Abstract
Objectives/Hypothesis
There is little known about how physical exercise may alter physiological parameters of voice production. In this investigation, vocal function and upper airway temperature were examined following a bout of submaximal exercise and compared with a resting breathing condition. It was hypothesized that phonation threshold pressure and perceived phonatory effort would increase, and pharyngeal temperature would decrease following an exercise bout.
Study Design
Using a within-participant repeated measures design, 18 consented participants (9 men, 9 women) completed the study.
Methods
A 20-minute equilibration task was immediately followed by 8 minutes of submaximal exercise on a stationary bike in a thermally neutral environment (25°C/40% RH). At the end of the equilibration trial and the exercise trial measures were taken in the following order: pharyngeal temperature, phonation threshold pressure, and perceived phonatory effort. Data were analyzed using paired t-tests with significance set at α<0.05.
Results
Significantly increased phonation threshold pressure and perceived phonatory effort and significantly decreased pharyngeal temperature (1.9°C) were found, supporting the initial hypotheses.
Conclusions
Findings from this investigation support the widely held belief that voice use associated with physical activity requires additional laryngeal effort and closure forces. The effect of the temperature reduction in the upper airway on voice function requires further study.
INTRODUCTION
Physical education teachers, military personnel, performers, and fitness instructors, represent a handful of the many individuals who are in occupations that require reliable, effective voice use during moderate to high intensity physical activity. While there is a general understanding that these professionals are at higher risk for development of occupationally-based voice disorders1,2 there is little known about the effect of moderate intensity exercise on voice production. Well-established exercise science methodology may be useful in examining this issue and has been applied in the current investigation.
Understanding voice function immediately following submaximal exercise requires knowledge of superficial vocal fold viscosity mechanisms and skeletal muscle tissue properties (biochemical, contractile, & bioenergetic) that may be altered with changes in tissue temperature secondary to increased ventilation rate. A recent review of vocal fold surface hydration mechanisms 3 described behavioral and environmental challenges as important variables for maintenance of superficial laryngeal viscosity. Behavioral challenges in the context of vocal function include mouth versus nose breathing and systemic hydration versus dehydration. Environmental challenges described for vocal function include manipulations of ambient relative humidity. Maintenance of superficial vocal fold viscosity, or airway surface liquid (ASL), may be influenced by mechanisms of evaporative water loss, the degree of fluid loss depending on many factors which could include ventilation rate and inspired air temperature and humidity.4 The upper airway, as a transitional structure between the ambient environment and the lower respiratory tract, serves an important role in thermoregulation and humidification of the air breathed. This role is often referred to as the conditioning process. Variations in the site of the conditioning process may change with increasing ventilation rate while sedentary and secondary to exercise, driving warming and humidification of the air below the level of the glottis.5–9 The shift in airway conditioning toward and below the level of the vocal folds may lower the temperature of the vibrating vocal fold surfaces and the deeper intrinsic laryngeal muscles. Even small changes (> 0.5°C) in skeletal muscle tissue temperatures have been shown to influence skeletal muscle physiology via changes in metabolic activity and contractile function.10–13
In addition to cooler upper airway tissue temperature that may occur with increased respiratory rate, core temperature increases consistent with moderate intensity exercise 14 may also affect laryngeal tissue temperature, potentially counteracting any lowering of tissue temperature that may occur with increased respiratory rate. Further, a change in upper airway tissue temperature may influence the thermoregulatory mechanisms of convective, conductive, and evaporative cooling. The latter of these mechanisms, evaporation, provides an important defense against overheating with heat loss attributed to both sweat and water vapor from the respiratory passages.15 How the water vapor loss through respiration during exercise influences laryngeal function is unknown. To date, little is known about laryngeal skeletal muscle temperature regulation and if those thermoregulation mechanisms mirror those of limb skeletal muscle. Environmental investigations of voice function using phonation threshold pressure (PTP) and perceived phonatory effort (PPE) measures have largely focused on hydration and ambient humidity manipulations 16–21 during sitting resting breathing. More recently, Sivasankar and Erickson 22 investigated the influence of sedentary accelerated breathing on voice function in smokers and nonsmokers, concluding that even short durations of accelerated breathing significantly increased PTP in both groups studied. While providing insight for certain environmental or behavioral conditions that may influence voice function, current evidence may not adequately describe vocal function that is required for individuals who use the voice professionally while the respiratory rate is elevated during physical activity. The purpose of this study was to determine if increased ventilation rate secondary to submaximal exercise influenced upper airway tissue temperature, phonation threshold pressure, and perceived phonatory effort to better understand voice use in the realistic scenario of physical activity, a previously unstudied condition. Specifically, it was hypothesized that phonation threshold pressure and perceived phonatory effort would increase, and pharyngeal temperature would decrease following a bout of submaximal exercise.
METHODS
Participants
Twenty volunteers (10 female and 10 male) between the ages of 19 and 35 were recruited from the local community following receipt of approval from the Auburn University Institutional Review Board (IRB) for human subjects. Based on the phonation threshold pressure literature, 16–18,23 previously published thermal studies, 24,25 and power analysis sample size described by Stevens, 26 10 participants were determined to be adequate for a robust degree of power (0.8) and effect size (0.89). Inclusion criteria included: ability to match pitch on a screening task and no evidence of laryngeal pathology as determined via videostroboscopic screening. Volunteers were excluded from participation for any of the following reasons: health conditions that prohibited exposure to cold or hot environments or interfered with the proper placement of the transnasal thermal probe, history of laryngeal pathology, diabetes, allergic rhinitis, respiratory disease, active smoker at the time of the investigation, and/or contraindicated medications known to dry the laryngeal mucosa or alter thermal responses.
Preliminary Procedures and Assessments
Voice Screening
Prior to inclusion in the study, each volunteer completed a videostroboscopic laryngeal structure and function screening (Digital Videostroboscopy System Model 9295 with a rigid 9106 endoscope, KayPENTAX), the images of which were then reviewed by a board-certified otolaryngologist and determined to be free of laryngeal disease. Modal frequency for use during PTP data collection was determined from repetition of the phrase, “The blue spot is on the key again,” a phrase that is balanced for both front and back vowels using Real Time Speech software (Multi-Speech Model 3700 Version 3.2, KayPENTAX). The frequency selected for the phonation threshold pressure elicitation task was modeled for the participants using a standard pitch pipe (WM. Kratt Co.) and pitch-matching ability was determined using Real Time Pitch software. The modal frequency determined was recorded for use during the study trial.
Work Rate Determination for Targeted Respiratory Rate
Pre-trial determination of submaximal cycle ergometer work rate for each participant was completed using a standardized cycle ergometer (Quinton Instrument Company, Howell, New Jersey) protocol beginning at 50 watts pedal resistance, from which a work rate was determined that averaged a respiratory rate of 20 breaths per minute for 8 minutes. Participants were asked to select and maintain the pedaling rate per minute (RPM), and both work rate and pedaling rate (RPM) were recorded for replication during the actual trial. Respiratory rate was determined objectively using standard exercise physiology gas analysis software (ParvoMedics, Provo, Utah) that counts respiratory rate per minute from the expired air sample that is collected from a mouthpiece designed for this purpose. A submaximal exercise target of 20 breaths per minute was chosen so that a wide range of individuals could participate, thus not limiting this research to only fit individuals. The 8-minute length of the aerobic cycling exercise was chosen because that would allow time for all participants to reach submaximal steady state with consistent breathing rate and stable heart rate. Additionally, the short duration of the exercise bout would allow for the increased respiratory rate manipulation without any substantive increase in core temperature. Target submaximal work rate and pedaling RPM varied between participants given variable levels of individual fitness as detailed in Table 1.
Table 1.
Participant cycle work rate.
| Work Rate (W) | Peddling Rate (RPM) | Age |
|---|---|---|
| Women | ||
| 0 | 55 | 20 |
| 5 | 30 | 23 |
| 25 | 60 | 21 |
| 40 | 48 | 23 |
| 45 | 65 | 21 |
| 50 | 45 | 21 |
| 55 | 70 | 24 |
| 70 | 45 | 20* |
| 75 | 75 | 21 |
| 140 | 40 | 21 |
| Men | ||
| 50 | 40 | 21 |
| 65 | 60 | 21* |
| 70 | 30 | 20 |
| 75 | 90 | 22 |
| 85 | 80 | 23 |
| 100 | 90 | 23 |
| 105 | 80 | 21 |
| 175 | 90 | 21 |
| 180 | 50 | 21 |
| 180 | 80 | 24 |
Denotes consented participants whose data were not included in data analysis. Large differences in both work rate (W) and RPM required to maintain an average of 20 breaths per minute are due to widely varying fitness levels of participants, e.g., sedentary individuals and college athletes.
Experimental Procedures
Environmental Conditions
All trials were conducted within an environmental chamber in which a thermally neutral temperature/relative humidity condition (25°C/40% RH) was maintained. Environmental parameters were closely monitored with a wet bulb global temperature device (QUEST°34 Thermal Environment Monitor, Quest Technologies, Oconomowoc, Wisconsin), a device commonly used in environmental and thermoregulation science.
Participants were scheduled to complete each of the trials at approximately the same time of day to avoid thermal differences secondary to circadian rhythm. 27 Participants were asked to refrain from caffeine, meals, hot/cold beverages, and exercise for at least one hour prior to the trial. All female participants were asked to schedule the trial during days 7–12 from the start of menstruation (prior to ovulation) to avoid any effects that hormone levels may have on the data collection. 28
Voice Function Assessment
Pre-trial familiarization training was completed for transnasal placement of the upper airway temperature probe (Exacon thermistor probes, T-F1345, Roskilde, Denmark), phonation threshold pressure (PTP) collection procedures, and perceived phonatory effort (PPE) procedures. The investigator advanced the flexible pharyngeal probe (diameter = 1.33 mm) approximately 12–15 cm from the tip of the nose to control for a depth that was close to the epilaryngeal structure, 29 but not deep enough to cause frequent gagging, throat clearing, coughing, or general discomfort. Depth was determined by centimeter markings on the probe and by visualization of the probe in the participant pharynx following insertion. Measurement of upper airway temperature (°C) was then recorded from a Squirrel Data Logger 2020 Series (Grant Instruments, Hillsborough, New Jersey).
Task elicitation training for phonation threshold pressure was scripted to standardize directions across participants and data was collected using the Phonatory Aerodynamic System (PAS) Model 6600 (KayPENTAX). Task familiarization included training of a 5 syllable train of the consonant-vowel combination /pi/ at a rate of 1.5 syllables per second (95 beats per minute), first above threshold, then below threshold, and at threshold. 17 Consistent rate production for the PTP task was maintained by providing a flashing light from a digital metronome (Korg, TM-40, Japan). Participants were allowed to practice until comfortable with the procedure and it was determined that the data produced met the following criteria: (1) frequency within one semitone of the participant’s modal frequency; (2) productions were perceptually judged as produced as quietly as possible18; (3) airflow measurements were negligible (considered to be less than 20 mL/s) to ensure appropriate lip closure 30,31; (4) all of the peaks within the PTP string were judged via visual inspection to be of equal height 32,33; and voicing was evident during vowel production.
Perceived phonatory effort (PPE) was determined after reading the Grandfather Passage at a comfortable loudness level. Participants were oriented to a 100 mm visual analog scale, with the left anchor labeled “no effort” and the right anchor labeled “maximum effort,” on which they were asked to place a mark indicating degree of PPE following the reading task. Following each participant trial, the distance from the left end of the perceived effort line was measured in millimeters (mm) and recorded.
Systemic hydration
Prior to the start of the experiment, each participant provided a urine sample for objective assessment of hydration level using a refractometer (ATAGO®, Tokyo, Japan) with hydration level set at less than or equal to 1.02 g/ml. 25 Participants were allowed to drink additional room temperature fluids to reach the hydration target as needed.
Data Collection
Once hydration criteria were achieved, the transnasal pharyngeal temperature probe was positioned. The participant then entered the thermal chamber to acclimate to the 25°C/40% RH environment for 20 minutes, using nasal breathing only. After the 20 minute acclimation period, upper airway temperature, baseline PTP data, and PPE were collected in that order. Participants then moved from a seated position to the cycle ergometer for the increased respiratory rate trial. Participants cycled for 8 minutes at the pre-determined submaximal aerobic work rate to elicit an average of 20 breaths per minute with oral breathing (nose clip in place) in the same thermally-neutral environment of 25°C/40% RH. Immediately after completion of the 8 minute cycle, measures of upper airway temperature, PTP, and perceived phonatory effort were repeated. Please refer to Table 2 for a complete list of measurements made before and after the submaximal exercise trial.
Table 2.
Trial design time points.
| Time Points | Measures |
|---|---|
| Pre-Trial | Submaximal work rate (Watts, breaths per minute) Hydration level (g/ml) |
| Resting nasal breathing (20 minutes) | Pharyngeal temperature (°C) Phonation threshold pressure (cm/H20) Perceived phonation effort (mm/100) |
| 8 minutes of submaximal cycling exercise |
Data Analysis
A within-participant repeated measures design was used. Paired t-tests were used to determine significance of differences measured during rest breathing versus increased ventilation secondary to submaximal aerobic exercise for PTP, pharyngeal temperature, and PPE. Significance was set at α<0.05. All data analyzed met normality assumptions based on visual inspection of the respective QQ plots and histograms. The data analysis for this paper was generated using SAS software, Version 9.2 of the SAS System for XP-Pro. To eliminate the possibility of phonation threshold pressure calculation error via manual PTP analysis, all pressure peak trains that met the study criteria for equal height, low intensity, negligible airflow during production of /p/, and pitch production within ½ semitone were measured and averaged via the automated measurement software for the PAS system.
RESULTS
Participants
Twenty (10 women; 10 men) volunteers met the study inclusion criteria and agreed to participate in the study. Data from 2 participants (1 male and 1 female) were excluded from data analysis due to post-data collection disclosure of history of asthma. The remaining 18 participants (9 women, 9 men) ranged in age from 20–24 (M = 21.72; SD = 1.27).
Effect of Submaximal Activity on Phonation Threshold Pressure
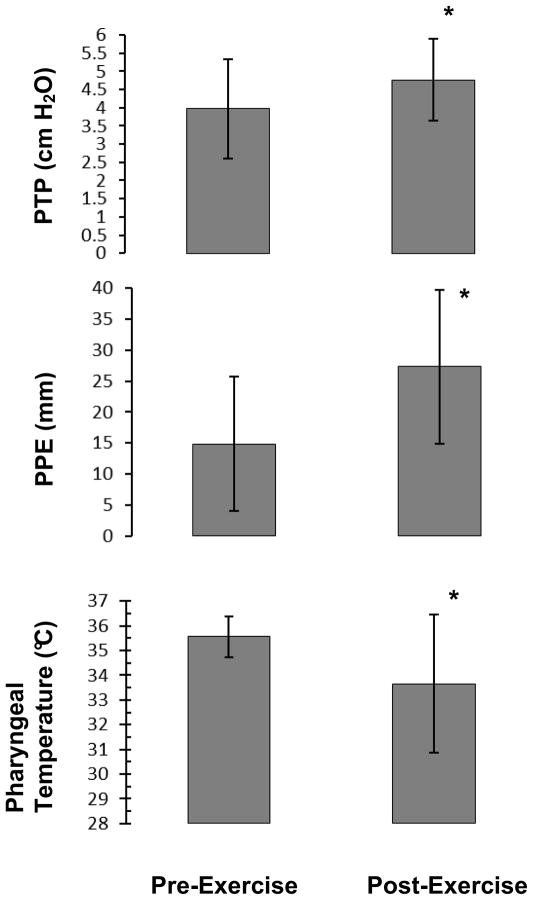
Accurate assessment of phonation threshold pressure (PTP) required adherence to specific methodology and a tightly regulated set of measurement parameters. 34 Of the 18 participants who met the criteria for inclusion in the data analysis, only 14 produced PTP targets that met the narrow criteria as described above for inclusion in data analysis. A paired-samples t-test was conducted to compare PTP before and after the exercise trial. There was a significant difference in the PTP values measured before exercise (M = 3.97 cm H2O, SD = 1.36) and after 8 minutes of submaximal exercise (M = 4.77 cm H2O, SD = 1.13) conditions; t(13) = −2.66, p = 0.019. Phonation threshold pressure data are presented in Figure 1.
Figure 1. Submaximal exercise effects on study variables.
Significant differences are denoted by the * (p< 0.05). Means and standard deviations are represented by the error bars.
Effect of Submaximal Activity on Perceived Phonatory Effort
Perceived phonatory effort (PPE) values were determined by measuring from the left anchor of the 100 mm visual analog scale to the mark made by the participant. A paired-samples t-test was conducted to compare PPE before and after the exercise trial. There was a significant difference in the effort values measured for the before exercise (M = 14.8 mm, SD = 10.8) and after 8 minutes of submaximal exercise (M = 27.3 mm, SD = 12.4) conditions; t(17) = −3.81, p = 0.001. These results indicate that with only moderate increases in activity, perception of effort for voicing tasks increases.
Effect of Submaximal Activity on Pharyngeal Temperature
One participant could not tolerate the transnasal temperature probe but completed the other measurements, therefore upper airway temperature data were analyzed for 17 participants. A paired-samples t-test was conducted to compare pharyngeal temperature before and after the exercise trial. There was a significant difference in the temperature values measured for the before exercise (M = 35.6 °C, SD = 0.83) and after 8 minutes of submaximal exercise (M = 33.7 °C, SD = 2.78) conditions; t(16) = 3.38, p = 0.004. These results demonstrate an average decrease of 1.9°C in upper airway temperature with a moderate increase in ventilation rate in young, healthy volunteers, a finding that is consistent with the upper airway conditioning literature. Temperature changes greater than 0.5°C are considered physiologically meaningful for muscle physiology.14,35–37
DISCUSSION
The primary goal of this investigation was to determine if measureable changes in vocal function and pharyngeal temperature could be identified following a bout of increased respiratory rate secondary to submaximal exercise. It was hypothesized that phonation threshold pressure (PTP) and perceived phonatory effort (PPE) would increase and pharyngeal temperature would decrease following a bout of submaximal exercise. The results of this investigation offer support for significant changes in all three of the study measures.
PTP was found to be higher after exercise because participants produced the target train of syllables immediately after cessation of cycling when the respiratory drive was increased from the resting condition. These findings extend previous work concerning elevated respiratory rate while sedentary 22 and offer a realistic representation of respiratory challenge and vocal function given that respiratory rate was actively increased through physical activity. Respiratory rate typically remains elevated for a period of time following cessation of exercise in the body’s effort to return to homeostasis. 14 This understanding is of importance when assessing vocal function of a fitness instructor, for example, who may not be exercising continuously while using the voice to offer direction but likely has elevated respiratory rate much of the time while teaching.
Partway through the course of data collection, it was learned that some of the participants typically talked to an exercise partner while working out, suggesting that some individuals may learn to overcome the increase in pulmonary drive during exercise to engage the larynx for speech. This may be hypothesized given the rule of specificity in exercise science – muscles become more efficient when trained for a specific task. If some of the volunteers already had muscle experience entraining the intrinsic laryngeal muscles for phonation while the respiratory drive was elevated, PTP values may be lower. Because talking during exercise was not discerned as a potential participant variable until data collection was nearly complete, this information was not available for all participants for further analyses.
The differences measured for PPE, while statistically significant, remained on the lower half of the 100 mm visual analog scale used for this study. If a mark in the middle of the scale (50 mm) was associated with perception of moderate voicing effort, then all participants perceived their effort as mild-moderate. This suggests that perception of voicing effort with submaximal exercise may not be a great physical stressor in young healthy individuals who are free of those health conditions that may promote less than optimal laryngeal tissue condition or use, e.g., pulmonary disease, laryngopharyngeal reflux, allergies, or use of anticholinergic medications. The average reduction in pharyngeal temperature (1.9°C) measured is potentially physiologically meaningful if the muscles of the upper airway, including the larynx, have metabolic and contractile features similar to those in the limb skeletal muscles. With temperature changes of >0.5°C the ability of muscle tissue to access stored fuel substrate and offload oxygen from the circulating blood supply is enhanced.36,38 Contractile function, both for speed and force production, is also enhanced with small increases in temperature.13,39 Therefore, it may be hypothesized that suboptimal muscle contractile function may occur with a reduction in upper airway tissue temperature, which may include intrinsic laryngeal skeletal muscle.
Additionally, the temperature change observed in the upper airway was more likely due to the increased respiratory rate than to any change to core temperature. The exercise bout, while it achieved steady state, was not extensive enough to cause a substantive increase to core temperature.40 To date, there is a large gap in the literature regarding bioenergetics, thermoregulation, and contractile function in vivo in the human larynx.
An additional aspect of this investigation, that cannot be directly assessed, is the state of superficial laryngeal viscosity following submaximal exercise. The pre-exercise superficial laryngeal viscosity was expected to be adequate secondary to the participant selection constraints as well as pre-trial constraints on exercise, diet, time of day scheduling, menstrual cycle limitations to days 7–12, and objective assurance of hydration status. It has been hypothesized that mouth breathing for a period of time may desiccate the superficial fluid layer of the laryngeal epithelium, with degree of desiccation increased with increased respiratory rate. 22,41,42 There are those investigators who believe, however, that the upper airway is remarkably well suited to adequately maintain respiratory epithelial surface liquid despite pulmonary challenge.4,9 Changes in viscosity may have influenced measures of PTP and PPE; however, reductions in tissue temperature and increased pulmonary drive could also account for increases in these measures.
A primary strength of this investigation was the use of well-established procedures used in exercise science to objectively discern the target work rate to match all volunteers for 20 breaths per minute. As can be seen from the values in Table 1, some participants achieved the target respiratory rate working with little to no resistance set on the cycle ergometer (watts) at a low RPM while other volunteers matched the same respiratory rate while pedaling at high resistance and high RPMs. This investigation also introduced the novel application of pharyngeal temperature, measured with transnasal thermistor probes, typically used in exercise science to measure core esophageal temperature. To date, temperature has not been considered in understanding laryngeal physiology with exercise. The technique for objective measurement of intrinsic laryngeal skeletal muscle temperature change in vivo has not been developed to date. The use of a pharyngeal thermistor probe, while a reasonable proxy for initial research efforts to discern upper airway thermoregulation and voice function, is not a substitute for direct measurement of intrinsic laryngeal skeletal muscle temperature change. Current direct measures of limb skeletal muscle temperature change with exercise are taken with a needle thermocouple, a methodology with many contraindications for use in the intrinsic laryngeal skeletal muscles in vivo. Investigations of temperature influences on laryngeal skeletal muscle tissue physiology and superficial laryngeal viscosity are needed.
A limitation of this study is the youthful age range of the participants recruited, rendering generalization of the findings to older adults difficult. In this initial effort to understand the vocal function perturbation of increased respiratory rate, the volunteer pool was limited to only those individuals who were free of several of the known medical conditions and medications that affect vocal function. The volunteer criteria were also designed to limit the age range to those individuals 35 and younger to exclude women who were nearing or past menopause. These limitations excluded many of the potential older volunteers from participation. An additional limitation of this study was exclusion of acoustic measures of voice function from the study protocol. The addition of acoustic measures would have strengthened this study in many ways. For example, it is not presently known if speaking fundamental frequency (sFo) changes with increased ventilation rate during submaximal exercise. A change in sFo could signal changes to laryngeal biomechanics for a more realistic understanding of vocal function during exercise. The study was conducted in an exercise science laboratory to allow ready access to gas analysis equipment, the cycle ergometer, and the environmental chamber and the ambient noise level in the laboratory made accurate collection of acoustic measures impractical.
With regard to the exercise challenge in this study, cycling was selected because it was the safest way to maintain the transnasal temperature probe for volunteer comfort while increasing respiratory rate. Cycling is not considered full body exercise, as is walking or running where the large muscle groups of the arms and legs are engaged. Use of full body exercise in this study would have better represented the type of physical activity that typically accompanies voice use for fitness trainers, military personnel, performers, and other individuals who require a durable voice while engaged in physical activity.
CONCLUSIONS
This investigation represented a novel effort to combine well-established physiological assessment tools used in exercise science with vocal function measures used in speech language pathology to better understand vocal function immediately following increased respiratory rate secondary to submaximal exercise. As hypothesized, measures of PTP and PPE increased and pharyngeal temperature decreased following the bout of submaximal exercise on the cycle ergometer in healthy young men and women. The increased vocal effort measured, both perceptually and objectively, in this study supports the hypothesis that exercise alters vocal function. Whether or not these data translate into increased risk for a voice disorder for individuals in occupations that require voice use during physical activity requires further study. Future research could discern vocal function differences following submaximal exercise for individuals with specific health conditions that are believed to contribute to the development of voice disorders, e.g., allergies and laryngopharyngeal reflux. Identification of potential risk factors for the development of voice disorders using realistic environmental and behavioral conditions will provide a more thorough understanding for the prevention of work-related voice disorders and aide in the development of voice habilitation and rehabilitation programs.
Acknowledgments
The work presented in this manuscript was supported by Award Number 1F31DC010946-01A1 from the National Institute on Deafness and Other Communication Disorders (NIDCD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mary J. Sandage, Auburn University
Nadine P. Connor, University of Wisconsin-Madison
David D. Pascoe, Auburn University
References
- 1.Smith E, Kirchner HL, Taylor M, Hoffman H, Lemke JH. Voice problems among teachers: Differences by gender and teaching characteristics. Journal of Voice. 1998;12(3):328–334. doi: 10.1016/s0892-1997(98)80022-2. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe V, Long J, Youngblood HC, Williford H, Olson MS. Vocal Parameters of Aerobic Instructors with and without Voice Problems. Journal of Voice. 2002;16(1):52–60. doi: 10.1016/s0892-1997(02)00072-3. [DOI] [PubMed] [Google Scholar]
- 3.Leydon C, Sivasankar M, Falciglia DL, Atkins C, Fisher KV. Vocal Fold Surface Hydration: A Review. Journal of Voice. 2009;23(6):658–665. doi: 10.1016/j.jvoice.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widdicombe JG. Airway Surface Liquid: Concepts and Measurements. In: Rogers DF, Lethem MI, editors. Airway Mucus; basic mechanisms and clinical perspectives. Basel: Birkhauser Verlag; 1997. pp. 1–17. [Google Scholar]
- 5.Cole P. Some aspects of temperature, moisture and heat relationships in the upper respiratory tract. Journal of Laryngology & Otology. 1953 Aug;67(8):449–456. doi: 10.1017/s0022215100048908. [DOI] [PubMed] [Google Scholar]
- 6.Cole P. Further observations on the conditioning of respiratory air. Journal of Laryngology & Otology. 1953 Nov;67(11):669–681. doi: 10.1017/s0022215100049161. [DOI] [PubMed] [Google Scholar]
- 7.Cole P. Temperature and humidity of respiratory air. Journal of Physiology. 1953;122(Suppl):51P. [PubMed] [Google Scholar]
- 8.Mcfadden ER, Pichurko BM, Bowman HF, et al. Thermal Mapping of the Airways in Humans. Journal of Applied Physiology. 1985;58(2):564–570. doi: 10.1152/jappl.1985.58.2.564. [DOI] [PubMed] [Google Scholar]
- 9.McFadden ER., Jr Respiratory heat and water exchange: physiological and clinical implications. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology. 1983 Feb;54(2):331–336. doi: 10.1152/jappl.1983.54.2.331. [DOI] [PubMed] [Google Scholar]
- 10.Febbraio MA, Carey MF, Snow RJ, Stathis CG, Hargreaves M. Influence of elevated muscle temperature on metabolism during intense, dynamic exercise. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1996 Nov;40(5):R1251–R1255. doi: 10.1152/ajpregu.1996.271.5.R1251. [DOI] [PubMed] [Google Scholar]
- 11.Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39(8):643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- 12.Dewhurst S, Graven-Nielsen T, De Vito G, Farina D. Muscle temperature has a different effect on force fluctuations in young and older women. Clinical Neurophysiology. 2007 Apr;118(4):762–769. doi: 10.1016/j.clinph.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Gray SR, De Vito G, Nimmo MA, Farina D, Ferguson RA. Skeletal muscle ATP turnover and muscle fiber conduction velocity are elevated at higher muscle temperatures during maximal power output development in humans. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2006 Feb;290(2):R376–R382. doi: 10.1152/ajpregu.00291.2005. [DOI] [PubMed] [Google Scholar]
- 14.Powers SK, Howley ET. Exercise physiology: theory and application to fitness and performance. 6. Boston: McGraw-Hill; 2007. [Google Scholar]
- 15.Katch VL, McArdle WD, Katch FI. Essentials of exercise physiology. 4. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2011. [Google Scholar]
- 16.Sivasankar, Erickson E, Schneider S, Hawes A. Phonatory Effects of Airway Dehydration: Preliminary Evidence for Impaired Compensation to Oral Breathing in Individuals With a History of Vocal Fatigue. J Speech Lang Hear Res. 2008 Dec 1;51(6):1494–1506. doi: 10.1044/1092-4388(2008/07-0181). [DOI] [PubMed] [Google Scholar]
- 17.Verdolini-Marston K, Sandage M, Titze IR. Effect of hydration treatments on laryngeal nodules and polyps and related voice measures. Journal of Voice. 1994;8(1):30–47. doi: 10.1016/s0892-1997(05)80317-0. [DOI] [PubMed] [Google Scholar]
- 18.Verdolini-Marston K, Titze IR, Druker DG. Changes in phonation threshold pressure with induced conditions of hydration. Journal of Voice. 1990;4(2):142–151. [Google Scholar]
- 19.Verdolini K, Titze IR, Fennell A. Dependence of Phonatory Effort on Hydration Level. J Speech Hear Res. 1994 Oct 1;37(5):1001–1007. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 20.Sivasankar M, Fisher KV. Oral breathing increases Pth and vocal effort by superficial drying of vocal fold mucosa. Journal of voice: official journal of the Voice Foundation. 2002 Jun;16(2):172–181. doi: 10.1016/s0892-1997(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 21.Sivasankar M, Fisher KV. Oral breathing challenge in participants with vocal attrition. J Speech Lang Hear Res. 2003 Dec;46(6):1416–1427. doi: 10.1044/1092-4388(2003/110). [DOI] [PubMed] [Google Scholar]
- 22.Sivasankar, Erickson E. Short-Duration Accelerated Breathing Challenges Affect Phonation. Laryngoscope. 2009 Aug;119(8):1658–1663. doi: 10.1002/lary.20530. [DOI] [PubMed] [Google Scholar]
- 23.Roy N, Tanner K, Gray SD, Blomgren M, Fisher KV. An evaluation of the effects of three laryngeal lubricants on phonation threshold pressure (PTP) Journal of voice: official journal of the Voice Foundation. 2003 Sep;17(3):331–342. doi: 10.1067/s0892-1997(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 24.Pascoe DD, Bellingar TA, McCluskey BS. Clothing and exercise. II. Influence of clothing during exercise/work in environmental extremes. Sports Med. 1994 Aug;18(2):94–108. doi: 10.2165/00007256-199418020-00003. [DOI] [PubMed] [Google Scholar]
- 25.Pascoe DD, Smith JW, Molloy JM. Regional surface temperature responses during a high intensity heat acclimation protocol. Medicine and science in sports and exercise. 2004 May;36(5):0573. [Google Scholar]
- 26.Stevens J. Applied multivariate statistics for the social sciences. 4. Mahwah, N.J: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- 27.Refinetti R. The circadian rhythm of body temperature. Front Biosci. 2010;15:564–594. doi: 10.2741/3634. [DOI] [PubMed] [Google Scholar]
- 28.Abitbol J, Abitbol P, Abitbol B. Sex hormones and the female voice. Journal of Voice. 1999;13(3):424–446. doi: 10.1016/s0892-1997(99)80048-4. [DOI] [PubMed] [Google Scholar]
- 29.McFadden ER, Jr, Pichurko BM, Bowman HF, et al. Thermal mapping of the airways in humans. J Appl Physiol. 1985 Feb 1;58(2):564–570. doi: 10.1152/jappl.1985.58.2.564. [DOI] [PubMed] [Google Scholar]
- 30.Solomon NP, DiMattia MS. Effects of a vocally fatiguing task and systemic hydration on phonation threshold pressure. Journal of voice: official journal of the Voice Foundation. 2000 Sep;14(3):341–362. doi: 10.1016/s0892-1997(00)80080-6. [DOI] [PubMed] [Google Scholar]
- 31.Morgan MD, Triana MA, Milroy TJ. The effect of auditory feedback on phonation threshold pressure measurement. Journal of voice: official journal of the Voice Foundation. 2004 Mar;18(1):46–55. doi: 10.1016/S0892-1997(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 32.Milbrath RL, Solomon NP. Do Vocal Warm-Up Exercises Alleviate Vocal Fatigue? Journal of Speech, Language & Hearing Research. 2003;46(2):422. doi: 10.1044/1092-4388(2003/035). [DOI] [PubMed] [Google Scholar]
- 33.Verdolini K, Titze IR, Fennell A. Dependence of phonatory effort on hydration level. J Speech Hear Res. 1994 Oct;37(5):1001–1007. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 34.Plexico LW, Sandage MJ, Faver KY. Assessment of Phonation Threshold Pressure: A Critical Review and Clinical Implications. Am J Speech Lang Pathol. 2011 Nov 1;20(4):348–366. doi: 10.1044/1058-0360(2011/10-0066). [DOI] [PubMed] [Google Scholar]
- 35.Rall JA, Woledge RC. Influence of temperature on mechanics and energetics of muscle contraction. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1990 Aug 1;259(2):R197–R203. doi: 10.1152/ajpregu.1990.259.2.R197. [DOI] [PubMed] [Google Scholar]
- 36.Brooks GA, Fahey TD, Baldwin KM. Exercise physiology: human bioenergetics and its applications. 4. Boston: McGraw-Hill; 2005. [Google Scholar]
- 37.Brooks G, Hittelman K, Faulkner J, Beyer R. Temperature, skeletal muscle mitochondrial functions, and oxygen debt. American Journal of Physiology -- Legacy Content. 1971 Apr 1;220(4):1053–1059. doi: 10.1152/ajplegacy.1971.220.4.1053. [DOI] [PubMed] [Google Scholar]
- 38.Starkie RL, Hargreaves M, Lambert DL, Proietto J, Febbraio MA. Effect of temperature on muscle metabolism during submaximal exercise in humans. Exp Physiol. 1999 Jul;84(4):775–784. [PubMed] [Google Scholar]
- 39.De Ruiter CJ, De Haan A. Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflügers Archiv European Journal of Physiology. 2000;440(1):163–170. doi: 10.1007/s004240000284. [DOI] [PubMed] [Google Scholar]
- 40.Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. Journal of Applied Physiology. 1966 Nov 1;21(6):1757–1762. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- 41.Man SF, Adams GK, 3rd, Proctor DF. Effects of temperature, relative humidity, and mode of breathing on canine airway secretions. J Appl Physiol. 1979 Feb 1;46(2):205–210. doi: 10.1152/jappl.1979.46.2.205. [DOI] [PubMed] [Google Scholar]
- 42.Freed AN, Davis MS. Hyperventilation with dry air increases airway surface fluid osmolality in canine peripheral airways. American Journal of Respiratory & Critical Care Medicine. 1999 Apr;159(4 Pt 1):1101–1107. doi: 10.1164/ajrccm.159.4.9802072. [DOI] [PubMed] [Google Scholar]