing in hippocampal CA3 neurons from spontaneously epileptic rats, and reduced excitatory post-synaptic potentials and responses to bath-applied glutamate, suggesting blockade of post-synaptic glutamate receptors,118 affecting kainate-evoked but not NMDA-evoked currents.119 Topiramate also inhibited both L-type and non L-type high voltage activated calcium currents120 and reduced bicarbonate production via inhibition of carbonic anhydrase,121 which may contribute to its antiseizure effect.
Topiramate (10 μM) enhanced chloride flux into cerebellar granule neurons stimulated by 10 μM GABA, but did not significantly increase chloride influx alone.122 It also enhanced GABA-evoked currents in cultured cortical neurons that were insensitive to the BZ, clonazepam, and clonazepam potentiated GABA currents in topiramate-insensitive neurons, confirming that topiramate’s site of action on GABAARs is independent of the BZ site.123 Subunit selectivity studies have been inconsistent. Topiramate (1-100 μM) reversibly inhibited Cl− currents evoked by 1-10 μM GABA in Xenopus oocytes expressing α1-β2-γ2S and α2-β2-γ2S GABAARs, and reduced the apparent desensitization (“current-fading rate”) in α1-β2-γ2S-expressing oocytes, but potentiated GABA-evoked Cl− currents and increased the desensitization rate in α6-β2-γ2S GABAARs, with no effect on α4-β2-γ2S receptors or mixed population GABAARs expressed from rat brain mRNA.124 In contrast, another study found that topiramate could both potentiate and directly activate β2 or β3-containing heteromeric GABAARs, with greatest effect on α4-β3-γ2S;125 positive or negative effects on β1-containing GABAARs depended on the co-expressed alpha subunit. Additional studies are needed to clarify the site and mechanism of action.
Felbamate
Felbamate was launched as a promising novel ASD in 1993 with an uncertain mechanism of action, but its use was curtailed after early reports of aplastic anemia126 and hepatic failure.127 Felbamate inhibited binding of the GABA antagonist [3H]T-BOB with a regional pattern different from that produced by GABA agonists, bicuculline, zinc or neurosteroids,128 and enhanced GABA-elicited Cl− currents in cultured cortical neurons. Felbamate enhancement was not blocked by flumazenil, and felbamate did not affect pentobarbital potentiation or PTX inhibition of GABA-evoked currents, suggesting an independent site of action. It prolonged the mean burst duration of GABA-activated single channel currents, suggesting a barbiturate-like effect.129 Felbamate also blocked N-methyl-D-aspartate (NMDA) receptor currents, an alternative possible antiseizure mechanism.130 Derivative compounds including fluorofelbamate and carisbamate that are not associated with hematopoietic or hepatic toxicity may revive interest in felbamate–like agents.131
Ezogabine
Ezogabine (formerly retigabine) is a novel ASD effective in a variety of animal models. Its primary effect is enhanced activation of heteromeric potassium channels composed of the KCNQ2 and KCNQ3 subunits132 which underlie the “M current” that is a major determinant of resting membrane potential and neuronal excitability. However, ezogabine also dose-dependently and reversibly potentiated GABAAR-dependent IPSC peak amplitude, decay times and total charge transfer.133 EPSCs were unaffected, and paired pulse depression was unchanged, suggesting a post-synaptic effect at GABAARs. Ezogabine potentiated GABA-induced currents in rat cortical neurons in a concentration-dependent fashion at 10 μM and above.132 This action was not antagonized by flumazenil, indicating a non-BZ site of action. Subunit dependence and binding site are not known.
Losigamone
Losigamone is a novel ASD which inhibited the persistent component of sodium currents in hippocampal neurons at depolarized potentials, suppressed sustained repetitive firing134 and decreased the frequency of spontaneous action potentials without affecting miniature post-synaptic current amplitudes.135 Losigamone stimulated 36Cl− influx into spinal cord neurons in the absence of GABA, and potentiated 36Cl− influx stimulated by submaximal GABA concentrations; both effects were blocked by bicuculline or picrotoxin.136 Losigamone did not affect the specific binding of [3H]GABA, [3H]flunitrazepam, or [35S]t-butyl-bicyclophosphorothionate (TBPS) to their receptors, and there was no difference in the effect of the + or – stereoisomers or the racemic mixture. The site of action at GABAARs is unknown.
Stiripentol
Stiripentol is as an adjunct ASD which was thought to act by inhibiting cytochrome P450 enzymes involved in metabolism of conventional ASDs. Recently, stiripentol was found to enhance recombinant GABAAR currents, with greater potentiation of α3-containing receptors and reduced potentiation with the β1 or ε subunits.137 It caused a leftward shift in the GABA concentration-response relationship without increasing maximal GABA-evoked currents, and did not involve sites associated with neurosteroid or loreclezole potentiation.137 Saturating barbiturate sites with pentobarbital occluded stiripentol enhancement, and stiripentol increased the duration but not the frequency of opening of GABAAR channels, suggesting a barbiturate-like mechanism.138
GABA-enhancing agents
An additional mechanism for enhancing GABAAR-mediated inhibition involves increasing the concentration or duration of GABA in the synaptic cleft and perisynaptic/extrasynaptic sites. Enhancing GABA synthesis or blocking its reuptake or catabolism could prolong GABA IPSPs and increase perisynaptic spill-over, increasing tonic/extrasynaptic GABA currents which likely play a major role in seizure prevention.
Gabapentin and Pregabalin
Gabapentin was designed as a GABA analog, and some studies have suggested that it modulates the action of the GABA synthetic enzyme, glutamic acid decarboxylase (GAD) and the glutamate synthesizing enzyme, branched-chain amino acid transaminase, resulting in increased GABA synthesis.139 Gabapentin increases non-synaptic GABA responses from neuronal tissues in vitro and increases GABA levels in brain.140 Its other (likely primary) mode of action involves binding to the α2-δ binding site on L- or P/Q-type presynaptic voltage-gated calcium channels, presumably inhibiting excessive neurotransmitter release141 by interfering with calcium channel functional expression or trafficking142. Pregabalin, which binds to the α2-δ calcium channel subunit with higher potency, may have similar mechanisms of action. Most studies emphasize the calcium channel as the primary site,143 though a contributory effect on GABA metabolism is possible.144
Valproic Acid
Valproic acid may affect GABA production, among other ASD mechanisms. Valproate caused non-significant increases in cerebral GABA levels but elevated brain GAD activity significantly.145 Valproic acid increased GABA synthesis and release in brain regions including substantia nigra,146 which is thought to be involved in the control of seizure generation and propagation.147 It also reduced the release of gamma-hydroxybutyric acid and attenuated neuronal excitation induced by NMDA-type glutamate receptors.147 Additional ASD mechanisms include enhancing sodium channel inactivation (like phenytoin) and reducing T-type Ca2+ channel currents (like ethosuximide),148 perhaps explaining its utility against both partial onset and absence seizures.
Tiagabine
Tiagabine is a derivative of nipecotic acid that binds to the presynaptic GAT-1 GABA transporter and blocks GABA reuptake, without acting as a “false neurotransmitter.” In control hippocampal slices, tiagabine alone induced a significant chloride conductance, suggesting that GAT-1 activity controls the basal level of extracellular GABA.149 Tiagabine prolonged hippocampal IPSC duration in a lamina-specific fashion, with greater effect in stratum radiatum (167%) than stratum oriens (115%).150 Tiagabine reduced the frequency of epileptiform discharges in hippocampal slices exposed to low Mg2+ or 4-aminopyridine.151 The ability of tiagabine to prolong GABA currents was preserved in hippocampal slices from pilocarpine-treated epileptic rats, hence the GAT-1 transporter remains a functional target for regulating GABA levels in this model of temporal lobe epilepsy.152 Tiagabine was effective as an adjunct agent against partial onset seizures, though its prolonged titration period, requirement for multiple daily doses and CNS side effect profile (dizziness, tremor, somnolence, mood disturbances and rare psychotic symptoms) have limited its use.153
Vigabatrin
Vigabatrin (γ-vinyl-GABA) has a unique mechanism of action, functioning as a “suicide inhibitor” of GABA transaminase, the primary enzyme for GABA catalysis. This leads to permanent inhibition of the affected enzyme, requiring synthesis of new GAT-1 and hence prolonging the biological half-life beyond its pharmacokinetic half-life of 8 hours.154 It is effective against complex partial seizures,155 Lennox-Gastaut syndrome and West Syndrome, for which it has become an alternative to ACTH156 particularly in children with tuberous sclerosis.157 Vigabatrin increases the extracellular concentrations of GABA both in vitro158 and in vivo.159 The anti-seizure effects of increasing GABA concentration may be complex. Biphasic responses to vigabatrin administration have been observed, with an early proconvulsant effect.160 A biphasic effect was also seen on after-discharge duration with an early facilitation of the limbic pattern of epileptiform discharge and later suppression at higher doses.161 Elevated GABA concentrations may increase neuronal synchronization, particularly of thalamic neurons in absence epilepsy, and there have been several reports of vigabatrin worsening absence seizures or precipitating absence status epilepticus.162
A major limitation to the use of vigabatrin is the relatively high incidence of vision disturbance associated with chronic use. In 21 of 30 children treated with vigabatrin who were screened for vision disturbances despite no report of visual symptoms, 4 had visual field constrictions, which did not improve after discontinuation of the drug.163 One study associated visual disturbances with infantile spasms rather than the drug.164 The prevalence of vision problems ranged from 10 to 40% of pediatric and adult patients exposed to vigabatrin.165 This adverse effect may be directly related to elevated GABA concentrations, as hippocampal neurons grown in depolarizing conditions (25 mM KCl) showed 20% loss of MAP-2-positive neurons in the presence of vigabatrin, which was mimicked by GABA (100 μM) and blocked by bicuculline or picrotoxin.166 The cellular mechanism of this toxicity is not entirely clear, but in situations in which GABA is depolarizing, excessive GABA could promote calcium entry through L-type (slowly desensitizing) voltage-gated calcium channels,167 which in turn could result in activation of caspases and cell death.168
Excitatory GABAA Currents
Early in CNS development, neurons express the Na+/K+/Cl− cotransporter, NKCC1, rather than the K+/Cl− cotransporter, KCC2, which is expressed in adult neurons. NKCC1 increases intracellular Cl− resulting in a depolarizing Cl− reversal potential, while KCC2 exports Cl− yielding the hyperpolarizing Cl− reversal potential found in adult neurons.169 As a result, activation of GABAARs may be excitatory during early development.170 Excitatory GABAAR currents may play a trophic role in neuronal migration and connectivity,171 but may also contribute to epileptogenesis.172 Endogenous GABA appears to be proconvulsant in early postnatal rat hippocampal slices, as GABAA antagonists blocked epileptiform activity induced by depolarization with high external [K+]173. However, BZ and barbiturate antiseizure efficacy appear to be intact, likely because persistent opening of GABAAR channels (in the presence of allosteric agents) may reduce the depolarizing chloride reversal potential, resulting in “shunt” inhibition. Alternatively, subthreshhold GABA-evoked depolarization may inactivate sodium channels and prevent action potential firing.174 The current through GABAAR channels can also be altered by changes in intracellular bicarbonate, [HCO3−],169 which can flow through the channel.175 Changes in [HCO3−] may underlie reduced synaptic GABA currents during development of BZ tolerance.176 Depolarizing GABAAR currents may also be a source of interictal spike activity, as observed in epileptic subiculum neurons in hippocampal brain slices from patients with temporal lobe epilepsy.177 Changes in the GABA current reversal potential might also explain why diazepam can be less effective in children with epileptic encephalopathies,178 and rarely can cause status epilepticus in patients with the Lennox Gastaut syndrome.179
Bumetanide, an inhibitor of NKCC-1 used clinically as a potent loop diuretic, has been proposed as an adjunctive agent to reduce intraneuronal chloride accumulation and reverse the depolarizing chloride gradient that makes GABAARs excitatory, particularly in the neonatal setting.170 Bumetanide improved the responsiveness of hippocampal slices to phenobarbital in reducing epileptiform discharges induced by low Mg2+,180 and also suppressed electrographic seizures in neonatal rats.172 Depolarizing GABA currents may occur in cortical more than subcortical brain regions, possibly explaining the electroclinical uncoupling that occurs with phenobarbital in neonatal seizures.181
Bumetanide has been used successfully in a single reported human neonate with seizures,182 and a clinical trial (NCT00830531) is in progress. However, elimination of the depolarizing Cl− gradient during development may have adverse consequences; a permanent reduction in AMPAR-mediated excitatory neurotransmission and sensorimotor gating deficits were observed after bumetanide exposure in neonatal rats.183 Such potential adverse effects will have to be weighed against the benefits associated with seizure termination or prevention.
GABAAR subunit changes and ASD efficacy in epilepsy and status epilepticus
There is considerable evidence that GABAAR composition and function change with epilepsy, both acutely in the setting of status epilepticus and in chronic epilepsy. During status epilepticus, there is a rapid change in GABAAR function that reduces BZ sensitivity,184 apparently due to activity-dependent internalization of GABAARs and replacement with BZ-insensitive receptors.185 Kainic acid-induced status epilepticus in young (postnatal day 9) rats altered the normal developmental pattern of GABAAR subunit maturation,186 preventing the normal switch from α2 to α1-containing GABAARs. These changes correlate with the finding in animal models15,184,187 and humans188,189 that BZs are effective early in the course of status epilepticus but lose their potency and efficacy for termination of status with longer seizure duration. These findings increase the urgency for treatment of status epilepticus early in its course, and also suggest the need to find alternative treatments that retain efficacy in refractory status epilepticus, or methods to prevent or reverse the GABAAR changes that occur with prolonged seizures.
In the pilocarpine model of temporal lobe epilepsy, the α1 subunit in dentate granule neurons was downregulated and α4 subunit was upregulated,190 while in younger (postnatal day 20) animals, the α1 subunit was increased after status epilepticus.191 GABAAR subunit changes after pilocarpine-induced status are associated with altered physiological properties including reduced neurosteroid sensitivity and an increase in α4-containing receptors colocalized with synapses rather than extracellular sites.112 Such changes might contribute to the propensity for spontaneous seizures or alternatively represent a functional adaptation to reduce seizure expression. Substitution of α1 with α4 might reduce synaptic (phasic) but increase extrasynaptic (tonic) inhibition, and BZ sensitivity would be lost in α4-containing receptors. Using an adenovirus vector to insert the α1 subunit gene driven by the α4 promoter resulted in a decrease in seizure frequency, suggesting that the GABAAR subunit changes were contributory toward epileptogenesis.192 Hence, altering GABAAR subunit expression in brain regions critical to seizure development may provide a new therapeutic strategy for seizure prevention. Alternatively, novel ASDs targeting the specific epilepsy-associated subunit composition might provide a less invasive approach toward seizure control.
Long term exposure to the GABAAR allosteric agents themselves can alter GABAAR composition and function. In rat pups exposed to therapeutic concentrations of diazepam or phenobarbital from postnatal day 10 through 40, then tapered for 2 weeks and euthanized on postnatal day 90 showed increased mRNA expression in dentate granule neurons for GAT-1, 3 and 4, GABAAR subunits α4, α6, β3, δ and θ and GABAB receptor subunit R1, and decreased mRNA expression for GAD65, GAD67 and GABAAR subunits α1 and α3.193 Tolerance- and dependence-associated changes in GABAAR subunit composition with chronic use of BZs194,195 and barbiturates196,197 have been reported, and may reflect tolerance to either the antiseizure or adverse CNS effects. However, these findings suggest that not only seizures, but their prolonged treatment with GABAergic agents, can alter inhibitory neuronal function for extended periods or even permanently, with possible cognitive and behavioral consequences that must be considered when contemplating chronic GABAergic ASD use.
Table 1.
Antiseizure Drugs and their GABAAR Effects
| Anti-Seizure Drug | Subunit Specificity |
Site of Action |
GABAAR Action | Other Mechanisms |
|---|---|---|---|---|
| Benzodiazepines | α1-3, α5; γ | α/γ interface, α1(H101) |
Left shift of GABA C/R curve, ↑ open frequency |
Possible increased GABAAR channel conductance |
| Zolpidem | α1>α2,3; γ | α/γ interface | “ “ | “ “ |
| Barbiturates | β subunits? | M3 residues? | ↑ channel open time & burst duration |
Use-dependent Na+ channel block |
| Loreclezole | β2, β3 | B2(N289) | Barbiturate-like? | inhibits GABAAR at ↑ concentration |
| Ganaxolone | α, β, δ, ↓ with ε |
α1(Q241) | Barbiturate-like, also ↑ open frequency |
Open channel block at high conc. |
| Topiramate | α6 >α4> α1- α2? |
unknown | Enhanced GABA currents |
Na+ /Ca2+ channel block, AMPA/kainate receptor block |
| Felbamate | unknown | unknown | Barbiturate-like | Blocks NMDA receptor currents |
| Retigabine | unknown | unknown | Increased GABA IPSCs |
Opens KCNQ2/3 potassium channels |
| Losigamone | unknown | unknown | Enhanced GABA receptor current |
Use-dependent Na+ channel block |
| Stiripentol | α3, ↓ with β1, ε |
unknown | Barbiturate-like | CYP450 inhibition |
| Vigabatrin | GABA transaminase |
– | increases [GABA] | – |
| Gabapentin, pregabalin |
Glutamic acid decarboxylase |
– | ↑ GABA synthesis? | α2-δ subunit of voltage-gated Ca2+ channel |
| Valproic Acid | Glutamic acid decarboxylase |
– | ↑ GABA synthesis? | block of Na+, T- type Ca2+ channels |
| Tiagabine | GAT-1 (GABA transporter) |
– | Blocks GABA reuptake |
– |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- 2.Olsen RW, DeLorey TM, Gordey M, Kang MH. GABA receptor function and epilepsy. Advances in Neurology. 1999;79:499–510. [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Seizures beget seizures: the quest for GABA as a key player. Crit Rev.Neurobiol. 2006;18:135–144. doi: 10.1615/critrevneurobiol.v18.i1-2.140. [DOI] [PubMed] [Google Scholar]
- 4.Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Advances in Experimental Medicine and Biology. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald RL, Olsen RW. GABAA receptor channels. Annual Reviews of Neuroscience. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 6.Nayeem N, Green TP, Martin IL, Barnard EA. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. Journal of Neurochemistry. 1994;62:815–818. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- 7.Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. Journal of Biological Chemistry. 1997;272:15346–15350. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- 8.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anesthetic agents conferred by a class of GABAA receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 9.Bonnert TP, McKernan RM, Le Bourdelles B, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford K, Whiting PJ. θ, a novel γ-aminobutyric acid type A subunit. Proc.Natl.Acad.Sci.U.S.A. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. Journal of Neuroscience. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks-Kayal AR, Jin H, Price M, Dichter MA. Developmental expression of GABAA receptor subunit mRNAs in individual hippocampal neurons in vitro and in vivo. Journal of Neurochemistry. 1998;70:1017–1028. doi: 10.1046/j.1471-4159.1998.70031017.x. [DOI] [PubMed] [Google Scholar]
- 12.Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 13.Wingrove PB, Wafford KA, Bain C, Whiting PJ. The modulatory action of loreclezole at the γ-aminobutyric acid type A receptor is determined by a single amino acid in the β2 and β3 subunit. Proc.Natl.Acad.Sci.U.S.A. 1994;91:4569–4573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 15.Jones-Davis DM, Macdonald RL. GABA(A) receptor function and pharmacology in epilepsy and status epilepticus. Curr.Opin.Pharmacol. 2003;3:12–18. doi: 10.1016/s1471-4892(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 16.Kokaia M, Pratt GD, Elmer E, Bengzon J, Fritschy JM, Kokaia Z, Lindvall O, Mohler H. Biphasic differential changes of GABAA receptor subunit mRNA levels in denate gyrus granule cells following recurrent kindling-induced seizures. Molecular Brain Research. 1994;23:323–332. doi: 10.1016/0169-328x(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 17.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nature Medicine. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 18.Loup F, Weiser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. Journal of Neuroscience. 2000;20(14):5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karle J, Woldbye DP, Elster L, Diemer NH, Bolwig TG, Olsen RW, Nielsen M. Antisense oligonucleotide to GABA(A) receptor gamma2 subunit induces limbic status epilepticus. Journal of Neuroscience Research. 1998;54(6):863–869. doi: 10.1002/(SICI)1097-4547(19981215)54:6<863::AID-JNR14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto A, Kumagai T, Miura K, Miyazaki S, Hayakawa C, Yamanaka T. Epilepsy in Angelman syndrome associated with chromosome 15q deletion. Epilepsia. 1992;33:1083–1090. doi: 10.1111/j.1528-1157.1992.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 21.DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. Journal of Neuroscience. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci. 2002;22(13):5321–5327. doi: 10.1523/JNEUROSCI.22-13-05321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme J-F, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nature Genetics. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 24.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nature Genetics. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 25.Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse Ml, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nature Genetics. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 26.Bowser DN, Wagner DA, Czajkowski C, Cromer BA, Parker MW, Wallace RH, Harkin LA, Mulley JC, Marini C, Berkovic SF, Williams DA, Jones MV, Petrou S. Altered kinetics and benzodiazepine sensitivity of a GABAA receptor subunit mutation [gamma 2(R43Q)] found in human epilepsy. Proc.Natl.Acad.Sci.U.S.A. 2002;99:15170–15175. doi: 10.1073/pnas.212320199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frugier G, Coussen F, Giraud MF, Odessa MF, Emerit MB, Boue-Grabot E, Garret M. A gamma 2(R43Q) mutation, linked to epilepsy in humans, alters GABAA receptor assembly and modifies subunit composition on the cell surface. Journal of Biological Chemistry. 2007;282:3819–3828. doi: 10.1074/jbc.M608910200. [DOI] [PubMed] [Google Scholar]
- 28.Hales TG, Tang H, Bollan KA, Johnson SJ, King DP, McDonald NA, Cheng A, Connolly CN. The epilepsy mutation, gamma2(R43Q) disrupts a highly conserved inter-subunit contact site, perturbing the biogenesis of GABAA receptors. Mol.Cell Neurosci. 2005;29:120–127. doi: 10.1016/j.mcn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Sancar F, Czajkowski C. A GABAA receptor mutation linked to human epilepsy (gamma2R43Q) impairs cell surface expression of alphabetagamma receptors. Journal of Biological Chemistry. 2004;279:47034–47039. doi: 10.1074/jbc.M403388200. [DOI] [PubMed] [Google Scholar]
- 30.Kang JQ, Macdonald RL. The GABAA receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum. Journal of Neuroscience. 2004;24:8672–8677. doi: 10.1523/JNEUROSCI.2717-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sternbach LH, Reeder E. Quinazolines and 1,4-benzodiazepines, IV: transformations of 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine-4-oxide. J Org Chem. 1961;26:4936–4941. [Google Scholar]
- 32.Sternbach LH, Fryer RI, Keller O, et al. Quinazolines and 1,4-benzodiazepines, X: nitro-substituted 5-phenyl-1,4-benzodiazepine derivatives. J Medicinal Chem. 1963;6:261–265. doi: 10.1021/jm00339a010. [DOI] [PubMed] [Google Scholar]
- 33.Gastaut H, Naquet R, Poire R, Tassarini CH. Treatment of status epilepticus with diazepam (valium) Epilepsia. 1965;6:167–182. doi: 10.1111/j.1528-1157.1965.tb03786.x. [DOI] [PubMed] [Google Scholar]
- 34.Sato S. Benzodiazepines, clonazepam. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic drugs. Raven Press; New York: 1995. pp. 725–734. [Google Scholar]
- 35.Chapman AG, Horton RW, Meldrum BS. Anticonvulsant action of a 1,5-benzodiazepine, clobazam, in reflex epilepsy. Epilepsia. 1978;19:293–299. doi: 10.1111/j.1528-1157.1978.tb04492.x. [DOI] [PubMed] [Google Scholar]
- 36.Munn R, Farrell K. Open study of clobazam in refractory epilepsy. Pediatric Neurology. 1993;9:465–469. doi: 10.1016/0887-8994(93)90026-9. [DOI] [PubMed] [Google Scholar]
- 37.Shannon M, Albers G, Burkhardt K. Safety and efficacy of flumazenil in the reversal of benzodiazepine-induced conscious sedation. Journal of Pediatrics. 1997;131:582–586. doi: 10.1016/s0022-3476(97)70066-0. [DOI] [PubMed] [Google Scholar]
- 38.Mullins ME. First-degree atrioventricular block in alprazolam overdose reversed by flumazenil. Journal of Pharmacy and Pharmacology. 1999;51:367–370. doi: 10.1211/0022357991772385. [DOI] [PubMed] [Google Scholar]
- 39.Turski L, Stephens DN, Jensen LH, Petersen EN, Meldrum BS, Patel S, Hansen JB, Loscher W, Schneider HH, Schmiechen R. Anticonvulsant action of the beta-carboline abecarnil: studies in rodents and baboon, Papio papio. Journal of Pharmacology and Experimental Therapeutics. 1990;253:344–352. [PubMed] [Google Scholar]
- 40.Zanotti A, Mariot R, Contarino A, Lipartiti M, Giusti P. Lack of anticonvulsant tolerance and benzodiazepine receptor downregulation with imidazenil in rats. British Journal of Pharmacology. 1996;117:647–652. doi: 10.1111/j.1476-5381.1996.tb15239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rundfeldt C, Wlaz P, Honack D, Loscher W. Anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. Comparison of diazepam, bretazenil and abecarnil. J.Pharmacol.Exp.Ther. 1995;275:693–702. [PubMed] [Google Scholar]
- 42.Hernandez TD, Heninger C, Wilson MA, Gallager DW. Relationship of agonist efficacy to changes in GABA sensitivity and anticonvulsant tolerance following chronic benzodiazepine ligand exposure. European Journal of Pharmacology. 1989;170(3):145–155. doi: 10.1016/0014-2999(89)90535-9. [DOI] [PubMed] [Google Scholar]
- 43.Haefely W, Kyburz E, Gerecke M, Mohler H. Recent advances in the molecular pharmacology of benzodiazepine receptors and in the structure-activity relationships of their agonists and antagonists. Adv.Drug Res. 1985;14:165–322. [Google Scholar]
- 44.Polc P. Electrophysiology of benzodiazepine receptor ligands: multiple mechanisms and sites of action. Progress in Neurobiology. 1988;31:349–423. doi: 10.1016/0301-0082(88)90014-7. [DOI] [PubMed] [Google Scholar]
- 45.Randall LO, Kappell B. Pharmacological activity of some benzodiazepines and their metabolites. In: Garattini S, Mussini E, Randall LO, editors. The Benzodiazepines. Raven Press; New York: 1973. pp. 27–51. [Google Scholar]
- 46.Rogawski MA, Porter RJ. Antiepileptic drugs: Pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacological reviews. 1990;42(No.3):223–286. [PubMed] [Google Scholar]
- 47.Swinyard EA, Castellion AW. Anticonvulsant properties of some benzodiazepines. J.Pharmacol.Exp.Ther. 1966;151:369–375. [PubMed] [Google Scholar]
- 48.Albertson TE, Stark LG, Derlet RW. Modification of amygdaloid kindling by diazepam in juvenile rats. Brain Research.Developmental Brain Research. 1990;51(2):249–252. doi: 10.1016/0165-3806(90)90282-4. [DOI] [PubMed] [Google Scholar]
- 49.Verdoorn TA, Draguhn A, Ymer S, Seeburg PH, Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- 50.Lüddens H, Korpi ER, Seeburg P. GABAA/benzodizaepine receptor heterogeneity: neurophysiological implications. Neuropharmacology. 1995;34(3):245–254. doi: 10.1016/0028-3908(94)00158-o. [DOI] [PubMed] [Google Scholar]
- 51.Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends in Pharmacological Sciences. 1995;16(5):162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 52.Dunn SMJ, Davies M, Muntoni AL, Lambert JJ. Mutagenesis of the rat α1 subunit of the γ-aminobutyric acidA receptor reveals the importance of residue 101 in determining the allosteric effects of benzodiazepine site ligands. Molecular Pharmacology. 1999;56:768–774. [PubMed] [Google Scholar]
- 53.Korpi ER, Kleingoor C, Kettenmann H, Seeburg PH. Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature. 1993;361:356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- 54.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subunit. Nature Neuroscience. 2000;3(6):529–530. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 55.Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. British Journal of Pharmacology. 2000;131(7):1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 57.Crestani F, Low K, Keist R, Mandelli M, Mohler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Molecular Pharmacology. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 58.Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc.Natl.Acad.Sci.U.S.A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–238. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. Journal of Neuroscience. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg HC, Tietz EI, Chiu TH. Tolerance to anticonvulsant effects of diazepam, clonazepam, and clobazam in amygdala-kindled rats. Epilepsia. 1989;30:276–285. doi: 10.1111/j.1528-1157.1989.tb05299.x. [DOI] [PubMed] [Google Scholar]
- 62.Macdonald R, Barker JL. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature. 1978;271:563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- 63.Tietz EI, Zeng XJ, Chen S, Lilly SM, Rosenberg HC, Kometiani P. Antagonist-induced reversal of functional and structural measures of hippocampal benzodiazepine tolerance. J.Pharmacol.Exp.Ther. 1999;291:932–942. [PubMed] [Google Scholar]
- 64.Study RE, Barker JL. Diazepam and (−)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of γ-aminobutyric acid responses in cultured central neurons. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rogers CJ, Twyman RE, Macdonald RL. Benzodiazepine and beta-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J.Physiol.(Lond) 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of γ-aminobutyric acid receptor channels by diazepam and phenobarbital. Annals of Neurology. 1989;25:213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- 67.Bianchi MT, Botzolakis EJ, Lagrange AH, Macdonald RL. Benzodiazepine modulation of GABA(A) receptor opening frequency depends on activation context: a patch clamp and simulation study. Epilepsy Research. 2009;85:212–220. doi: 10.1016/j.eplepsyres.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. Journal of Physiology. 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory post-synaptic currents in hippocampal neurons. Proc.Natl.Acad.Sci.USA. 1992;78:7180–7184. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 70.Eghbali M, Curmi JP, Birnir B, Gage PW. Hippocampal GABA(A) channel conductance increased by diazepam. Nature. 1997;388:71–75. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- 71.Eghbali M, Birnir B, Gage PW. Conductance of GABAA channels activated by pentobarbitone in hippocampal neurons from newborn rats. J.Physiol. 2003;552:13–22. doi: 10.1113/jphysiol.2003.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaul S, Ozsarac N, Liu L, Fink RH, Gage PW. The neuroactive steroids alphaxalone and pregnanolone increase the conductance of single GABAA channels in newborn rat hippocampal neurons. J.Steroid Biochem.Mol.Biol. 2007;104:35–44. doi: 10.1016/j.jsbmb.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 73.Eghbali M, Gage PW, Birnir B. Effects of propofol on GABAA channel conductance in rat-cultured hippocampal neurons. European Journal of Pharmacology. 2003;468:75–82. doi: 10.1016/s0014-2999(03)01641-8. [DOI] [PubMed] [Google Scholar]
- 74.Birnir B, Eghbali M, Cox GB, Gage PW. GABA concentration sets the conductance of delayed GABAA channels in outside-out patches from rat hippocampal neurons. Journal of Membrane Biology. 2001;181:171–183. doi: 10.1007/s00232-001-0021-5. [DOI] [PubMed] [Google Scholar]
- 75.Everitt AB, Luu T, Cromer B, Tierney ML, Birnir B, Olsen RW, Gage PW. Conductance of recombinant GABA (A) channels is increased in cells co-expressing GABA(A) receptor-associated protein. Journal of Biological Chemistry. 2004;279:21701–21706. doi: 10.1074/jbc.M312806200. [DOI] [PubMed] [Google Scholar]
- 76.Breitwieser GE. G protein-coupled receptor oligomerization: implications for G protein activation and cell signaling. Circulation Research. 2004;94:17–27. doi: 10.1161/01.RES.0000110420.68526.19. [DOI] [PubMed] [Google Scholar]
- 77.Kwan P, Brodie MJ. Phenobarbital for the treatment of epilepsy in the 21st century: a critical review. Epilepsia. 2004;45:1141–1149. doi: 10.1111/j.0013-9580.2004.12704.x. [DOI] [PubMed] [Google Scholar]
- 78.Macdonald RL, McLean MJ. Anticonvulsant drugs: mechanisms of action. In: Delgado-Escueta AV, Ward AA, Woodbury DM, Porter RJ, editors. Advances in neurology. Raven Press; New York: 1986. pp. 713–736. [PubMed] [Google Scholar]
- 79.Rehberg B, Duch DS, Urban BW. The voltage-dependent action of pentobarbital on batrachotoxin-modified human brain sodium channels. Biochimica et Biophysica Acta. 1994;1194:215–222. doi: 10.1016/0005-2736(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 80.Macdonald RL, Rogers CJ, Twyman RE. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J.Physiol. 1989;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinbach JH, Akk G. Modulation of GABA(A) receptor channel gating by pentobarbital. J.Physiol. 2001;537:715–733. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rho JM, Donevan SD, Rogawski MA. Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons. J.Physiol. 1996;497(Pt 2):509–522. doi: 10.1113/jphysiol.1996.sp021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krishek BJ, Moss SJ, Smart TG. Homomeric beta 1 gamma-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Molecular Pharmacology. 1996;49:494–504. [PubMed] [Google Scholar]
- 84.Davies PA, Kirkness EF, Hales TG. Modulation by general anaesthetics of rat GABAA receptors comprised of alpha 1 beta 3 and beta 3 subunits expressed in human embryonic kidney 293 cells. Br.J Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng HJ, Bianchi MT, Macdonald RL. Pentobarbital differentially modulates alpha1beta3delta and alpha1beta3gamma2L GABAA receptor currents. Molecular Pharmacology. 2004;66:988–1003. doi: 10.1124/mol.104.002543. [DOI] [PubMed] [Google Scholar]
- 86.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 87.Neelands TR, Fisher JL, Bianchi M, Macdonald RL. Spontaneous and gamma-aminobutyric acid (GABA)-activated GABA(A) receptor channels formed by epsilon subunit-containing isoforms. Molecular Pharmacology. 1999;55:168–178. doi: 10.1124/mol.55.1.168. [DOI] [PubMed] [Google Scholar]
- 88.Greenfield LJ, Jr., Zaman SH, Sutherland ML, Lummis SC, Niemeyer MI, Barnard EA, Macdonald RL. Mutation of the GABAA receptor M1 transmembrane proline increases GABA affinity and reduces barbiturate enhancement. Neuropharmacology. 2002;42:502–521. doi: 10.1016/s0028-3908(01)00196-4. [DOI] [PubMed] [Google Scholar]
- 89.Serafini R, Bracamontes J, Steinbach JH. Structural domains of the human GABAA receptor 3 subunit involved in the actions of pentobarbital. J.Physiol. 2000;524(Pt 3):649–676. doi: 10.1111/j.1469-7793.2000.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amin J. A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Molecular Pharmacology. 1999;55:411–423. [PubMed] [Google Scholar]
- 91.Ashton D, Fransen J, Heeres J, Clincke GH, Janssen PA. In vivo studies on the mechanism of action of the broad spectrum anticonvulsant loreclezole. Epilepsy Research. 1992;11:27–36. doi: 10.1016/0920-1211(92)90018-o. [DOI] [PubMed] [Google Scholar]
- 92.Zhang CL, Heinemann U. Effects of the triazole derivative loreclezole (R72063) on stimulus induced ionic and field potential responses and on different patterns of epileptiform activity induced by low magnesium in rat entorhinal cortex-hippocampal slices. Naunyn-Schmiedebergs Archives of Pharmacology. 1992;346:581–587. doi: 10.1007/BF00169016. [DOI] [PubMed] [Google Scholar]
- 93.Wafford KA, Bain CJ, Quirk K, McKernan RM, Wingrove PB, Whiting PJ, Kemp JA. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron. 1994;12:775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 94.Wingrove PB, Wafford KA, Bain C, Whiting PJ. The modulatory action of loreclezole at the gamma-aminobutyric acid type A receptor is determined by a single amino acid in the beta 2 and beta 3 subunit. Proc.Natl.Acad.Sci.U.S.A. 1994;91:4569–4573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fisher JL, Macdonald RL. Functional properties of recombinant GABA(A) receptors composed of single or multiple beta subunit subtypes. Neuropharmacology. 1997;36:1601–1610. doi: 10.1016/s0028-3908(97)00133-0. [DOI] [PubMed] [Google Scholar]
- 96.Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR. Effect of alpha subunit on allosteric modulation of ion channel function in stably expressed human recombinant gamma-aminobutyric acid(A) receptors determined using (36)Cl ion flux. Molecular Pharmacology. 2001;59:1108–1118. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]
- 97.Neelands TR, Macdonald RL. Incorporation of the pi subunit into functional gamma-aminobutyric Acid(A) receptors. Molecular Pharmacology. 1999;56:598–610. doi: 10.1124/mol.56.3.598. [DOI] [PubMed] [Google Scholar]
- 98.Donnelly JL, Macdonald RL. Loreclezole enhances apparent desensitization of recombinant GABAA receptor currents. Neuropharmacology. 1996;35:1233–1241. doi: 10.1016/s0028-3908(96)00053-6. [DOI] [PubMed] [Google Scholar]
- 99.Thomet U, Baur R, Dodd RH, Sigel E. Loreclezole as a simple functional marker for homomeric rho type GABA(C) receptors. European Journal of Pharmacology. 2000;408:R1–R2. doi: 10.1016/s0014-2999(00)00778-0. [DOI] [PubMed] [Google Scholar]
- 100.Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J.Pharmacol.Exp.Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- 101.Monaghan EP, Mcauley JW, Data JL. Ganaxolone: a novel positive allosteric modulator of the GABA(A) receptor complex for the treatment of epilepsy. Expert.Opin.Investig.Drugs. 1999;8:1663–1671. doi: 10.1517/13543784.8.10.1663. [DOI] [PubMed] [Google Scholar]
- 102.Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J.Pharmacol.Exp.Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- 103.McEwen BS, Parsons B. Gonadal steroid action on the brain: neurochemistry and neuropharmacology. Annual Review of Pharmacology and Toxicology. 1982;22:555–598. doi: 10.1146/annurev.pa.22.040182.003011. [DOI] [PubMed] [Google Scholar]
- 104.Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev.Neurobiol. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- 105.Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- 106.Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J.Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu WJ, Wang JF, Krueger KE, Vicini S. Delta subunit inhibits neurosteroid modulation of GABAA receptors. Journal of Neuroscience. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc.Natl.Acad.Sci.U.S.A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. Journal of Neuroscience. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors.Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- 111.Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 112.Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the alpha4 subunit of GABA(A) receptors in an animal model of epilepsy. Journal of Neuroscience. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hosie AM, Clarke L, da SH, Smart TG. Conserved site for neurosteroid modulation of GABA A receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 114.Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Molecular Pharmacology. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bracamontes JR, Steinbach JH. Steroid interaction with a single potentiating site is sufficient to modulate GABA-A receptor function. Molecular Pharmacology. 2009;75:973–981. doi: 10.1124/mol.108.053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zona C, Ciotti MT, Avoli M. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Neuroscience Letters. 1997;231:123–126. doi: 10.1016/s0304-3940(97)00543-0. [DOI] [PubMed] [Google Scholar]
- 117.McLean MJ, Bukhari AA, Wamil AW. Effects of topiramate on sodium-dependent action-potential firing by mouse spinal cord neurons in cell culture. Epilepsia. 2000;41(Suppl 1):S21–S24. [PubMed] [Google Scholar]
- 118.Hanaya R, Sasa M, Ujihara H, Ishihara K, Serikawa T, Iida K, Akimitsu T, Arita K, Kurisu K. Suppression by topiramate of epileptiform burst discharges in hippocampal CA3 neurons of spontaneously epileptic rat in vitro. Brain Research. 1998;789:274–282. doi: 10.1016/s0006-8993(98)00116-4. [DOI] [PubMed] [Google Scholar]
- 119.Gibbs JW, III, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl 1):S10–S16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X, Velumian AA, Jones OT, Carlen PL. Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia. 2000;41(Suppl 1):S52–S60. doi: 10.1111/j.1528-1157.2000.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 121.Mirza N, Marson AG, Pirmohamed M. Effect of topiramate on acid-base balance: extent, mechanism and effects. British Journal of Clinical Pharmacology. 2009;68:655–661. doi: 10.1111/j.1365-2125.2009.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Research. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 123.White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(Suppl 1):S17–S20. [PubMed] [Google Scholar]
- 124.Gordey M, DeLorey TM, Olsen RW. Differential sensitivity of recombinant GABA(A) receptors expressed in Xenopus oocytes to modulation by topiramate. Epilepsia. 2000;41(Suppl 1):S25–S29. [PubMed] [Google Scholar]
- 125.Simeone TA, Wilcox KS, White HS. Subunit selectivity of topiramate modulation of heteromeric GABA(A) receptors. Neuropharmacology. 2006;50:845–857. doi: 10.1016/j.neuropharm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 126.Pennell PB, Ogaily MS, Macdonald RL. Aplastic anemia in a patient receiving felbamate for complex partial seizures. Neurology. 1995;45:456–460. doi: 10.1212/wnl.45.3.456. [DOI] [PubMed] [Google Scholar]
- 127.O’Neil MG, Perdun CS, Wilson MB, McGown ST, Patel S. Felbamate-associated fatal acute hepatic necrosis. Neurology. 1996;46:1457–1459. doi: 10.1212/wnl.46.5.1457. [DOI] [PubMed] [Google Scholar]
- 128.Kume A, Greenfield LJ, Jr., Macdonald RL, Albin RL. Felbamate inhibits [3H]t-butylbicycloorthobenzoate (TBOB) binding and enhances Cl-current at the gamma-aminobutyric AcidA (GABAA) receptor. J.Pharmacol.Exp.Ther. 1996;277:1784–1792. [PubMed] [Google Scholar]
- 129.Rho JM, Donevan SD, Rogawski MA. Barbiturate-like actions of the propanediol dicarbamates felbamate and meprobamate. J.Pharmacol.Exp.Ther. 1997;280:1383–1391. [PubMed] [Google Scholar]
- 130.Subramaniam S, Rho JM, Penix L, Donevan SD, Fielding RP, Rogawski MA. Felbamate block of the N-methyl-D-aspartate receptor. J.Pharmacol.Exp.Ther. 1995;273:878–886. [PubMed] [Google Scholar]
- 131.Landmark CJ, Johannessen SI. Modifications of antiepileptic drugs for improved tolerability and efficacy. Perspect.Medicin.Chem. 2008;2:21–39. [PMC free article] [PubMed] [Google Scholar]
- 132.Rundfeldt C, Netzer R. Investigations into the mechanism of action of the new anticonvulsant retigabine. Interaction with GABAergic and glutamatergic neurotransmission and with voltage gated ion channels. Arzneimittelforschung. 2000;50:1063–1070. doi: 10.1055/s-0031-1300346. [DOI] [PubMed] [Google Scholar]
- 133.Otto JF, Kimball MM, Wilcox KS. Effects of the anticonvulsant retigabine on cultured cortical neurons: changes in electroresponsive properties and synaptic transmission. Molecular Pharmacology. 2002;61:921–927. doi: 10.1124/mol.61.4.921. [DOI] [PubMed] [Google Scholar]
- 134.Gebhardt C, Breustedt JM, Noldner M, Chatterjee SS, Heinemann U. The antiepileptic drug losigamone decreases the persistent Na+ current in rat hippocampal neurons. Brain Research. 2001;920:27–31. doi: 10.1016/s0006-8993(01)02863-3. [DOI] [PubMed] [Google Scholar]
- 135.Draguhn A, Jungclaus M, Sokolowa S, Heinemann U. Losigamone decreases spontaneous synaptic activity in cultured hippocampal neurons. European Journal of Pharmacology. 1997;325:245–251. doi: 10.1016/s0014-2999(97)00121-0. [DOI] [PubMed] [Google Scholar]
- 136.Dimpfel W, Chatterjee SS, Noldner M, Ticku MK. Effects of the anticonvulsant losigamone and its isomers on the GABAA receptor system. Epilepsia. 1995;36:983–989. doi: 10.1111/j.1528-1157.1995.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 137.Fisher JL. The anti-convulsant stiripentol acts directly on the GABA(A) receptor as a positive allosteric modulator. Neuropharmacology. 2009;56:190–197. doi: 10.1016/j.neuropharm.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quilichini PP, Chiron C, Ben Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006;47:704–716. doi: 10.1111/j.1528-1167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 139.Taylor CP. Mechanisms of action of gabapentin. Revue Neurologique (Paris) 1997;153(Suppl 1):S39–S45. [PubMed] [Google Scholar]
- 140.Loscher W, Honack D, Taylor CP. Gabapentin increases aminooxyacetic acid-induced GABA accumulation in several regions of rat brain. Neuroscience Letters. 1991;128:150–154. doi: 10.1016/0304-3940(91)90249-s. [DOI] [PubMed] [Google Scholar]
- 141.Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol.Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 142.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc.Natl.Acad.Sci.U.S.A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr.Opin.Pharmacol. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 144.Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacology Bulletin. 2003;37:133–146. [PubMed] [Google Scholar]
- 145.Loscher W. Anticonvulsant and biochemical effects of inhibitors of GABA aminotransferase and valproic acid during subchronic treatment in mice. Biochemical Pharmacology. 1982;31:837–842. doi: 10.1016/0006-2952(82)90471-3. [DOI] [PubMed] [Google Scholar]
- 146.Loscher W. Valproate enhances GABA turnover in the substantia nigra. Brain Research. 1989;501:198–203. doi: 10.1016/0006-8993(89)91044-5. [DOI] [PubMed] [Google Scholar]
- 147.Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Progress in Neurobiology. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 148.Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36(Suppl 2):S2–12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- 149.Frahm C, Engel D, Draguhn A. Efficacy of background GABA uptake in rat hippocampal slices. Neuroreport. 2001;12:1593–1596. doi: 10.1097/00001756-200106130-00016. [DOI] [PubMed] [Google Scholar]
- 150.Engel D, Schmitz D, Gloveli T, Frahm C, Heinemann U, Draguhn A. Laminar difference in GABA uptake and GAT-1 expression in rat CA1. J.Physiol. 1998;512(Pt 3):643–649. doi: 10.1111/j.1469-7793.1998.643bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Richter D, Luhmann HJ, Kilb W. Intrinsic activation of GABA receptors suppresses epileptiform activity in the cerebral cortex of immature mice. Epilepsia. 2010 doi: 10.1111/j.1528-1167.2010.02591.x. [DOI] [PubMed] [Google Scholar]
- 152.Frahm C, Stief F, Zuschratter W, Draguhn A. Unaltered control of extracellular GABA-concentration through GAT-1 in the hippocampus of rats after pilocarpine-induced status epilepticus. Epilepsy Research. 2003;52:243–252. doi: 10.1016/s0920-1211(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 153.Adkins JC, Noble S. Tiagabine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the management of epilepsy. Drugs. 1998;55:437–460. doi: 10.2165/00003495-199855030-00013. [DOI] [PubMed] [Google Scholar]
- 154.Richens A. Pharmacology and clinical pharmacology of vigabatrin. Journal of Child Neurology. 1991;(Suppl 2):S7–10. [PubMed] [Google Scholar]
- 155.Gram L, Klosterskov P, Dam M. gamma-Vinyl GABA: a double-blind placebo-controlled trial in partial epilepsy. Annals of Neurology. 1985;17:262–266. doi: 10.1002/ana.410170307. [DOI] [PubMed] [Google Scholar]
- 156.Riikonen R. ACTH therapy of West syndrome: Finnish views. Brain and Development. 2001;23:642–646. doi: 10.1016/s0387-7604(01)00306-0. [DOI] [PubMed] [Google Scholar]
- 157.Parisi P, Bombardieri R, Curatolo P. Current role of vigabatrin in infantile spasms. Eur.J.Paediatr.Neurol. 2007;11:331–336. doi: 10.1016/j.ejpn.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 158.Gram L, Larsson OM, Johnsen AH, Schousboe A. Effects of valproate, vigabatrin and aminooxyacetic acid on release of endogenous and exogenous GABA from cultured neurons. Epilepsy Research. 1988;2:87–95. doi: 10.1016/0920-1211(88)90024-1. [DOI] [PubMed] [Google Scholar]
- 159.Petroff OA, Behar KL, Mattson RH, Rothman DL. Human brain gamma-aminobutyric acid levels and seizure control following initiation of vigabatrin therapy. Journal of Neurochemistry. 1996;67:2399–2404. doi: 10.1046/j.1471-4159.1996.67062399.x. [DOI] [PubMed] [Google Scholar]
- 160.Stuchlik A, Kubova H, Mares P. Single systemic dose of vigabatrin induces early proconvulsant and later anticonvulsant effect in rats. Neuroscience Letters. 2001;312:37–40. doi: 10.1016/s0304-3940(01)02195-4. [DOI] [PubMed] [Google Scholar]
- 161.Mares P, Slamberova R. Biphasic action of vigabatrin on cortical epileptic after-discharges in rats. Naunyn-Schmiedebergs Archives of Pharmacology. 2004;369:305–311. doi: 10.1007/s00210-004-0865-1. [DOI] [PubMed] [Google Scholar]
- 162.Panayiotopoulos CP, Agathonikou A, Sharoqi IA, Parker AP. Vigabatrin aggravates absences and absence status. Neurology. 1997;49:1467. doi: 10.1212/wnl.49.5.1467. [DOI] [PubMed] [Google Scholar]
- 163.Iannetti P, Spalice A, Perla FM, Conicella E, Raucci U, Bizzarri B. Visual field constriction in children with epilepsy on vigabatrin treatment. Pediatrics. 2000;106:838–842. doi: 10.1542/peds.106.4.838. [DOI] [PubMed] [Google Scholar]
- 164.Hammoudi DS, Lee SS, Madison A, Mirabella G, Buncic JR, Logan WJ, Snead OC, Westall CA. Reduced visual function associated with infantile spasms in children on vigabatrin therapy. Invest Ophthalmol.Vis.Sci. 2005;46:514–520. doi: 10.1167/iovs.04-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wild JM, Ahn HS, Baulac M, Bursztyn J, Chiron C, Gandolfo E, Safran AB, Schiefer U, Perucca E. Vigabatrin and epilepsy: lessons learned. Epilepsia. 2007;48:1318–1327. doi: 10.1111/j.1528-1167.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- 166.Lukasiuk K, Pitkanen A. GABA(A)-mediated toxicity of hippocampal neurons in vitro. Journal of Neurochemistry. 2000;74:2445–2454. doi: 10.1046/j.1471-4159.2000.0742445.x. [DOI] [PubMed] [Google Scholar]
- 167.Lorsignol A, Taupignon A, Dufy B. Short applications of gamma-aminobutyric acid increase intracellular calcium concentrations in single identified rat lactotrophs. Neuroendocrinology. 1994;60:389–399. doi: 10.1159/000126773. [DOI] [PubMed] [Google Scholar]
- 168.Kahraman S, Zup SL, McCarthy MM, Fiskum G. GABAergic mechanism of propofol toxicity in immature neurons. J.Neurosurg.Anesthesiol. 2008;20:233–240. doi: 10.1097/ANA.0b013e31817ec34d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 170.Brumback AC, Staley KJ. Thermodynamic regulation of NKCC1-mediated Cl-cotransport underlies plasticity of GABA(A) signaling in neonatal neurons. Journal of Neuroscience. 2008;28:1301–1312. doi: 10.1523/JNEUROSCI.3378-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kriegstein AR, Owens DF. GABA may act as a self-limiting trophic factor at developing synapses. Sci STKE. 2001;2001:E1. doi: 10.1126/stke.2001.95.pe1. [DOI] [PubMed] [Google Scholar]
- 172.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat.Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 173.Dzhala VI, Staley KJ. Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus. Journal of Neuroscience. 2003;23:1840–1846. doi: 10.1523/JNEUROSCI.23-05-01840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang SJ, Jackson MB. GABAA receptor activation and the excitability of nerve terminals in the rat posterior pituitary. J.Physiol. 1995;483(Pt 3):583–595. doi: 10.1113/jphysiol.1995.sp020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. Journal of Physiology. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zeng XJ, Tietz EI. Role of bicarbonate ion in mediating decreased synaptic conductance in benzodiazepine tolerant hippocampal CA1 pyramidal neurons. Brain Res. 2000;868:202–214. doi: 10.1016/s0006-8993(00)02330-1. [DOI] [PubMed] [Google Scholar]
- 177.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 178.Shorvon SD. Its Clinical Features and Treatment in Children and Adults. Cambridge University Press; New York: 1994. Status Epilepticus. [Google Scholar]
- 179.Tassinari CA, Dravet C, Roger J, Cano JP, Gastaut H. Tonic status epilepticus precipitated by intravenous benzodiazepine in five patients with Lennox-Gastaut syndrome. Epilepsia. 1972;13:421–435. doi: 10.1111/j.1528-1157.1972.tb04582.x. [DOI] [PubMed] [Google Scholar]
- 180.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Annals of Neurology. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 181.Glykys J, Dzhala VI, Kuchibhotla KV, Feng G, Kuner T, Augustine G, Bacskai BJ, Staley KJ. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009;63:657–672. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kahle KT, Barnett SM, Sassower KC, Staley KJ. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na(+)-K(+)-2Cl(−) cotransporter NKCC1. Journal of Child Neurology. 2009;24:572–576. doi: 10.1177/0883073809333526. [DOI] [PubMed] [Google Scholar]
- 183.Wang DD, Kriegstein AR. Blocking Early GABA Depolarization with Bumetanide Results in Permanent Alterations in Cortical Circuits and Sensorimotor Gating Deficits. Cereb.Cortex. 2010 doi: 10.1093/cercor/bhq124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Goodkin HP, Sun C, Yeh JL, Mangan PS, Kapur J. GABA(A) receptor internalization during seizures. Epilepsia. 2007;48(Suppl 5):109–113. doi: 10.1111/j.1528-1167.2007.01297.x. [DOI] [PubMed] [Google Scholar]
- 186.Lauren HB, Lopez-Picon FR, Korpi ER, Holopainen IE. Kainic acid-induced status epilepticus alters GABA receptor subunit mRNA and protein expression in the developing rat hippocampus. Journal of Neurochemistry. 2005;94:1384–1394. doi: 10.1111/j.1471-4159.2005.03274.x. [DOI] [PubMed] [Google Scholar]
- 187.Feng HJ, Mathews GC, Kao C, Macdonald RL. Alterations of GABA A-receptor function and allosteric modulation during development of status epilepticus. Journal of Neurophysiology. 2008;99:1285–1293. doi: 10.1152/jn.01180.2007. [DOI] [PubMed] [Google Scholar]
- 188.Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O’Neil N, Neuhaus JM, Segal MR, Lowenstein DH. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. New England Journal of Medicine. 2001;345:631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 189.Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Calabrese VP, Uthman BM, Ramsay RE, Mamdani MB. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. New England Journal of Medicine. 1998;339(12):792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 190.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat.Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 191.Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABA(A)-receptor alpha1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia. 2006;47:1665–1673. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 192.Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. Journal of Neuroscience. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Raol YH, Zhang G, Budreck EC, Brooks-Kayal AR. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience. 2005;132:399–407. doi: 10.1016/j.neuroscience.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 194.Kang I, Miller LG. Decreased GABAA receptor subunit mRNA concentrations following chronic lorazepam administration. British Journal of Pharmacology. 1991;103:1285–1287. doi: 10.1111/j.1476-5381.1991.tb09781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen S, Huang X, Zeng XJ, Sieghart W, Tietz EI. Benzodiazepine-mediated regulation of alpha1, alpha2, beta1-3 and gamma2 GABA(A) receptor subunit proteins in the rat brain hippocampus and cortex. Neuroscience. 1999;93:33–44. doi: 10.1016/s0306-4522(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 196.Tseng YT, Wellman SE, Ho IK. In situ hybridization evidence of differential modulation by pentobarbital of GABAA receptor alpha 1- and beta 3-subunit mRNAs. Journal of Neurochemistry. 1994;63:301–309. doi: 10.1046/j.1471-4159.1994.63010301.x. [DOI] [PubMed] [Google Scholar]
- 197.Klein RL, Harris RA. Regulation of GABAA receptor structure and function by chronic drug treatments in vivo and with stably transfected cells. Japanese Journal of Pharmacology. 1996;70:1–15. doi: 10.1254/jjp.70.1. [DOI] [PubMed] [Google Scholar]
- 198.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J.Mol.Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 199.Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB Journal. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- 200.Macdonald RL, Kang JQ, Gallagher MJ. GABAA Receptor Subunit Mutations and Genetic Epilepsies. 2012. [PubMed]