Abstract
Background
Much remains to be learned about the effect of the APOE ε4 allele on the trajectory of cognitive aging including the onset of terminal decline and rates of decline before and after, particularly in the presence of Alzheimer’s disease (AD) brain pathology.
Objective
To examine the association of APOE ε4 allele with the late-life cognitive trajectory and test the hypothesis that association of ε4 with cognitive decline is explained by AD neuropathology.
Methods
Participants (N=581) came from two longitudinal clinical-pathologic studies of aging and dementia, the Religious Orders Study and the Memory and Aging Project, which involve uniform annual cognitive assessments and brain autopsy. Longitudinal measures of cognition were derived from detailed annual neuropsychological testing. Participants with 1 or more copies of ε4 allele (ε2/4 excluded) were considered ε4 carriers. Global AD pathology was summarized based on counts of neuritic plaques, diffuse plaques and neurofibrillary tangles. Separate measures of amyloid load and tangle density were assessed using immunohistochemistry. A uniform examination was conducted to document chronic cerebral infarctions. Lewy bodies were identified using alpha-synuclein immunostained sections of substantia nigra, limbic, and neocortical regions. Random change point models were applied to examine the association of ε4 allele with onset of terminal decline as well as pre-terminal and terminal slopes.
Results
On average, the onset of terminal decline occurred around 3 years before death and the rate of terminal decline was 8-fold faster than the pre-terminal decline. The presence of ε4 allele was associated with an earlier onset of terminal decline and faster rates of decline before and after the onset. After adjusting for global AD pathology, the ε4 allele was no longer associated with onset of terminal decline or pre-terminal slope, and the association with terminal slope became marginal. Similarly, ε4 allele was not associated with trajectory of cognitive aging after replacing global AD pathology with the more molecularly-specific measures of amyloid and tau tangles. The result was essentially unchanged after controlling for other common age-related brain pathologies.
Conclusion
The APOE ε4 allele is an important determinant of the change in late-life cognition, including terminal decline. The association is primarily working through AD pathology.
INTRODUCTION
Cognitive aging is generally characterized by a mild but progressive cognitive decline followed by a precipitous drop in the years just prior to death, known as terminal decline [Kleemeier 1962]. The trajectory of cognitive aging is determined by a broad range of age- and disease-related processes attributable to distinct sets of risk factors [Steinerman et al 2010]. Consequently, a risk factor may have different effects along the trajectory of cognitive aging and may influence either the onset of terminal decline or rates of pre-terminal or terminal decline. Identifying the dissociable effects of risk factors on the components of the cognitive trajectory will help to decipher the underlying biological mechanisms and facilitate the development of targeted therapies to reduce the burden of cognitive aging.
The presence of the APOE ε4 allele is unequivocally the strongest known genetic risk factor for late-onset AD, and ε4 carriers often exhibit accelerated cognitive decline compared with non-carriers. However, little is known regarding when ε4 allele exerts its effect over the course of cognitive aging and in particular whether it impacts terminal decline. Previous findings are mixed. Some studies have shown that the effect of APOE ε4 allele on cognition or cognitive decline was evident long before the onset of dementia [Yaffe et al 1997, Bondi et al 1999, Caselli et al 2009], and some reported a lack of ε4 association with cognitive decline in ‘predemented’ or nondemented individuals [Bunce et al 2004] or after the occurrence of clinical symptoms [Jonker et al 1998, Slooter et al 1999]. A new study of older community-dwelling populations in Australia has shown that ε4-related cognitive decline is due to a higher risk of preclinical dementia among carriers [Batterham et al 2012]. Thus, these findings may suggest that separate mechanisms contribute to the development and progression of dementia. On the other hand, other studies have found that ε4 allele remained as a moderate-to-strong predictor of progression to AD after subjects had cognitive impairment [Petersen et al 1995, Elias-Sonnenschein et al 2011], and recent data from our cohorts have shown that, among participants who were dementia free at enrollment and developed incident AD, ε4 carriers had more rapid cognitive decline both before and after the onset of AD dementia [Yu et al 2012]. Further, ε4 carriers also had a faster cognitive decline during the terminal period [Wilson et al 2007]. Taken together, these findings raise an interesting question as to whether the presence of APOE ε4 allele primarily influences the initial development of dementia or contributes to the progression of the disease from cognitive health all the way through dementia.
What remains less clear is whether common age-related neuropathologies account for the association of ε4 allele with cognitive decline. Cross-sectional models shows that AD pathology lies in the causal pathway between APOE ε4 and clinical AD such that the association of ε4 allele with AD dementia is diminished after controlling for AD pathology [Bennett et al 2003; Mortimer et al 2009; Nicoll et al 2011]. However, other studies suggest that the effect of ε4 on cognitive aging is likely mediated by mechanisms independent of AD [Deary et al 2002], and that ε4 might also contribute to non-pathological decline in verbal memory and abstract reasoning [Schiepers et al 2012]. Recent data support the role of brain pathology in not only the terminal decline phase, but also pre-terminal decline [Wilson et al 2010]. Another study suggests that cerebrovascular risk factors contribute to accelerated age-related memory decline in APOE ε4 homozygotes [Caselli et al 2011]. We are not aware of any study that has directly investigated the role of neuropathology in the association of APOE ε4 with terminal decline.
In the present analyses, we expand on previous findings to examine the association of APOE ε4 with the onset of terminal decline as well as rates of decline before and after its onset, and test the hypothesis that the associations would be attenuated by common age-related neuropathologies known to cause cognitive impairment in old age. The data came from almost 600 deceased participants from the Religious Orders Study and the Memory and Aging Project, two longitudinal clinical-pathologic studies of aging and dementia. Participants completed up to 18 years of annual clinical evaluations including detailed cognitive testing and underwent brain autopsy at the time of death. Measures of AD pathology, cerebral infarctions and Lewy bodies were evaluated through a uniform postmortem examination of the brain. Random change point models were applied to capture the different components of cognitive trajectory. The onset of terminal decline relative to the time of death, and slopes before and after its onset were estimated and compared by ε4 status. In subsequent models, indices of neuropathology were added to examine whether the effect of the APOE ε4 allele on the cognitive trajectory works through neuropathology.
METHODS
Participants
The participants came from two ongoing longitudinal clinical-pathologic studies of aging and dementia, the Religious Orders Study (ROS) and the Memory and Aging Project (MAP). The Religious Orders Study, started in 1994, enrolled older Catholic priests, nuns and brothers from about 40 groups in 12 states across the United States [Bennett et al 2012]. The Memory and Aging project, started in 1997, enrolled community-based elderly from retirement communities and other housing units in the Chicago area [Bennett et al 2012]. The studies were approved by the Institutional Review Board of Rush University Medical Center. Participants were enrolled without known dementia and agreed to annual clinical evaluations and brain donation upon death. At the time of these analyses, 1,151 participants had died (593 ROS, 558 MAP), of whom 967 (84%) had autopsy data.
The diagnosis of dementia and AD was determined by clinicians following the recommendation of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, as previously described [Bennett et al 2006]. The criteria require evidence of cognitive decline in memory and at least one other domain of cognitive function [McKhann et al 1984].
Cognitive function
Both the Religious Orders Study and the Memory and Aging Project implement uniform and structured neuropsychological performance testing at baseline and subsequent follow-up waves. Cognitive function was assessed via a battery of 19 tests from which 17 were combined into a composite measure of global cognition [Wilson et al 2007]. These include immediate and delayed story recall of story A from the logical memory subtest of the Wechsler Memory Scale-Revised [Wechsler 1987], and of the East Boston story [Albert et al 1991, Wilson et al 2002], word list memory, word list recall, and word list recognition [Morris et al 1989]; Boston naming test [Morris et al 1989, Kaplan et al 1983], national adult reading [Nelson 1982], and verbal fluency [Morris et al 1989], digit span forward and backward [Weshler 1987], and digit ordering [Cooper and Sager 1993], symbol digit modalities test [Smith 1982], number comparison [Ekstrom et al 1976], and judgment of line orientation [Benton et al 1994] and standard progressive matrices [Raven et al 1992]. Tests are scored such that higher scores indicate better performance. To compute the composite measure, raw scores on each of the individual tests were converted to z-scores using the baseline mean and standard deviation, and the z-scores of all 17 tests were averaged. Psychometric properties of the summary score were discussed previously [Wilson et al 2002, Wilson et al 2005].
APOE genotyping
Blood was collected at each site, using BD Vacutainer CPT cell preparation tubes containing sodium citrate and stored at room temperature. DNA was extracted from approximately 2–3 million mononuclear cells using Flexigene DNA extraction kits (Qiagen, Valencia, Calif., USA), and quantified using Quant-iT™ PicoGreen® dsDNA detection assay kits (Molecular Probes, Eugene, Oreg., USA). In most cases, DNA was extracted from blood, and in some cases DNA from frozen post-mortem brain tissue (cerebellum) was used. Genotyping was done at the Agencourt Bioscience Corporation (Beverly, Mass., USA), utilizing high-throughput sequencing of codon 112 (position 3937) and codon 158 (position 4075) of exon 4 of the APOE gene [Boyle et al 2010].
Participants with at least one copy of ε4 allele (i.e. ε3/4, ε4/4) were considered ε4 carriers. All the others (i.e. ε2/2, ε2/3, or ε3/3) were considered non-carriers. A very small proportion of ε2/4 carriers (<2%) were excluded from the study.
Neuropathology measures
Brains were removed, weighed, and processed following a standard procedure as previously described [Bennett et al 2005]. One hemisphere was fixed in 4% paraformaldehyde and cut coronally into 1 cm slabs. We dissected five cortical regions (frontal, temporal, parietal, entorhinal and hippocampal cortices) from the fixed slabs and stained six micron sections with Bielschowsky silver stain to detect neuritic plaques, diffuse plaques and neurofibrillary tangles [Bennett et al 2006]. A composite measure of AD pathology was constructed based on separate counts of neuritic plaques, diffuse plaques and neurofibrillary tangles in a 1mm2 area visualized to have the greatest density of each index. The raw counts for each neuropathologic measure in each of the five regions were standardized and averaged across the regions. Global AD pathology was derived by averaging the summary scores of neuritic plaques, diffuse plaques and neurofibrillary tangles. Separate measures of global cortical amyloid load and PHF-tangle density were quantified using immunohistochemical methods, image analysis (Image J 1.42 g (http://rsbweb.nih.gov/ij/), and systematic sampling using Stereo Investigator 8.0 software program (MicroBrightField Inc., Williston, VT), as previously reported [Bennett et al 2005]. The amyloid measure included both plaque and vascular amyloid. Gross cerebral infarcts were identified by examining the fixed slabs and/or pictures from both hemispheres; and verified after dissection and histologic review [Schneider et al 2003]. Micro cerebral infarcts were identified by the examination of hematoxylin and eosin stained paraffin-embedded sections from at least nine regions, including six cortical, two subcortical regions and midbrain [Arvanitakis et al 2011]. Only chronic infarcts were considered in the present analyses. Lewy bodies were identified using alpha-synuclein immunohistochemistry on six micron sections from the neocortex, limbic cortices, and substantia nigra [Schneider et al 2007].
Statistical analysis
Change in late-life cognition was modeled by a piecewise linear trajectory with a change point reflecting the onset of terminal decline relative to the time of death [Hall et al 2000, McArdle and Wang 2008]. Random effects were introduced to account for subject-specific deviations from the mean trajectory. We first estimated the location of the change point, the slope before and after the change point, and examined how ε4 allele influenced each of these three components. This allows us to test whether there are different effects of ε4 along the trajectory. In a subsequent model, we added in a term for global AD pathology and tested the hypothesis that the association of ε4 with cognitive decline was attenuated by the overall burden of AD pathology in the brain. Since prior literature shows cross-sectionally that neurofibrillary tangles mediate the association of amyloid with AD and level of cognition [Bennett et al 2004], and amyloid further mediates the association of ε4 with cognition [Bennett et al 2005], we replaced global AD pathology with the more molecularly-specific measures of amyloid load and tangle density, separately and then combined, to examine whether such a mediation pathway could be indentified in the trajectory of cognitive aging. Next, we examined the role of chronic cerebral infarctions in the association of ε4 with the cognitive trajectory. Finally, we retested the association by adjusting for both global AD pathology and other common age-related pathologies of chronic cerebral infarction and Lewy bodies. In any of these models, an attenuated association of ε4 after controlling for the postmortem indices would suggest that ε4’s association with late-life cognitive change is likely mediated by neuropathology.
Throughout the analyses, we restricted participants to those who had at least 5 cognitive evaluations in order to best capture the trajectory of cognitive change and allow for stable estimates both before and after the onset of terminal decline. Statistical inference was drawn from posterior distribution under the Bayesian approach. The posterior distribution combines prior beliefs with sample information from the data to create updated knowledge about the parameters of interest. Mathematically, the posterior distribution is proportional to the product of prior distributions (prior beliefs) and the model likelihood (data) through Bayes’ rule. In our analyses, defused conventional conjugate priors, which reflect our little knowledge about the parameters, were applied such that the posterior distribution was largely determined by the model likelihood. Bayesian Monte Carlo Markov Chain (MCMC) method was used to sample from the posterior distribution. The posterior means and credible intervals were approximated using sampling statistics, where the means were calculated by averaging a sufficiently large number of simulated samples; and 95% credible intervals were defined as 2.5th and 97.5th quantiles of the samples. The credible intervals were used to test the statistical significance of the means such that a credible interval that didn’t cover zero would indicate that the corresponding mean was statistically different from zero. Further details on Bayesian analysis can be found in Gelman et al [1995]. All the analyses were implemented in OpenBUGS [Lunn et al 2000].
RESULTS
Description of the participants
Among 967 participants with autopsy data, 330 had fewer than 5 waves of cognitive assessment, and we further excluded 21 participants who died more than 2 years after their last evaluation, 9 participants on whom APOE was not yet available, 15 who were diagnosed with non-AD dementias, and 11 with ε2/4 genotyping. This left 581 participants eligible for the analyses. Their average age was 88.9 years (SD=6.5, range 71 to 108), 381 were female (65.6%) and 138 (23.8%) were ε4 carriers. The mean follow-up length was about 8 years but went up to 17 years. Post-mortem review of clinical data resulted in a diagnosis of AD for 266 (45.9%) participants. The mean global AD pathology measure was 0.7 standardized unit (SD=0.6, range 0 to 3.0), 277 (47.7%) had either gross or micro chronic cerebral infarction, and Lewy bodies were present in 113 (19.5%) persons.
Change in cognition and ε4 allele
We first examined the association of ε4 and the onset of terminal decline, controlling for age, sex, and education. The result is summarized in Table 1. On average, the terminal decline started around 3 years prior to the time of death (onset of terminal decline for non-carriers, EST = −3.20 years). APOE ε4 carriers had an earlier onset of terminal decline by about 9 months (onset of terminal decline for carriers, EST = −0.75 years).
Table 1.
Association of APOE ε4 with trajectory of cognitive decline
| EST (SD) | 95% CI | |
|---|---|---|
| Mean trajectory for non-carriers | ||
| Onset of terminal decline | −3.20 (0.16) | −3.51, −2.89 |
| Pre-terminal decline slope | −0.04 (0.005) | −0.05, −0.03 |
| Terminal decline slope | −0.30 (0.02) | −0.35, −0.27 |
| Additional burden for carriers | ||
| Onset of terminal decline | −0.75 (0.22) | −1.18, −0.32 |
| Pre-terminal decline slope | −0.03 (0.008) | −0.05, −0.01 |
| Terminal decline slope | −0.12 (0.03) | −0.18, −0.06 |
EST: estimated posterior mean; SD: standard deviation of the sampling distribution; CI: credible interval; Onset of terminal decline: the change point in years prior to death when the cognitive decline accelerated; Pre-terminal decline slope: annual rate of decline in cognition before the change point; Terminal decline slope: annual rate of decline in cognition after the change point. The model was adjusted for age, sex and education.
Next, we examined how ε4 was associated with rates of decline before and after the onset of terminal decline. For non-carriers, the estimated mean rate of decline during the pre-terminal period was about 0.04 standardized unit per year (pre-terminal decline slope for non-carriers, EST = −0.04), and the rate of decline during the terminal period was almost 8-fold faster (terminal decline slope for non-carriers, EST = −0.30). The rates of decline in cognition among ε4 carriers were about 75% faster before (pre-terminal decline slope for carriers, EST = −0.03), and 40% faster after the onset (terminal decline slope for carriers, EST = −0.12), compared to non-carriers (Table 1).
Change in cognition and ε4 allele, adjusted for global AD pathology
Since cross-sectional findings suggest that the ε4 allele affects the clinical manifestation of AD through AD pathology, we first investigated the association of the ε4 allele with the trajectory of late-life cognition after controlling for global AD pathology and examined whether AD pathology attenuated the association of ε4 with cognitive decline. The overall burden of AD pathology was significantly associated with the late-life change in cognition such that for every 1 standard deviation increase of AD pathology, the onset of terminal decline was shifted earlier by about 8 months (EST = −0.69 years, 95% CI=[−0.88, −0.49]), and participants with more AD pathology also had faster rates of decline before (EST = −0.03, 95% CI=[−0.04, −0.02]) and after the onset (EST = −0.06, 95% CI=[−0.09, −0.03]). The difference between ε4 carriers and non-carriers in the trajectory of cognitive decline was attenuated after controlling for global AD pathology, such that the presence of the ε4 allele was no longer associated with the onset of terminal decline or the pre-terminal slope, and the estimate of ε4 with the terminal slope was reduced by about 50% and became marginally significant (Table 2, Global AD pathology).
Table 2.
Attenuation of the ε4 association by common neuropathologies
| Controlled for | Global AD pathology | Macro/Micro Infarctions | AD, Infarctions, and Lewy bodies | |||
|---|---|---|---|---|---|---|
| EST (SD) | 95% CI | EST (SD) | 95% CI | EST (SD) | 95% CI | |
| Association of ε4 | ||||||
| Onset of terminal decline | −0.13 (0.22) | −0.57, 0.30 | −0.73 (0.21) | −1.15, −0.31 | −0.07 (0.23) | −0.52, 0.37 |
| Pre-terminal decline slope | −0.004 (0.008) | −0.02, 0.01 | −0.028 (0.008) | −0.04, −0.01 | −0.002 (0.008) | −0.02, 0.01 |
| Terminal decline slope | −0.06 (0.03) | −0.12, −0.006 | −0.11 (0.03) | −0.17, −0.05 | −0.06 (0.03) | −0.11, 0.005 |
EST: estimated posterior mean; SD: standard deviation of the sampling distribution; CI: credible interval; Onset of terminal decline: the change point in years prior to death when the cognitive decline accelerated; Pre-terminal decline slope: annual rate of decline in cognition before the change point; Terminal decline slope: annual rate of decline in cognition after the change point. The model was also adjusted for age, sex and education.
Change of cognition and ε4 allele, adjusted for amyloid and tangles
Prior cross-sectional data have also shown that amyloid deposition and subsequent tangle formation mediate the effect of ε4 allele on cognition. In a follow-up analysis we investigated the association of ε4 allele with change in late-life cognition after controlling for individual measures of amyloid load and tangle density, separately and then combined. Amyloid was associated with an earlier onset of terminal decline (EST = −0.37, 95% CI=[−0.56, −0.18]) as well as a faster rate of pre-terminal decline (EST = −0.014, 95% CI=[−0.022, −0.007]), and the association with terminal decline was a trend (EST = −0.027, 95% CI=[−0.055, 0.002]). After controlling for amyloid, ε4 allele remained significantly associated with all the three components of the cognitive trajectory (Table 3, Amyloid). In comparison, tangles were associated with both an earlier onset of terminal decline (EST = −0.57, 95% CI=[−0.74, −0.40]) and faster rates of decline in pre-terminal (EST = −0.037, 95% CI=[−0.047, −0.026]) and terminal periods (EST = −0.055, 95% CI=[−0.081, −0.030]). After controlling for tangles, however, ε4 was only weakly associated with the terminal decline (Table 3, Tangles).
Table 3.
Attenuation of the ε4 association by amyloid and tangles pathologies
| Controlled for | Amyloid | Tangles | Amyloid and Tangles | |||
|---|---|---|---|---|---|---|
| EST (SD) | 95% CI | EST (SD) | 95% CI | EST (SD) | 95% CI | |
| Association of ε4 | ||||||
| Onset of terminal decline | −0.58 (0.22) | −1.01, −0.16 | −0.28 (0.21) | −0.69, 0.15 | −0.25 (0.22) | −0.67, 0.19 |
| Pre-terminal decline slope | −0.02 (0.009) | −0.04, −0.005 | −0.007 (0.009) | −0.03, 0.01 | −0.006 (0.009) | −0.02, 0.01 |
| Terminal decline slope | −0.09 (0.03) | −0.15, −0.02 | −0.07 (0.03) | −0.13, −0.003 | −0.06 (0.03) | −0.12, 0.01 |
EST: estimated posterior mean; SD: standard deviation of the sampling distribution; CI: credible interval; Onset of terminal decline: the change point in years prior to death when the cognitive decline accelerated; Pre-terminal decline slope: annual rate of decline in cognition before the change point; Terminal decline slope: annual rate of decline in cognition after the change point. The model was also adjusted for age, sex and education.
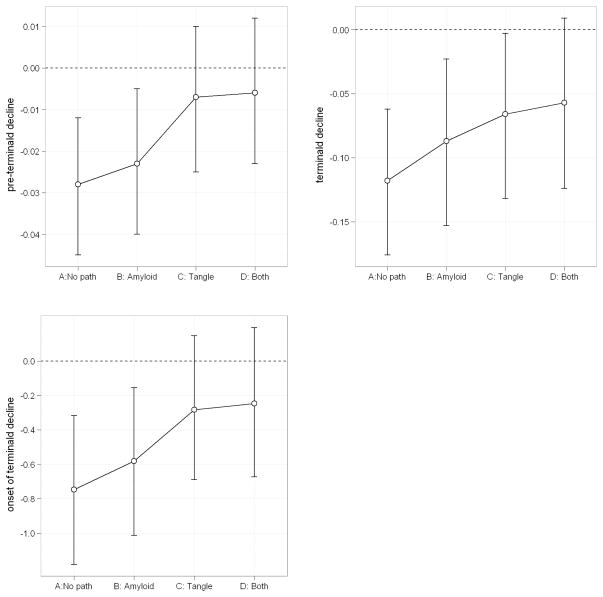
There is also evidence showing that tangles mediate the effect of amyloid and APOE on cognition. Thus, with both amyloid and tangles included in the model, we anticipated that tangles would remain significant but the effects of both APOE and amyloid would be reduced. In this model adjusted for both amyloid and tangles, amyloid was only associated with an earlier onset of terminal decline. By contrast, tangles were significantly associated with all three components of the trajectory. Consistent with the result using global AD pathology, the effect of ε4 allele on late-life cognitive trajectory was diminished after controlling for amyloid and tangles and became non-significant (Table 3, Amyloid and Tangles). Figure 1 further illustrates the attenuation of ε4 effect in the model comparing the model not adjusted for any AD pathology to the ones adjusted for amyloid and tangles separately, as well as both amyloid and tangles.
Figure 1.
Attenuation of ε4 association by AD pathologies
On the x-axis, “A: No path” refers to the model without adjusting for AD pathology; “B: Amyloid” refers to the model only adjusted for amyloid pathology; “C: Tangle” refers to the model adjusted for tangles pathology; “D: both” refers to the model adjusted for both amyoid and tangles pathologies. On the y-axis, estimated means (open circle) and 95% credible intervals for the association of the ε4 allele with pre-terminal slope (upper left), terminal slope (upper right) and onset of terminal decline (lower left).
Change in cognition and ε4 allele, adjusted for potential protective effect of ε2
Recent studies have reported that the ε2 allele is associated with heavier burden of AD pathology among the oldest old, but not with the clinical manifestation of dementia or AD. [Berlau et al 2012]. Thus, to further investigate our findings of ε4 allele with late-life cognitive change while considering the potential effect of ε2, we re-examined the association by adjusting for the presence of ε2 allele. About 14.3% of the subjects in this analysis had at least 1 ε2 allele (2/2, 2/3). Our analysis found no strong evidence supporting the protective effect of ε2 allele. Comparing to 3/3 carriers, the presence of ε2 was not associated with either the rates of pre-terminal decline (EST =0.02, 95% CI = [−0.003, 0.04]), terminal decline (EST =0.004, 95% CI = [−0.077, 0.081]), or the onset of the terminal decline (EST =0.29, 95% CI= [−0.30, 0.90]). Further, all previous findings on the association of ε4 allele with cognitive trajectory and the attenuation of such association after adjustment for AD pathologies remain unchanged (results not shown).
Change in cognition and ε4 allele, adjusted for cerebrovascular disease
AD pathology is commonly associated with other age-related pathologies [Neuropathology Group. Medical Research Council Cognitive Function and Aging Study 2001, Schneider et al 2007], and prior studies in this and other cohorts have shown that ε4 allele is also associated with increased odds of chronic cerebral infarction at autopsy [Schneider et al 2005] and vascular dementia [Chuang et al 2010]. While cross-sectional data does not support a mediation effect for infarctions [Li et al 2007, Mortimer et al 2009], it is unclear whether the relationship between ε4 and AD is attributable to cerebrovascular diseases. Therefore, we next investigated whether the presence of macro- and microscopic chronic infarctions influenced the association of ε4 allele with the cognitive trajectory. Macroscopic chronic infarction was not associated with onset of terminal decline (EST =−0.06, 95% CI = [−0.47, 0.35]), but was associated with faster rates of decline before (EST =−0.02, 95% CI = [−0.03, −0.003]) and after the onset (EST =−0.06, 95% CI = [−0.12, −0.01]). In comparison, microscopic chronic infarction was associated with an earlier onset of terminal decline (EST =−0.57, 95% CI = [−1.00, −0.14]), but not the rates of decline before or after the onset (pre-terminal: EST =−0.004, 95% CI = [−0.02, 0.01]; terminal: EST =0.04, 95% CI = [−0.02, 0.10]). Comparing the models with and without terms for cerebral infarctions, the estimates for the effect of ε4 are very close, suggesting that the ε4 association was not accounted for by infarctions (Table 2, Macro/Micro Infarctions).
Finally we augmented the model by simultaneously examining global AD pathology, cerebral infarctions and Lewy bodies. Global AD pathology was again significantly associated with change in cognition, including an earlier onset of terminal decline and faster rates of decline before and after the onset. The presence of chronic cerebral infarctions was independently associated with an earlier onset of terminal decline (microscopic infarction) and faster rates of pre-terminal and terminal decline (macroscopic infarction). Participants with Lewy bodies had accelerated rates of decline in both pre-terminal (EST =−0.03, 95% CI = [−0.04, −0.01) and terminal stages (EST =−0.14, 95% CI = [−0.21, −0.07]), while Lewy bodies were not associated with the onset of terminal decline (EST =0.02, 95% CI = [−0.44, 0.48]). After controlling for these postmortem indices, the presence of ε4 allele was not associated with the onset of terminal decline, nor was it associated with the pre-terminal or terminal slopes (Table 2, AD, Infarctions, Lewy bodies).
DISCUSSION
Cognitive aging over many years prior to death can be efficiently modeled by a piecewise trajectory consisting of a change point with a pre-terminal period followed by a terminal period. A distinct risk factor might have different effects along this trajectory. The presence of ε4 allele has long been recognized as the primary genetic risk factor for accelerated cognitive decline. Many studies have confirmed that ε4 starts to affect cognition as early as in the preclinical stage [Yaffe et al 1997, Bondi et al 1999, Caselli et al 2009]. However, consensus has not been reached with respect to the role of the APOE polymorphism in late-life cognition including the terminal decline. Some reported a lack of ε4 association after the appearance of clinical symptoms [Jonker et al 1998, Slooter et al 1999], suggesting that ε4 serves only as a trigger or some “initiating factor” [Roses 1996], followed by a separate mechanism.
Using data from nearly 600 participants who completed up to 18 years of annual cognitive assessment, died and underwent brain autopsies, we applied random change point models to characterize the cognitive trajectory and demonstrated that the ε4 allele was associated with the onset of terminal decline, pre-terminal slope, and terminal slope. Non-AD dementia cases were removed from the analyses in order to focus primarily on modeling the association APOE ε4 with AD-related cognitive decline. The result suggests that APOE ε4 allele does not simply contribute to the initial, pre-terminal phase of cognitive decline but also expedites the progression of dementia, influencing cognition throughout the last years of life. Our result is consistent with the prior report that ε4 remains a strong predictor for AD dementia even after participants exhibit cognitive impairment [Petersen et al 1995, Elias-Sonnenschein et al 2011]. The persistent association of ε4 with decline also provides supporting evidence for the existence of a constant pathogenetic process.
Using cross-sectional data, we and others have previously reported that the ε4 allele is associated with clinical manifestation of AD through neuropathology [Bennett et al 2003, Bennett et al 2006, Nicoll et al 2011]. In this study we further found that controlling for the overall burden of AD pathology, ε4 was no longer associated with the onset of terminal decline or pre-terminal slope, and the association with terminal slope became marginal. Replacing global AD pathology with the two individual hallmarks of AD pathologies (amyloid and tangles) yielded consistent results. On the other hand, the effect of the ε4 allele was not affected by the presence of cerebral infarctions. These findings offer further evidence that the biological pathway that links APOE ε4 allele and late-life cognitive decline works primarily through AD pathology. In addition, amyloid or tangles alone was associated with the pre-terminal decline, terminal decline and onset of terminal decline. When both amyloid and tangles were included in the same model, our result shows a diminished effect of amyloid on the trajectory of decline. This is consistent with a previous report showing that tangles mediate the effect of amyloid which attenuates the association of ε4 with clinical AD and cognition, supporting the idea of a neuropathologic pathway whereby tangle formation is preceded or catalyzed by amyloid deposition.
There are several possible mechanisms that account for the association of ε4 with cognitive aging. A popular proposition is the association of APOE with brain amyloid deposition. Neuropathological studies have shown a positive correlation of ε4 allele with β-amyloid (Aβ) proteins in the cortex, regardless of dementia status [Polvikoski et al 1995], and recent studies have found that the APOE isoforms differentially regulate Aβ accumulation through clearance or synthesis [Castellano et al 2011], and ε4 was the least effective in facilitating the proteolytic degradation of Aβ in the brain [Jiang et al 2008]. Alternatively, isoforms of APOE are also likely to have a dosage effect (ε4< ε3< ε2) on the formation and maintenance of synaptic function. One study has shown that the concentration of synaptic proteins in superior temporal cortex of normal brains was significantly lower in ε4 carriers [Love et al 2006]. Other mechanisms reported in the literature include the potential role of APOE in hippocampal response to neural damage [Poirior J 1994], as well as the decreased antioxidant activity of ε4 [Miyata and Smith 1996]. Further studies at the molecular level are necessary to elucidate the underlying biological mechanisms at play.
The present analyses also revealed some interesting dissociable patterns regarding the effect of different postmortem indices on the longitudinal trajectory of cognition. AD pathology, gross cerebral infarction and the presence of Lewy bodies all independently led to a faster rate of decline during the pre-terminal period. Similar to a previous report [Wilson et al 2010], this finding connects pre-terminal decline, traditionally thought to be primarily age-related, to the pathophysiological processes known to cause dementia in old age. AD pathology, gross cerebral infarction and Lewy bodies were also found to be associated with a faster rate of decline during the terminal period, and AD pathology and micro infarction were associated an earlier onset of terminal decline. Follow-up studies are required to confirm the different effects of these pathologic indices on the trajectory of cognitive aging and to clarify the underlying mechanisms.
Understanding where risk factors operate in the trajectory of cognitive decline has important public health implications. Increasing evidence suggests that interventions and treatments administered after the onset of overt dementia are insufficient to effectively modify the course of the disease or reduce the considerable pathologic changes that have accumulated in the brain. Thus, in order to effectively prevent and treat cognitive aging, we need to have a better understanding of the pathogenesis of the disease, neurobiological pathways that link various risk factors such as APOE ε4 to cognitive function, and a better understanding of where in the cognitive trajectory risk factors operate. This will allow for the administration of targeted interventions precisely when needed in the course of the disease and could inform the conduct of drug studies so they are implemented more effectively and in populations most likely to benefit.
Strengths and limitations of this study should be noted. Our data came from two longitudinal cohorts of aging and dementia that evaluate participants’ cognitive performance annually and persons in these analyses underwent evaluation for up to 18 years prior to death. Trajectories of late life cognitive change derived from these assessments have provided objective evidence in support of the terminal decline hypothesis. Availability of APOE and neuropathology data among a fairly large number of participants further provide us with unique opportunities to test hypotheses regarding the biological pathway that links genetic risk factors to clinical endophenotypes through neuropathology. The implementation of random change point models for the longitudinal cognitive data enhanced our ability to capture the different effects of risk factors along the trajectory [Yu et al 2012]. One limitation is that the subjects used in the analyses are selected and this may limit the generalizability of our findings.
Acknowledgments
We are indebted to all the participants of the Religious Order Study and Rush Memory and Aging Project, as well as the staff at the Rush Alzheimer’s Disease Center for this work. This research was supported by National Institute on Aging grants R01 AG17917, R01 AG34374, R01 AG33678, R01 AG15819, and P30 AG10161.
References
- 1.Albert M, Smith L, Scherr P, Taylor J, Evans D, Funkenstein H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batterham PJ, Bunce D, Cherbuin N, Christensen H. Apolipoprotein E ε4 and Later-Life Decline in Cognitive Function and Grip Strength. Am J Geriatr Psychiatry. 2012 Sep 19; doi: 10.1016/j.jagp.2013.01.035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003 Jan 28;60(2):246–52. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005 Sep 27;65(6):953–5. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Wilson RS, Bienias J, Berry-Kravis E, Arnold S. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005 Sep;76(9):1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Tang Y, et al. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet neurology. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and Findings from the Religious Orders Study. Curr Alzheimer Res. 2012 Jul 1;9(6):628–45. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and Findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012 Jul 1;9(6):646–63. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychologial assessment. 2. New York: Oxford University Press; 1994. [Google Scholar]
- 13.Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychology and Aging. 1999 Jun;14(2):295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- 14.Boyle PA, Buchman AS, Wilson RS, et al. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2010;34(1):43–9. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunce D, Fratiglioni L, Small BJ, Winblad B, Bäckman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004 Sep 14;63(5):816–21. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- 16.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009 Jul 16;361(3):255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caselli RJ, Dueck AC, Locke DE, Sabbagh MN, Ahern GL, Rapcsak SZ, Baxter LC, Yaari R, Woodruff BK, Hoffman-Snyder C, Rademakers R, Findley S, Reiman EM. Cerebrovascular risk factors and preclinical memory decline in healthy APOE ε4 homozygotes. Neurology. 2011 Mar 22;76(12):1078–84. doi: 10.1212/WNL.0b013e318211c3ae. Epub 2011 Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellano JM, Kim J, Stewart FR, et al. Human apoE Isoforms differentially regulate brain amyloid-beta peptide clearance. Science Translational Medicine. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang Y, Hayden KM, Norton MC, et al. Association between APOE ε4 Allele and Vascular Dementia: The Cache County Study. Dement Geriatr Cogn Disord. 2010;29(3):248–253. doi: 10.1159/000285166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper JA, Sager HJ. Incidental and intentional recall in Parkinson’s disease: an account based on diminished attentional resources. J Clin Exp Neuropsychol. 1993;15:713–731. doi: 10.1080/01688639308402591. [DOI] [PubMed] [Google Scholar]
- 21.Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ. Cognitive change and the APOE epsilon 4 allele. Nature. 2002 Aug 29;418(6901):932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- 22.Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, Verhey FR, Visser PJ. Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011 Oct;82(10):1149–56. doi: 10.1136/jnnp.2010.231555. Epub 2011 Apr 14. [DOI] [PubMed] [Google Scholar]
- 23.Ekstrom RB, French JW, Harman HH, Kermen D. Manual for Kit of Factor-Referenced Cognitive Tests. Princeton: Educational Testing Service; 1976. [Google Scholar]
- 24.Gelman A, Carlin JB, Stern HS, et al. Bayesian Data Analysis. Chapman & Hall; London: 1995. [Google Scholar]
- 25.Hall CB, Lipton R, Sliwinski M, et al. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Statistics in Medicine. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonker C, Schmand B, Lindeboom J, et al. Association between apolipoprotein E epsilon4 and the rate of cognitive decline in community-dwelling elderly individuals with and without dementia. Arch Neurol. 1998;55(8):1065–9. doi: 10.1001/archneur.55.8.1065. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 29.Kleemeier RW. Proceedings of the Social Statistics Section of the American Statistical Association. Washington, DC: American Statistical Association; 1962. Intellectual change in the senium; pp. 290–295. [Google Scholar]
- 30.Lane RM, Farlow MR. Lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer’s disease. J Lipid Res. 2005 May;46(5):949–68. doi: 10.1194/jlr.M400486-JLR200. Epub 2005 Feb 16. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Schneider JA, Bennett DA. Estimation of the mediation effect with a binary mediator. Stat Med. 2007;26(18):3398–414. doi: 10.1002/sim.2730. [DOI] [PubMed] [Google Scholar]
- 32.Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging. 2006;27(6):797–803. doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Lunn DJ, Thomas A, Best N, et al. WinBUGS -- a Bayesian modeling framework: concepts, structure, and extensibility. Statistics and Computing. 2000;10:325–337. [Google Scholar]
- 34.McArdle JJ, Wang L. Modeling age-based turning points in longitudinal life-span growth curves of cognition. In: Cohen P, editor. Applied data analytic techniques for turning points research. Routledge; 2008. [Google Scholar]
- 35.McKhann G, Drachmann D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease. Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 36.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and betaamyloid peptides. Nat Genet. 1996;14(1):55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 37.Morris J, Heyman A, Mohs R, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). 1. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 38.Mortimer JA, Snowdon DA, Markesbery WR. The Effect of APOE-ε4 on Dementia is Mediated by Alzheimer Neuropathology. Alzheimer Dis Assoc Disord. 2009;23(2):152–157. doi: 10.1097/wad.0b013e318190a855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson HE. National Adult Reading Test (NART): Test Manual. Windsor: NFER Nelson; 1982. [Google Scholar]
- 40.Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357(9251):169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 41.Nicoll JA, Savva GM, Stewart J, et al. Association between APOE genotype, neuropathology and dementia in the older population of England and Wales. Neuropathol Appl Neurobiol. 2011 Apr;37(3):285–94. doi: 10.1111/j.1365-2990.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- 42.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 43.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci. 1994 Dec;17(12):525–30. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 44.Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinistö L, Halonen P, Kontula K. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333(19):1242–7. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 45.Raven JC, Court JH, Raven J. Manual for Raven’s Progressive Matrices and Vocabulary: Standard Progressive Matrices. Oxford, UK: Oxford Psychologists Press; 1992. [Google Scholar]
- 46.Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- 47.Schiepers OJ, Harris SE, Gow AJ, et al. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17(3):315–24. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- 48.Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 49.Schneider JA, Bienias JL, Wilson RS, et al. The apolipoprotein E ε4 allele increases the odds of chronic cerebral infarction detected at autopsy in older persons. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- 50.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007 Dec 11;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 51.Smith A. Symbol Digit Modalities Test manual–revised. Los Angeles, CA: Western Psychological Press; 1982. [Google Scholar]
- 52.Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, Cruts M, Van Broeckhoven C, Breteler MM, et al. Apolipoprotein E genotype and progression of Alzheimer’s disease: the Rotterdam Study. J Neurol. 1999;246:304–308. doi: 10.1007/s004150050351. [DOI] [PubMed] [Google Scholar]
- 53.Steinerman JR, Hall CB, Sliwinski MJ, Lipton RB. Modeling Cognitive Trajectories Within Longitudinal Studies: A Focus on Elders. J Am Geriatr Soc. 2010 Oct;58 (Suppl 2):S313–S318. doi: 10.1111/j.1532-5415.2010.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weshler D. Wechsler Memory Scale-Revised Manual. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- 55.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75(12):1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Psychosomatic medicine. 2007;69(1):47–53. [Google Scholar]
- 57.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychology and aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 58.Wilson RS, Barnes LL, Krueger KR, et al. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–407. [PubMed] [Google Scholar]
- 59.Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997;54:1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 60.Yu L, Boyle PA, Wilson RS, Segawa E, Leurgans S, De Jager PL, Bennett DA. A random change point model for cognitive decline in Alzheimer’s disease and mild cognitive impairment. Neuroepidemiology. 2012 doi: 10.1159/000339365. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]