Abstract
Cellulose acetate phthalate (CAP) and Pluronic F-127 combined together (70:30 wt:wt) create a rigid, surface eroding association polymer. To impart flexibility into the polymer system and allow for a drug delivery film that can contour to varying wound shapes, plasticizers were added. Triethyl citrate (TEC) or tributyl citrate (TBC) was combined with CAP and Pluronic F-127 at 0, 10, or 20 wt%. Mechanical analysis was performed on the films as they were prepared and following a 2 hour incubation in phosphate-buffered saline. Tensile tests showed that higher plasticizer content increased the % elongation but decreased the elastic modulus (E) and ultimate tensile strength (UTS). The effect TEC had on the % elongation was twice as much than that of TBC. After incubation, % elongation, E, and UTS all increased because plasticizer leached out of the films. MicroCT and SEM were performed on the samples both before and after incubation to determine how erosion and leaching of plasticizer affected the interior and exterior structure of the films. Porosity increased as plasticizer content increased, however, plasticizer content did not have a significant effect on the rate of erosion. The mechanical properties of CAP-Pluronic films can be adjusted by the type and amount of plasticizer added to the system and therefore can be tailored for different drug delivery applications.
Keywords: drug delivery film, plasticizer, cellulose acetate phthalate, erosion, tensile testing
Introduction
While convenient, medications taken orally must make a long journey, traveling from the intestines, through the portal vein, into the liver, through the bloodstream until finally reaching their intended therapeutic target. During this convoluted process, many drug molecules are eliminated from circulation by the liver and kidneys, metabolized and excreted from the body [1–4]. Because of this, oftentimes drugs must be given at doses far exceeding what the target site needs for treatment. This excessive concentration coupled with presence of the drug throughout all of the body systems results in a scenario where systemic side effects are guaranteed [5–7].
As a result, there is a need for a degradable, localized, drug delivery system capable of delivering drug to soft tissue so unnecessary sites are not medicated, reducing the risk of side effects, and drug is not wasted during clearance. Most of the current drug delivery systems are composed of slowly eroding polymer networks, including Eudragit and poly(sebacic anhydride), from which drug diffuses out to provide therapy [8–13]. This kind of device leaves behind material that may cause a foreign body reaction or cause inflammation from the byproducts as it slowly degrades. Also, many of the other currently researched systems are composed of rigid polymers, such as chitosan and Eudragit, that cannot contour to the shape of the treated site [13–15]. The rigidity or stiffness of materials is related to their elastic modulus [16]. Rigid polymers implanted in soft tissue will cause discomfort and possible fibrous capsule formation [17–19].
Plasticizers can be added to polymers to make them more flexible [20, 21]. Plasticizers are small lubricating molecules that can change polymer properties and processability [21]. In this study, the effect of plasticizers on the mechanical properties of an association polymer was explored. The system formed from cellulose acetate phthalate (CAP) and Pluronic F-127 has been proven to be a surface-eroding system that would release drug as the device eroded so no material would be left behind to cause an inflammatory reaction [22–24]. Without plasticizer, however, the polymer is rigid and cannot be readily contoured. Two plasticizers, triethyl citrate (TEC) and tributyl citrate (TBC), have been included, and the system as a whole has been evaluated through mechanical analysis and degradation studies. Because these materials are ultimately intended to be implanted, their properties and structure when wet were also determined [25, 26].
Materials and Methods
Film Production
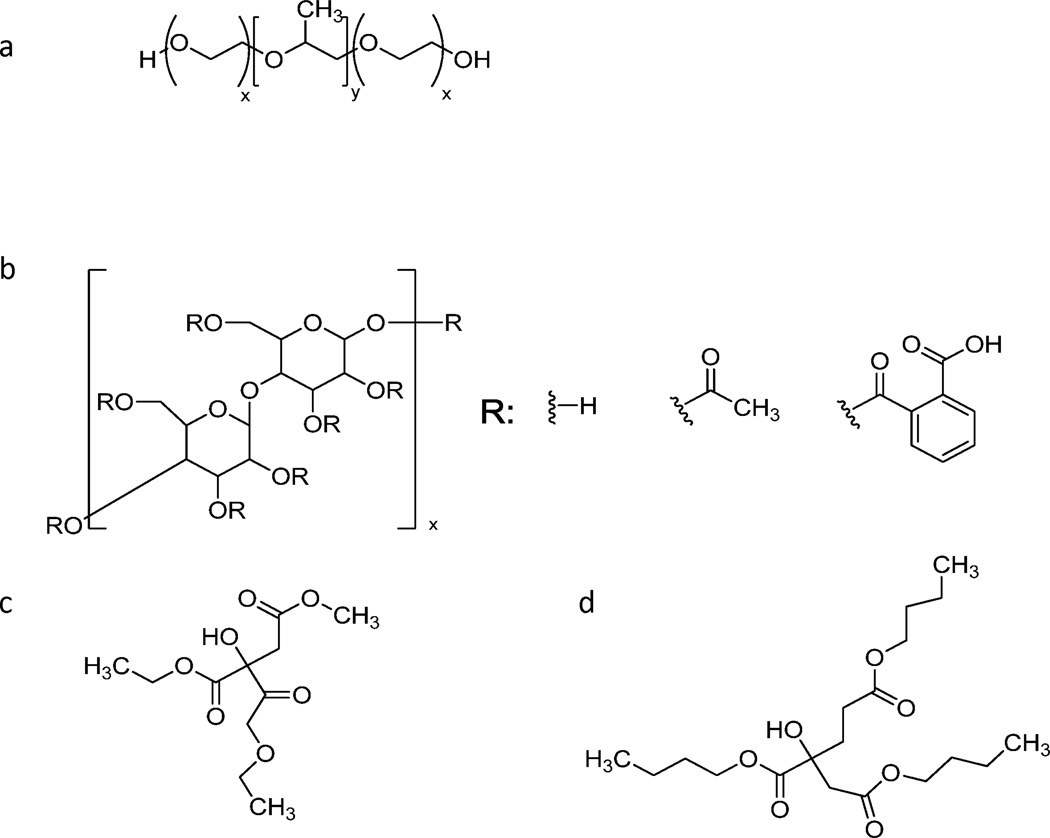
Films were made with a 70:30 weight percent ratio of cellulose acetate phthalate (CAP; Sigma-Aldrich, St. Louis, MO) and Pluronic F-127 (Sigma-Aldrich), respectively (Figure 1). Plasticizer, either tributyl citrate (Sigma-Aldrich) or triethyl citrate (Sigma-Aldrich), was added to the CAP-Pluronic mixture at either 0, 10, or 20 wt%. Acetone was added to make a 25% (w/v) solution. After vortexing the solution to ensure homogeneity, it was cast into Teflon dishes and then let stand in a 10°C refrigerator overnight. Films were desiccated overnight before testing.
Figure 1.
Chemical structures of (a) Pluronic F127, (b) cellulose acetate phthalate, (c) triethyl citrate, and (d) tributyl citrate.
Erosion Studies
Films were cut into discs using a circular punch measuring 5 mm in diameter. After determining their initial mass, samples were placed individually in a 24-well plate, and 1.85 ml of phosphate-buffered saline (PBS), pH 7.4, was added to each well. Plates were incubated on an orbital shaker plate 37°C. Half of the supernatant was replaced with fresh PBS every 12 hours. Three samples were collected and dried at different time points ranging from every 3 to 5 hours. Dried samples were weighed to determine final mass. The average mass loss was determined. The erosion rates were found by calculating the slope of average mass loss with time. Supernatants were collected from both TEC and TBC films after being incubated in PBS for 2 hours. The supernatants were pipetted into a 96-well plate and UV absorbance was measured at 630 nm using the BioTek PowerWave HT and Gen5 software to determine the opacity. PBS was used as a control.
Mechanical Properties
Films were cut to make microtensile test samples using a dog bone die (ASTM D 1708). Wet samples were incubated in 4 ml of PBS for two hours. Cross-sectional areas were measured for each film, both wet and dry, using calipers. Samples were mechanically tested to failure in tensile mode using the BOSE ELF 3300 with a ramp at a displacement rate of 0.5 mm/sec. Stress and strain were calculated, and elastic modulus (E), percent elongation, and ultimate tensile strength (UTS) were determined. The percent elongation was normalized by the cross-sectional area so this value would not be influenced by thickness variations in the cast films.
Scanning Electron Microscopy (SEM)
After dog bone samples were incubated in PBS for two hours, they were lyophilized to better preserve microarchitecture for morphological assessment. Dried polymeric films were then frozen and frozen at −80°C for 30 min before being fractured so the cross-sectional area could be analyzed using a model S-3200-N Hitachi scanning electron microscope. The samples were coated with gold, and the microscope was operated at 20 kV and digital images were collected.
Microcomputerized Tomography (microCT)
To determine the interior porosity, microCT scans were performed using the SCANCO Medical AG µCT 40. Samples were cut to fit 12.3 mm tubes and were batch scanned. Scans were run in high resolution having 1000 projections with 2048 samples, a current of 177 µA, a potential of 70 kVp, and a 0° angle. Samples were contoured and evaluated with a program that distinguished between the polymer material and vacancies. The uniformly biased lower threshold was set to 20 so any part of the scan with a density below that was considered a void. The slices were reconstructed into a 3-dimensional representation of the scanned samples, from which the total volume, porosity volume, average pore size, and average spacing between pores were calculated using a morphometry script. Samples were scanned before and after being incubated in PBS for two hours.
Mass Spectrometry
Analyses were conducted in the University of Kentucky Mass Spectrometry Facility. Supernatants from erosion studies were mixed with an equal volume of acetonitrile before analysis. Electrospray ionization mass spectra were obtained on a ThermoFinnigan LTQ (ion trap mass spectrometer), with sample introduction by direct infusion at 3 µL/min. Full scan mass spectra were recorded in positive ion mode. Instrument parameters included: spray voltage, 3.5kV; capillary temperature, 185°C; capillary voltage, 50V; and tube lens voltage, 80V.
Statistics
Statistical analysis was performed using Instat (GraphPad Software). All of the samples with the same weight percentage of plasticizer or the same type of plasticizer were compared against each other. Results were analyzed using one-way ANOVA, and a p-value < 0.05 was considered statistically significant.
Results
Films
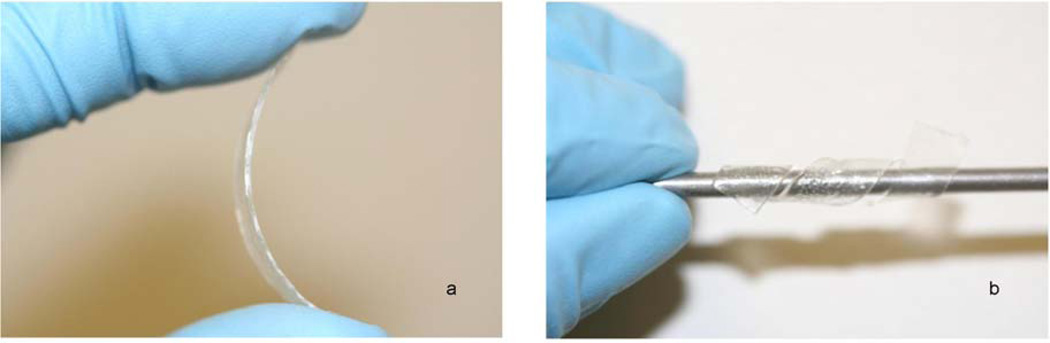
Plasticized films increased in flexibility as plasticizer content increased, while the unplasticized films remained rigid and could not be plastically deformed without failure (Figure 2). Qualitatively, films plasticized with TEC were more flexible than those plasticized with TBC at an equivalent weight percentage.
Figure 2.
A) Unplasticized, inflexible CAP-Pluronic film and B) plasticized CAP-Pluronic film able to conform to any shape.
Erosion Studies
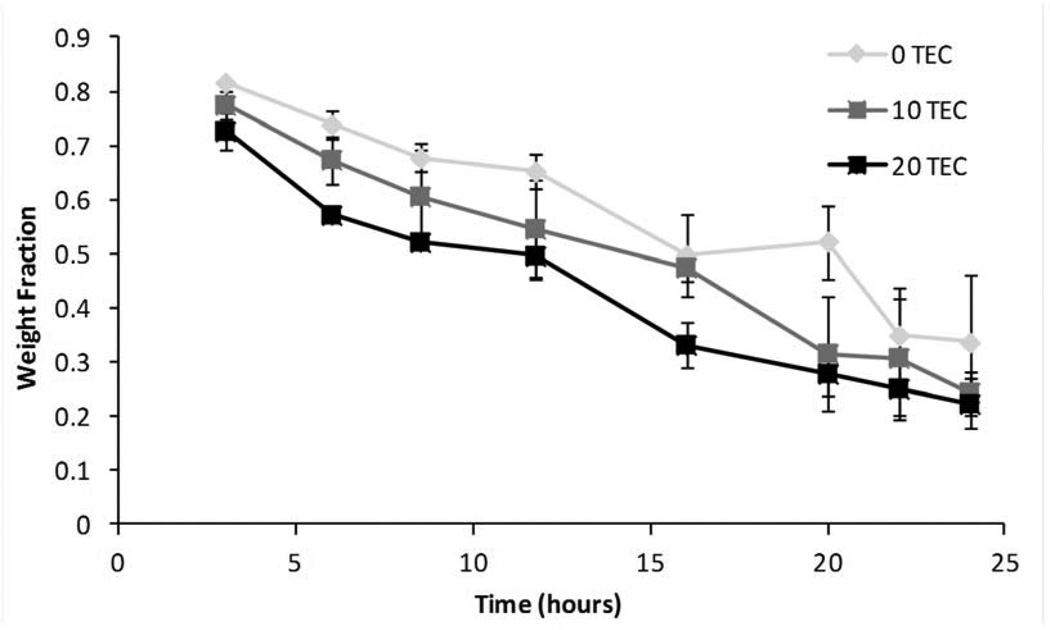
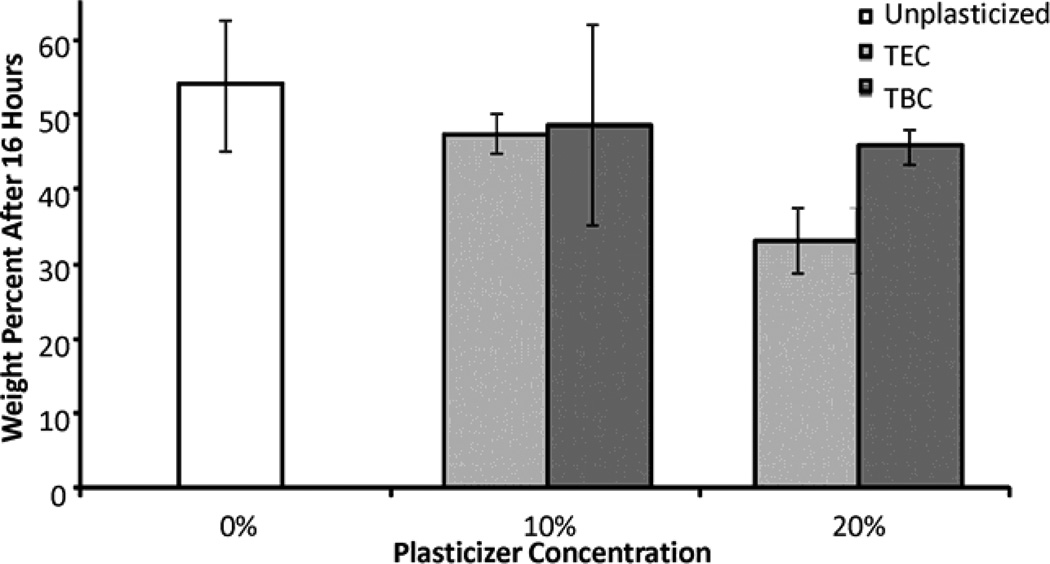
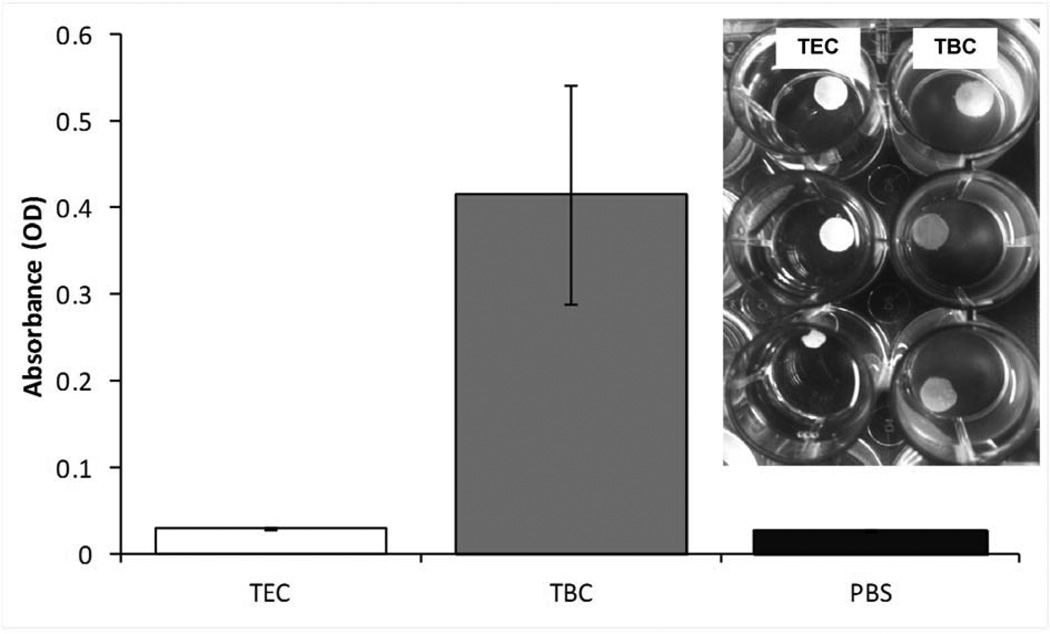
Figure 3 shows typical mass loss curves for the control (unplasticized) and plasticized films. The addition of plasticizer did not significantly affect the erosion of the films. As the weight percentage of plasticizer increased, the magnitude of rate of erosion slightly increased from 2.2 wt% to 2.4 wt% per hour but the differences between the three rates were not significant (Figure 3). As the films eroded, collecting the residual polymer to measure mass loss became a challenge so samples were only collected for the first 24 hours. The remaining mass of all of the films, plasticized with either TEC or TBC, after 16 hours was also statistically the same (Figure 4). Sixteen hours was chosen because at that point the initial mass loss due to plasticizer leaching had already occurred and the supernatant had been replenished with fresh PBS resulting in close to sink conditions. As TBC films eroded, the PBS became milky in appearance, whereas the supernatant remained transparent as TEC films eroded (Figure 5). Quantitatively, the opacity of the supernatant from the TBC sample was over 10 times as high as the TEC sample. The supernatant from the TEC sample was the same as PBS alone. Analysis of two hour erosion supernatants by mass spectrometry showed higher relative abundance of positive ion mass numbers corresponding to TEC and TBC than any other peak.
Figure 3.
Example of typical degradation curves. Data are mean ± standard deviation (n=3).
Figure 4.
Remaining mass after 16 hours of incubation in PBS at 37°C. Data are mean ± standard deviation (n=3). No differences were statistically significant.
Figure 5.
Absorbance at 630 nm of supernatants from TEC and TBC films after 2 hours of incubation compared to PBS. Data are mean ± standard deviation (n=3). Inset: Images showing appearance of supernatants from TEC (top) and TBC (bottom) films.
Mechanical Properties as Prepared
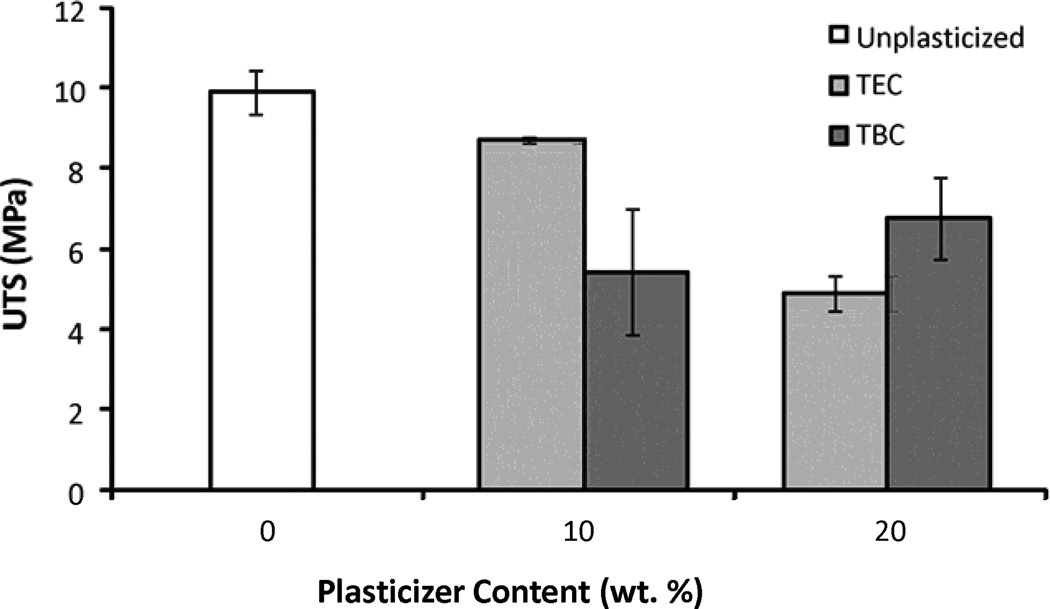
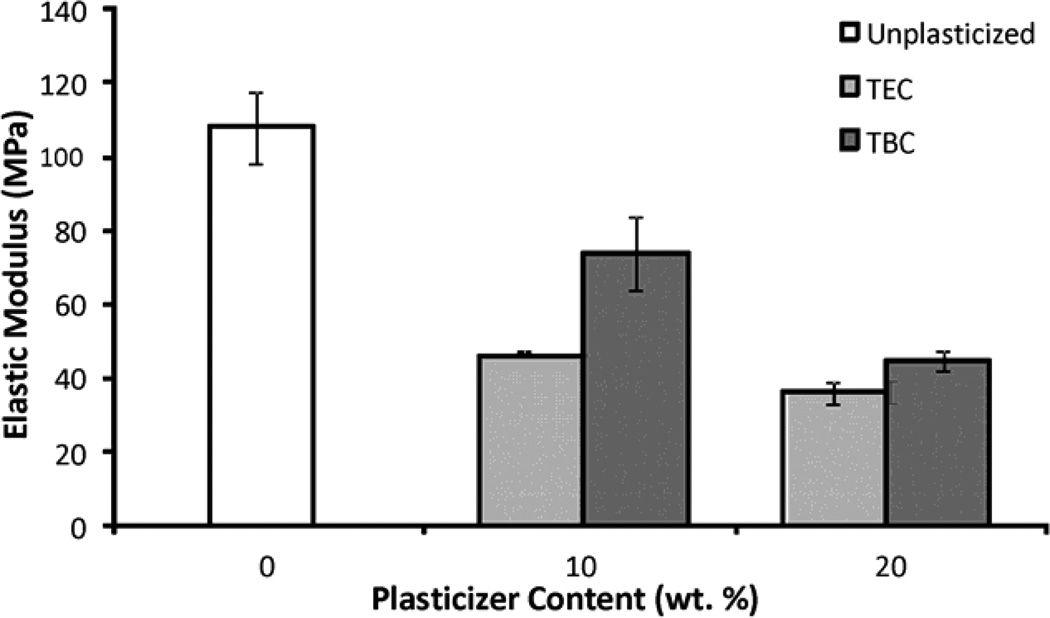
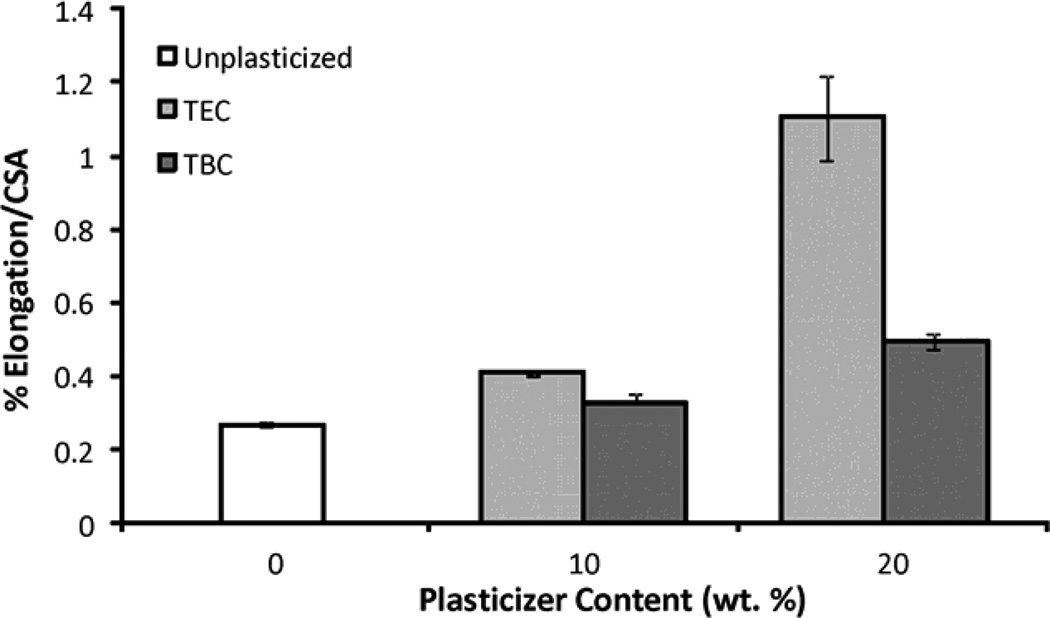
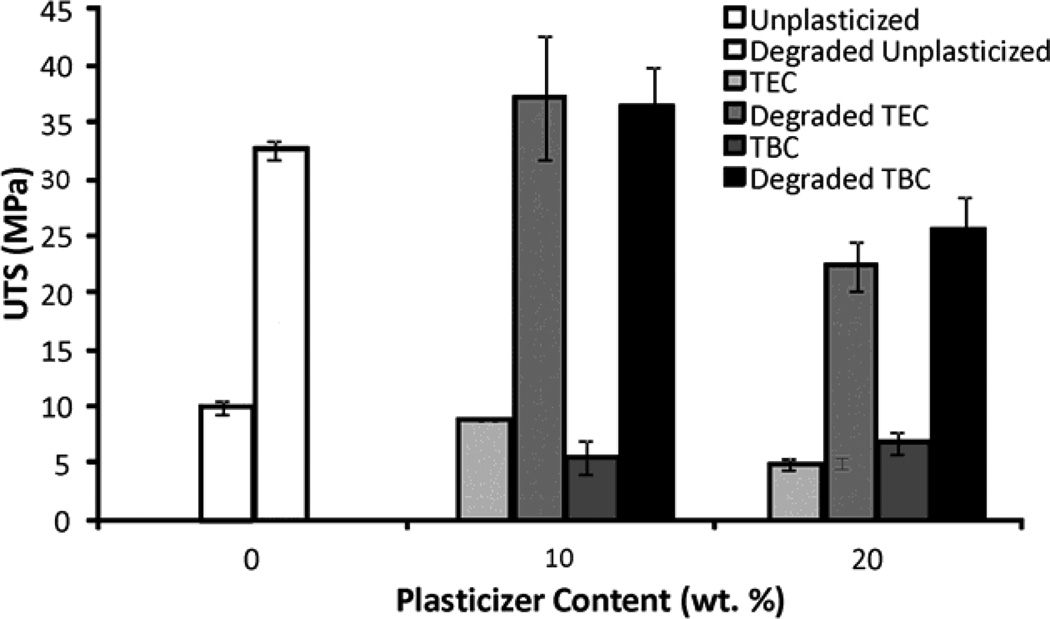
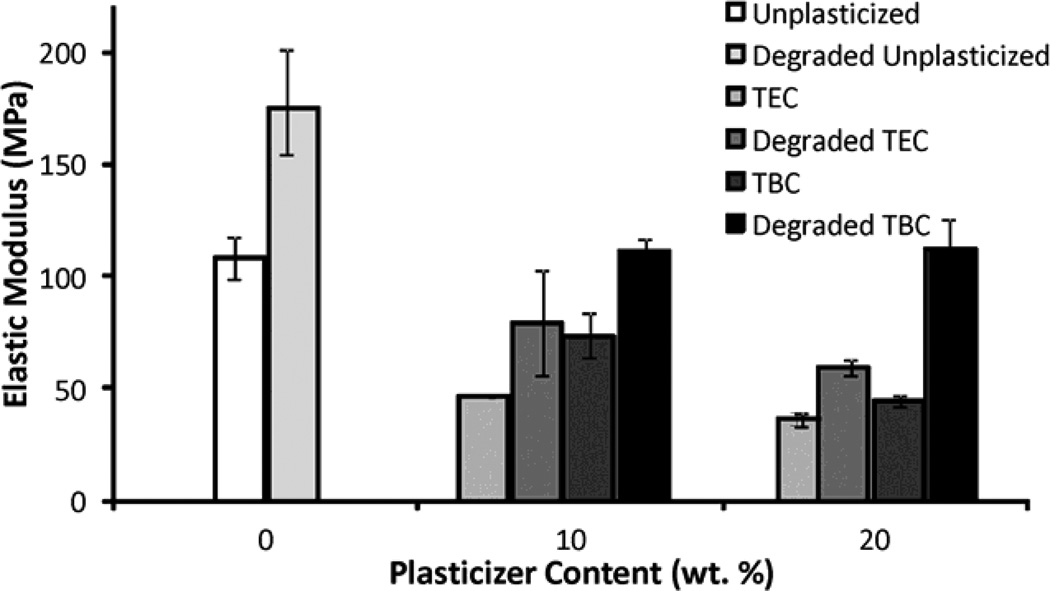
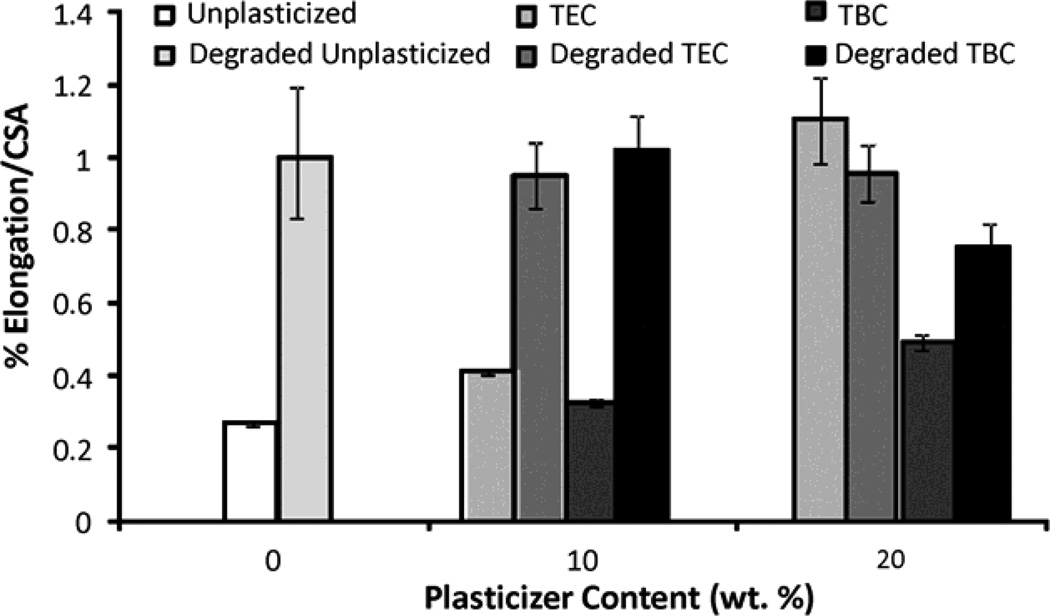
Increasing the plasticizer content increased the effects on the mechanical properties; more plasticizer decreased the E and UTS and increased the percent elongation. Compared to unplasticized films, the 20 wt% samples plasticized with TEC had a lower E and UTS (Figures 6a & 6b), by about half, but had a 5 times higher percent elongation (Figure 6c). Addition of TEC to the films always significantly (p ≤ 0.05) affected the mechanical properties except between the ultimate tensile strength of the 10 wt% TEC and unplasticized samples. Adding 10 wt% TBC to the films decreased the UTS by roughly half, but addition of more TBC did not further affect the UTS. The 0 wt% TBC films had an elongation that was statistically the same as the 10 wt% TBC films, which had an elongation that was comparable to that of the 20 wt% TBC films. However, 0 and 20 wt% TBC films had elongations significantly (p ≤ 0.05) different from each other. The elastic modulus did significantly (p ≤ 0.001) decrease as more TBC was added to the films. For the concentrations examined, as plasticizer increased, the UTS of the TEC films decreased. With the TBC films, increasing plasticizer concentration past 10 wt% did not significantly affect the UTS. For similar plasticizer content, TEC caused greater changes to elongation, UTS, and E. For the 20 wt% samples, the TEC and TBC films had statistically similar UTS and E values, but their elongations were statistically different (p < 0.001), with and the 20 wt% TEC films plastically deforming over twice as much as the TBC films.
Figure 6.
A) UTS of TBC and TEC films. 0 and 20 TEC were statistically different, p < 0.001; 0 and 20 TBC were statistically different, p < 0.01. B) Elastic modulus of TBC and TEC films. 0, 10, and 20 TBC were all statistically different from each other, p < 0.01; both 10 and 20 TEC were statistically different from 0 TEC, p < 0.001 but 10 and 20 TEC were statistically the same. C) Elongation of TBC and TEC films normalized by the cross-sectional areas (CSA). 0 TBC samples were statistically different from 20 TBC samples, p < 0.05; 0 TEC was statistically different from 10 TEC, p < 0.05; 20 TEC was statistically different from both 0 and 10 TEC, p < 0.001. Data are mean ± standard deviation (n=3).
Mechanical Properties after Incubation
Allowing the films to soak in PBS for 2 hours drastically changed their properties, with the magnitude of some properties being five times different from those of the dry samples. With both TEC and TBC films, the UTS, E, and elongation (except for the 20 wt% TEC film) all increased after films soaked in PBS for 2 hours. When comparing the dry samples to their wet counterparts, the differences were always significant (p < 0.001) for all UTS data for both TEC and TBC samples (Figure 7a). The elongation significantly decreased for 0 and 10 wt% TEC samples when they became wet, but the 20 wt% had an elongation that was the same when wet or dry (Figure 7c). For the TEC samples, the elastic modulus was significantly (p < 0.05) affected by the duration in PBS for only the 0 wt% films (Figure 7b). Elastic moduli for the 0 and 20 wt% TBC films were significantly (p < 0.05) higher after incubation in PBS. For both TEC and TBC films, the elongation increased (p < 0.001) for 0 wt% and 10 wt% films, but not 20 wt%.
Figure 7.
A) UTS of dry films and films incubated in PBS for 2 hours. Dry values compared to degraded values were all significantly different, p < 0.001. B) Elastic modulus of dry films and films incubated in PBS for 2 hours. 0 TEC and TBC films were statistically different than their degraded counterparts, p < 0.05; 20 TBC were statically different from their wet counterparts, p < 0.05. C) Elongation of dry films and films incubated in PBS for 2 hours. Dry 0 and 10 TBC and TEC films were statistically different from their wet counterparts, p < 0.001. Data are mean ± standard deviation (n=3).
Morphology
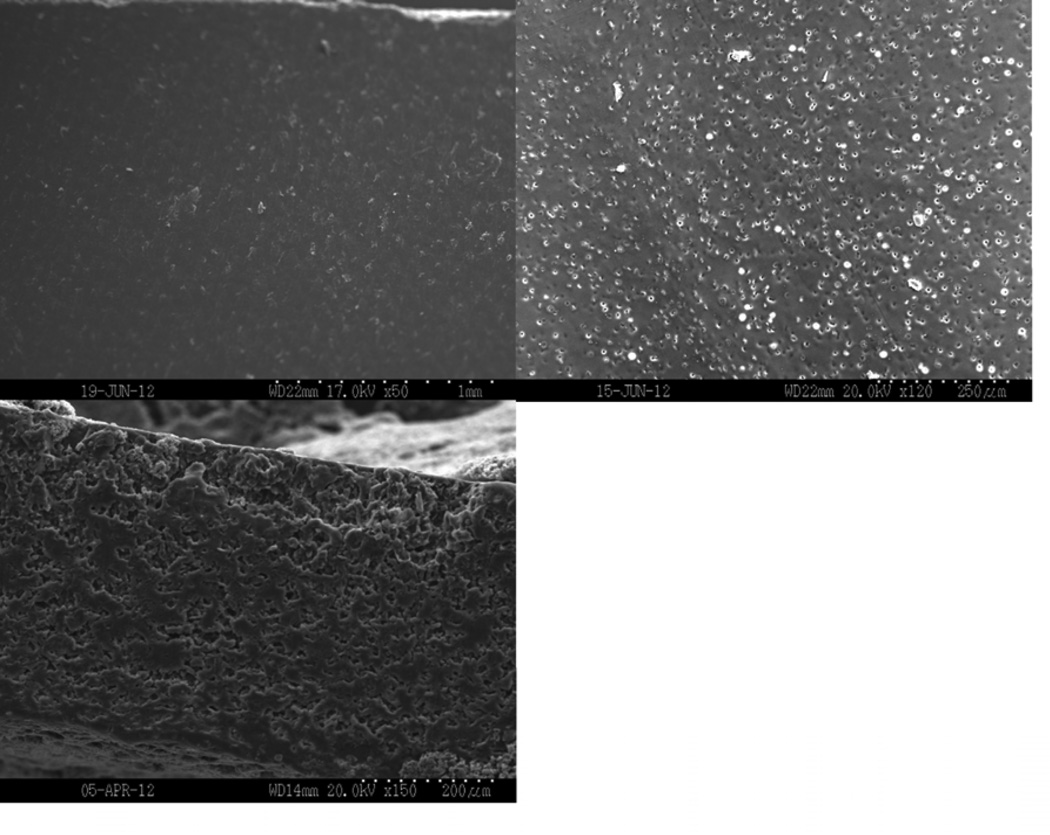
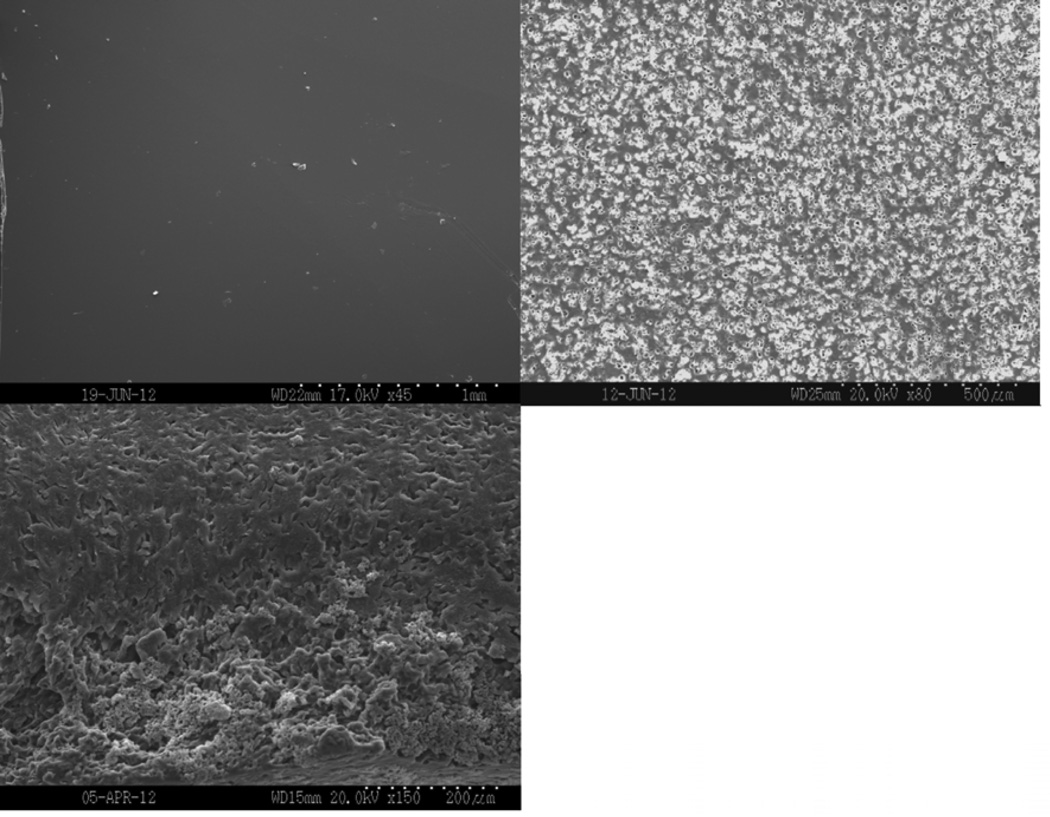
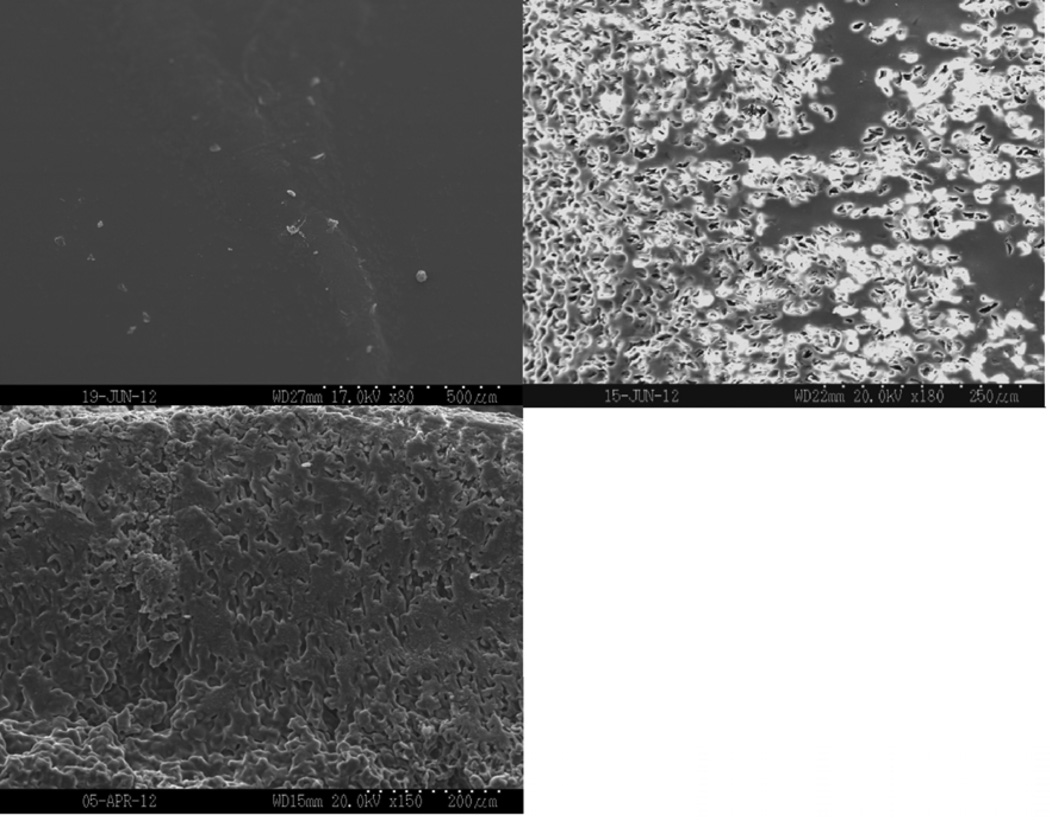
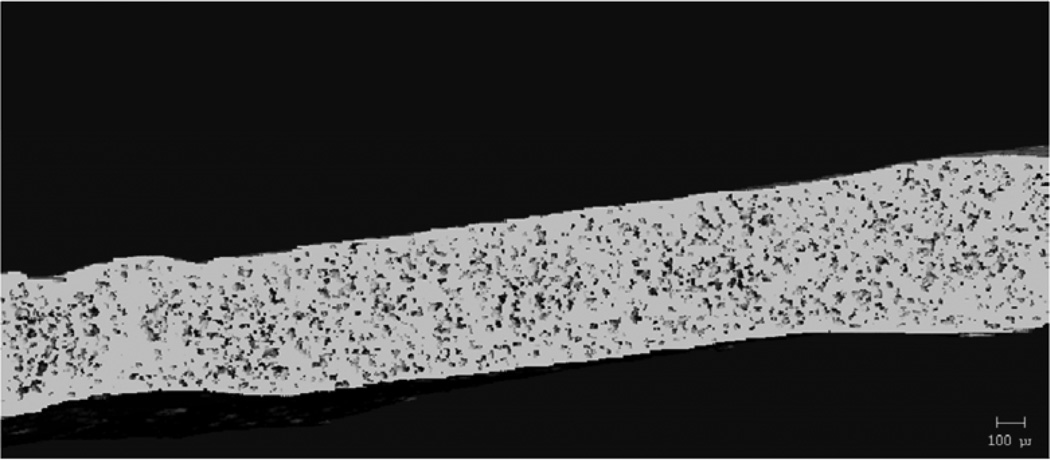
Figure 8 shows representative SEM images of dry film surfaces and surfaces and cross-sectional areas after incubation in PBS for two hours. Samples appeared smooth and had no porosity present before incubation. All of the samples had begun eroding after incubating in PBS for two hours. Porosity was seen both on the surfaces and in the cross-sections. As plasticizer content increased, more erosion occurred both on the exterior and interior of the film, resulting in increased porosity.
Figure 8.
Scanning electron micrographs of the as-prepared surface (top left), the surface (top right), and the cross-section (bottom) of films incubated in PBS for 2 hours. A) 0, B) 10, and C) 20 wt% TEC films.
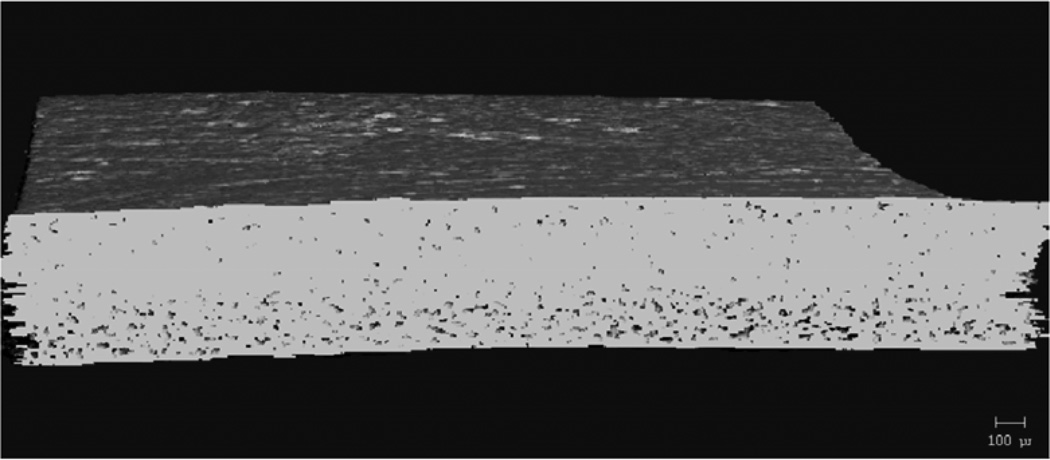
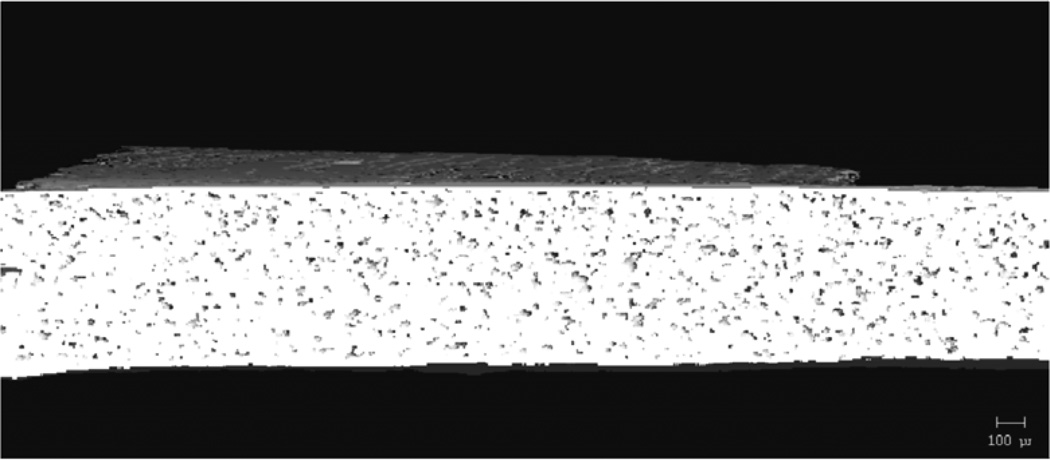
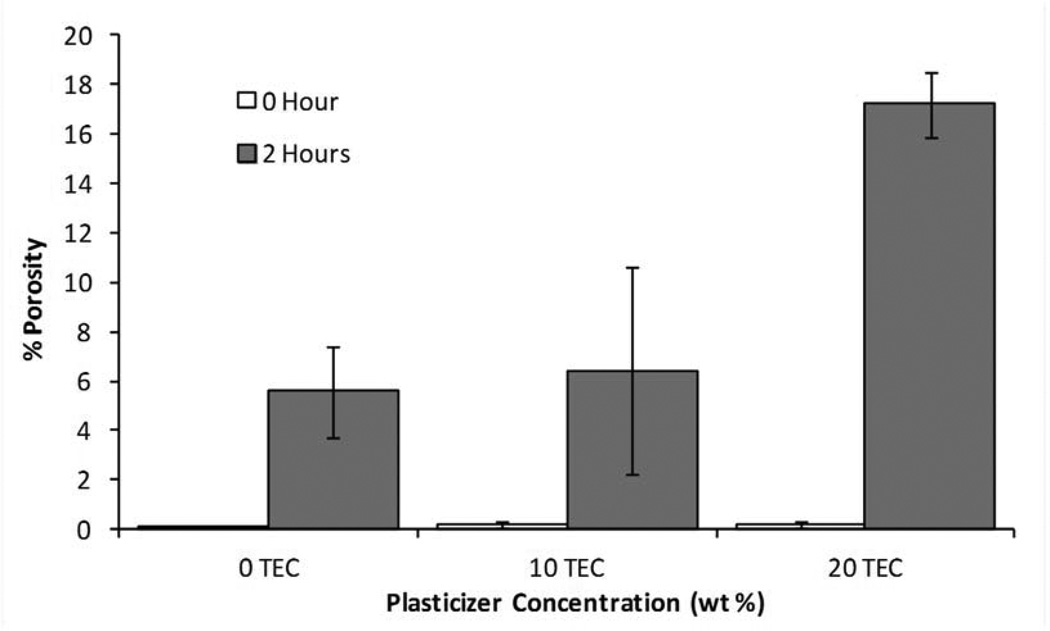
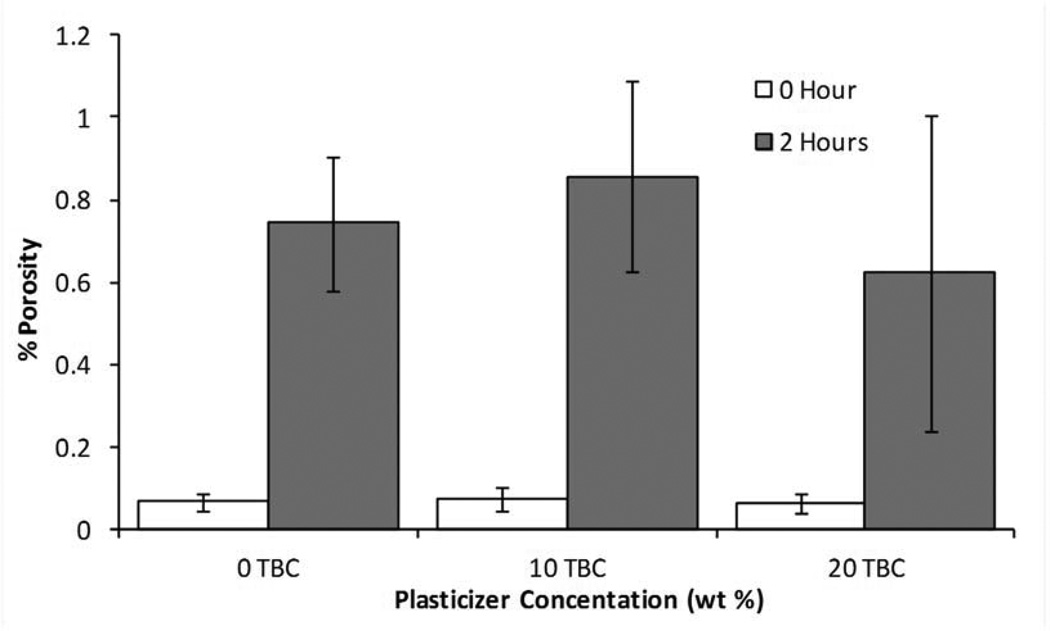
MicroCT analysis quantitatively confirmed what was seen in the SEM images. Figure 9 shows cross-sectional slices of representative films before and after two hours in PBS, and the porosity of each film type is plotted in Figure 10. For TEC films, as plasticizer content increased, so did the porosity. The 20 wt% films increased to 17% porosity during incubation. The incubated samples were always statistically (p ≤ 0.05) different from their dry counterparts. Incubated 0 and 10 wt% samples were statistically the same, but both were significantly (p < 0.001) different than the porosity measured in the 20 wt% films. With TBC films, plasticizer content did not affect the porosity after 2 hours in PBS. Porosity increased uniformly to just under 1%. Pore sizes for both TEC and TBC films were found to be as small as 6 µm. The majority of the accessible volume in the films after erosion was from pores with a diameter of less than 20 µm. There were almost no pores present that were larger than 40 µm in either TBC or TBC plasticized films.
Figure 9.
MicroCT images showing A) 0, B) 10, and C) 20 wt% TEC films incubated in PBS for 2 hours.
Figure 10.
Porosity measurements for as-prepared films and films incubated in PBS for 2 hours. A) TEC-containing films. Films with 20 wt% TEC were statistically different than those without TEC, p < 0.05. B) TBC-containing films. There were no statistical difference between incubated samples and no statistical difference between non-incubated samples.
Discussion
CAP-Pluronic films are rigid and unable to conform to the varying topology of soft tissue defects that may occur in motor vehicle accidents or war wounds. In order to aid in placement of the films, imparting flexibility and allow the films to contour to any shape, plasticizers were added. With the aim of making the FDA approval process quicker, plasticizers that were already deemed safe were chosen for the CAP-Pluronic films. Both triethyl citrate and tributyl citrate are common plasticizers used in pharmaceutical and biomedical devices ([20, 27–29]. The United States Pharmacoepia deems both TEC and TBC appropriate to be used in pharmaceutical dosage forms [29]. They are used in gelatin capsules, enteric coatings on pills, and transdermal drug delivery patches [27, 28, 30]. They are also used in poly(vinyl chloride) and poly(vinyl acetate) components of medical devices, including tubing and films [31]. Toxicology studies showed that, when taken orally, TEC was toxic to rats in high doses corresponding to over half a liter in a 70 kg man. TBC was non-toxic in rats even at extremely high doses [32]. If a multilayered, plasticized film were applied to a wound that is 10 by 10 centimeters, it would contain approximately 5 mL, which is well within the toxicity limits for a 70 mg man.
Triethyl citrate and tributyl citrate have molecular weights of 276.28 g/mol and 360.45 g/mol, respectively. This means that, for the same weight percent, more TEC molecules are present in the films than TBC molecules (TEC: 0.74 mol per film for 10 wt%, TBC 0.58 mol per film for 10 wt%). The same molar concentration was not compared between TBC and TEC since plasticizers are most commonly added as a weight percent of the whole polymer. However, the 20 wt% films had 1.57× more plasticizing molecules than the 10 wt% TEC and they had very similar effects on the % elongation, UTS, and E, which shows that even with fewer molecules present, TEC plasticizes to a greater degree than TBC. Plasticizers with a lower molecular weight cause more flexibility in the material they are incorporated into because they have a higher mobility and allow polymer chains to more easily slide across one another [30]. With more lubricating plasticizer added to the films, a greater number of molecules were present between the polymer chains, which thereby increased the molecular separation and decreased the packing order of CAP and Pluronic F-127 [29]. Because of this, for the same weight percent of plasticizer added, the TEC had more of an impact on the mechanical properties of the CAP-Pluronic F-127 films.
Similar to what has been previously reported for poly(lactic acid) and poly(lactic-co-glycolic acid) [33], the mechanical properties of CAP-Pluronic films changed rapidly after incubation in PBS. In the present case, however, properties were altered because much of the plasticizer leached out in the first two hours of erosion. In addition to inferring leaching from effects on mechanical properties, mass spectrometry confirmed the abundance of both plasticizers in the erosion supernatant after only two hours of incubation. The TEC may leach out more quickly than the TBC because it is more hydrophilic than the TBC, and having smaller molecules also allowed it to travel down its chemical gradient faster. Triethyl citrate is a water soluble plasticizer up to 65 g/L, while TBC is a water-insoluble plasticizer [34, 35]. Differences in the solubility of TBC and TEC explain the differences in the supernatant’s appearance during degradation. The milky appearance was likely due to the TBC separating out from the PBS solution as the molecules leached out since it is insoluble in water, while the supernatant from the TEC films remained clear since it had not reached it solubility limit. Plasticizer leach out will not be an issue since the polymer will be in the shape of the wound and the film will only become stronger. After the film has been laid in place, there will no longer be a need for flexibility and the increased strength of the film will cause it to be a protective barrier during healing.
Pluronic F-127 may be eroding at a faster rate than CAP causing the porosity seen in the 0 wt% films. CAP is commonly used in enteric coatings because of its low water solubility. It dissolves at higher pH and therefore more slowly than Pluronic F-127 [36]. This, in addition to the plasticizer leaching out, created the nanometer- and micron-sized voids seen in the SEM and microCT images. The pores increased the surface area and allowed the films to degrade at a faster rate. The films containing more plasticizer may allow the Pluronic degradation to occur more quickly. As voids are created in the film, PBS can penetrate the films more deeply. Water causes disassociation of the hydrogen bonds linking the ether oxygens from the Pluronic F-127 to the carboxylic acid groups on CAP, leaving behind a material that contains increasing amounts of CAP relative to all of the other materials. Because CAP is a stronger polymer than Pluronic and the association polymer they form together, erosion actually increased the strength of the material [37, 38]. The ultimate tensile strength and elastic modulus increased since the polymer chains could not stretch and begin to slide past each other as easily as before incubation. But interestingly, the elongation also increased. This is likely due to the small holes that were created in the polymer which allowed it to behave and stretch more like a sponge. As tension was applied to the wet material, the holes collapsed and caused the remaining chains to elongate. CAP is a stiffer, slower eroding material than Pluronic F-127, so even the 0 wt% samples had an increase in the elastic modulus and ultimate tensile strength as CAP became the predominant material in the films.
Before plasticization, the CAP-Pluronic system is less rigid than other drug delivery polymers such as Eudragit, which has a modulus of 500 MPa or chitosan, which has a modulus of over 1000 MPa without plasticizer (14, 39). CAP-Pluronic F-127 is a competitive system for drug delivery applications because it is surface eroding so drug is released as the device erodes, and since it erodes, no material is left behind to cause inflammation. Another beneficial property is its lower elastic modulus allowing it to be more flexible and contour and set to the shape of varying wounds when it is plasticized.
Conclusion
The degradation behavior and mechanical properties of CAP-Pluronic films can be varied by the type and amount of plasticizer incorporated into the system. The material properties will change shortly after the CAP-Pluronic drug delivery films are exposed to fluid. These changes do not affect the integrity of the system, since the material only gets stronger and will maintain the shape formed in the tissue. The CAP-Pluronic system is attractive for a variety of soft tissue drug delivery applications because it could be tailored to have different properties.
Acknowledgements
This research was supported in part by the NIH (DE019645 and AR060964) and NSF (EPS-0814194 and EEC-0851716). CR was supported by NSF IGERT (DGE-0653710).
References
- 1.Thummel KE, O'Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59(5):491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 2.Thummel KE, Kunze KL, Shen DD. Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Advanced Drug Delivery Reviews. 1997;27(2–3):99–127. doi: 10.1016/s0169-409x(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 3.Chan LMS, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. European Journal of Pharmaceutical Sciences. 2004;21(1):25–51. doi: 10.1016/j.ejps.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Glare PA, Walsh TD. Clinical Pharmacokinetics of Morphine. Ther Drug Monit. 1991;13:1–23. doi: 10.1097/00007691-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Jeu L, Piacenti FJ, Lyakhovetskiy AG, Fung HB. Voriconazole. Clinical Therapeutics. 2003;25(5):1321–1381. doi: 10.1016/s0149-2918(03)80126-1. [DOI] [PubMed] [Google Scholar]
- 6.Polisson R. Nonsteroidal anti-inflammatory drugs: Practical and theoretical considerations in their selection. The American Journal of Medicine. 1996;100(2) Supplement 1:31S–36S. doi: 10.1016/s0002-9343(97)89544-7. [DOI] [PubMed] [Google Scholar]
- 7.Yano H, Hirayama F, Kamada M, Arima H, Uekama K. Colon-specific delivery of prednisolone-appended α-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. Journal of Controlled Release. 2002;79(1–3):103–112. doi: 10.1016/s0168-3659(01)00532-6. [DOI] [PubMed] [Google Scholar]
- 8.Shelke NB, Aminabhavi TM. Synthesis and characterization of novel poly(sebacic anhydride-co-Pluronic F68/F127) biopolymeric microspheres for the controlled release of nifedipine. Int J Pharm. 2007;10(345):51–58. doi: 10.1016/j.ijpharm.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Zhu YMK, McGinity JW. Influence of plasticizer level on the drug release from sustained release film coated and hot-melt extruded dosage forms. Pharm Dev Technol. 2006;11(3):285–294. doi: 10.1080/10409230600767551. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri GF, Michelini S, Di Martino P, Martelli S. Polymers with pH-dependent solubility: Possibility of use in the formulation of gastroresistant and controlled-release matrix tablets. Drug Development and Industrial Pharmacy. 2000;26(8):837–845. doi: 10.1081/ddc-100101307. [DOI] [PubMed] [Google Scholar]
- 11.Kumar AR, Grewal NS, Chung TL, Bradley JP. Lessons From the Modern Battlefield: Successful Upper Extremity Injury Reconstruction in the Subacute Period. Journal of Trauma. 2009;67(4):752–757. doi: 10.1097/TA.0b013e3181808115. [DOI] [PubMed] [Google Scholar]
- 12.Huang SJ, Sun SL, Feng TH, Sung KH, Lui WL, Wang LF. Folate-mediated chondroitin sulfate-Pluronic 127 nanogels as a drug carrier. Eur J Pharm Sci. 2009;12(38):64–73. doi: 10.1016/j.ejps.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Tan Q, Liu W, Guo C, Zhai G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int J Nanomedicine. 2011;6:1621–1630. doi: 10.2147/IJN.S22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elgindy N, Samy W. Evaluation of the mechanical properties and drug release of cross-linked Eudragit films containing metronidazole. Int J Pharm. 2009;376(1–2):1–6. doi: 10.1016/j.ijpharm.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Zhou S, Yu X, Li X, Feng B, Qu S, et al. Effect of in vitro degradation of poly(D,L-lactide)/beta-tricalcium composite on its shape-memory properties. J Biomed Mater Res B Appl Biomater. 2008;86(1):170–180. doi: 10.1002/jbm.b.31003. [DOI] [PubMed] [Google Scholar]
- 16.Baumgart F. Stiffness - an unknown world of mechanical science? Injury. 2000;31:14–23. [PubMed] [Google Scholar]
- 17.Bruggeman JP, de Bruin B-J, Bettinger CJ, Langer R. Biodegradable poly(polyol sebacate) polymers. Biomaterials. 2008;29(36):4726–4735. doi: 10.1016/j.biomaterials.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemperle G, Morhenn V, Charrier U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Aesthetic Plastic Surgery. 2003;27(5):354–366. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JM. Biological responses to materials. Annual Review of Materials Research. 2001;31:81–110. [Google Scholar]
- 20.Wypach G. Handbook of Plasticizers. Toronto, Ontario, Canada: ChemTec Publishing, William Andrew Inc; 2004. [Google Scholar]
- 21.Platzer N. The technology of plasticizers. Journal of Polymer Science: Polymer Letters Edition. 1982;20(8):459. [Google Scholar]
- 22.Jeon JH, Thomas MV, Puleo DA. Bioerodible devices for intermittent release of simvastatin acid. International Journal of Pharmaceutics. 2007;340(1–2):6–12. doi: 10.1016/j.ijpharm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon JH, Puleo DA. Alternating release of different bioactive molecules from a complexation polymer system. Biomaterials. 2008;29(26):3591–3598. doi: 10.1016/j.biomaterials.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Lee PI. Programmable Drug-Delivery from an Erodible Association Polymer System. Pharmaceutical Research. 1993;10(8):1144–1152. doi: 10.1023/a:1018960016756. [DOI] [PubMed] [Google Scholar]
- 25.Wu L, Zhang J, Jing D, Ding J. "Wet-state" mechanical properties of three-dimensional polyester porous scaffolds. J Biomed Mater Res A. 2006;76(2):264–271. doi: 10.1002/jbm.a.30544. [DOI] [PubMed] [Google Scholar]
- 26.Bodmeier R, Paeratakul O. Dry and Wet Strengths of Polymeric Films Prepared from an Aqueous Colloidal Polymer Dispersion, Eudragit RS0D. International Journal of Pharmaceutics. 1993;96(1–3):129–138. [Google Scholar]
- 27.Sastri VR. Plastics in Medical Devices: Properties, Requirements, and Applications. Burlington, MA: Elsevier Inc; 2010. [Google Scholar]
- 28.El-Gendy NA. Pharmaceutical Plasticizers for Drug Delivery Systems. Current Drug Delivery. 2012;9(2):148–163. doi: 10.2174/156720112800234602. [DOI] [PubMed] [Google Scholar]
- 29.Snejdrova E, Dittrich M. Pharmaceutically Used Plasticizers. In: Luqman M, editor. Recent Advances in Plasticizers: InTech. 2012. pp. 45–68. [Google Scholar]
- 30.Güngör S, Erdal MS, Özsoy Y. Plasticizers in Transdermal Drug Delivery Systems. In: Luqman M, editor. Recent Advances in Plasticizers: InTech. 2012. pp. 91–112. [Google Scholar]
- 31.Rahman M, Brazel CS. The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Progress in Polymer Science. 2004;29(12):1223–1248. [Google Scholar]
- 32.Finkelstein M, Gold H. Toxicology of the citric acid esters: Tributyl citrate, acetyl tributyl citrate, triethyl citrate, and acetyl triethyl citrate. Toxicology and Applied Pharmacology. 1959;1(3):283–298. doi: 10.1016/0041-008x(59)90113-9. [DOI] [PubMed] [Google Scholar]
- 33.Kranz H, Ubrich N, Maincent P, Bodmeier R. Physicomechanical properties of biodegradable poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) films in the dry and wet states. Journal of Pharmaceutical Sciences. 2000;89(12):1558–1566. doi: 10.1002/1520-6017(200012)89:12<1558::aid-jps6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Triethyl Citrate. [cited 2012 April 2, 2012]; Available from: http://www.thegoodscentscompany.com/data/rw1012771.html.
- 35.Tributyl Citrate. [cited 2012 April 2, 2012]; Available from: http://www.sciencelab.com/msds.php?msdsId=9925302.
- 36.Béchard SR, Levy L, Clas S-D. Thermal, mechanical and functional properties of cellulose acetate phthalate (CAP) coatings obtained from neutralized aqueous solutions. International Journal of Pharmaceutics. 1995;114(2):205–213. [Google Scholar]
- 37.Liu J, Williams RO., III Properties of heat-humidity cured cellulose acetate phthalate free films. European Journal of Pharmaceutical Sciences. 2002;17(1–2):31–41. doi: 10.1016/s0928-0987(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 38.Chaibundit C, Ricardo NMPS, Ricardo NMPS, Muryn CA, Madec M-B, Yeates SG, et al. Effect of ethanol on the gelation of aqueous solutions of Pluronic F127. Journal of Colloid and Interface Science. 2010;351(1):190–196. doi: 10.1016/j.jcis.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Azeredo HMC, Mattoso LHC, Avena-Bustillos RJ, Filho GC, Munford ML, Wood D, et al. Nanocellulose Reinforced Chitosan Composite Films as Affected by Nanofiller Loading and Plasticizer Content. Journal of Food Science. 2010;75(1):N1–N7. doi: 10.1111/j.1750-3841.2009.01386.x. [DOI] [PubMed] [Google Scholar]