Abstract
Background
Percent body fat equations are usually developed in specific populations and have low generalizability.
Objectives
To use a nationally representative sample of the American youth population (8–17 years old) from the 1999–2004 NHANES data to develop gender-specific percent body fat equations.
Methods
Percent body fat equations were developed for girls and boys using information on weight, height, waist circumference, triceps skinfolds, age, race/ethnicity, and menses status compared to dual-emission X-ray absorptiometry (DXA). Terms were selected using forward and backward selection in regression models and in a 2/3 development sample and were cross-validated in the remaining sample. Final coefficients were estimated in the full sample.
Results
Final equations included 10 terms in girls and 8 terms in boys including interactions with age and race/ethnicity. In the cross-validation sample the adjusted R2 was 0.818 and the root mean squared error (RMSE) was 2.758 in girls. Comparable estimates in boys were 0.893 and 2.525. Systematic bias was not detected in the estimates by race/ethnicity or by BMI categories.
Conclusion
Gender-specific percent body fat equations were developed in youth with a strong potential for generalizability and utilization by other investigators studying adiposity-related issues in youth.
Keywords: anthropometry, percent body fat, NHANES, dual-emission X-ray absorptiometry, children, adolescents
Introduction
Obesity is a condition in which excess adipose tissue has accumulated to the extent that it has adverse effects on health1. Although the classic definition of obesity emphasizes adiposity, in practice body mass index (BMI) is the measure most often used to characterize obesity. BMI was constructed with the intention to provide an index of weight independent of height, but it is sometimes viewed as a simple prediction equation for percentage body fat. Nevertheless, since BMI does not distinguish fat from lean tissue, some misdiagnosis of obesity is inevitable. Factors that influence body composition in ways not detected by BMI include gender, puberty, age, race/ethnicity, and physical activity2–3.
As an alternative to methods that require relatively expensive equipment, trained technicians and high subject burden and cooperation, investigators often measure percent body fat using equations that are derived from more field-friendly assessments such as height, weight, skinfold thickness, bioelectric impedance and/or circumferences. Nevertheless, identifying an age, gender and race/ethnicity appropriate equation is often challenging4–34. Most body composition equations to date have been derived from a convenience or purposive sample with investigators intentionally recruiting such that a range of body sizes was studied. Internal validity (an equation that performed well in those subjects being studied) is the focus rather than external validity (an equation with high generalizability), which impedes researchers from using these equations for other study populations. We know of only one study that has developed an equation using a sample representative of the United States35. That study concentrated on testing mathematical functions of height, weight and BMI (such as the square root) and did not use either circumferences or skinfolds.
The objective of this study was to use extant data from a nationally representative sample to derive and validate gender-specific percent body fat equations that are composed of demographic and anthropometric variables and can be applied to youth (8–17 years old). This age range was selected in order to develop equations for use in the Childhood Obesity Prevention and Treatment Research (COPTR) Consortium field centers at Case Western Reserve University and Stanford University. Our goal was to produce precise, accurate and unbiased percent body fat equations that use the variables collected by the COPTR investigators (gender, age, height, weight, triceps skinfold, waist circumference and menses in girls).
METHODS
The NHANES uses a complex multistage probability design to provide a representative sample of US non-institutionalized children and adults36. NHANES oversampled African Americans, Mexican Americans, low income Whites (beginning in 2000), adolescents aged 12–19 and adults aged 60+ years. Data for this study were from the 1999- 2004 NHANES.
Race and ethnicity were self-reported and categorized as non-Hispanic Whites, non-Hispanic Blacks, Mexican Americans, other Hispanics and other race/ethnicities. The other race/ethnicities group includes other non-Hispanic race groups and non-Hispanic multiracial groups. Maturation status (e.g. Tanner stages) was not collected in the 1999–2004 NHANES. Girls over 12 years of age were asked the age when their first menstrual period occurred. Using this information, we created a dichotomous variable indicating presence or absence of menarche.
Weight, height, waist circumference and triceps skinfolds were measured using standardized procedures in the mobile examination centers36. For all anthropometrics, two trained and certified staff members performed and recorded the measurements. Weight was measured to the nearest 0.1 kilogram in an examination gown without shoes. Standing height (without shoes) was measured to the nearest 0.1 centimeter using a stadiometer with a fixed vertical backboard and adjustable head piece. Waist was measured just above the iliac crest to the nearest 0.1 centimeter and triceps skinfolds were measured to the nearest 0.1 millimeter using Holtain skinfold calipers.
DXA measurements were obtained on participants 8 years of age or older using a Hologic QDR-4500A fan-beam densitometer (Hologic, Inc., Bedford, Massachusetts). Details of the DXA procedures are described in technical documents37. The DXA data were adjusted as described by Schoeller et al.38. Participants were excluded from DXA measurement if pregnant, had a self-reported history of radiographic contrast material use in past 7 days or participation in nuclear medicine studies in the past 3 days, had amputations other than fingers and toes, weighted over 300 pounds or had a height over 6’5”. Pregnancy tests were performed for all females 12 to 59 years of age and menstruating 8 to 11 year olds. Unresolved IRB issues concerning the reporting of pregnancy test results to minors resulted in no DXA data in females 8 to 17 years of age in 1999. Since NHANES data were weighted by two-year increments, there are no DXA data available for girls 8–17 years from the 1999–2000 survey. We used the 4-year (2001–2004) and 6-year (1999–2004) sampling weights in females and males, respectively.
Analytic sample
There are 7,730 children 8 –17 years old in the 1999–2004 NHANES datasets. We excluded children who had missing height, weight, waist circumference or triceps skinfolds (n=614), had an anthropometric flag (e.g. non-standard clothing, not standing straight) (n=28), had biologically implausible values for height, weight or BMI (n=49), were missing all measured DXA data (n=1,599), had given birth in the last year or were breastfeeding (n=13), and were missing menarche status (girls only) (n=53). The full analytic sample included 5,374 children (3,334 boys and 2,040 girls)
Analysis plan
Our goal was to produce equations that maximized the percent of variance explained (R2 and adjusted R2) while minimizing the root mean squared error (RMSE) and the bias (mean signed difference, MSD). Age, race/ethnicity, menarche status (girls only), weight, waist, height, and triceps were our base variables. These variables were selected because they were measured by the COPTR investigators and are correlated with percent body fat. Here we distinguish variables (e.g. race/ethnicity, weight) from terms (e.g. squared terms, interaction terms). We selected the terms to study based on our review of terms used in published equations4–33 and our own exploratory analyses. We tested a total of 76 terms in girls and 65 terms in boys. These terms included squared terms, height as a reciprocal and 2-way interactions of age, age squared, race/ethnicity and menses with the anthropometric variables.
The following steps outline our approach:
Step 1. Create a development and cross-validation dataset
We used the PROC SURVEYSELECT Procedure in SAS (SAS/STAT® 9.2 User’s Guide, 2011) to create the development dataset containing a random sample of 2/3 of the children. The remaining 1/3 of the children constituted the cross-validation dataset. All analyses were stratified by gender and all continuous variables were centered on the mean prior to analysis to improve the interpretability and decrease multi-colinearity39–40. Race/ethnicity was a categorical variable with non-Hispanic Whites as the referent.
Step 2. Generate models in development dataset
We started with a base model (age, race/ethnicity, menarche status (girls only), weight, waist, height, and triceps), and then used PROC SURVEYREG with the appropriate sample weights to determine which additional terms to add to the model using forward selection to best estimate percent body fat measured by DXA. Because of the large sample size several terms were significant (p<0.05) even though their addition to the model only changed the adjusted R2 negligibly. We did not use p-values to determine which variables to select, but instead we used a criterion that an equation must increase the adjusted R2 over a simpler equation by at least 0.005 or decrease RMSE by at least 0.025 in order for the change to be judged non-trivial. We used the same criteria to confirm the models using backward selection. This created our models including the base terms plus selected terms. We also tested three additional models: 1) BMI only; 2) BMI z-score only and 3) base terms only.
Step 3. Evaluate models in the cross-validation datasets
The intercept and coefficients for all of the terms in each model from the development dataset were used to calculate the predicted percent body fat in the cross-validation dataset. Gender-specific univariate regression models were run using the estimated percent body fat as the only independent variable and DXA as the dependent variable. R2, RMSE and MSD were calculated. Since there was only one independent term in these models adjusted R2 was not calculated. MSD were calculated overall and by race/ethnicity and weight status groups. as the predicted percent body fat from an equation minus the percent body fat measured by DXA.
Step 4. Obtain and examine final equations in full dataset
To estimate the coefficients with greater precision, we ran the models by gender in the full dataset and calculated the same parameters shown in step 3. As a final check, an independent programmer calculated percent body fat for 200 randomly chosen girls and boys in a Microsoft Excel spreadsheet using our final formula and compared results to those predicted by our SAS program and the observed DXA values.
RESULTS
Table 1 shows descriptive information on the girls and boys included in the analytic sample. The majority of the sample was non-Hispanic White. Girls had a lower mean weight and height, and higher percent body fat from DXA compared to boys. Over half of the girls had reached menarche.
Table 1.
Descriptive information on analysis sample from the 1999– 2004 NHANES
| Girls (n=2,040) |
Boys (n=3,334) |
|||
|---|---|---|---|---|
| Mean or % | SE | Mean or % | SE | |
| Age (years) | 12.4 | 0.09 | 12.4 | 0.08 |
| Ethnicity (%) | ||||
| Non-Hispanic White | 63.3 | 61.1 | ||
| Non-Hispanic Black | 14.0 | 14.8 | ||
| Mexican American | 10.4 | 11.6 | ||
| Other Hispanic | 6.7 | 6.6 | ||
| Other | 5.7 | 5.9 | ||
| Height (cm) | 152.9 | 0.35 | 157.1 | 0.51 |
| Weight (kg) | 50.5 | 0.50 | 53.5 | 0.53 |
| Triceps skinfold (mm) | 17.5 | 0.25 | 13.6 | 0.23 |
| Waist (cm) | 74.1 | 0.50 | 74.3 | 0.36 |
| Menstruating (%) | 56.7 | ---- | ---- | |
| DXA % body fat | 32.27 | 0.24 | 25.66 | 0.27 |
| BMI (kg/m2) | 21.18 | 0.16 | 20.97 | 0.13 |
| BMI z-score | 0.49 | 0.04 | 0.49 | 0.03 |
| BMI percentile categories (%) | ||||
| < 5th %tile | 2.9 | 3.5 | ||
| ≥5th to < 85th %tile | 64.6 | 62.6 | ||
| ≥85th to < 95th %tile | 17.5 | 16.7 | ||
| ≥95th %tile | 15.0 | 17.2 | ||
The forward and backward selection procedures added 3 terms for girls and 2 terms for boys. In girls, the base plus selected terms model included triceps squared, menses * triceps interaction and menses * triceps square interaction in addition to age, race/ethnicity, weight, height, waist, triceps and menses. In boys, the base plus selected terms model also included triceps squared and an age * weight interaction. In both genders, triceps skinfold explained more variance than any other anthropometric variable. Table 2 shows evidence that the adjusted R2 and RMSE for BMI and BMI Z-score were inferior to the other models tested for the prediction of percent body fat. Although the difference was not substantial, the addition of selected terms to the base model improved performance. The overall MSD was not different from zero in all the models tested, and the base plus selected terms model had the tightest confidence intervals around the MSD.
Table 2.
Adjusted R2, RMSE and mean signed mean difference (MSD) from percent body fat prediction models in the development and cross-validation datasets1
| Girls (n = 2,040) |
Boys (n = 3,334) |
|||||||
|---|---|---|---|---|---|---|---|---|
| BMI2 | BMI z-score3 |
Base terms4 |
Base + selected terms5 |
BMI2 | BMI z-score3 |
Base terms6 |
Base + selected terms7 |
|
|
Development dataset | ||||||||
| R2 | 0.615 | 0.628 | 0.805 | 0.832 | 0.346 | 0.522 | 0.865 | 0.884 |
| Adjusted R2 | 0.615 | 0.628 | 0.804 | 0.831 | 0.346 | 0.522 | 0.865 | 0.884 |
| RMSE | 4.114 | 4.041 | 2.940 | 2.732 | 6.368 | 5.442 | 2.894 | 2.678 |
| Mean Signed Difference (95% Confidence Interval) |
0.000 (−0.335, 0.335) |
0.000 (−0.330, 0.330) |
0.000 (−0.233, 0.233) |
−0.015 (−0.239, 0.209) |
0.000 (−0.481, 0.481) |
0.000 (−0.398, 0.398) |
0.000 (−0.246, 0.246) |
0.000 (−0.239, 0.209) |
|
Cross-validation dataset | ||||||||
| R2 | 0.622 | 0.608 | 0.786 | 0.818 | 0.348 | 0.506 | 0.874 | 0.893 |
| RMSE | 3.978 | 4.055 | 2.991 | 2.758 | 6.233 | 5.428 | 2.746 | 2.525 |
| Mean Signed Difference (95% Confidence Interval) |
0.000 (−0.521, 0.521) |
0.000 (−0.434, 0.434) |
−0.063 (−0.430, 0.304) |
−0.078 (−0.394, 0.239) |
0.000 (−0.561, 0.561) |
0.000 (−0.468, 0.468) |
−0.055 (−0.320, 0.210) |
−0.078 (−0.315, 0.160) |
– Intercept and coefficient values were calculated using the development dataset.
– BMI model included only BMI (wt/ht2)
– BMI z-score model included only BMI z-score
– Base terms model for girls included age, race/ethnicity (5 categories), weight, height, waist, triceps and menses
– Base plus selected terms model for girls included all terms in the base model plus triceps squared, menses-triceps interaction and menses-triceps squared interaction.
– Base terms model for boys included age, race/ethnicity (5 categories), weight, height, waist, and triceps
– Base plus selected terms model for boys included all terms in the base model plus triceps squared and age-weight interaction
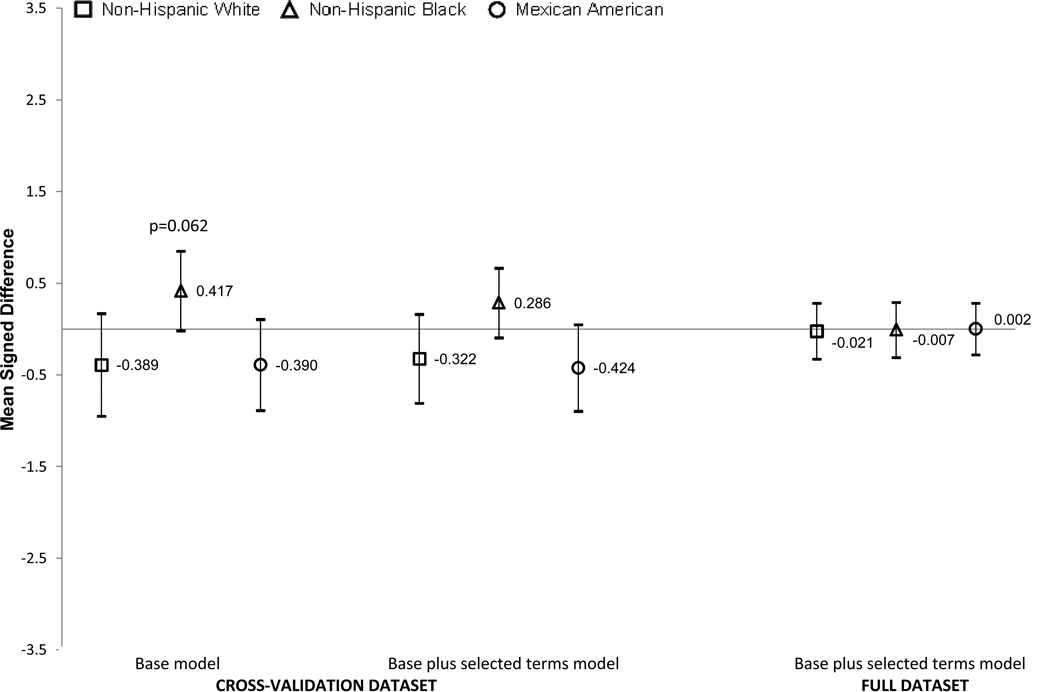
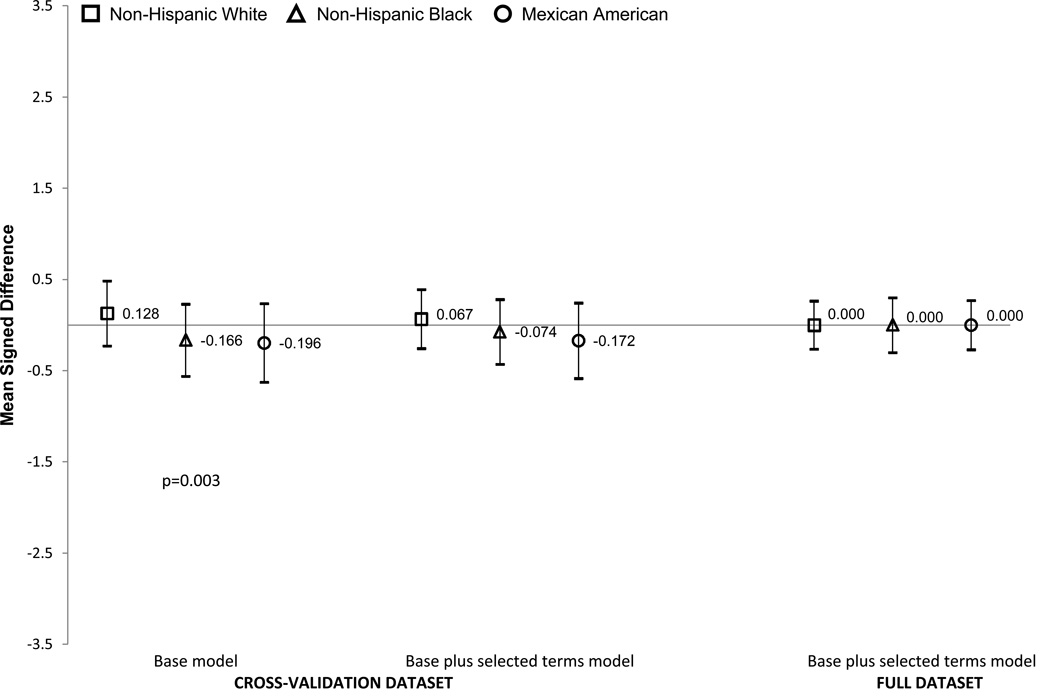
Figures 1 and 2 show the MSDs by race/ethnicity for the base terms model and the base plus selected terms model in the cross-validation. Results for the base plus selected terms are also shown in the full dataset using coefficients calculated using the full dataset rather than the development data set (final equation). We elected not to show the results for the other Hispanic and other race/ethnic groups due small sample sizes and wide confidence intervals. In girls (Figure 1), the MSDs were small (all less than half a body fat percentage point) and not statistically significant, indicating little systematic bias by race/ethnicity. Similar results were seen for non-Hispanic White, non-Hispanic Black and Mexican American boys (Figure 2). None of the prediction equation results were significantly different from zero. As expected, confidence intervals were narrower when the full dataset was used.
Figure 1. GIRLS.
Signed mean difference (SMD) and 95% Confidence Intervals for the base model and base plus selected terms model in the cross validation dataset and base plus selected terms model in the full dataset by race/ethnicity in girls. The base model included age, race/ethnicity (5 categories), weight, height, waist, triceps and menses. The selected terms are triceps squared, menses-triceps interaction and menses-triceps squared interaction. The y-axis is the SMD difference (prediction equation percent body fat minus DXA percent body fat). Positive values mean that the percent body fat equation overestimated the DXA percent body fat and negative values indicated that the estimation equation underestimated the DXA body fat percentage.
Figure 2. BOYS.
Signed mean difference (SMD) and 95% Confidence Intervals for the base model and base plus selected terms model in the cross validation dataset and base plus selected terms model in the full dataset by race/ethnicity in boys. The base model included age, race/ethnicity (5 categories), weight, height, waist, and triceps. The selected terms are triceps squared, age-weight interaction. The y-axis is the SMD difference (prediction equation percent body fat minus DXA percent body fat). Positive values mean that the percent body fat equation overestimated the DXA percent body fat and negative values indicated that the estimation equation underestimated the DXA body fat percentage.
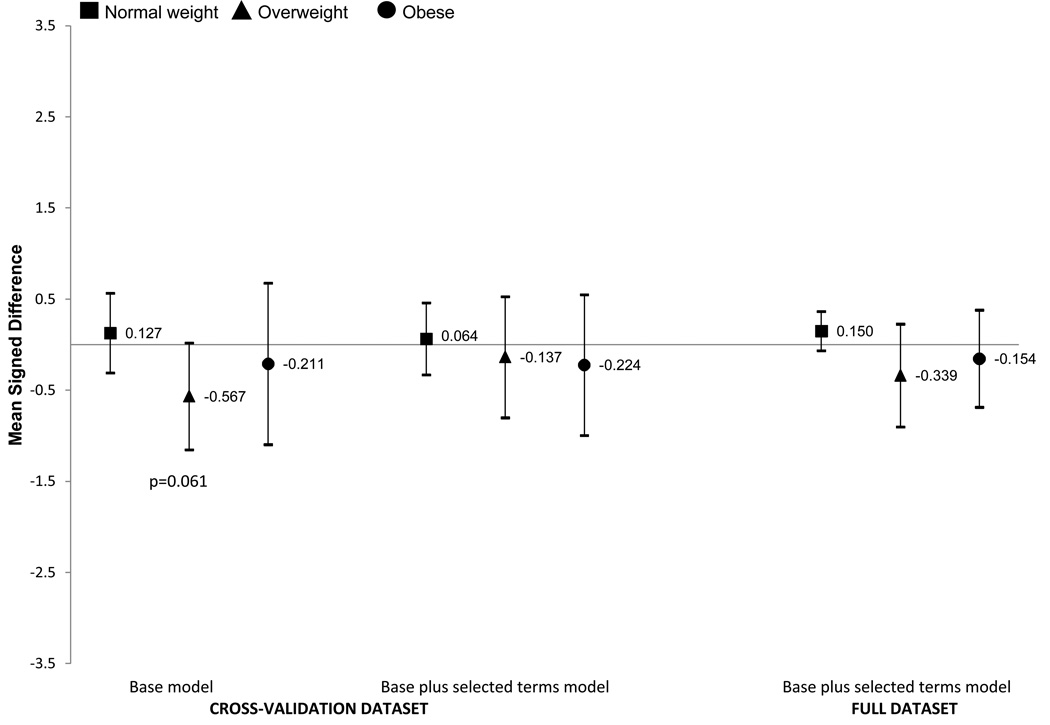
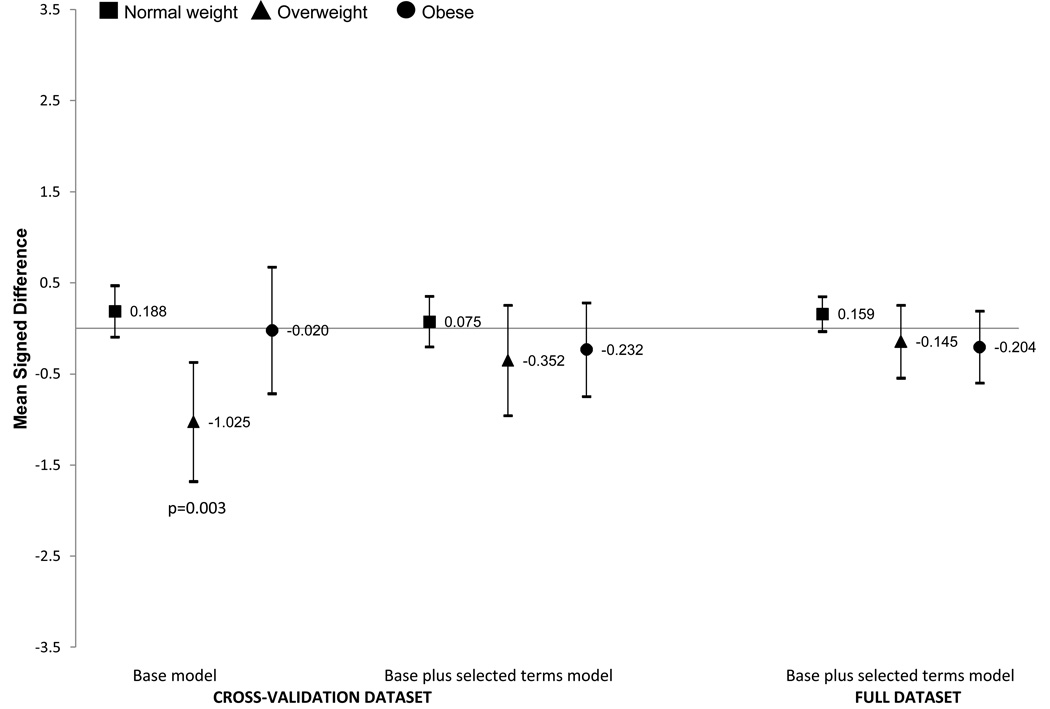
Across weight status groups (Figures 3 and 4) the prediction equations tended to slightly overestimate percent body fat in normal weight girls and boys and underestimate in overweight and obese girls and boys. These differences were small and not statistically significant for the base plus selected terms model. The base model underestimated percent body fat by −0.567 percentage points in overweight girls (p=0.061) and −1.025 in overweight boys (p=0.003). We do not show the underweight group as the confidence intervals were very wide, likely due to small sample sizes (n = 16 in girls and 42 in boys in the cross-validation dataset). The large confidence intervals for MSD did include zero for underweight boys but not underweight girls.
Figure 3. GIRLS.
Signed mean difference (SMD) and 95% Confidence Intervals for the base model and base plus selected terms model in the cross validation dataset and base plus selected terms model in the full dataset by weight status in girls. The base model included age, race/ethnicity (5 categories), weight, height, waist, triceps and menses. The selected terms are triceps squared, menses-triceps interaction and menses-triceps squared interaction. The y-axis is the SMD difference (prediction equation percent body fat minus DXA percent body fat). Positive values mean that the percent body fat equation overestimated the DXA percent body fat and negative values indicated that the estimation equation underestimated the DXA body fat percentage.
Figure 4. BOYS.
Signed mean difference (SMD) and 95% Confidence Intervals for the base model and base plus selected terms model in the cross validation dataset and base plus selected terms model in the full dataset by weight status groups in boys. The base model included age, race/ethnicity (5 categories), weight, height, waist, and triceps. The selected terms are triceps squared, age-weight interaction. The y-axis is the SMD difference (prediction equation percent body fat minus DXA percent body fat). Positive values mean that the percent body fat equation overestimated the DXA percent body fat and negative values indicated that the estimation equation underestimated the DXA body fat percentage.
Table 3 shows the final (base plus selected terms from the full dataset) percent body fat equations and the corresponding R2, adjusted R2, and RMSE in girls and boys in the full dataset. Overall, the final equations performed better in boys than girls. In girls, the base plus selected terms had a R2 of 0.829 and slightly underestimated percent body fat overall, although not significantly (MSD: −0.013, 95% CI: −0.227, 0.201). In boys, the R2 was 0.888 and the MSD was 0.000 (95% CI: −0.187, 0.187).
Table 3.
Base plus selected terms percent body fat prediction equation for girls and boys and corresponding model fit values in the full dataset1
| Gender | Equation2 | R2 | Adj R2 | RMSE |
|---|---|---|---|---|
| Girls | %BF = 31.836841 - 0.609018 * (menses) + 0.003317 * (age - 161) - 0.975391 * (Race1) + 0.499227 * (Race2) + 0.602171 * (Race3) + 0.173877 * (Race4) + 0.053756 * (weight - 56) - 18.641446 * (height – 1.58) + 0.218830 * (waist - 76) + 0.744310 * (triceps - 15) - 0.018648 * (triceps -15)2 – 0.194114 * (menses) * (triceps-15) + 0.005748 * (menses)*(triceps)2 | 0.829 | 0.828 | 2.744 |
| Boys | %BF = 28.009373 - 0.038460 * (age - 161) - 0.425327 * (Race1) + 0.350376 * (Race2) - 0.238080 * (Race3) - 0.106154 * (Race4) - 0.113560 * (weight - 56) - 10.010607 * (height – 1.58) + 0.353623 * (waist - 76) + 0.690984 * (triceps - 15) - 0.016657 * (triceps - 15)2 - 0.000852 * (age - 161) * (weight - 56) | 0.888 | 0.888 | 2.624 |
Intercept and coefficient values were calculated using the full dataset.
Menses = menarche status (girls) is 0 if have not started period and 1 if started periods; Race1 = 1 if non-Hispanic Black and 0 if not non-Hispanic Black; Race2 = 1 if Mexican American and 0 if not Mexican American; Race3 = 1 if Other Hispanic and 0 if not Other Hispanic; Race4 = 1 if Other non-Hispanic race group including non-Hispanic multiracial and 0 if not other non-Hispanic race group; weight = weight in kilograms; height = height in meters; waist = waist circumference in centimeters; triceps = triceps skinfolds in millimeters.
In additional work (data not shown) we used forward and backward selection to choose terms without forcing the base variables. Those models included only 5 terms in both girls (triceps, triceps squared, waist, race/ethnicity, and height) and boys (triceps, triceps squared, waist, age and weight). In the cross-validation dataset the adjusted R2 and RMSE estimates were comparable to those of the base plus selected terms model shown in Table 2, but the bias associated with race/ethnicity was larger and for some points statistically significant. We also explored the use of lower limits for term selection into models (R2 > 0.001 instead of 0.005 or RMSE > 0.01 instead of 0.025). This resulted in many more terms in the prediction equations (28 in girls and 29 in boys), but only trivial effects on the R2, RMSE and MSD compared to the base plus selected terms models.
DISCUSSION
We successfully created equations for the prediction of percent body fat that produced an adjusted R2 > 0.83 and used variables that are feasible to measure in the field. The final equations performed better in boys than in girls, despite the inclusion of a puberty-related variable in girls, but not boys. We have no ready explanation for this difference. Both equations far outperformed BMI as an indicator of percent body fat for which the R2 was only 0.62 in girls and 0.35 in boys. The addition of terms using pre-determined criteria of R2 and RMSE resulted in an equation that provided a less biased estimate in overweight boys (and to a lesser degree, also in overweight girls) compared to the base model.
Our analyses showed that triceps skinfold added more to the predictive ability of the equations than the more commonly measured variables weight and height. Triceps skinfold alone, with no other variables in the model, resulted in an R2 for percent body fat of 0.73 for boys (RMSE= 4.06) and 0.71 for girls (RMSE=3.58). Thus, this skinfold measurement alone performed better than BMI alone (see Table 2), even though skinfold measurements are known to be prone to larger measurement errors than height and weight41. For comparison, waist alone only reached an R2 of 0.34 (RMSE= 6.39) in boys and an R2 of 0.58 (RMSE= 4.31) in girls. Other studies in children have also shown that skinfolds make an important contribution to the prediction of percent body fat3, 16, 42–44. Freedman et al. showed that the addition of triceps skinfolds to a model that included BMI z-score increased the R2 value by 0.07 or more and decreased prediction errors by 20–30% in 5–18 year old children compared to DXA measurements16. Given the large number of participants and the resources of NHANES, it is likely that anthropometrics were obtained by technicians who were more experienced than those used in many other studies. However, the Pathways42 and Trial of Activity in Adolescent Girls (TAAG)3 studies cited above used local, trained and certified staff43–44 temporarily assigned to the study to obtain skinfold measurements (communication from Coordinating Center Principal Investigator, coauthor JS). Thus, in community research settings it is feasible to train technicians to collect skinfolds that add importantly to the prediction of percent body fat, and their contribution to validity appears to more than compensate for lower levels of repeatability41. In some clinical settings obtaining reliable measurements may be more problematic as periodic training and certification of data collectors and on-going quality control may be needed to insure high quality.
A judgment that is fundamental to the selection of an appropriate prediction equation is the level of accuracy and precision needed in order for it to successfully substitute for measurements obtained using more direct, but more difficult methods. Results from observational studies in children indicate that physical activity is associated with percent body fat calculated from prediction equations, but not with BMI. In the Pathways trial44, higher levels of accelerometry-measured physical activity in 2nd grade normal weight American Indian elementary school children were associated with lower levels of calculated percent body fat in 5th grade, but there was no association with BMI42. In children who were overweight and obese in 2nd grade, physical activity was positively associated with BMI (the opposite of the expected direction). In TAAG43 accelerometry-measured minutes of moderate to vigorous physical activity in 6th grade girls was associated with lower percent body fat in 8th grade, but again there was no association with BMI. In the Pathways study investigators measured percent body fat using prediction equations that included demographic and anthropometric variables and BIA19. The TAAG prediction equation did not include BIA25. R2 values when compared to a criterion measure of percent body fat were 0.843 in Pathways and 0.88 in TAAG; levels similar to those found here (0.829 in girls and 0.888 in boys).
The randomized controlled trial literature in children has shown that some interventions aiming at increasing physical activity had no effect on BMI, but showed a meaningful impact on percent body fat45. A study by Pudar et al.46 examined the impact of a school-based multidimensional lifestyle intervention in predominantly migrant preschool children living in Switzerland. They found no difference in BMI between the control and intervention groups (−0.07 kg/m2, −0.19 to 0.06; p=0.31), but the intervention group had a lower percent body fat (−1.1%, −2.0 to −0.2; p=0.02). In this research percent body fat was measured using a prediction equation that included BIA measurements29. Given these consistent findings across observational studies and randomized trials, a calculated percent body fat may prove useful for detection of effects associated with physical activity.
To our knowledge, only one other published paper has used DXA percent body fat measures from the NHANES to explore associations with anthropometric variables. Dugas et al.35 examined non-Hispanic White, non-Hispanic Black and Mexican American adolescents 12 to 20 years of age in the 1999–2004 NHANES. Their primary goal was to investigate whether percent body fat was equivalent at comparable BMI’s across three race/ethnic groups of adolescents in the United States. Their main analysis compared the mean percent fat levels as measured by DXA within the normal weight, overweight and obese BMI categories35 in gender-stratified analyses in the 3 ethnic groups studied and found several ethnic differences. Relevant to the work presented here, they also examined prediction equations for percent body fat. These analyses were not stratified by gender, but gender was included as a variable in all models in addition to ethnicity and age. BMI with exponents of ½, −1, and −2 and body weight were explored in selected combinations. The authors indicated that the model with gender, ethnicity, age, weight and BMI1/2 explained the most variance (R2 = 0.786). No evidence of potential systematic differences between the observed and the predicted values from their equation was shown for race/ethnic groups or for weight status groups.
Differences in body composition between White and African American populations have been noted for decades47–48. It is well known that at the same BMI African Americans tend to have more lean mass and skeletal mass than Whites. African Americans with the same subscapular skinfold as White children have a smaller triceps skinfold. Researchers have shown that published equations have either underestimated or overestimated percent body fat in different ethnic groups6, 8, 19, 25–27, 34–35, 48. Our final equations included race/ethnicity and did not systematically under or over-estimate percent body fat in African Americans, Mexican Americans or Whites. We tested numerous interactions between race/ethnicity and our candidate anthropometric variables, but none contributed substantially to the prediction equations, and, therefore, they were not retained.
Investigators have used several approaches to determine optimal models for the prediction of percent body fat in children, but details on the criteria used for variable selection are not always given. As was done here, other investigators have formed models around a base of preselected variables and then tested the contribution of adding additional terms17, 24. Many authors use regression analyses (often stepwise) with terms selected either according to p values5, 9, 11–12, 20, 23, 26, 29, 33 or R2 (or adjusted R2 or r)8, 18. The criterion p value used is usually provided, but criterion levels for R2 are not stated. Similarly, investigators seek to minimize RMSE (or SEE) but do not state limits. It is a strength of this work that the steps used in equation development are clearly articulated and a priori criteria for model selection are presented.
Our final equations are not very convenient for hand calculations. Nevertheless, they are simple to implement using a computerized spreadsheet or statistical software. The equations developed met our goals of providing a means for the COPTR investigators to calculate percent body fat from a limited number of relatively easily obtained measurements using a valid equation with relatively low error and bias. Given that the equations were developed in a sample of boys and girls assembled to be representative of the United States population, the potential generalizability is strong and we anticipate that these equations will be of use to other investigators studying adiposity in youth.
What is already known about the subject
It is often not feasible to measure percent body fat using precise methods such as dual-emission X-ray absorptiometry (DXA).
Equations developed to estimate percent body fat in youth using measures that are feasible to collect in a variety of settings usually have been developed in focused samples and therefore have low generalizability.
What this study adds
First equations developed in a representative sample of American youth that estimate percent body fat using self-reported demographic variables as well as measured skinfolds and other anthropometric variables that are feasible to collect in a variety of settings.
Equations were demonstrated to have low levels of bias by BMI category and by race/ethnicity in non-Hispanic Whites, Mexican Americans and African Americans.
Acknowledgements and author contributions
JS and JC conceived the project, KT and AR analyzed the data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
The project described was supported by Award Number 1U01HL103561 and 3U01HL103561-01S1 from the National Heart, Lung, And Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Development and the Office of Behavioral and Social Sciences Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Conflicts of Interest statement:
None
References
- 1.Bray GA, Bouchard C, James WPT. Handbook of obesity. New York: M. Dekker; 1998. [Google Scholar]
- 2.Jackson AS, Ellis KJ, McFarlin BK, Sailors MH, Bray MS. Body mass index bias in defining obesity of diverse young adults: the Training Intervention and Genetics of Exercise Response (TIGER) study. Br J Nutr. 2009;102:1084–1090. doi: 10.1017/S0007114509325738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens J, Murray DM, Baggett CD, et al. Objectively assessed associations between physical activity and body composition in middle-school girls: the Trial of Activity for Adolescent Girls. Am J Epidemiol. 2007;166:1298–1305. doi: 10.1093/aje/kwm202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball SD, Altena TS, Swan PD. Comparison of anthropometry to DXA: a new prediction equation for men. Eur J Clin Nutr. 2004;58:1525–1531. doi: 10.1038/sj.ejcn.1602003. [DOI] [PubMed] [Google Scholar]
- 5.Bray GA, DeLany JP, Harsha DW, Volaufova J, Champagne CC. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton Rouge Children's Study. Am J Clin Nutr. 2001;73:687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, DeLany JP, Volaufova J, Harsha DW, Champagne C. Prediction of body fat in 12-y-old African American and white children: evaluation of methods. Am J Clin Nutr. 2002;76:980–990. doi: 10.1093/ajcn/76.5.980. [DOI] [PubMed] [Google Scholar]
- 7.Brook CG. Determination of body composition of children from skinfold measurements. Arch Dis Child. 1971;46:182–184. doi: 10.1136/adc.46.246.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron N, Griffiths PL, Wright MM, et al. Regression equations to estimate percentage body fat in African prepubertal children aged 9 y. Am J Clin Nutr. 2004;80:70–75. doi: 10.1093/ajcn/80.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Deurenberg P, van der Kooy K, Leenen R, Weststrate JA, Seidell JC. Sex and age specific prediction formulas for estimating body composition from bioelectrical impedance: a cross-validation study. Int J Obes. 1991;15:17–25. [PubMed] [Google Scholar]
- 10.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 11.Dezenberg CV, Nagy TR, Gower BA, Johnson R, Goran MI. Predicting body composition from anthropometry in pre-adolescent children. Int J Obes Relat Metab Disord. 1999;23:253–259. doi: 10.1038/sj.ijo.0800802. [DOI] [PubMed] [Google Scholar]
- 12.Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21:681–689. doi: 10.1079/bjn19670070. [DOI] [PubMed] [Google Scholar]
- 13.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 14.Eisenmann JC, Heelan KA, Welk GJ. Assessing body composition among 3- to 8-year-old children: anthropometry, BIA, and DXA. Obes Res. 2004;12:1633–1640. doi: 10.1038/oby.2004.203. [DOI] [PubMed] [Google Scholar]
- 15.Eston RG, Rowlands AV, Charlesworth S, Davies A, Hoppitt T. Prediction of DXA-determined whole body fat from skinfolds: importance of including skinfolds from the thigh and calf in young, healthy men and women. Eur J Clin Nutr. 2005;59:695–702. doi: 10.1038/sj.ejcn.1602131. [DOI] [PubMed] [Google Scholar]
- 16.Freedman DS, Wang J, Ogden CL, et al. The prediction of body fatness by BMI and skinfold thicknesses among children and adolescents. Annals of human biology. 2007;34:183–194. doi: 10.1080/03014460601116860. [DOI] [PubMed] [Google Scholar]
- 17.Garcia AL, Wagner K, Hothorn T, Koebnick C, Zunft HJ, Trippo U. Improved prediction of body fat by measuring skinfold thickness, circumferences, and bone breadths. Obes Res. 2005;13:626–634. doi: 10.1038/oby.2005.67. [DOI] [PubMed] [Google Scholar]
- 18.Gartner A, Dioum A, Delpeuch F, Maire B, Schutz Y. Use of hand-to-hand impedancemetry to predict body composition of African women as measured by air displacement plethysmography. Eur J Clin Nutr. 2004;58:523–531. doi: 10.1038/sj.ejcn.1601839. [DOI] [PubMed] [Google Scholar]
- 19.Going S, Nichols J, Loftin M, et al. Validation of bioelectrical impedance analysis (BIA) for estimation of body composition in Black, White and Hispanic adolescent girls. Int J Body Compos Res. 2006;4:161–167. [PMC free article] [PubMed] [Google Scholar]
- 20.Goran MI, Driscoll P, Johnson R, Nagy TR, Hunter G. Cross-calibration of bodycomposition techniques against dual-energy X-ray absorptiometry in young children. Am J Clin Nutr. 1996;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- 21.Guo SM, Roche AF, Houtkooper L. Fat-free mass in children and young adults predicted from bioelectric impedance and anthropometric variables. Am J Clin Nutr. 1989;50:435–443. doi: 10.1093/ajcn/50.3.435. [DOI] [PubMed] [Google Scholar]
- 22.Hodgdon JA, Friedl K, Naval Health Research C. United States. Navy Dept. Bureau of M, Surgery. Development of the DoD body composition estimation equations. San Diego, CA: Naval Health Research Center; 1999. [Google Scholar]
- 23.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 24.Kwok T, Woo J, Lau E. Prediction of body fat by anthropometry in older Chinese people. Obes Res. 2001;9:97–101. doi: 10.1038/oby.2001.12. [DOI] [PubMed] [Google Scholar]
- 25.Loftin M, Nichols J, Going S, et al. Comparison of the validity of anthropometric and bioelectric impedance equations to assess body composition in adolescent girls. Int J Body Compos Res. 2007;5:1–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman TG, Caballero B, Himes JH, et al. Estimation of body fat from anthropometry and bioelectrical impedance in Native American children. Int J Obes Relat Metab Disord. 2000;24:982–988. doi: 10.1038/sj.ijo.0801318. [DOI] [PubMed] [Google Scholar]
- 27.Lohman TG, Caballero B, Himes JH, et al. Body composition assessment in American Indian children. Am J Clin Nutr. 1999;69:764S–766S. doi: 10.1093/ajcn/69.4.764S. [DOI] [PubMed] [Google Scholar]
- 28.Peterson MJ, Czerwinski SA, Siervogel RM. Development and validation of skinfold-thickness prediction equations with a 4-compartment model. Am J Clin Nutr. 2003;77:1186–1191. doi: 10.1093/ajcn/77.5.1186. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer F, Georgi M, Zieger A, Scharer K. Usefulness of bioelectric impedance and skinfold measurements in predicting fat-free mass derived from total body potassium in children. Pediatr Res. 1994;35:617–624. [PubMed] [Google Scholar]
- 30.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 31.van der Ploeg GE, Gunn SM, Withers RT, Modra AC. Use of anthropometric variables to predict relative body fat determined by a four-compartment body composition model. Eur J Clin Nutr. 2003;57:1009–1016. doi: 10.1038/sj.ejcn.1601636. [DOI] [PubMed] [Google Scholar]
- 32.Weststrate JA, Deurenberg P. Body composition in children: proposal for a method for calculating body fat percentage from total body density or skinfold-thickness measurements. Am J Clin Nutr. 1989;50:1104–1115. doi: 10.1093/ajcn/50.5.1104. [DOI] [PubMed] [Google Scholar]
- 33.Yavari R, McEntee E, McEntee M, Brines M. Anthropometric variables accurately predict dual energy x-ray absorptiometric-derived body composition and can be used to screen for diabetes. PLoS One. 2011;6:e24017. doi: 10.1371/journal.pone.0024017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor DP, Bray MS, McFarlin BK, Sailors MH, Ellis KJ, Jackson AS. Generalized equations for estimating DXA percent fat of diverse young women and men: the TIGER study. Med Sci Sports Exerc. 2010;42:1959–1965. doi: 10.1249/MSS.0b013e3181dc2e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugas LR, Cao G, Luke AH, Durazo-Arvizu RA. Adiposity Is Not Equal in a Multi-Race/Ethnic Adolescent Population: NHANES 1999–2004. Obesity (Silver Spring) 2011;19:2099–2101. doi: 10.1038/oby.2011.52. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Examination Protocol. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 37.National Health and Nutrition Examination Survey: Technical Documentation for the 1999–2004 Dual Energy X-Ray Absorptiometry (DXA) Multiple Imputation Data files. 2008
- 38.Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–1025. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 39.Judd CM, McClelland GH. Data analysis: A model-comparison approach. 1989 [Google Scholar]
- 40.West SG, Aiken LS. Multiple regression: Testing and interpreting interactions. Sage Publications, Incorporated; 1991. [Google Scholar]
- 41.Stevens J, Taber DR, Murray DM, Ward DS. Advances and controversies in the design of obesity prevention trials. Obesity (Silver Spring) 2007;15:2163–2170. doi: 10.1038/oby.2007.257. [DOI] [PubMed] [Google Scholar]
- 42.Stevens J, Suchindran C, Ring K, et al. Physical activity as a predictor of body composition in American Indian children. Obes Res. 2004;12:1974–1980. doi: 10.1038/oby.2004.248. [DOI] [PubMed] [Google Scholar]
- 43.Webber LS, Catellier DJ, Lytle LA, et al. Promoting physical activity in middle school girls: Trial of Activity for Adolescent Girls. Am J Prev Med. 2008;34:173–184. doi: 10.1016/j.amepre.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caballero B, Clay T, Davis SM, et al. Pathways: a school-based, randomized controlled trial for the prevention of obesity in American Indian schoolchildren. Am J Clin Nutr. 2003;78:1030–1038. doi: 10.1093/ajcn/78.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutin B, Yin Z, Johnson M, Barbeau P. Preliminary findings of the effect of a 3-year afterschool physical activity intervention on fitness and body fat: the Medical College of Georgia Fitkid Project. Int J Pediatr Obes. 2008;3(Suppl 1):3–9. doi: 10.1080/17477160801896457. [DOI] [PubMed] [Google Scholar]
- 46.Puder JJ, Marques-Vidal P, Schindler C, et al. Effect of multidimensional lifestyle intervention on fitness and adiposity in predominantly migrant preschool children (Ballabeina): cluster randomised controlled trial. BMJ. 2011;343:d6195. doi: 10.1136/bmj.d6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merz AL, Trotter M, Peterson RR. Estimation of skeleton weight in the living. Am J Phys Anthropol. 1956;14:589–609. doi: 10.1002/ajpa.1330140405. [DOI] [PubMed] [Google Scholar]
- 48.Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr. 2000;71:1392–1402. doi: 10.1093/ajcn/71.6.1392. [DOI] [PubMed] [Google Scholar]