Abstract
Munc13-1 is a presynaptic active-zone protein essential for neurotransmitter release and involved in presynaptic plasticity in brain. Ethanol, butanol and octanol quenched the intrinsic fluorescence of the C1 domain of Munc13-1 with EC50s of 52 mM, 26 mM and 0.7 mM, respectively. Photoactive azialcohols photolabeled Munc13-1 C1 exclusively at Glu-582, which was identified by mass spectrometry. Mutation of Glu-582 to alanine, leucine and histidine reduced the alcohol binding two- to five-fold. Circular dichroism studies suggested that binding of alcohol increased the stability of the wild type Munc13-1 compared with the mutants. If Munc13-1 plays some role in the neural effects of alcohol in vivo, changes in the activity of this protein should produce differences in the behavioral responses to ethanol. We tested this prediction with a loss-of-function mutation in the conserved Dunc-13 in Drosophila melanogaster. The Dunc-13P84200/+ heterozygotes have 50% wild type levels of Dunc-13 mRNA and display a very robust increase in ethanol self-administration. This phenotype is reversed by the expression of the rat Munc13-1 protein within the Drosophila nervous system. The present studies indicate that Munc13-1 C1 has binding site(s) for alcohols and Munc13-1 activity is sufficient to restore normal self-administration to Drosophila mutants deficient in Dunc-13 activity.
Keywords: Munc13-1, presynaptic, protein kinase C, alcohol, diazirine, circular dichroism, photolabeling, mass spectrometry, fluorescence, homology model, alcohol self-administration, Drosophila
Introduction
The molecular mechanisms by which alcohols exert their actions remain poorly understood. Defining the target(s) is the primary step in elucidating the mechanism of alcohol action. Alcohols have been shown to alter the function of numerous types of voltage- and ligand-gated ion channels in the central nervous system and to affect multiple intracellular signaling pathways (Diamond & Gordon 1997, Harris 1999). However, no single target is well defined and fully characterized. It is believed that alcohol could act on multiple targets in the brain (Harris et al. 2008).
At relatively low concentrations, ethanol directly affects the function of many central and peripheral synapses (Liu 1999). Postsynaptically, ethanol modulates the functions of a number of neurotransmitter receptors, such as GABA, glutamate and serotonin (Diamond & Gordon 1997). Although clear effects of ethanol have been identified in postsynaptic compartments, recently there has been increasing evidence of a significant effect of ethanol on presynaptic function at concentrations well below 100 mM (Diamond & Gordon 1997). Evidence has emerged indicating that ethanol may be directly affecting synaptic transmission by altering vesicle fusion. A single amino acid polymorphism in the Munc18-SNARE complex associated protein correlates with strong differences in ethanol preference in a two-bottle choice assay (Fehr et al. 2005). When this analogous Unc18 mutation was created in the Caenorhabditis elegans, the resulting animals were resistant to both stimulatory and sedative effects of ethanol (Graham et al. 2009). This missense mutation in Unc18 also lengthened the duration of quantum release and slowed the frequency of release, indicating that ethanol may act by affecting synaptic vesicle exocytosis (Graham et al. 2009). However, the mechanism by which ethanol may affect synaptic release is unknown.
Munc13-1 is a presynaptic active zone-protein essential for synaptic vesicle fusion (Betz et al. 1997, Sassa et al. 1999) and neurotransmitter release, (Betz et al. 1998, Brose et al. 2000) and interact with both syntaxin and Munc18 proteins in mammalian brain. It is also implicated in modulating short-term presynaptic plasticity (Rosenmund et al. 2002).
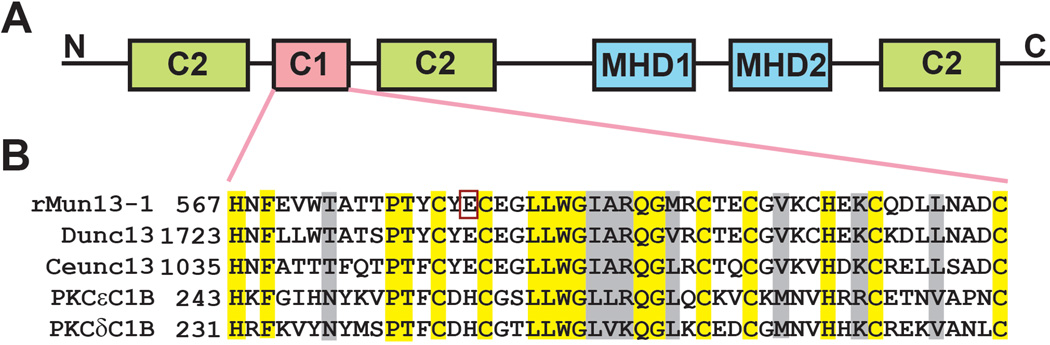
Structurally, Munc13-1 is a large ~ 200 kDa peripheral membrane protein with multiple regulatory domains. These domains include an N-terminal Ca2+ binding C2 domain, a high-affinity diacylglycerol (DAG)/phorbol ester binding C1 domain next to another C2 domain, two Munc13 homology domains (MHD) and a C-terminal C2 domain (Fig. 1) (Aravamudan et al. 1999). The binding of DAG or phorbol ester to the Munc13-1 C1 domain stimulates synaptic vesicle priming as well as the Munc13-1 translocation from the cytoplasm to the plasma membrane (Andrews-Zwilling et al. 2006). There are strong sequence similarities among the C1 domains of Munc13, Dunc-13 and Unc13 and the C1 domain of PKCs. The Munc13-1 and Dunc-13 C1 domains are particularly well conserved: 90% identical and 96% similar. Structurally, the C1 domain of the Munc13-1 proteins are similar to that of protein kinase C (PKC), both having two β-sheets, a short C-terminal α-helix and two Zn+2 binding sites (Shen et al. 2005, Zhang et al. 1995). DAG binds inside a groove formed by two “rabbit-ear” loops of Unc13, similar to the binding sites in PKCδ. The binding of DAG to the Unc13 C1 domain lowers the energy barrier for vesicle fusion and promotes neurotransmitter release (Basu et al. 2007). The H567K mutation at the beginning of the C1 domain prevents the binding of phorbol esters, thereby inhibiting the activation of vesicle fusion (Betz et al. 1998, Basu et al. 2007).
Fig. 1. Munc13-1 domains and sequences.
A) Schematic representation of the domains of Munc13-1. The C1 regulatory domain binds to lipids, diacylglycerol and phorbol esters; the C2 regulatory domain binds to anionic lipids and Ca2+; MHD1 and MHD2 represent Munc13 homology domains and suggested to binds syntaxin, the vesicle fusion protein. B) Sequence comparison of different C1 domains. Residues that are conserved in each protein are highlighted in yellow. Residues that are conserved in all family members using the BLOSUM amino acid matrix criteria (Henikoff & Henikoff 1992) are highlighted in grey. The alcohol binding residue found in the present study is marked with a red colored box.
PKCε knockout mouse consumes 75% less alcohol compared with the wild type mouse (Hodge et al. 1999). We recently identified the PKCε C1 domain as a site of alcohol binding (Das et al. 2009). In PKCε, alcohol molecules bind to the His-248 and Tyr-250 residues, resulting in the inhibition of the PKCε activity, presumably by interfering with DAG binding and activation. The binding of alcohol with the PKCε C1 domain suggested that the Munc13-1 C1 domain may likewise be a target for alcohol binding. In addition, Unc13, the C. elegans homolog of Munc13-1, has been implicated for its sensitivity to volatile anesthetics (Metz et al. 2007). To explore if alcohol-binding site could be present in other proteins expressed in brain containing structurally related C1 domains, we chose to investigate the alcohol binding to the C1 domain of Munc13-1.
In the present study, we expressed the C1 domain of Munc13-1 in E. coli, purified and studied it’s interaction with alcohols by fluorescence, photolabeling and mass spectrometry and circular dichroism spectroscopy. Our results show that ethanol, butanol and octanol interact with Munc13-1 C1 domain and the diazirine derivatives of butanol and octanol photoincorporate into a primary site consisting of Glu-16 in the C1 domain corresponding to the Glu-582 of the full-length protein. Mutation of this residue with alanine, leucine or histidine affected the alcohol binding, indicating the role of glutamate residue in alcohol binding of Munc13-1 C1. We further test the hypothesis that Munc13-1 impacts the neural effects of alcohol using Drosophila melanogaster. Flies with reduced activity of the Dunc-13 ortholog display significantly increased preference for ethanol containing food compared to wild type controls. This increased preference for ethanol is rescued by the expression of the rat Munc13-1 within the Drosophila nervous system. The functional complementation of the Dunc-13 alcohol self-administration phenotype by the vertebrate Munc13-1 indicates that this protein functions in vivo to modulate ethanol related behaviors. Together, these data point to Munc13-1 as an impactful mediator of the presynaptic effects of ethanol.
Materials and Methods
Materials
Phorbol ester (TPA) was purchased from LC Laboratories, MA; TPCK treated trypsin and chymotrypsin were obtained from Roche (Indianapolis, IN); glutathione sepharose 4B was from GE Healthcare Biosciences. All other reagents were obtained from Sigma. Protein concentration was determined using a Bradford protein assay reagent kit from BioRad Laboratories Inc., CA. The Munc13-1 C1 domain construct in pGEX-KG vector was kindly provided by Dr. Josep Rizo of the University of Texas Southwestern Medical Center at Dallas, Texas.
Site-directed mutagenesis
Site-specific mutations in the Munc 13-1 C1 domain were introduced with PCR by presenting the mutation in oligonucleotide primers. The Quickchange II site-directed mutagenesis kit (Stratagene) was utilized to carry out this experiment. Sequencing of the PCR product was performed by Seqwright (Houston, TX). Primers were designed using Sim Vector and Gene Runner and were synthesized from Sigma.
Bacterial expression and purification
The Munc 13-1 C1 domain wild-type or mutants fused with glutathione S-transferase (GST) were expressed in BL21 DE3 gold E. coli (Stratagene). The proteins were purified following the methods described earlier (Das et al. 2009)
The purity of the affinity-purified and thrombin-cleaved protein was characterized by 10% or 15% SDS-PAGE.
Fluorescence measurements
Steady-state fluorescence measurements were carried out using PTI fluorimeter (Photon Technology Instruments, Princeton, NJ). The intrinsic fluorescence of Munc13-1 C1 (WT) and mutants (E582A, E582H, and E582L) was measured as a function TPA and alcohol concentration at 25°C. Protein solutions (1 μM) in buffer containing 50 mM Tris,100 mM NaCl, pH 7.4 were excited at 280 nm and the emission spectra were recorded from 300 nm to 500 nm in the presence of varying concentration of TPA (1–40 µM) or alcohols (ethanol, 1–250 mM; butanol, 1–150 mM and octanol, 1–5 mM). TPA or alcohols were incubated with the protein for 15 minutes at room temperature before recoding the spectra. For quenching studies with alcohols, ethanol (neat), butanol (neat) and octanol (0.25 M in DMSO) were used. DMSO did not show any significant effect at this concentration. Each experiment was repeated three times, and the data were corrected for buffer background. Data were analyzed using the equation as described earlier (Das et al. 2004, Das et al. 2006). The highest emission intensity (Fmax) values were found to be 1.02×106 for WT, 1.18×106 for E582A, 0.9×106 for E582H and 0.84×106 for E582L. The data set for the wild type and mutant proteins were normalized and presented separately for each alcohol.
Mass spectrometry and circular dichroism spectroscopy
Methods are followed as described earlier (Das et al. 2009) and in the supplemental section.
Expression of mammalian Unc13-1 in Drosophila
The coding regions of the Munc13-1—EGFP fusion construct (Betz et al., 1998) was cloned it into the pBRETU vector (Roman et al. 1999). Transgenic UAS-Munc13-1-EGFP flies were generated by ry506 embryo injection. The insertion selected demonstrated strong EGFP expression throughout the brain when driven by elav-GeneSwitch (Roman et al. 2001).
Fly strains and husbandry and gene expression analysis, CAFE assay, proboscis extension reflex (PER) assay, inducing GeneSwitch activity assay and immunohistochemistry have been described in the supplemental section. Drosophila, as an invertebrate, does not require Institutional approval. The molecular work has been approved by the Institutional Biosafety Committee.
Statistical analysis
All statistical analysis on behavioral experiments were performed using Statview (SAS Inst., USA). In the CAFE assay, food consumption or preference index was analyzed using Student’s t-test, or one-way ANOVA. Bonferonni correction was performed when more than two groups were compared.
Results
Protein purification and characterization
Munc13-1 C1 is a non-kinase DAG/phorbol ester receptor (Betz et al. 1998). Munc13-1C1 (WT), E582A, E582L, E582H and E582K were expressed in E. coli. The expressed Munc13-1 C1 contained an extra twenty residues, thirteen (GSPGISGGGGGIQ) at the N terminus and eight (QDLLNADC) at the C terminus, added for the cloning purposes. While the wild-type protein and the first three mutants were purified with reasonable yield (2–5 mg/liter), the E582K could not be purified from the soluble cell extract, highlighting the fact that the substitution of a negatively charged residue to a positively charged residue affected the solubility and stability of the protein. Purified proteins were thoroughly characterized by SDS-PAGE (Fig.S1A for WT), mass spectrometry (Fig. S1B for WT), their ability to bind TPA (Fig. S1C for WT) and circular dichroism spectroscopy (Fig. S2).
Binding of alcohol with Munc13-1 C1
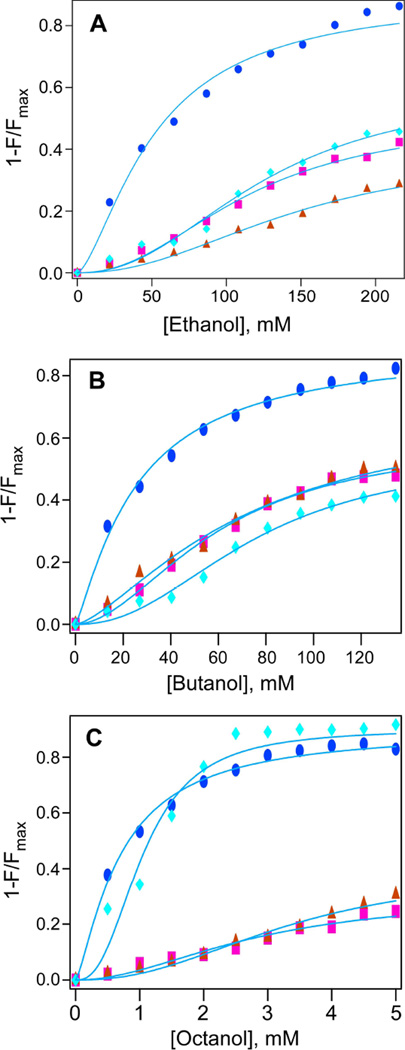
Quenching of intrinsic protein fluorescence has been used previously as a quantitative method to estimate alcohol and anesthetics binding to proteins (Das et al. 2006, Johansson 1997). Unlike the PKC subdomains C1A and C1B, which contain a single tryptophan, Munc13-1 has two tryptophans (Trp-272 and Trp-588) and two tyrosines (Tyr-579 and Tyr-581) that are responsible for its emission maximum at 347 nm, when excited at 280 nm. To measure alcohol binding, quenching of the intrinsic fluorescence of Munc13-1 C1 was examined in presence of alcohols, and EC50 values were calculated using the logistic equation as described in the “Methods” section. The EC50 values of fluorescence quenching were found to be 51.7±2.8 mM (nH=1.5) for ethanol, 25.8±1.1 mM (nH=1.2) for butanol and 0.7±0.03 mM (nH= 1.3) for octanol (Fig. 2 and Table 1). Quenching of Munc13-1 C1 fluorescence by alcohols suggested that alcohols interact with it like other homologous C1 domains of PKC (Das et al. 2004).
Fig. 2. Effect of alcohol on the intrinsic fluorescence of Munc13-1 C1 (w) and its mutants.
Plot of fluorescence intensity and alcohol concentration for A) ethanol, B) butanol, and C) octanol for the quenching of WT(●), E582A(■), E582L(▲) and E582H(♦) Curved solid lines represent nonlinear least square fits to the logistic equation (see Materials and Methods). F and Fmax are the average fluorescence intensities at 348–350 nm in the presence and absence of alcohol respectively. Protein (1µM) and varying concentration of alcohols were incubated for 15min. Then emission spectra were recorded from 300–500 nm and exciting at 280 nm.
Table 1.
Summary of binding parameters obtained from fluorescence studies.
| Protein | Ethanol | Butanol | Octanol | |||
|---|---|---|---|---|---|---|
| EC50 (mM) | nH | EC50 (mM) | nH | EC50 (mM) | nH | |
| WT | 51.7 ± 2.8 | 1.5 ± 0.1 | 25.8 ± 1.1 | 1.2 ± 0.06 | 0.7 ± 0.03 | 1.3± 0.07 |
| E582A | 113.7 ± 2.3 | 2.2 ± 0.1 | 59.9 ± 0.9 | 1.8 ± 0.06 | 2.6 ± 0.2 | 1.6 ± 0.31 |
| E582L | 148.7 ± 3.5 | 2.1 ±0.1 | 70.5 ± 1.8 | 1.4 ± 0.07 | 3.3 ± 0.06 | 2.2 ± 0.19 |
| E582H | 125.3 ± 3.6 | 2.2 ± 0.1 | 75.3 ± 1.9 | 2.2 ± 0.1 | 1.1 ± 0.07 | 1.9 ± 0.1 |
Our observation is that alcohols interact with the Munc13-1 C1 domain with EC50 values that were dependent on the alcohol-chain length. Similar effects were also observed for other alcohol-binding proteins (Das et al. 2009, Dildy-Mayfield et al. 1996, Mascia et al. 1996). The EC50 value for ethanol is 52 mM, which is higher than the blood alcohol level for social drinking (10–20 mM) but in the range for profound to severe intoxication (32–65 mM) value. The EC50 values for butanol and octanol are 26 mM and 0.7 mM, respectively, which are also higher than their respective anesthetic potencies of 10.8 mM and 57 µM (Alifimoff et al. 1989).
To determine the Munc13-1 C1-alcohol interactions site(s), we used the diazirine derivatives alcohols to photolabel the protein and determine the site of photolabeling by mass spectrometry. A similar photolabeling technique has been used earlier for detecting the alcohol- and anesthetic-binding sites of several soluble proteins (Das 2011). The photolabeling was carried out with two azialcohols, 3-azibutanol and 3-azioctanol, to identify the precise sites of alcohol binding. Like butanol and octanol, these two azialcohols also quenched the fluorescence of Munc13-1C1 (Fig S3). The diazirine analog of ethanol has not been synthesized yet because of the inherent instability associated with the molecule. 3-azibutanol acts as an excellent surrogate for aziethanol.
Photoincorporation of azialcohols into Munc13-1 C1
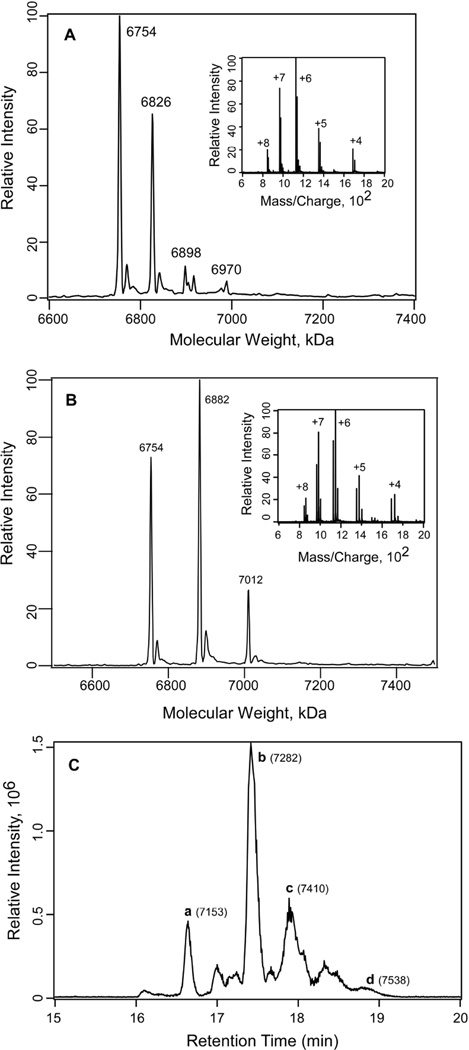
The charge envelope for the photolabeled sample of Munc13-1 C1 and 1 mM 3-azibutanol can be seen in Fig. 3A. Deconvolution of the mass/charge envelope yielded four major peaks with molecular weights 6754, 6826, 6898 and 6970 Da for the incorporation of 0, 1, 2 and 3 molecules of azibutanol, respectively. The observed difference between the two major peaks was 72 ± 1 Da, corresponding to photoincorporation of a single 3-azibutanol molecule in Munc13-1 C1. Similarly, for the azioctanol-photolabeled sample, peaks at 6754, 6882 and 7012 Da were observed. These peaks correspond to the photoincorporation of 0, 1 and 2 molecules of azioctanol to the Munc13-1 C1. In this case the observed difference between the two peaks is 128 ± 1, corresponding to the photoincorporation of a single 3-azioctanol molecule (Fig. 3B). Minor peaks observed at masses which were 16 Da higher than the main peaks could be because of the oxidation of the single methionine present in the protein (Fig. 3A & 3B). The protein–azioctanol complexes were separated after the 3-azioctanol photolabeled sample was reduced by DTT, alkylated with iodoacetamide and run through the C18 microcapillary column coupled to the mass spectrometer (Fig. 3C). The unlabeled protein, with seven cysteine residues modified with iodoacetamide (+57 Da for each cysteine modification), showed the peak at 7154 Da (a, in Fig. 3C). The peaks at 7282, 7410 and 7538 labeled as b, c, and d correspond to the incorporation of 1, 2, and 3 molecules of azioctanol respectively. This experiment demonstrated the power of the online mass spectrometric technique in separating labeled and unlabeled proteins from a mixture.
Fig. 3. Photoincorporation of azialcohols into Munc13-1 C1.
A) Deconvoluted mass spectrum of the charge envelope of Munc13-1 C1 photolabeled with 1 mM 3-azibutanol. The peaks at 7654 Da, 6826 Da, 6898 Da and 6970 correspond to the photoincorporation of 0, 1, 2 and 3 molecules of azibutanol (addition of 72 Da for each molecule) respectively, to Munc13-1 C1. Inset: the charge envelop of the photolabeled Munc13-1 C1 obtained after the sample was infused into the LTQ linear ion trap mass spectrometer. B) Deconvoluted mass spectrum of the charge envelope of Munc13-1 C1 photolabeled with 1 mM 3-azioctanol. The peaks at 7654 Da, 6882 Da and 7012 Da correspond to the photoincorporation of 0, 1 and 2 molecules of azioctanol (addition of 128 Da for each molecule) respectively to Munc13-1 C1. Inset: the corresponding charge envelop of the photolabeled Munc13-1 C1. C) LC-MS analysis of the 3-azioctanol (5 mM) photolabeled Munc13-1 C1 sample after reduction with DTT and alkylation by iodoacetamide. Total ion chromatogram for the 3-azioctanol labeled Munc13-1 C1. The peaks a, b, c and d and the corresponding masses in the bracket represent the photoincorporation of 0, 1, 2 and 3 molecules 3-azioctanol respectively to Munc13-1 C1.
Identification of photolabeled amino acid residues by LC/MS/MS
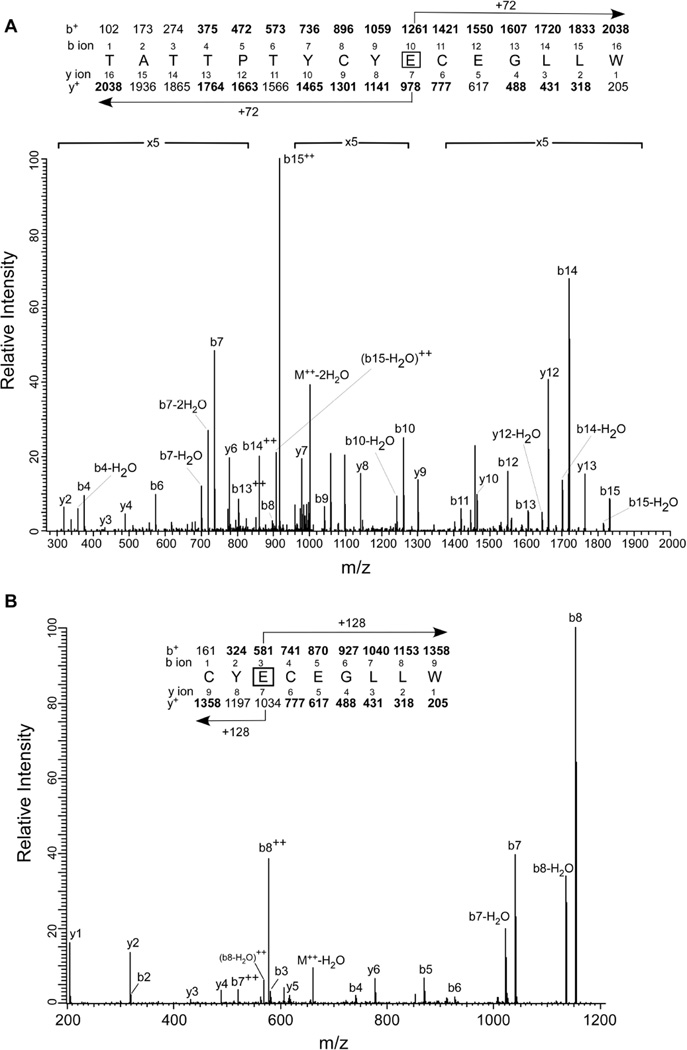
To identify the site of photolabeling, the photomixture was reduced with DTT, alkylated with iodoacetamide, digested with trypsin/chymotrypsin and analyzed by tandem mass spectrometry. Peptides generated by trypsin/chymotrypsin digestion of photolabeled Munc13-1 C1 were analyzed by LC/MS/MS. Digested samples were eluted directly from the online HPLC column into the mass spectrometer, and MS/MS spectra were acquired under automatic acquisition of the most intense ion from each MS scan. At 100 µM, 3-azibutanol labeled the Munc13-1 C1 at the Glu-582 site. No other residues were detected at this concentration, indicating that this residue was the most favored residue in the protein for photolabeling. The MS/MS spectrum of the 3-azibutanol labeled doubly charged 16-mer peptide TATTPTYCYECEGLLW at m/z 1019.6 is shown in Fig. 4A. In the b ion series, b4 through b15 (except the b5) were observed of which b10 to b15 ions have the extra mass of 72 Da for the addition of a molecule of 3-aziobutanol. In the y ion series, y2–y4, y6–y10, y12–y13 were observed. The detection of y7 and the higher y ions with an extra mass of 72 Da indicated that E10 of the peptide, i. e. E582 of the full length protein, is modified. In the doubly charged ion series, b13++, b14++ and b15++ were also detected-with b15++ having the highest intensity among all the detected ions. Several peaks corresponding to the loss of water also were observed. These doubly charged ions, with the most intense b15++, also were observed in the MS/MS data of the unlabeled peptide. These observations confirmed our assignment that the labeled residue was the E10.
Fig. 4. Identification of the photolabeled residues in Munc13-1 C1 by LC/MS/MS after tryptic digest.
A) Glu-582 as the site for 3-azibutanol: MS/MS data for the 3-azibutanol (0.5 mM) modified 16-mer peptide TATTPTYCYECEGLLW. In addition to the singly charged ions, a doubly charged b8++ (m/z 549) ion is also observed. B) Identification of Glu-582 as the site for 3-azioctanol: MS/MS data for the 3-azioctanol (3 mM) modified heptamer peptide CYECEGLLW. In addition to the singly charged ions, two doubly charged b7++ (m/z 521) and b8++ (m/z 577) ions are also observed. At the top of the each figure, the predicted charge/mass ratio of N-terminal ions (b ions) and C-terminal ions (y ions) are shown above and below the sequence, respectively. They are singly charged unless noted otherwise. The horizontal arrows show which m/z values for the b ions (above) and y ions (below) have a mass of 72 Da for 3-azibutanol and 128 Da for 3-azioctanol added to them. Observed values are shown in bold.
Furthermore, a smaller fragment of the above peptide CYECEGLLW with m/z 651.9 was detected in the digest of 3-azibutanol (1 mM)–labeled Munc13-1 C1 (Fig. S4). This peptide, with a modification at the same residue E582, also was detected in the azioctanol-labeled sample. The collision-activated dissociation MS/MS spectrum of the doubly charged, 3-azioctanol modified peptide (m/z 504) CYECEGLLW is shown in Fig. 4B. Photoincorporation of azioctanol caused a mass shift of 128 Da for all fragment ions containing the modified amino acid. All the b ions (from b1 to b6) and the y ions (y1 to y6) were observed in the MS/MS spectrum. In this case, b2 was unmodified, but b3 through b6 contained an additional mass of 128 Da. In the y ion series, all the y1–y6 ions were observed and were unmodified. This confirms that the azioctanol modification is with the glutamic acid at position 3 in the peptide. Several doubly charged ions such as y7++ (m/z 521) and y8++ (m/z 577), also were detected.
The higher efficiency of photolabeling and the use of a highly sensitive LC-MS with the capillary column have made it possible to separate the photolabeled product from the unlabeled protein. Because aliphatic diazirines tend to label amino acids with nucleophilic side chains(Das 2011), in the photolabeling experiments, the protein was titrated with azialcohol to track down the most reactive residue which was found to be the Glu-582 (Table 2). Even at as low as 100 µM, 3-azibutanol labeled the protein at this site. Even after increasing azialcohol concentrations, Glu-582 remained the major photolabeled residue along with other residues indicating that Glu-582 represents the highest affinity site for alcohols. Additional labeled residues seen at higher concentration could be either part of low affinity sites or nonspecific labeling, not unusual for photolabeling techniques.
Table 2.
Concentration dependent photolabeling of Munc13-1 C1 analyzed by mass spectrometry
| Protein | Alcohol | Concentration (mM) |
Labeled residues |
|---|---|---|---|
| Munc13-1 C1 | 3-azibutanol | 0.1 | E582 |
| 1 | E582, Y581, D615 | ||
| 5 | E582, E584, Y581, D615 | ||
| 10 | E582, Y581, E584, Y579, D615 | ||
| Munc13-1 C1 | 3-azioctanol | 0.1 | E582 |
| 1 | E582, D615 | ||
| 3 | E582, E599, D615 | ||
| 5 | E582, E599, D615 | ||
To determine the importance of the Glu-582 residue in alcohol binding, three mutant proteins E582A, E582L and E582H were designed, expressed in E. coli and purified and characterized by gel electrophoresis, mass spectrometry and CD spectroscopy. As mentioned earlier, the E582K was poorly expressed and could not be purified in significant amounts.
Effect of mutation on Munc13-1 C1 Structure
To determine the overall structure and stability of Munc13-1 C1 and the mutants, the intrinsic fluorescence emission and circular dichroism spectra were examined. The emission maxima for Munc13-1C1, E582A, E582L and E582H were 347 nm, 347 nm, 348 nm and 346 nm, respectively, indicating no significant change in the emission upon mutation at the 582 site. This finding also indicates that there was no significant conformational or structural destabilization because of point mutation made at this putative alcohol-binding region. In addition, circular dichroism spectra (shown in Supplemental Fig. S2) also suggested that the relative secondary structure remained unaltered for mutants compared with the wild type protein.
Effect of mutation of Munc13-1 C1 at the site 582 on alcohol binding and photolabeling
After establishing structural tolerance of Munc13-1C1 upon mutation at the 582 site, we studied the effect of these mutations on alcohol binding (Fig. 2). When fluorescence quenching studies were performed on the mutant proteins, the extent of fluorescence quenching was found to be much less as compared with the wild-type protein. The mutation at the 582 site caused about two- to five-fold lowering in alcohol-binding affinity compared with wild-type protein (Fig. 2 and Table 1). The chain-length dependence on the binding affinity follows the order octanol>butanol>ethanol, similar to that of the wild-type protein. For ethanol binding, the most pronounced effect was observed in E582L, where binding affinity was decreased by three-fold as compared with the wild-type protein; for octanol the decrease was five-fold. For butanol, a three-fold decrease in alcohol affinity was observed in E582H. Overall, for ethanol and octanol, alcohol affinities followed the order E582L<E582H<E582A<WT and E582L<E582A<E582H<WT; whereas, for butanol the order is E582H <E582L <E582A<WT. Because there were no significant differences in the wild-type and the mutant structures, it is safe to infer that the observed reduction in alcohol affinity is because of the point mutation only. While it is expected that, for E582A or E582L, ethanol and butanol binding would be affected much more than that of relatively more hydrophobic octanol because of the disruption of the hydrogen bond network upon mutation, the lowest affinity of octanol for E582L suggests that hydrophobicity is also an important factor in alcohol binding. Substitution of glutamate by histidine is expected to retain the hydrogen-bonding ability of the parent residue; however, it could reverse the polarity of the binding site. This is reflected in the lowest binding affinity for butanol in E582H. Therefore, this differential in affinities of three different types of alcohols for their binding to the mutant protein demonstrate the relative importance of the hydrogen bond and hydrophobic/van der Waals interactions and/or the polarity of the alcohol-binding site. Overall, our results indicate that mutations at the Glu-582 affect the alcohol binding and that there exists a subtle balance of charge and hydrophobicity in its vicinity.
In the mass spectral analysis of the photolabeled mutants E582A, E582L and H582H with 3-azibutanol (1 mM), a 100% protein sequence was detected. At 1 mM 3-azibutanol, no photoincorporation was observed at alanine, leucine or histidine at the position 582 of E582A, E582L and E582H (Table S1). However, Glu-3 was found to be labeled in the tryptic digest of 3-azibutanol-labeled mutant proteins. The doubly charged peptide CTECGVK (m/z, 463.2) was identified for E582A and E582H and the same peptide with a single charge was observed for E582L. Photoincorporation at E3 generated fragment ions b3 to b6, y5 and y6 with an additional mass of 72 Da for a molecule of 3-azibutanol. In the doubly charged peptide, all the fragment ions except for the b3 or y4 (m/z 463.2) were observed. The b3 and/or b4 have a mass identical to that of the precursor peptide and therefore, are not observed in the MS/MS spectrum. It is possible that the C4 also could be a modified residue. However, with the observation that the cysteine at position 4 was found to be modified with iodoacetamide, it is reasonable to assign E3 in the peptide or E599 in the full-length protein as the labeled residue (Fig. S5). In the singly charged peptide (m/z 925.4) for E582L, however, the b3 or y4 was observed. In all the three mutant proteins the unlabeled peptide also was identified. In addition, there are high probabilities that the Y581 in E582A, the Y579 and E584 in E582L, and the E579 and E584 in E582H are labeled. However, because of the non-observation of several fragment ions, this prediction could not be confirmed.
Is Munc13-1 a possible target of alcohol?
The fact that the alcohol-binding residue Glu-582 is conserved among the mammalian, C. elegans and Drosophila orthologs (Fig. 1B), and that the C. elegans Unc13.1 regulates anesthetic sensitivity, Munc13-1 is likely to be considered as a functional target of alcohol and anesthetics.
In order to begin testing this hypothesis, we examined the effect of reducing Dunc-13 activity in Drosophila on alcohol self-administration. If the binding of ethanol to Dunc-13 has functional consequences for neurophysiology, reducing the level of Dunc-13 should modify the level of ethanol self-administration (Xu et al. 2012). The Dunc-13P84200 allele is a loss-of-function insertion into the Dunc-13 locus (Aravamudan et al. 1999). This allele is recessive lethal, and homozygotes fail to display neurotransmission in the embryonic neuromuscular junction (Aravamudan et al. 1999). The heterozygotes have approximately 50% wild type levels of Dunc-13 mRNA (Fig. 6A). For these reasons, we examined these Dunc-13P84200/+ heterozygotes as a genetically sensitized strain for a self-administration defect.
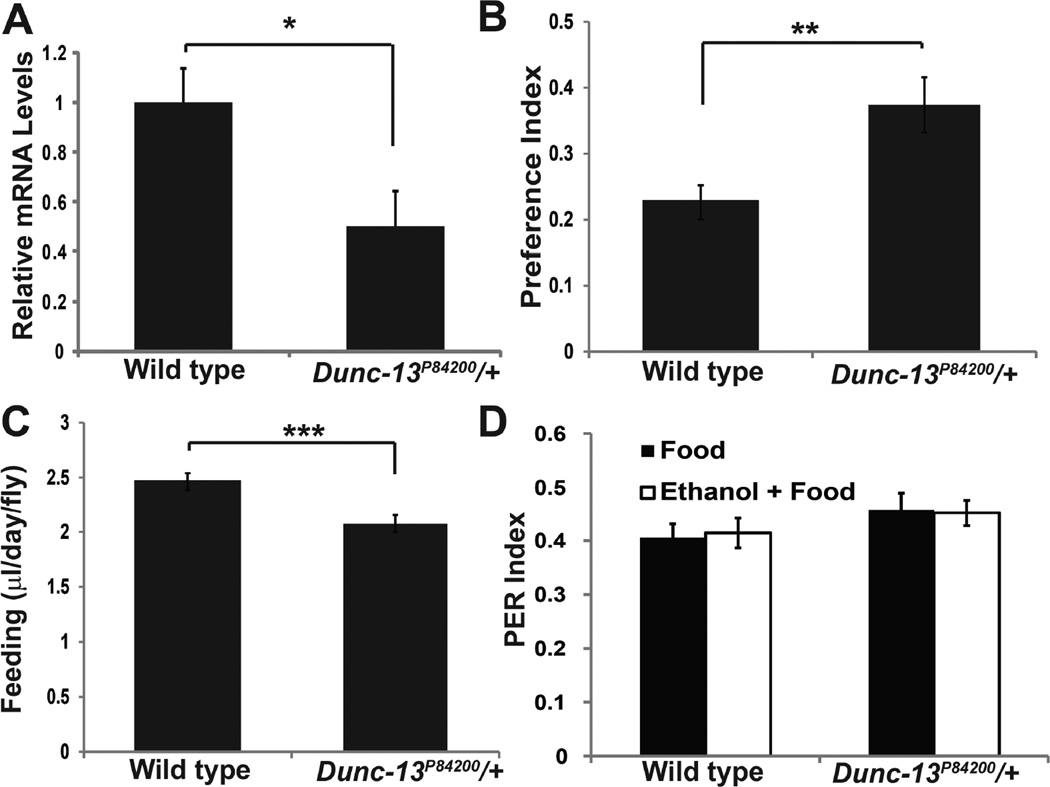
Fig. 6. Dunc-13P84200/+ heterozygotes display an increased preference for ethanol.
A. Dunc-13P84200/+ heterozygotes have 50% wild type levels of mRNA as measured by quantitativertPCR. (n = 6 each group, t(10) =2.54, * p < 0.05) B. Dunc-13P84200/+ heterozygotes were analyzed in the two-choice CAFE assay for ethanol preference. The preference index is a measure of the excess ethanol containing food consumed compared to the consumption of non-ethanol containing food (Ja et al. 2007). Dunc-13P84200/+ heterozygotes had a significantly greater preference index than wild type control Drosophila (n = 44–45 each group, t(87) =2.965, ** p < 0.005). C. The Dunc-13P84200/+ heterozygotes consume less food than the wild type control genotype (n = 44–45, t(87) = 5.597, *** p < 0.001). D. The PER elicited by liquid food or liquid food +10% ethanol were determined for both wild type controls genotype and the Dunc-13P84200/+ heterozygotes. Ethanol did not change the response in either control (t(20) = 0.235, p =0.82) or Dunc-13P84200/+ (t(19) = 0.154, p =0.88) genotypes. The difference between wild type and mutant heterozygotes was not significant (t(41) =1.686, p =0.099). n > 8 for each group.
In Drosophila, ethanol preference is measured using the CAFÉ assay, which is analogous to the rodent two-bottle choice self-administration assay (Ja et al. 2007, Devineni & Heberlein 2009, Xu et al. 2012). In this paradigm, Dunc-13P84200/+ flies display a significant increase in ethanol preference (Fig. 6B). These heterozygotes consumed slightly less liquid food overall compared to wild type control flies (Fig. 6C). The increased preference for ethanol is not due to a change in naïve gustatory salience since both the food and the food plus ethanol elicits the same proboscis extension reflex in the Dunc-13P84200/+ heterozygotes (Fig. 6D). These data support a role for Dunc-13 in modifying ethanol self-administration.
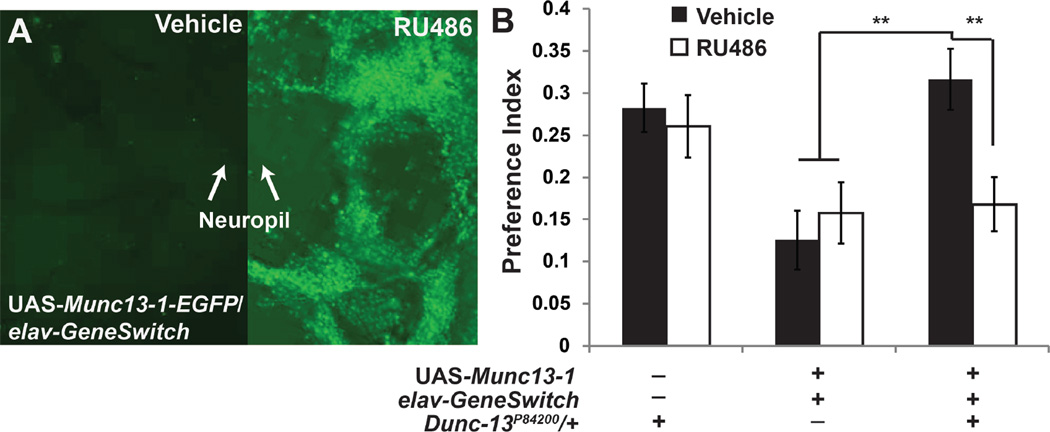
We next examined the ability of the rat Munc13-1 to functionally rescue the Dunc-13P84200/+ self-administration phenotype (Fig. 7A). In this experiment, we utilized the pan-neural elav-GeneSwitch driver and a UAS-Munc13-1-EGFP responder transgene (Roman et al. 2001). In the w+; UAS-Munc13.1-EGFP/+; elav-GeneSwitch/+ genotype, RU486 induces the transcription of the Munc13-1-EGFP fusion within the nervous system (Fig. 7A). Induction of Munc13-1–EGFP rescues the Dunc-13P84200/+ ethanol self-administration phenotype (Fig. 7B). Munc13-1-EGFP expression in a wild type control background does not measurably alter ethanol self-administration, indicating the effect of this expression on self-administration is specific to the Dunc13 haploinsufficient condition. These data indicate that Munc13-1 functionally complements Dunc-13 haploinsufficiency for ethanol preference.
Fig. 7. Munc13-1 rescues the Dunc-13P84200/+ self-administration phenotype.
A. Munc13-1-EGFP is expressed throughout the Drosophila nervous system after activating GeneSwitch with RU486. The fusion protein is detected by immunohistochemistry with anti-GFP antibodies. Most fusion protein is found within the neuronal cell bodies, but increases in Munc13-1-EGFP are also detected in the neuropil. Images were acquired under identical conditions. B. The self-administration phenotype of Dunc-13P84200/+ flies is reversed after post-developmental, pan-neural induction of Munc13-1-EGFP within the nervous system using the elav-GeneSwitch driver (Osterwalder et al. 2001). F(5,260) = 5.044. n = 44–45 for each group, ** <0.005, significant with Bonferonni correction.
Discussion
The major finding of the present study is that presynaptic Munc13-1 binds alcohol at Glu-582 of its C1 domain and its partial knock down alters alcohol self administration in Drosophila.
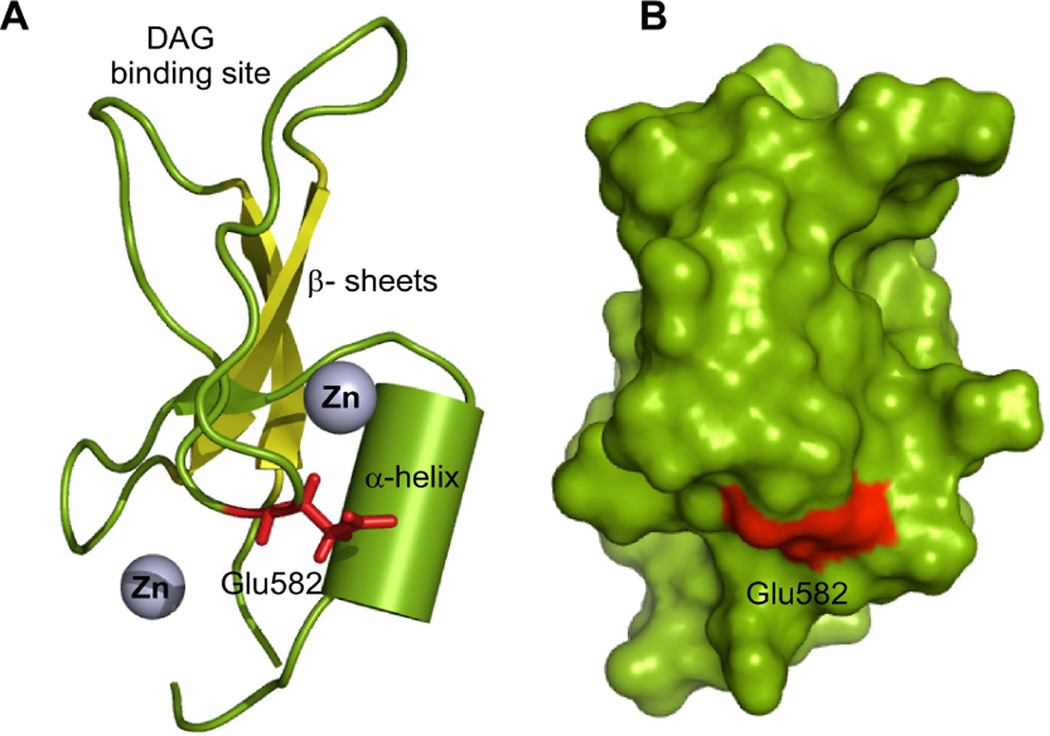
Inspection of Glu-582 in the Munc13-1 C1 structure (Shen et al. 2005) reveals that it is located near an α-helix formed by residues 608CQDLL612 and on the tip of the β-turn formed by residues 579YCYECE584 (Fig. 5). The hydrophilic Glu-582 is surrounded by hydrophobic residues such as Tyr-581, Tyr-583 of the β-turn and Leu-611, Leu-612 of the α-helix and hydrophilic residues such as Glu-584 of β-turn and Gln-609, Asp-610 of α-helix indicating that the alcohol-binding site is amphiphilic in nature (Fig. 5). Some of these residues are located within the possible interaction radius (3Å) of Glu-582, raising the possibility of formation of a hydrogen bond network. The presence of the alcohol-binding residue in a turn or loop segment that is adjacent to an α-helix is one of the characteristic features of an alcohol-binding motif (Dwyer & Bradley 2000). The alcohol molecule could be involved in polar and non-polar interaction within an amphiphilic binding cavity, including the presence of weak hydrogen-bond interaction with an amino acid and a water molecule (Dwyer & Bradley 2000). Detection of several photolabeled residues in the loop 579YCYECE584, other than Glu-582 (Table 2), is another indication for the existence of this alcohol-binding motif. In addition to this, our observation that the increase in melting temperature (monitored at 222 nm) in the presence of alcohols (Fig S6, Table S2) suggests that there may be a change in the α-helix conformational state and azialcohol labeling resulting in the multiple residues of the loop and the helix including Glu-582.
Fig. 5. Putative alcohol-binding site in Munc 13 C1.
Location of Glu-582 in the C1 domain. A) Ribbon diagram showing of the Munc13-1 C1 domain and alcohol-binding residue Glu-582 (red). α-helix important for alcohol recognition are depicted as a cylinder (split pea). Two zinc atoms that coordinated with cysteine and histidine residues are shown as spheres (grey). The β-sheets, important structural element are shown in yellow color. B) Surface diagram of Munc13-1 C1 showing the photolabeled residue Glu-582 (red). The models are generated using the solution structure of Munc13-1 C1 (Shen et al. 2005) and PyMol molecule visualization software.
Glu-582 is not a conserved residue among the proteins containing C1 domains (Fig. 1B). While for PKCεC1B the alcohol binding site is composed of His-248 and Tyr-250, for PKCδC1B it is the Tyr-236, which is homologous to His-248 of PKCε. The 1.3Å crystal structure of PKCδC1B-cyclopropane methanol complex (Shanmugasundararaj et al. 2012) revealed that the alcohol-binding groove consists of Tyr-236, Met-239 and Ser-240. The hydroxyl group of cyclopropane methanol is hydrogen-bonded to the nearby Tyr-236 and a water molecule with distances of 2.8Å and 3.0Å, respectively. Meanwhile, the methylene groups of cyclopropane ring interact (van der Waals interactions) with the methylene groups of Met-239 and Ser-240. Although Glu-582 in Munc13-1 and Tyr-236 in PKCδC1B may be involved in a similar type of hydrogen bond and similar hydrophobic interactions, a distinct difference is that-unlike in Munc13-1, where the zinc atom is only 3.3 Å away from the Glu-582, the closest distance between the zinc atom and Tyr-236 in PKCδC1B is about 13.8 Å, which rules out any possibility of zinc-alcohol interactions. The other difference is that-whereas Glu-582 in Munc13-1C1 is located far from the phorbol ester binding loops-Tyr-236 in PKCδ is located close to it.
The involvement of glutamic acid in alcohol binding previously has been highlighted for several proteins, including Glu-262 for acetylcholine receptor (Pratt et al. 2000); Glu-33 for L1 cell adhesion molecule (Arevalo et al. 2008); Glu-163 and Glu-193 for Rho GDP dissociation inhibitor (Ho et al. 2008); Glu-146 for lignin peroxidase (Ambert-Balay et al. 1998); and Glu-13 for pepsin (Andreeva et al. 1984).
Recent studies indicate that Munc13-1 interacts with other proteins, such as Munc 18 and PKC directly or indirectly in regulating vesicle fusion and trafficking (Guan et al. 2008, Betz et al. 1997, Rizo & Rosenmund 2008). That PKC (Newton & Ron 2007, Qi et al. 2007) and Munc 18/Unc18 (Graham et al. 2009) are known to regulate alcohol actions and interact with Munc13-1 directly or indirectly, Munc13-1 can act as a key component in the protein milieu regulating the alcohol’s action in the synapse. Furthermore, the close proximity of Glu-582 to the His-567 (8.8Å), which is known to affect the vesicle transfer and neurotransmitter release in presynaptic neurons (Betz et al. 1998), indicate that alcohol binding could also affect these processes.
A role for Munc13-1 as an effector of alcohol’s presynaptic actions is supported by the Dunc-13 haploinsufficiency for ethanol self-administration. Dunc-13 has a conserved function in regulating presynaptic release and the C1 domain of Mun13-1 and Dunc-13 are strongly conserved (Aravamudan et al. 1999). Ethanol preference for Drosophila is thought to arise from the hedonistic properties of this drug (Devineni & Heberlein 2009, Devineni & Heberlein 2010, Xu et al. 2012). The functional complementation of the reduced Dunc-13 activity by the rat Munc13-1-EGFP fusion protein strongly suggests that the increased ethanol preference found in the Dunc-13P84200/+ heterozygotes is due to the reduced Dunc-13 activity. Hence, Dunc-13 activity regulates ethanol self-administration. Interestingly, even though a reduction of Dunc-13 leads to an increase in ethanol preference, the reversal of this phenotype depends on the activity on Munc13-1, suggesting that Drosophila can be used as an in vivo model for measuring Munc13-1 activity and the impact of ethanol binding to the C1 domain.
Supplementary Material
Acknowledgements
We thank Dr. J. Rizo of the University of Texas Southwestern Medical Center for providing the Munc13-1 C1 construct and Dr. N. Brose of Max Planck Institute, Germany for providing the EGFP-Munc13 construct. This work was funded by NIH 7R21AA016140 and the start-up fund from the University of Houston.
Abbreviations
- GABA
gamma amino butyric acid
- DAG
diacylglycerol
- PKC
protein kinase C
- TPA
12-O-tetradecanoylphorbol 13-acetate
- PCR
polymerase chain reaction
- CD
circular dichroism
- SAVS
Structural Analysis and Verification Server
- SDS-PAGE
sodium dodecyl sulfonate-polyacrylamide gel electrophoresis
- GRP
Ras guanyl-releasing protein
- GPCR
G-protein coupled receptor
- Tm
melting temperature
Footnotes
The authors have no conflict of interest.
References
- Alifimoff JK, Firestone LL, Miller KW. Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br. J. Pharmacol. 1989;96:9–16. doi: 10.1111/j.1476-5381.1989.tb11777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambert-Balay K, Fuchs SM, Tien M. Identification of the veratryl alcohol binding site in lignin peroxidase by site-directed mutagenesis. Biochem. Biophys. Res. Commun. 1998;251:283–286. doi: 10.1006/bbrc.1998.9454. [DOI] [PubMed] [Google Scholar]
- Andreeva NS, Zdanov AS, Gustchina AE, Fedorov AA. Structure of ethanol-inhibited porcine pepsin at 2-A resolution and binding of the methyl ester of phenylalanyl-diiodotyrosine to the enzyme. J. Biol. Chem. 1984;259:11353–11365. [PubMed] [Google Scholar]
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J. Biol. Chem. 2006;281:19720–19731. doi: 10.1074/jbc.M601421200. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat. Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- Arevalo E, Shanmugasundararaj S, Wilkemeyer MF, Dou X, Chen S, Charness ME, Miller KW. An alcohol binding site on the neural cell adhesion molecule L1. Proc. Natl. Acad. Sci. USA. 2008;105:371–375. doi: 10.1073/pnas.0707815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Betz A, Brose N, Rosenmund C. Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. J. Neurosci. 2007;27:1200–1210. doi: 10.1523/JNEUROSCI.4908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J. Biol. Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr. Opin. Neurobiol. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Das J. Aliphatic diazirines as photoaffinity probes for proteins: recent developments. Chem. Rev. 2011;111:4405–4417. doi: 10.1021/cr1002722. [DOI] [PubMed] [Google Scholar]
- Das J, Addona GH, Sandberg WS, Husain SS, Stehle T, Miller KW. Identification of a general anesthetic binding site in the diacylglycerol-binding domain of protein kinase Cdelta. The J. Biol. Chem. 2004;279:37964–37972. doi: 10.1074/jbc.M405137200. [DOI] [PubMed] [Google Scholar]
- Das J, Pany S, Rahman GM, Slater SJ. PKC epsilon has an alcohol-binding site in its second cysteine-rich regulatory domain. Biochem. J. 2009;421:405–413. doi: 10.1042/BJ20082271. [DOI] [PubMed] [Google Scholar]
- Das J, Zhou X, Miller KW. Identification of an alcohol binding site in the first cysteine-rich domain of protein kinase Cdelta. Protein Sci. 2006;15:2107–2119. doi: 10.1110/ps.062237606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr. Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Addiction-like behavior in Drosophila. Communicative & integrative biology. 2010;3:357–359. doi: 10.4161/cib.3.4.11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol. Rev. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Mihic SJ, Liu Y, Deitrich RA, Harris RA. Actions of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. Br. J. Pharmacol. 1996;118:378–384. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DS, Bradley RJ. Chemical properties of alcohols and their protein binding sites. Cell. Mol. Life Sci. 2000;57:265–275. doi: 10.1007/PL00000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Shirley RL, Crabbe JC, Belknap JK, Buck KJ, Phillips TJ. The syntaxin binding protein 1 gene (Stxbp1) is a candidate for an ethanol preference drinking locus on mouse chromosome 2. Alcohol. Clin. Exp. Res. 2005;29:708–720. doi: 10.1097/01.alc.0000164366.18376.ef. [DOI] [PubMed] [Google Scholar]
- Graham ME, Edwards MR, Holden-Dye L, Morgan A, Burgoyne RD, Barclay JW. UNC-18 modulates ethanol sensitivity in Caenorhabditis elegans. Mol. Biol. Cell. 2009;20:43–55. doi: 10.1091/mbc.E08-07-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Dai H, Rizo J. Binding of the Munc13-1 MUN domain to membrane-anchored SNARE complexes. Biochemistry. 2008;47:1474–1481. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- Harris RA. Ethanol actions on multiple ion channels: which are important? Alcohol. Clin. Exp. Res. 1999;23:1563–1570. [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. Ethanol's molecular targets. Sci. Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. U S A. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C, Shanmugasundararaj S, Miller KW, Malinowski SA, Cook AC, Slater SJ. Interaction of anesthetics with the Rho GTPase regulator Rho GDP dissociation inhibitor. Biochemistry. 2008;47:9540–9552. doi: 10.1021/bi800544d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat. Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JS. Binding of the volatile anesthetic chloroform to albumin demonstrated using tryptophan fluorescence quenching. J. Biol. Chem. 1997;272:17961–17965. doi: 10.1074/jbc.272.29.17961. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hunt WA. The Drunken Synapse: Studies of Alcohol-Related Disorders. New York: Klewer Academic/Plenum Publishing Corp.; 1999. [Google Scholar]
- Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br. J. Pharmacol. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz LB, Dasgupta N, Liu C, Hunt SJ, Crowder CM. An evolutionarily conserved presynaptic protein is required for isoflurane sensitivity in Caenorhabditis elegans. Anesthesiology. 2007;107:971–982. doi: 10.1097/01.anes.0000291451.49034.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Ron D. Protein kinase C and alcohol addiction. Pharmacol. Res. 2007;55:570–577. doi: 10.1016/j.phrs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt MB, Husain SS, Miller KW, Cohen JB. Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J. Biol. Chem. 2000;275:29441–29451. doi: 10.1074/jbc.M004710200. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, et al. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J. Biol. Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, He J, Davis RL. New series of Drosophila expression vectors suitable for behavioral rescue. BioTechniques. 1999;27:54–56. doi: 10.2144/99271bm09. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Sigler A, Augustin I, Reim K, Brose N, Rhee JS. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron. 2002;33:411–424. doi: 10.1016/s0896-6273(02)00568-8. [DOI] [PubMed] [Google Scholar]
- Sassa T, Harada S, Ogawa H, Rand JB, Maruyama IN, Hosono R. Regulation of the UNC-18-Caenorhabditis elegans syntaxin complex by UNC-13. J. Neurosci. 1999;19:4772–4777. doi: 10.1523/JNEUROSCI.19-12-04772.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundararaj S, Das J, Sandberg WS, Zhou X, Wang D, Messing RO, Bruzik KS, Stehle T, Miller KW. Structural and functional characterization of an anesthetic binding site in the second cysteine-rich domain of protein kinase cdelta(*) Biophys. J. 2012;103:2331–2340. doi: 10.1016/j.bpj.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N, Guryev O, Rizo J. Intramolecular occlusion of the diacylglycerol-binding site in the C1 domain of munc13-1. Biochemistry. 2005;44:1089–1096. doi: 10.1021/bi0476127. [DOI] [PubMed] [Google Scholar]
- Xu S, Chan T, Shah V, Zhang S, Pletcher SD, Roman G. The propensity for consuming ethanol in Drosophila requires rutabaga adenylyl cyclase expression within mushroom body neurons. Genes Brain Behav. 2012;11:727–739. doi: 10.1111/j.1601-183X.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Kazanietz MG, Blumberg PM, Hurley JH. Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.