Abstract
Emerging evidence suggests that fibroblast growth factor 23 (FGF23) levels are elevated in patients with acute kidney injury (AKI). In order to determine how early this increase occurs we used a murine folic acid nephropathy model and found that plasma FGF23 levels increased significantly from baseline already after 1 hour of AKI, with an 18-fold increase at 24 hours. Similar elevations of FGF23 levels were found when AKI was induced in mice with osteocyte-specific parathyroid hormone receptor ablation or the global deletion of parathyroid hormone or vitamin D receptor, indicating that the increase in FGF23 was independent of parathyroid hormone and vitamin D signaling. Furthermore, FGF23 levels increased to a similar extent in wild-type mice maintained on normal or phosphate-depleted diets prior to induction of AKI, indicating that the marked FGF23 elevation is at least partially independent of dietary phosphate. Bone production of FGF23 was significantly increased in AKI. The half-life of intravenously administered recombinant FGF23 was only modestly increased. Consistent with the mouse data, plasma FGF23 levels rose 15.9-fold by 24 hours following cardiac surgery in patients who developed AKI. The levels were significantly higher than in those without postoperative AKI. Thus, circulating FGF23 levels rise rapidly during AKI in rodents and humans. In mice this increase is independent of established modulators of FGF23 secretion.
Keywords: AKI, fibroblast growth factor 23, phosphate, PTH, vitamin D
Introduction
Fibroblast growth factor 23 (FGF23) is produced by osteocytes and possibly osteoblasts and acts on the kidney to increase renal phosphate excretion and to decrease 1,25-dihydroxyvitamin D (1,25D) production1. Enhanced FGF23 production is observed after dietary phosphate loading2,3 and administration of parathyroid hormone (PTH)4 or 1,25D5. However, the most dramatic increases in FGF23 levels occur in patients with chronic kidney disease (CKD), in whom elevated FGF23 levels are independently associated with greater risks of death, cardiovascular events, progression to end-stage renal disease, and premature allograft loss after kidney transplant6-12. FGF23 increases during the early stages of CKD and its levels are hundreds- to thousands-fold above the normal range in patients with end-stage renal disease7,13,14.
While increased FGF23 levels early in the course of human and animal CKD are well documented14-17, the mechanisms triggering and perpetuating its rise are poorly understood. Traditional factors that stimulate FGF23 production, including intestinal phosphate absorption2,5, high levels of 1,25D, and elevated PTH levels4,18,19,20-22, may occur in CKD but rarely antedate the initial rise in FGF23. Deficiency of Klotho, the FGF23 co-receptor, in the diseased kidney23 may theoretically lead to FGF23 resistance and thus elevated FGF23 levels, although a temporal relationship whereby reduced Klotho expression precedes the rise in FGF23 levels has not been established in CKD.
Recent small studies reported FGF23 elevations in patients with acute kidney injury (AKI)24-26. In the one study that examined FGF23 and outcomes in AKI, elevated FGF23 levels were associated with significantly increased risk of death or need for dialysis26. However, those studies measured FGF23 after AKI was already established and thus could not provide insights into the mechanisms that might govern the FGF23 rise in AKI, including the effects of established modulators of FGF23 secretion, such as PTH, activated vitamin D metabolites, and phosphate.
Using wild-type and genetically manipulated mice, we investigated the time course of changes in FGF23 levels following induction of AKI and whether AKI-induced elevations of FGF23 are dependent on concomitant changes in serum phosphate or PTH- or 1,25D-dependent signaling events. Furthermore, we validated the rodent AKI data by examining the time course of changes in FGF23 levels in human AKI by measuring FGF23 levels before and at several time points after patients underwent cardiac surgery that either was or was not complicated by AKI.
Results
Folic acid induces AKI
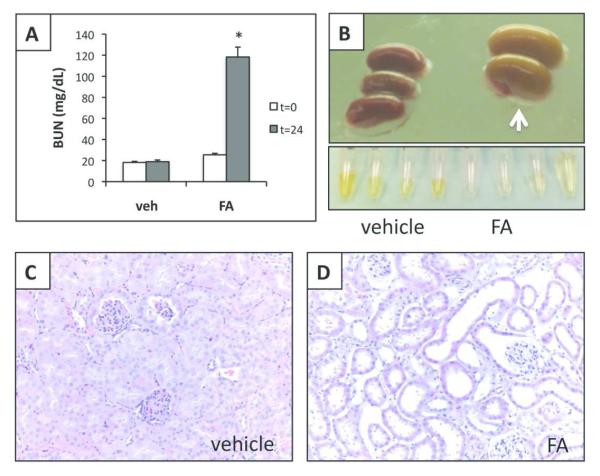
Administration of high-dose, intraperitoneal folic acid (FA) causes AKI27,28. In the current study, FA injection into CD1 mice led to an increase in blood urea nitrogen (BUN) from 21±1 to 118±9 mg/dl (p<0.01) after 24 hours compared to no change in vehicle-injected animals (Figure 1A and Table 1). Consistent with prior reports, the FA-injected animals remained non-oliguric, and excreted apparently dilute urine (Figure 1B); they had an average weight loss of 4 ± 1% by 24 hours.
Figure 1.
Folic acid (FA)-induced acute kidney injury model. A. Plasma BUN levels (mg/dl) at time 0 and 24 hours after FA or vehicle injection, N=6-8, mean±SEM (two independent experiments) B. Upper panel: gross view of kidneys 24 hours after vehicle or FA injection; white arrow points to paler swollen kidneys after FA injection; lower panel: urine collections from 4 vehicle or FA injected animals; note the pale urine from FA injected mice. C and D. Renal histology of kidneys harvested 24 hours after vehicle (panel C) or FA injection (panel D); H&E 20× magnification.
Table 1.
Effects of FA-induced acute kidney injury on animals maintained on a 3 standard phosphate diet.
| T=0 | T=24 hours vehicle |
T=24 hours FA |
P value T=24 vehicle vs FA |
|
|---|---|---|---|---|
| BUN (mg/dL) | 21±1 (N=13) |
19±2 (N=6) |
118±9 (N=8) |
<0.01 |
| Phosphate (mg/dL) |
6.7±0.2 (N=21) |
6.4±0.3 (N=11) |
11.2±1.4 (N=12) |
<0.05 |
| Calcium (mg/dL) |
8.1±0.3 (N=8) |
8.4±0.5 (N=4) |
8.2±0.8 (N=4) |
NS |
| cFGF23 (pg/mL) |
245±22 (N=17) |
307±19 (N=13) |
4500±562 (N=16) |
<0.01 |
|
iFGF23
(pg/mL) |
136±6
(N=9) |
121±7
(N=5) |
2651±510
(N=5) |
<0.01 |
| PTH (pg/mL) | 127±33 (N=10) |
85±38 (N=7) |
1359±320 (N=7) |
<0.05 |
Shown are mean±SEM (number of animals). Means for BUN, phosphate, 7 cFGF23 and PTH are from two or more independent experiments. P value 8 shown is for vehicle-injected versus FA-injected animals at 24 hours post9 injection.
FA = folic acid; BUN = blood urea nitrogen; PTH = parathyroid hormone
Consistent with severe renal injury, kidneys from FA-injected animals appeared pale and swollen (Figure 1B), and showed tubular dilation, flattening and loss of tubular cells and interstitial edema (Figure 1D). Kidneys from control animals showed no changes (Figure 1C). FA-induced AKI resulted in hyperphosphatemia and elevated PTH levels, although total calcium levels remained unchanged in both groups (Table 1).
FGF23 levels increase in AKI
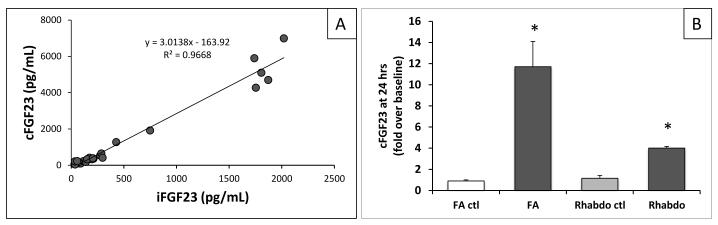
Plasma FGF23 levels were measured with a mouse-specific assay detecting intact FGF23 and C-terminal fragments (cFGF23 assay)29. cFGF23 levels rose from 245±22 to 4500±562 pg/mL by 24 hours after AKI induction, but remained unchanged in control animals (307±19 pg/mL; Table 1). Similarly, FGF23 levels also rose from 136±6 to 2651±510 pg/mL when measured with an assay that detects only the intact hormone (iFGF23 assay) (Table 1). Since results obtained with cFGF23 and iFGF23 assays were highly correlated (r=0.97; p<0.01, Figure 2A), all subsequent analyses used the cFGF23 assay except where indicated.
Figure 2.
FGF23 levels increase after induction of AKI independent of mode of renal injury A. Comparison of values obtained from plasma samples measured with a mouse-specific cFGF23 ELISA (cFGF23) and an intact ELISA that detects human and mouse FGF23 (iFGF23). R2 coefficient = 0.967, p<0.01; N=25, 6 samples with AKI. B. FGF23 levels increase in different models of AKI. FA=folic acid nephropathy, N=13-16; Rhabdo=pigment nephropathy, N=2-3. Shown are means of fold increase over an average baseline of t=0 FGF23 levels for the model. FA control shows average change in FGF23 levels in vehicle-injected animals. *Indicates p value <0.05 compared to FA at t=0.
To rule out non-specific effects of FA, we measured cFGF23 levels in C57BL/6 mice following induction of pigment nephropathy, another established rodent model of AKI30. Pigment nephropathy led to an increase in cFGF23 levels from 561±212 pg/ml to 2249±140 pg/mL by 24 hours, but levels remained unchanged in vehicle-injected animals (Figure 2B). The different mouse strains likely account for the difference in baseline cFGF23 levels between mice with FA-versus pigment-induced nephropathy. These data indicate that the rise in FGF23 is related to AKI rather than its specific etiology.
FGF23 rises acutely following AKI
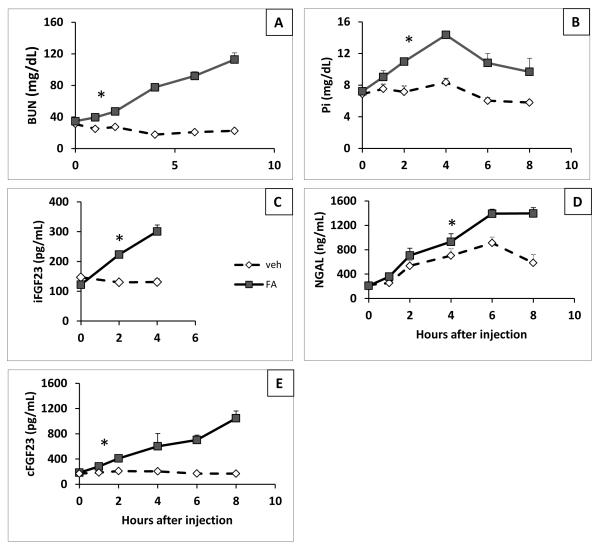
Since we found significant changes in FGF23 and other mineral metabolites by 24 hours, we investigated earlier changes in BUN, phosphate, and cFGF23 levels (Figure 3). PTH levels were not assessed due to bleeding volume limitations. BUN and FGF23 levels first became significantly higher than control mice one hour after FA injection (cFGF23: 282±19 vs 186±20 pg/mL, p=0.004; iFGF23: two hours after FA injection 223±8 vs 130±8 pg/mL, p<0.001; Figure 3A, C, E). In contrast, serum phosphate levels first became significantly higher than controls at 2 hours (Figure 3B) and plasma neutrophil gelatinase-associated lipocalin (NGAL), an established biomarker of renal injury31,32, first became significantly higher 6 hours after AKI induction (Figure 3D). NGAL also increased in control animals, which may reflect inflammation due to intra-peritoneal injection33. Based on these data, FGF23 rises earlier in the course of AKI than phosphate or NGAL.
Figure 3.
FGF23 levels rises early after renal injury. Time course data were obtained from time 0, 1, 2, 4, 6, and 8 hours after FA or vehicle injection. N=7-9 for time-points 0-4, and N=3-5 for time points 6 and 8; shown are mean values ±SEM from two independent experiments. *Indicates the earliest time point at which a significant difference between FA (solid line) and vehicle (dotted line) injected animals was observed. A. Plasma BUN levels (mg/dL). B. Plasma phosphate levels (mg/dL). C. Plasma iFGF23 levels (pg/mL). D. Plasma NGAL levels (ng/mL). E. Plasma cFGF23 levels (pg/mL).
Bone production of FGF23 increases in FA-induced AKI
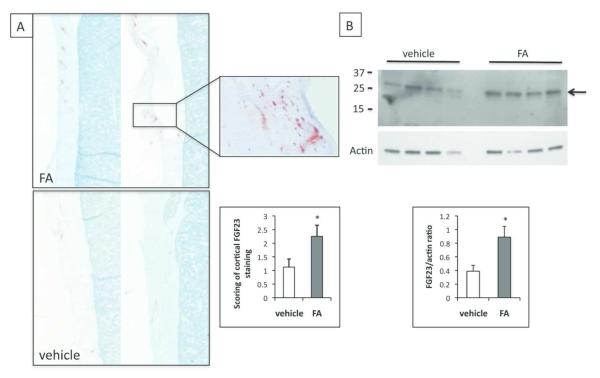
To determine whether increased FGF23 levels in AKI are due to increased production, we assessed bone FGF23 levels 24 hours after inducing AKI. Immunohistochemical analysis revealed increased FGF23 staining in the femoral cortex of AKI animals compared to controls (2.25±0.41 vs. 1.13±0.30, p<0.05; Table 2, Figure 4A inset). Staining was patchy, which is similar to wild-type mice34 and humans with increased bone FGF23 production due to CKD or genetic defects35,36. There was no correlation, however, between cortical scores and plasma cFGF23 levels.
Table 2.
Summary of FGF23 protein expression by immunohistochemistry 1 of 2 plastic-embedded femur sections from FA- and vehicle-injected animals.
| Femur area examined |
Treatment | p value | |
|---|---|---|---|
| Folic acid | Vehicle | ||
| Cortex | |||
| Average score |
2.25±0.41 | 1.125±0.30 | <0.05 |
| Positive samples |
7/8 | 6/8 | |
|
| |||
| Trabeculae | |||
| Average score |
1.25±0.67 | 0.5±0.19 | 0.30 |
| Positive samples |
3/8 | 4/8 | |
Cortical staining was scored on a scale of 0 to 3+. Trabecular staining was scored by counting the number of osteocytes positive for FGF23 in the whole section. Two animals had neither cortical nor trabecular staining (both vehicle-injected); all others had either cortical or trabecular staining or both detectable (i.e. at least a 1+ score in cortex or 1 positive osteocyte in the trabecular area). Average staining shown are mean±SEM. P value is for vehicle-injected versus FA-injected animals. (N=8 animals for each group).
Figure 4.
FGF23 levels are increased in bone of AKI mice. A. Immunohistochemistry of femurs using an anti-FGF23 antibody. Shown are low-power images of cortical bone from FA injected (upper panel) or vehicle-injected (lower panel) animals. 4× magnification. Inset shows 40× magnification of cortical bone. N=8 in each group. Second inset shows average cortical staining (scale of 0-3+) for FGF23 in vehicle or FA-injected animals. N=6-7 *p<0.05. B. Western blot of femur lysates after immunoprecipitation with anti-FGF23 antibody. Upper panel, anti-FGF23 antibody, lower panel, actin antibody. 2 independent experiments, N=6 in each group, (vehicle, FA). Graph shows band densitometry of FGF23/actin.
To directly assess FGF23 expression in bone, we analyzed FGF23 protein by Western blot after immunoprecipitation from femur lysates of vehicle or FA-injected mice29 (Figure 4B). When using actin in the lysate as a loading control for densitometry evaluation, bone FGF23 levels in mice with AKI were approximately 2-fold elevated compared to bones from control mice, which is consistent with the cortical scoring above (p=0.03).
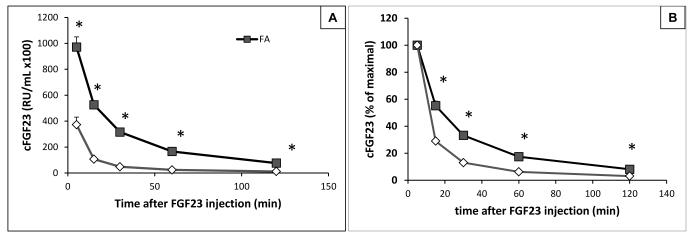
FGF23 has a modestly longer half-life in AKI
To determine whether impaired FGF23 elimination contributes to the elevated levels observed in AKI, recombinant human FGF23 (rhFGF23) was injected intravenously into the tail vein of AKI and control mice 24 hours after intraperitoneal injection of FA or vehicle, and plasma FGF23 was measured 5, 15, 30, 60, and 120 minutes thereafter using a human assay that does not cross-react with mouse FGF23. rhFGF23 disappeared less rapidly from the circulation in AKI than in control animals, but the half-life was only 50% prolonged (33 vs. 22 minutes, Figure 5A, p=0.01). Furthermore, although higher absolute levels of immunoreactive FGF23 were initially found in the circulation of AKI mice (97,200±7,800 vs 37,300±5,850 RU/mL in control), most of the injected rhFGF23 had been cleared by 120 minutes post-injection (8% vs 3% remaining, Figure 5B).
Figure 5.
Half-life of recombinant human FGF23 in animals with AKI or control mice. Left panel shows hFGF23 levels at different time points after IV rhFGF23 injection. Right panel shows percent remaining of the initial value. N=3 ctl, N=5 AKI, *p<0.05 compared with vehicle-injected mice.
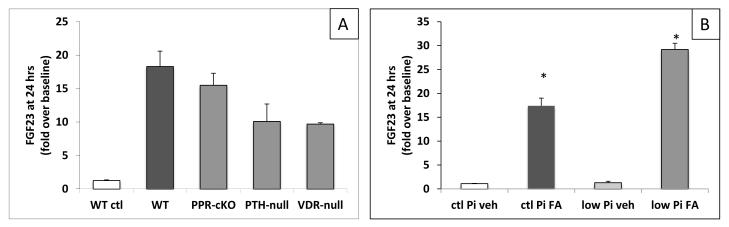
FGF23 levels rise in AKI independent of PTH receptor signaling
In a rat model of early CKD, the PTH-associated increase in FGF23 production was prevented by parathyroidectomy 4. To determine whether PTH signaling at the PTH/PTHrP receptor (PPR) is required for the AKI-induced increase in plasma FGF23 levels, we injected FA into mice with osteocyte-specific ablation of the PPR (PPR-cKO), which show no obvious changes in mineral ion homeostasis at baseline 37-39. 24 hours after FA-injection, the increase in FGF23 levels was indistinguishable in PPR-cKO and control littermates (5863±187 vs 5013±627 pg/mL) (Figure 6A); the changes in BUN and phosphate were also indistinguishable between groups (Table 3). These results indicate that the AKI-induced increase in plasma FGF23 levels occurs independently of PPR activation in osteocytes.
Figure 6.
FGF23 elevation in AKI under different conditions. Shown are means of fold increase over an average baseline of t=0 FGF23 levels for the genotype used or condition used. Shown are average increases in FGF23 levels at 24hrs over baseline. A. FGF23 levels in genetically modified animals. PPR-cKO=animals with osteocyte-specific deletion of the PPR, N=7; PTH-null=animals with deletion of the coding region for the mature PTH protein, N=5; VDR-null=animals with deletion of exon 3 of the vitamin D receptor, N=4. For comparison, WT control shows average change in FGF23 levels in vehicle injected WT animals. B. FGF23 levels in animals on control and low phosphate diets (ctl Pi and low Pi). N=4-10. *p<0.05 compared with baseline.
Table 3.
Effects of AKI on genetically 1 modified animals
| Experimental condition |
Parameter | T=0 | T=24 hours AKI |
P value T=24 vs t=0 |
|---|---|---|---|---|
| PPR-cKO | BUN (mg/dL) | 14±2 (N=6) |
135±8 (N=7) |
<0.01 |
| Phosphate (mg/dL) |
5.49±0.5 (N=7) |
21.1±1.9 (N=7) |
<0.01 | |
| cFGF23 (pg/mL) |
324±28 (N=7) |
5013±626 (N=7) |
<.01 | |
| VDR-null | BUN (mg/dL) | 20.2±4.3 (N=3) |
175±9.2 (N=4) |
<0.01 |
| Phosphate (mg/dL) |
6.2±0.7 (N=3) |
16.6±2.8 (N=4) |
<0.05 | |
| cFGF23 (pg/mL) |
584±135 (N=4) |
5912±149 (N=4) |
<0.01 | |
| PTH-null | BUN (mg/dL) | 27.2±12.2 (N=5) |
154±69 (N=5) |
<0.01 |
| Phosphate (mg/dL) |
NA | 25±11.2 (N=4) |
NA | |
| cFGF23 (pg/mL) |
185±30 (N=8) |
1860±489 (N=5) |
<0.01 |
Shown are mean±SEM (number of animals). P value shown is for baseline (t=0) 4 versus 24 hours post-injection in FA-injected animals. NA=not available. 5 FA = folic acid; BUN = blood urea nitrogen
To investigate whether PTH signaling in cells other than osteocytes may contribute to increased FGF23 levels during AKI, we analyzed PTH-null mice receiving high-calcium intake to maintain normal serum calcium levels. When treated with FA, FGF23 levels rose in these animals from 185±30 to 1860±489 pg/mL (Table 3, Figure 6A). Thus, PTH-ablation did not prevent the major elevation of FGF23 levels in response to AKI induction.
FGF23 levels rise in AKI independent of vitamin D levels and signaling
1,25D treatment increases bone FGF23 production5,40,41. To test whether the AKI-induced increase in FGF23 is 1,25D-dependent, we measured 1,25D levels in mice with and without AKI, and repeated the FA experiments in vitamin D receptor (VDR)-null mice. There was no difference in mean plasma 1,25D levels between AKI and control animals 24 hours after FA-injection (144±18 vs 126±23 pg/mL) when FGF23 levels were already more than 10-fold higher. VDR-null mice maintained on a rescue diet showed normal serum phosphate, calcium, and PTH levels, but their iFGF23 levels were previously found to be 2- to 3-times higher than those of WT littermates42,43. FGF23 levels increased from 614±129 pg/mL to 5972±150 pg/ml (p<0.001) with AKI in the VDR-null mice (Table 3, Figure 6A). These data indicate that the rise in FGF23 levels in AKI occurs independently of 1,25D levels and VDR activation.
FGF23 levels rise in AKI independent of dietary phosphate
Dietary phosphate loading increases FGF23 levels in mice and humans2,3,43,44. To test whether a low phosphate intake and resulting hypophosphatemia can prevent the dramatic increase in FGF23 levels in AKI, we examined animals maintained on a normal (0.4% Pi) or low phosphate diet (0.02% Pi) for 7 days prior to induction of AKI. By the end of this induction period, serum phosphate and plasma cFGF23 levels were significantly lower in mice fed the low phosphate diet (phosphate: 4.0±0.3 vs. 6.2±0.3 mg/dL, p<0.01; cFGF23: 92±13 pg/mL vs. 226±16 pg/mL, p<0.01; Table 4).
Table 4.
Effects of AKI on animals on low and normal 1 phosphate diets
| Experimental condition |
Parameter | T=0 | T=24 hours AKI |
P value T=0 vs. T=24 |
|---|---|---|---|---|
| Control phosphate diet |
BUN (mg/dL) | 26±1 (N=4) |
117±13 (N=4) |
<0.01 |
| Phosphate (mg/dL) |
6.2±0.3 (N=9) |
13.5±1.3 (N=9) |
<0.01 | |
| cFGF23 (pg/mL) |
226±16 (N=9) |
4530±417 (N=9) |
<0.01 | |
| Low phosphate diet |
BUN (mg/dL) | 23±2 (N=6) |
98±11 (N=9) |
<0.01 |
| Phosphate (mg/dL) |
4.0±0.28* (N=12) |
7.6±0.83* (N=14) |
<0.01 | |
| cFGF23 (pg/mL) |
90±13* (N=13) |
2682±296* (N=14) |
<0.01 |
Shown are mean±SEM (number of animals). Low phosphate results represent 3 4 independent experiments; control phosphate 2 experiments. Diets have 5 controlled protein source and are otherwise equivalent except for the phosphorus 6 levels of 0.4% (control) or 0.02% (low). P value shown is for baseline (t=0) 7 versus 24 hours post-injection in FA-injected animals.
=p<0.01 comparing 8 between-group differences between mice on a control vs. low phosphate diet. A 9 = folic acid; BUN = blood urea nitrogen
Twenty-four hours after inducing AKI, phosphate levels rose in the low phosphate diet group to 7.6±0.8 mg/dL, which was accompanied by an almost 30-fold increase in plasma cFGF23 levels to 2682±296 pg/ml. This relative increase in plasma cFGF23 concentration was greater than that observed in animals on the normal phosphate diet (17-fold) (Table 4, Figure 6B). The increases in cFGF23 and iFGF23 levels were comparable, and could not be prevented by switching mice to the low phosphate diet after FA injection (data not shown). Thus, neither a low phosphate diet nor a low serum phosphate level abrogated the major increase in plasma FGF23 levels in AKI.
FGF23 levels rise in humans with AKI following cardiac surgery
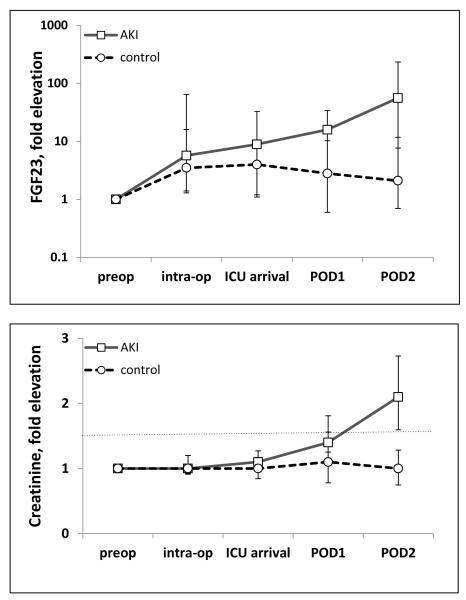
Finally, we aimed to validate the human relevance of our finding of early and marked increases in FGF23 levels in mice with AKI. Elective cardiac surgery is the optimal clinical setting to prospectively assess biomarkers of human AKI since kidney function is routinely assessed pre- and post-operatively, and rates of AKI are high. We measured cFGF23 levels before and after cardiac surgery in 14 patients, 4 of whom developed AKI, which was defined as a ≥50% increase in serum creatinine (sCr) within 5 days post-operatively.
Baseline clinical characteristics of cases and controls are presented in Table 5. cFGF23 levels rose significantly during the post-operative period in both AKI cases and controls (Figure 7), but were significantly higher in the AKI cases beginning at 24 hours post-operatively and at all subsequent time-points. Although sCr also increased significantly by 24 hours, the observed 1.4-fold change was of substantially smaller magnitude than the simultaneous 15.9-fold increase in cFGF23. Consistent with our animal data, these results suggest that FGF23 levels rise early in the course of AKI following cardiac surgery, often before major decrements in renal function are detected.
Table 5.
Baseline characteristics of BWH-AKI cardiac surgery cohort. For details on eligibility criteria see methods.
| AKI N=4 |
Controls N=10 |
|
|---|---|---|
| Age (years ±SD) | 75±8 | 75±8 |
| % Female (n) | 25 (1) | 10 (1) |
| Baseline creatinine (median mg/dL, range) |
1.45 (0.5-1.96) | 1.0 (0.42-1.2) |
| Baseline FGF23 (median RU/mL, range) |
98 (56-130) | 100 (42-2465) |
| % CABG + valve (n) | 50 (2) | 20 (2) |
| % Valve (n) | 25 (1) | 70 (7) |
| % CABG (n) | 0 (0) | 10 (1) |
| % Other (n) | 25 (1) | 0 (0) |
| Bypass time (min ± SD) | 150 ± 44 | 144 ± 64 |
Some patients had both CABG and valve surgery. Surgeries were either elective or urgent; none were emergent.
AKI = acute kidney injury; CABG = coronary artery bypass graft
Figure 7.
Plasma FGF23 levels rise in surgical patients with AKI. Shown are fold elevation of FGF23 levels (upper panel) and fold elevation of creatinine (lower panel) measured pre-operatively, intra-operatively, on ICU arrival, and daily for 2 days post-operatively in 14 cardiac surgery patients at the Brigham and Women’s Hospital AKI Cohort. In comparison to the findings in 10 control patients, FGF23 levels rose significantly in all 4 participants who developed AKI (p<0.05). The dashed line in the lower panel represents 50% increase in creatinine from baseline, defining AKI.
Discussion
Acute kidney injury, which affects 5-7% of hospital admissions in the United States, is associated with increased risk of in-hospital mortality,45,46 and confers greater risks of developing CKD, ESRD, and death long after the initial AKI episode has resolved47-49. In the present study, we showed that there is a rapid, early increase in circulating FGF23 levels in two animal models of toxin-induced AKI. In FA-induced AKI, the increase in FGF23 occurred independent of PTH, dietary and serum phosphate, plasma 1,25D levels, VDR activation, and PPR signaling in osteocytes. These observations were supported by complementary human data, which demonstrated that FGF23 levels rose early in patients who developed AKI following cardiac surgery compared to those who remained free of AKI.
Circulating FGF23 levels began to increase within one hour of AKI induction in mice. This was earlier than the increase in NGAL, which is an established biomarker of AKI. Since FGF23 levels correlate with eGFR50,51 and are lower in dialysis patients with residual renal function,52,53 one could hypothesize that the early increase in FGF23 may be due to an acute reduction in renal clearance of FGF23.13 However, the modestly increased half-life of intravenously administered rhFGF23 in mice with AKI compared to controls cannot explain the markedly elevated circulating levels. This suggests that despite the strong inverse correlation between FGF23 and GFR, impaired renal FGF23 clearance is not a primary mechanism for elevated FGF23 levels in states of reduced kidney function, as has been previously suggested52. In fact, immunohistochemical and Western blot analyses of bones confirmed increased bone production of FGF23, as observed in previous human and animal studies of CKD16,35. Collectively, these data suggest that increased production rather than decreased elimination accounts for elevated FGF23 levels in AKI and likely CKD.
Although bone production of FGF23 was significantly increased in AKI by 24 hours, the magnitude of increase in circulating FGF23 levels was out of proportion to the change in bone expression. This has been observed previously in other mouse models of kidney disease.16,54 Perhaps relatively small increases in FGF23 expression by individual osteocytes distributed throughout the skeleton account for the substantial increases in systemic levels. Alternatively, kidney disease could theoretically activate production of FGF23 by other tissues that are known to produce FGF23 in the fetal period, such as liver, spleen, heart, and brain, as suggested by the finding of small amounts of FGF23 mRNA in extraskeletal sites55. Furthermore, the extremely rapid increase in FGF23 within 1 hour is unlikely to be solely due to increased FGF23 transcription. This raises the possibility that stored, preformed hormone could have been released. Alternatively, perhaps AKI acutely reduces intracellular degradation of constitutively transcribed FGF23 within osteocytes. This potential mechanism could explain the high correlation between cFGF23 and iFGF23 levels in this study and the absence of circulating C-terminal FGF23 fragments in a previous study of patients with end-stage renal disease56. These findings contrast sharply with those from healthy individuals and in states of iron deficiency in which enhanced FGF23 transcription is matched by intracellular degradation leading to normal iFGF23 but high levels of C-terminal fragments.3056
Our findings that the FGF23 elevation in AKI occurs independent of PTH-signaling may seem at odds with recent data showing that parathyroidectomy prevents the rise in circulating FGF23 in early CKD models (4-7 days on adenine diet).4,19 However, the mechanisms leading to the rise in FGF23 hours after AKI induction may be different from those that occur over days to weeks, in animal models of CKD induced by adenine or 5/6 nephrectomy. Furthermore, acute parathyroidectomy may have effects on bone metabolism that alter signaling in osteocytes and osteoblasts that produce FGF2357,58, but are not observed in genetically modified animals on stable rescue diets and compensated bone physiology.
Our observation that AKI increases FGF23 levels in the absence of VDR signaling suggests that vitamin D-dependent mechanisms do not contribute significantly to the transcriptional regulation of FGF23 when renal function is acutely impaired. We were unable to investigate whether other pathways shown to regulate FGF23 expression on other settings, such as the Wnt59 or FGF signaling pathways54, may also contribute to FGF23 elevation in AKI, which is unknown. Recently, Klotho deficiency was described in AKI in both rodents and humans31 and Klotho protein levels decreased in plasma and kidney by 3 hours after ischemia-reperfusion injury in rats.31 Thus, while Klotho deficiency may contribute to the FGF23 elevation in AKI, it is unlikely to contribute to the very early increases that we detected already at 1 hour after FA injection.
A low phosphate diet did not prevent the increase in serum phosphate levels in AKI. However, despite lower levels of phosphate and FGF23 at baseline, FGF23 increased ~30-fold within 24 hours after induction of AKI in the low phosphate group, which was significantly greater than the ~17-fold increase in mice on the control diet. These data suggest that the absolute level of serum phosphate does not govern FGF23 levels in AKI, since the peak serum phosphate levels in the low phosphate group (7.6 mg/dL) were similar to the baseline level in the normal phosphate group (6.8 mg/dL). It is nevertheless possible that the relative increase but not the baseline serum phosphate level modulates FGF23 production.
By using an AKI model, and thus a novel physiological state of renal dysfunction, we have been able for the first time to show that in addition to the currently known regulators of FGF23 (PTH, vitamin D, phosphate), other mechanisms and regulators likely play a role in controlling FGF23 production or stability in AKI and possibly in CKD.
Consistent with the experimental data, FGF23 levels rose acutely in patients undergoing cardiac surgery who developed AKI, even prior to the rise in serum creatinine. Interestingly, FGF23 levels also rose post-operatively in patients who did not develop AKI, albeit to a lesser extent. While further investigation is needed to determine the mechanisms underlying the acute post-operative rise in FGF23 levels in patients who did not develop AKI, we hypothesize that a systemic inflammatory response to surgery may have contributed60. We recently reported that FGF23 levels are increased in patients with critical illness, with and without AKI, and that elevated FGF23 is strongly associated with higher levels of inflammatory markers in CKD patients26,61.
Our studies have several limitations. Changes in PTH beyond the 24-hour period, or Klotho protein in circulation or urine were not assessed partly due to limitations in the amount of blood volume that can be collected from mice. Additionally, our experiments with PTH-null mice did not allow us to determine if the FGF23 levels in mice with AKI were partially attenuated and only a small cohort of patients undergoing elective cardiac surgery was investigated for AKI-induced changes in FGF23 levels. We were also unable to determine whether the epidemiologic association between elevated FGF23 and mortality in AKI26 is driven by elevated FGF23 acting as a marker of disease severity or whether it exerts end-organ toxicity that contributes directly to adverse clinical outcomes.
Despite these limitations, our work suggests that FGF23 could serve as a novel biomarker of AKI. Additional studies are needed to dissect mechanisms contributing to changes in FGF23 secretion in AKI and to define the predictive utility of FGF23 as a novel early biomarker of AKI and perhaps, novel risk factor underlying the transition from AKI to CKD.
Materials and Methods
Experimental Animals
Male animals were studied. CD1 and C57BL/6 mice at 11-13 weeks of age (Charles River Laboratories, Wilmington, MA) were fed regular chow (1%Ca, 0.44%non-phytate phosphorus) or a low phosphate diet (0.6%Ca, 0.02%Pi) or a control diet (0.6%Ca, 0.4%Pi; Harlan Laboratories, Madison, WI). Animals were housed in the Center for Comparative Medicine at the Massachusetts General Hospital, and experiments were done in accordance with the hospital’s Subcommittee on Research Animal Care standards.
Additional animal strains, crossed into the C57BL/6 background, and genotyped using published protocols37,38,43 included: 1) Mice homozygous for floxed exon 1 of PPR (fl/fl-PPR) and expressing the Cre recombinase under the control of the 8-kb DMP1 promoter resulting in ablation of the PPR in osteocytes (PPR-cKO); fl/fl-PPR littermates served as controls. 2) Mice with homozygous ablation of exon 3 of PTH; animals ate regular chow and drinking water was supplemented with 1% calcium gluconate. 3) Mice with homozygous ablation of exon 3 of the VDR; animals ate a rescue diet (20% lactose, 1.25%Pi, and 1%Ca, Harlan Laboratories); wild-type littermates served as controls.
AKI induction
AKI was induced by a single intraperitoneal injection (IP) of FA (240mg/kg in vehicle: 0.15M NaHCO3, pH 7.4). Control animals were injected with vehicle. Animals were sacrificed after 24 hours by isofluorane inhalation and cervical dislocation, and organs harvested within minutes of sacrifice. For the rhabdomyolysis experiment, animals were injected intramuscularly into the hind legs with 50% glycerol in PBS or PBS according to published protocols30, and sacrificed as above.
Histological and Western blot analysis
Kidneys were fixed in 4% paraformaldehyde and processed for paraffin embedding. Femurs were dissected and fixed in 70% ethanol for fixation and subsequent embedding into methylmethacrylate. Immunohistochemical detection of FGF23 in bone62 was detected using an affinity-purified polyclonal goat anti-mouse FGF23(225–244) (Immutopics, San Clemente, CA)35. A single blinded investigator scored low-power images using a scale of 0 to 3+.
Whole femur lysates were obtained from animals 24 hours after FA or vehicle injection and analyzed for FGF23 expression using the protocol of Farrow et al.29. Densitometry of FGF23 or actin bands was performed using Alphaimager (ProteinSimple, Santa Clara, CA).
Biochemical analysis for animal studies
Biochemical analyses were done on blood samples collected from the tail-vein or intracardiac puncture. Phosphate was measured by the Phospha-C test (Wako, Osaka, Japan);BUN by a colorimetric assay (Stanbio Laboratories, Boerne, TX). ELISAs were used for PTH and mouse cFGF23 (Immutopics, San Clemente, CA), iFGF23 (Kainos, Tokyo, Japan), 1,25D (IDS, Fountain Hills, AZ), and plasma NGAL (R&D systems, Minneapolis, MN).
FGF23 injection
RhFGF23 (R&D systems) in PBS was injected at 40ug/kg via tail vein injection into animals 24 hours after either FA or vehicle injection. Plasma samples were collected 5, 15, 30, 60, and 120 minutes after injection. FGF23 was measured after 1:100 dilution using a human cFGF23 ELISA (Immutopics, San Clemente, CA).
Statistical analysis for animal experiments
Differences between treatment groups were performed using the two-tailed Student’s t-test. Results were considered significant if p<0.05. Values are expressed as means ±SEM.
Human AKI study
The Brigham and Women’s Hospital-AKI study is a prospective cohort of study patients undergoing cardiac surgery. Inclusion criteria include age ≥18 years and ≥2 of the following: baseline eGFR <60 ml/min/1.73m2; diabetes; ejection fraction ≤40%; previous cardiac surgery; combined bypass and valve procedure; urgent procedure; and pre-operative balloon pump. Exclusion criteria include preexisting AKI (>0.5 mg/dl increase in sCr over baseline); sCr >4.5 mg/dl; dialysis; renal transplantation; pregnancy; and recent aminoglycoside use. Daily samples plasma samples were collected before and up to 8 days after AKI onset. The study was approved by the institutional review board at Brigham and Women’s Hospital and all patients provided written informed consent.
We performed a nested case-control study, defining AKI as a ≥50% rise from baseline in sCr within 5 days. We measured cFGF23 in plasma samples obtained pre-operatively, intra-operatively, on ICU arrival; and on post-operative days 1 and 2. Statistical analysis of cFGF23 levels of patients who did or did not develop AKI was done using the Mann-Whitney test (MedCalc Software, Mariakerke, Belgium).
Acknowledgements
This work was supported by grants from the NIH; K08 DK93608 to MC and by PO1 DK11794, subproject IV to HJ. MC would like to thank Drs. R.I. Thadhani and J.T. Potts, Jr. for their invaluable support and A. Maeda and B. Yu for technical assistance.
Footnotes
Disclosure: HJ declares that he is a named co-inventor on a patent describing the measurement of FGF23; all other authors declared no conflict of interest
References
- 1.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004 Feb;113(4):561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006 Aug;21(8):1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 3.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006 Aug;91(8):3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 4.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010 Oct;299(4):F882–889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 5.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011 Jan;79(1):112–119. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 6.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011 Oct;22(10):1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008 Aug 7;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011 Jun 15;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011 May;22(5):956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009 Sep;24(9):2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 11.Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, Moyses RM. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011 Feb;6(2):241–247. doi: 10.2215/CJN.04250510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesseling-Perry K, Tsai EW, Ettenger RB, Jüppner H, Salusky IB. Mineral abnormalities and long-term graft function in pediatric renal transplant recipients: a role for FGF-23? Nephrol Dial Transplant. 2011 Nov;26(11):3779–3784. doi: 10.1093/ndt/gfr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003 Dec;64(6):2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 14.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011 Jun;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005 Jul;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 16.Stubbs JR, He N, Idiculla A, Gillihan R, Liu S, David V, Hong Y, Quarles LD. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. 2011 Sep 28; doi: 10.1002/jbmr.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Husen M, Fischer AK, Lehnhardt A, Klaassen I, Moller K, Muller-Wiefel DE, Kemper MJ. Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int. 2010 Jul;78(2):200–206. doi: 10.1038/ki.2010.107. [DOI] [PubMed] [Google Scholar]
- 18.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007 Oct;18(10):2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 19.Lopez I, Rodriguez-Ortiz ME, Almaden Y, Guerrero F, de Oca AM, Pineda C, Shalhoub V, Rodriguez M, Aguilera-Tejero E. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011 Sep;80(5):475–482. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 20.Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res. 2009 Oct;24(10):1681–1685. doi: 10.1359/JBMR.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesseling-Perry K, Harkins GC, Wang HJ, Elashoff R, Gales B, Horwitz MJ, Stewart AF, Jüppner H, Salusky IB. The calcemic response to continuous parathyroid hormone (PTH)(1-34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH(7-84) J Clin Endocrinol Metab. 2010 Jun;95(6):2772–2780. doi: 10.1210/jc.2009-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sridharan M, Cheung J, Moore AE, Frost ML, Fraser WD, Fogelman I, Hampson G. Circulating fibroblast growth factor-23 increases following intermittent parathyroid hormone (1-34) in postmenopausal osteoporosis: association with biomarker of bone formation. Calcif Tissue Int. 2010 Nov;87(5):398–405. doi: 10.1007/s00223-010-9414-8. [DOI] [PubMed] [Google Scholar]
- 23.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001 Feb 2;280(4):1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 24.Leaf DE, Wolf M, Stern L. Elevated FGF-23 in a patient with rhabdomyolysis-induced acute kidney injury. Nephrol Dial Transplant. 2010 Apr;25(4):1335–1337. doi: 10.1093/ndt/gfp682. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M. FGF-23 and PTH levels in patients with acute kidney injury: A cross-sectional case series study. Annals of Intensive Care. 2011;1(21) doi: 10.1186/2110-5820-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leaf DE, Wolf M, Waikar S, Chase H, Christov M, Cremers S, Stern L. FGF-23 levels in patients with acute kidney injury and risk of adverse outcomes. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.00550112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, Yancopoulos GD, Rudge JS, Woolf AS. Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int. 2008 Aug;74(3):300–309. doi: 10.1038/ki.2008.179. [DOI] [PubMed] [Google Scholar]
- 28.Koziolek MJ, Muller GA, Zapf A, Patschan D, Schmid H, Cohen CD, Koschnick S, Vasko R, Bramlage C, Strutz F. Role of CX3C-chemokine CX3C-L/fractalkine expression in a model of slowly progressive renal failure. Nephrol Dial Transplant. 2010 Mar;25(3):684–698. doi: 10.1093/ndt/gfp602. [DOI] [PubMed] [Google Scholar]
- 29.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):E1146–1155. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Q, Hill WD, Su Y, Huang S, Dong Z. Heme oxygenase-1 induction contributes to renoprotection by G-CSF during rhabdomyolysis-associated acute kidney injury. Am J Physiol Renal Physiol. 2011 Jul;301(1):F162–170. doi: 10.1152/ajprenal.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010 Dec;78(12):1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010 Mar;42(1):141–150. doi: 10.1007/s11255-009-9608-z. [DOI] [PubMed] [Google Scholar]
- 33.Oikonomou KA, Kapsoritakis AN, Theodoridou C, Karangelis D, Germenis A, Stefanidis I, Potamianos SP. Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel disease: association with pathophysiology of inflammation, established markers, and disease activity. J Gastroenterol. 2011 Dec 27; doi: 10.1007/s00535-011-0516-5. [DOI] [PubMed] [Google Scholar]
- 34.Ubaidus S, Li M, Sultana S, de Freitas PH, Oda K, Maeda T, Takagi R, Amizuka N. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc (Tokyo) 2009 Dec;58(6):381–392. doi: 10.1093/jmicro/dfp032. [DOI] [PubMed] [Google Scholar]
- 35.Pereira RC, Jüppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009 Dec;45(6):1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makitie O, Pereira RC, Kaitila I, Turan S, Bastepe M, Laine T, Kroger H, Cole WG, Jüppner H. Long-term clinical outcome and carrier phenotype in autosomal recessive hypophosphatemia caused by a novel DMP1 mutation. J Bone Miner Res. 2010 Oct;25(10):2165–2174. doi: 10.1002/jbmr.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest. 2002 May;109(9):1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell WF, Jr., Barry KJ, Tulum I, Kobayashi T, Harris SE, Bringhurst FR, Pajevic PD. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011 Apr;209(1):21–32. doi: 10.1530/JOE-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012 Feb 3; doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004 Mar;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006 May;17(5):1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 42.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998 Oct;139(10):4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 43.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005 Jun;36(6):971–977. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Fujita T. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25(6):419–422. doi: 10.1007/s00774-007-0779-3. [DOI] [PubMed] [Google Scholar]
- 45.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 46.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006 Apr;17(4):1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 47.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009 Jan;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010 Feb;21(2):345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012 Mar;81(5):477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 50.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010 Apr;95(4):1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 51.Filler G, Liu D, Huang SH, Casier S, Chau LA, Madrenas J. Impaired GFR is the most important determinant for FGF-23 increase in chronic kidney disease. Clin Biochem. 2011 Apr;44(5-6):435–437. doi: 10.1016/j.clinbiochem.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Isakova T, Xie H, Barchi-Chung A, Vargas G, Sowden N, Houston J, Wahl P, Lundquist A, Epstein M, Smith K, Contreras G, Ortega L, Lenz O, Briones P, Egbert P, Ikizler TA, Jueppner H, Wolf M. Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol. 2011 Nov;6(11):2688–2695. doi: 10.2215/CJN.04290511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Jüppner H, Salusky IB. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009 Feb;94(2):511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wohrle S, Bonny O, Beluch N, Gaulis S, Stamm C, Scheibler M, Muller M, Kinzel B, Thuery A, Brueggen J, Hynes NE, Sellers WR, Hofmann F, Graus-Porta D. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J Bone Miner Res. 2011 Oct;26(10):2486–2497. doi: 10.1002/jbmr.478. [DOI] [PubMed] [Google Scholar]
- 55.Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. Mineralized tissue cells are a principal source of FGF23. Bone. 2007 Jun;40(6):1565–1573. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010 Feb;95(2):578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yajima A, Ogawa Y, Takahashi HE, Tominaga Y, Inou T, Otsubo O. Changes of bone remodeling immediately after parathyroidectomy for secondary hyperparathyroidism. Am J Kidney Dis. 2003 Oct;42(4):729–738. doi: 10.1016/s0272-6386(03)00909-0. [DOI] [PubMed] [Google Scholar]
- 58.Yajima A, Inaba M, Tominaga Y, Nishizawa Y, Ikeda K, Ito A. Increased osteocyte death and mineralization inside bone after parathyroidectomy in patients with secondary hyperparathyroidism. J Bone Miner Res. 2010 Nov;25(11):2374–2381. doi: 10.1002/jbmr.126. [DOI] [PubMed] [Google Scholar]
- 59.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011 Oct;49(4):636–643. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunt I, Day J. Cardiac Surgery and Inflammation: The Inflammatory Response and Strategies to Reduce the Systemic Inflammatory Response Syndrome. Current Cardiology Reviews. 2007;3(1):91–98. [Google Scholar]
- 61.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M. Fibroblast Growth Factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012 May 17; doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes SA, dos Reis LM, de Oliveira IB, Noronha IL, Jorgetti V, Heilberg IP. Usefulness of a quick decalcification of bone sections embedded in methyl methacrylate[corrected]: an improved method for immunohistochemistry. J Bone Miner Metab. 2008;26(1):110–113. doi: 10.1007/s00774-007-0788-2. [DOI] [PubMed] [Google Scholar]