Abstract
Objective
To assess chlorhexidine absorption and skin tolerability in premature infants following skin antisepsis with 2% aqueous chlorhexidine gluconate (CHG) prior to peripherally inserted central catheter (PICC) placement.
Study Design
Neonates less than 32 weeks gestation had skin cleansed with CHG prior to PICC placement. CHG concentrations were measured on serial blood samples. Skin integrity was evaluated for 2 weeks after CHG exposure.
Results
Twenty infants were enrolled; median gestational age 28 2/7 weeks (range 24 3/7–31 4/7). Ten infants had detectable serum chlorhexidine concentrations (range 1.6–206 ng/ml). Seven of these infants had their highest serum concentration 2 to 3 days following exposure. No CHG-related skin irritation occurred in any infant.
Conclusion
CHG was detected in the blood of preterm infants receiving CHG skin antisepsis for PICC insertion. Highest serum concentrations occurred 2 to 3 days after exposure. Further investigation is needed to determine the clinical relevance of CHG absorption in preterm infants.
Keywords: Chlorhexidine gluconate, prematurity, neonates, drug safety, drug toxicity
Introduction
Chlorhexidine gluconate (CHG) is a topical antiseptic widely used for skin antisepsis in adults and older children (1). The Centers for Disease Control and Prevention recommend cleaning the skin with chlorhexidine before placement of central venous catheters because numerous studies have demonstrated reduced catheter infection rates following CHG skin preparation compared with alternatives such as povidone iodine (2). However, “no recommendation can be made for the safety or efficacy of chlorhexidine in infants aged <2 months” due to limited safety data (2).” Despite this, CHG is frequently used in Neonatal Intensive Care Units (NICU) (3). The U.S. Food and Drug Administration (FDA) recently approved changes to the safety labeling of chlorhexidine products for use in infants, now stating “use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns (4).” It is not known how this labeling change will impact the use of CHG in neonates.
Trace amounts of CHG can be absorbed through the skin of adults and term neonates (5–11). There are no reported adverse consequences as a result of this absorption (12). Preterm infants, especially in the first 2 weeks of life, may be at increased risk of skin irritation and absorption due to immature skin with increased permeability. The objective of this study was to assess CHG absorption into the bloodstream following skin antisepsis for central venous catheter placement in preterm infants.
Methods
Subjects
This pilot study was performed to measure CHG absorption into the blood of preterm infants following topical exposure. The Institutional Review Board approved the study. Informed consent was obtained from the parents of participating infants. Eligible subjects included any infant born less than 32 weeks gestation admitted to the Johns Hopkins Hospital NICU or the Johns Hopkins Bayview Medical Center NICU who was anticipated to have a PICC placed while the infant was at least 48 hours old and less than 14 days of age. Infants were excluded if they had a preexisting skin condition, significant congenital anomalies, or a planned PICC insertion in the scalp. The study was performed under an investigator-held U.S. FDA investigational new drug license (IND 99,754).
Skin Preparation
Enrolled infants had their skin cleansed prior to placement of a PICC with a 2% aqueous CHG-impregnated cloth (Sage Products Inc., Cary, IL, USA). Each cloth contains 500 mg CHG. The infant’s extremity used for PICC insertion was first washed with soap (Gentle Rain® Extra Mild, Coloplast Corp., Minneapolis, MN, USA) and water as part of our standard protocol. A CHG cloth was folded into quarters and one quarter was used to cleanse the infant’s extremity in order to limit the total dose exposure. The extremity was cleansed with the CHG cloth using an up and down motion. The skin site was then allowed to dry for one minute prior to PICC insertion attempt. The CHG was not wiped or washed off of the skin prior to PICC insertion attempt. The nurse placing the PICC changed sterile gloves after cleansing the skin with the CHG cloth and before PICC insertion to prevent cross contamination of the PICC with CHG. If the PICC attempt on the first extremity was unsuccessful, a second extremity was cleansed with a new CHG cloth in the same fashion for a subsequent PICC insertion attempt. The second attempt had to be within 1 hour of the first PICC attempt if the CHG cloth was also used on the second extremity. No more than two extremities on any infant were exposed to CHG. If two PICC attempts were unsuccessful, subjects were considered exposed and maintained in the study, but the standard povidone-iodine skin preparation was used for any further PICC insertion attempts. Of note, CHG is not used in our NICUs for any purpose in preterm infants who are less than 4 weeks of age.
Skin Assessment
The skin site or sites exposed to CHG were inspected daily by the bedside nurse through the transparent PICC dressing and assessed for any erythema or irritation. In addition, a study team member inspected the site at least once in the first 48 hours after exposure, and at least once in the 72 hours following the first inspection. Skin sites were monitored until the first catheter dressing change or for 14 days if catheter insertion attempt was unsuccessful at that site. A contact dermatitis score was assigned daily for each skin site using a five point scale (7). A study team member was notified for any skin erythema or irritation and a skin reaction form was completed. If any erythema was noted, its size and location were documented and then monitored daily by a study team member.
Data and Sample Collection
Data collected on each infant included gestational age, birth weight, gender, date and time of CHG exposure and catheter insertion attempt, number of skin sites exposed, catheter location, number attempts of catheter placement, and laboratory values including AST, ALT and creatinine both before and 3–5 days after CHG exposure. Infants were followed for any evidence of a bloodstream infection until their PICCs were removed.
Blood samples were collected 1–2 hours and 6–12 hours after CHG exposure. Residual samples from routine blood draws before CHG exposure and >48 hours after CHG exposure were also collected. Samples were stored at −80 degrees Celsius and analyzed in two groups. An interim analysis was requested by the FDA and performed on Group 1 (11 infants). Following this interim analysis, an additional blood sample was drawn at 48 hours after CHG exposure in Group 2 (9 infants) and a residual sample was collected >72 hours after exposure. To prevent cross-contamination of blood samples with residual CHG on the skin, blood was drawn from an extremity that was not exposed to CHG.
Serum Chlorhexidine Gluconate Concentrations
Serum CHG concentrations were determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (William Clarke, PhD, Johns Hopkins University School of Medicine). Samples from Group 1 infants were analyzed using a 3-point calibration curve. The limit of quantitation was 12.5 ng/ml using a 0.25 ml sample of whole blood. Based on concentrations detected in Group 1 infants, samples from Group 2 infants were analyzed using a 6-point calibration curve with a limit of quantitation of 1.0625 ng/ml using a 0.25 ml sample of whole blood.
Results
Twenty preterm neonates were exposed to CHG and 11 were male. The median gestational age was 28 2/7 weeks (range 24 3/7 to 31 4/7) and median birth weight was 925 grams (range 630–1735). The median chronologic age (CA) at time of exposure was 5 days (range 2–11). None of the pre-exposure samples had chlorhexidine detected. A total of 64 post-exposure blood samples were analyzed. Overall, 10 of the 20 infants had detectable chlorhexidine concentrations in their blood; 4 infants in Group 1 and 6 infants in Group 2 (Table 1). In Group 1, 5 of 30 samples had detectable chlorhexidine (Table 1) and concentrations ranged from 16 to 274 ng/ml with a limit of quantitation of 12.5 ng/ml. In Group 2, 13 of 34 samples had detectable chlorhexidine (Table 2) and concentrations ranged from 1.6 to 54.4 ng/ml with a limit of quantitation of 1.0625 ng/ml. For 7 of the 10 infants with detectable chlorhexidine concentrations, their highest measured concentrations occurred between 2 to 3 days after exposure (Figures 1A and 1B). There was no correlation between chlorhexidine serum concentrations and gestational age, birth weight or chronologic age at time of exposure.
Table 1.
Chlorhexidine Gluconate (CHG) Concentrations in 20 Preterm Infants <32 weeks gestation
| Clinical Characteristics | CHG concentration (ng/ml)1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||||
| Subject # | Gestational Age (weeks) | Birth Weight (grams) | Chronologic age at time of exposure (days) | Sex | Number of Extremities Exposed | Pre- exposure | 1–2 hrs | 6–12 hrs | 48 hrs | >48 hrs | >72 hrs |
| 1 | 26 0/7 | 740 | 11 | M | 1 | ND | ND | ND | --- | ND | --- |
| 2 | 27 2/7 | 750 | 3 | M | 1 | ND | ND | 104 | --- | 274 | --- |
| 3 | 27 4/7 | 970 | 5 | F | 2 | ND | ND | ND | --- | QNS | --- |
| 4 | 27 6/7 | 800 | 6 | F | 1 | ND | ND | 16 | --- | QNS | --- |
| 5 | 27 6/7 | 1070 | 7 | F | 1 | ND | ND | ND | --- | ND | --- |
| 6 | 28 4/7 | 770 | 7 | F | 1 | ND | ND | ND | --- | ND | --- |
| 7 | 29 0/7 | 630 | 3 | F | 1 | ND | ND | ND | --- | ND | --- |
| 8 | 29 5/7 | 1460 | 5 | F | 1 | ND | ND | 18 | --- | ND | --- |
| 9 | 30 0/7 | 1510 | 4 | M | 1 | ND | ND | ND | --- | 206 | --- |
| 10 | 31 0/7 | 1480 | 4 | F | 1 | ND | ND | ND | --- | ND | --- |
| 11 | 31 4/7 | 1540 | 8 | F | 1 | ND | ND | ND | --- | QNS | --- |
| Group 2 | |||||||||||
| 12 | 24 3/7 | 720 | 5 | M | 1 | ND | 18.9 | 8 | ND | --- | 42.8 |
| 13 | 25 2/7 | 820 | 5 | M | 1 | ND | ND | ND | ND | --- | 2 |
| 14 | 25 3/7 | 880 | 5 | M | 2 | SNA | SNA | ND | ND | --- | ND |
| 15 | 27 3/7 | 710 | 7 | F | 2 | ND | 17.7 | 6 | 4.7 | --- | 54.4 |
| 16 | 28 1/7 | 700 | 2 | M | 1 | ND | ND | ND | ND | --- | ND |
| 17 | 29 4/7 | 1350 | 3 | M | 1 | ND | ND | ND | 7.8 | --- | QNS |
| 18 | 30 0/7 | 1735 | 2 | M | 1 | ND | ND | ND | ND | --- | SNA |
| 19 | 30 2/7 | 1150 | 3 | M | 1 | ND | 17.1 | ND | 3.6 | --- | 21.2 |
| 20 | 31 3/7 | 1030 | 7 | M | 1 | QNS | 1.6 | ND | ND | --- | ND |
ND – Not detected, QNS- Quantity not sufficient; SNA – Sample not available for analysis.
Limit of Quantitation (LOQ) was 12.5 ng/ml for infants in Group 1 and 1.0625 ng/ml for infants in Group 2.
Figure 1.
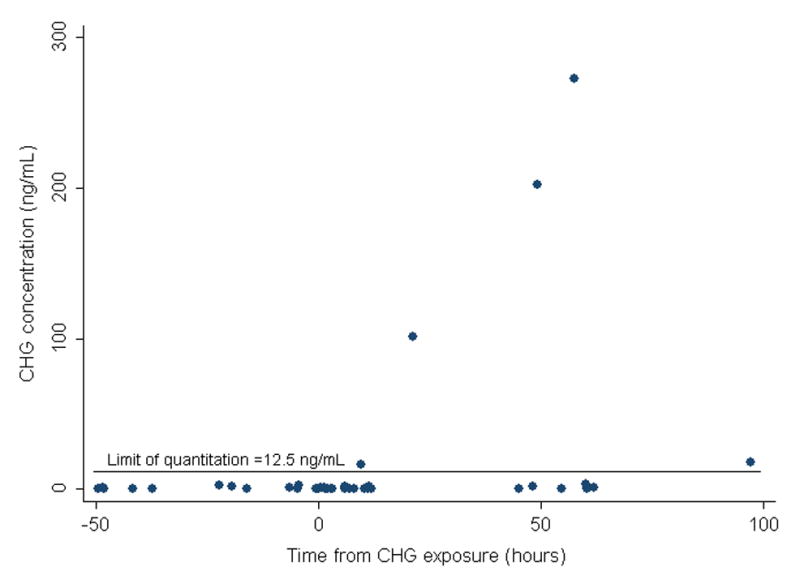
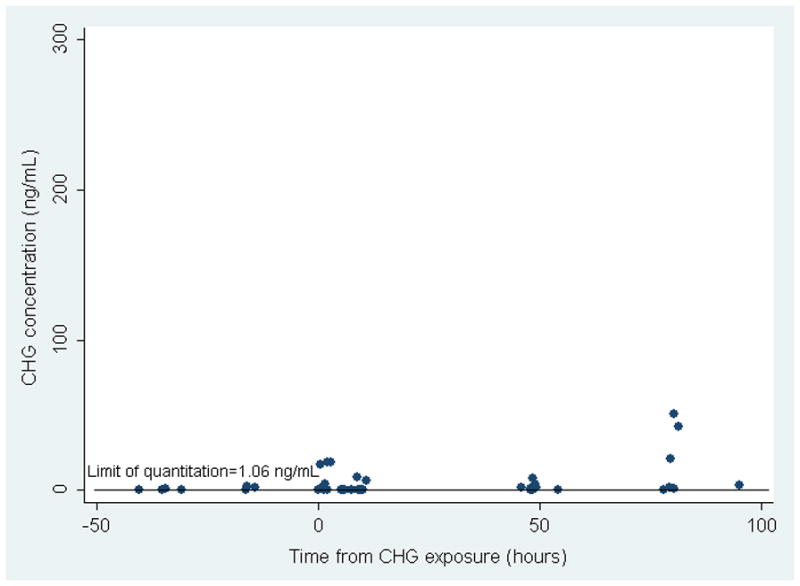
Figure 1A. CHG Serum Concentrations in 11 Preterm Infants in Group 1 at Varying Times from CHG Exposure.
The solid line depicts the Limit of Quantitation (LOQ) which was calculated to be 12.5 ng/ml for infants in Group 1.
Figure 1B. CHG Serum Concentrations in 9 Preterm Infants in Group 2 at Varying Times from CHG Exposure.
The solid line depicts the Limit of Quantitation (LOQ) which was calculated to be 1.0625 ng/ml for infants in Group 2.
Only 3 infants had 2 PICC placement attempts with 2 extremities exposed to CHG. The remaining 17 infants had only one extremity exposed and one PICC placement attempt. Two of the 3 infants with 2 extremities exposed had no detectable chlorhexidine concentrations and one had detectable concentrations at all time points following exposure. PICC placement was not successful in 4 of the 20 infants. Three of these infants had no detectable concentrations, including 2 who had 2 PICC attempts and 2 extremities exposed, and the fourth infant had the highest reported levels after only one extremity was exposed.
There was no evidence of increased transaminases (AST and ALT) following CHG exposure. One infant had an increase in creatinine from 0.9 mg/dl pre-exposure to 1.6 mg/dl 4 days after exposure, however this infant had been treated for sepsis and hypotension just prior to CHG exposure and subsequently developed acute renal failure with anuria and reversal of diastolic flow in the left renal artery. None of the other 19 infants showed increased creatinine levels from pre- to post-exposure.
There were no catheter-associated bloodstream infections in any infant. One infant born to a Group B Streptococcus (GBS) positive mother developed late-onset GBS bacteremia 13 days after PICC placement and CHG exposure. One infant had a positive blood culture for Coagulase-negative Staphylococcus 7 days after PICC placement. The blood culture was drawn secondary to cardiorespiratory events and a repeat blood culture prior to starting antibiotics was negative.
There was no evidence of CHG-related skin toxicity in any infant. One infant had a small area of mild erythema just lateral to the bioclusive PICC dressing, but not under the dressing or on any other surrounding skin that had been exposed to CHG. This infant required multiple PICC dressing changes within the first few hours after PICC placement secondary to line positioning and irritation was thought to be secondary to repeated bioclusive dressing removal.
Discussion
Our results show that CHG is absorbed into the bloodstream of some preterm infants after a single exposure to CHG and that higher concentrations for an individual infant tend to occur 2 to 3 days after exposure. There are only 3 other published studies documenting CHG absorption in preterm infants less than 32 weeks gestation after topical exposure (5–7). Collectively these 3 studies report chlorhexidine concentrations in 17 preterm infants less than 32 weeks gestation with concentrations ranging from 0 to 214 ng/ml (13). However, none of these infants were less than 27 weeks gestation and only 5 had levels drawn within the first 2 weeks of age. The current study provides absorption and tolerability data for infants less than 32 weeks gestation exposed to CHG within the first two weeks of life.
Our study and previous studies in preterm infants used different CHG concentrations and formulations, different methods of exposure (e.g. whole body bathing vs. umbilical cord application), different laboratory assays for detecting chlorhexidine, and a varied number of exposures making it difficult to compare the studies. Despite these differences, all studies found low concentrations of chlorhexidine in the blood of preterm infants following topical exposure.
While there is evidence that CHG can be absorbed following topical exposure in neonates, there are no data on the clinical relevance of trace CHG absorption. There are no safety data exploring what is a tolerable blood chlorhexidine concentration and no data demonstrating above what level there may be adverse consequences. There are several in vitro studies demonstrating toxicity to various cell lines following direct exposure to CHG (13). One in vitro study reports L1-mediated neurite outgrowth inhibition after direct exposure to CHG concentrations that have been detected in the bloodstream of neonates following topical exposure(14). The in vivo implication of these in vitro studies is unclear. Lethal doses of chlorhexidine following oral ingestion and intravenous administration have been reported in animals (15), however there are no reported serum concentrations of chlorhexidine that correspond with these lethal doses. There are several studies in full term infants and adults demonstrating safety and tolerance after chlorhexidine exposure and no reports of adverse consequences following chlorhexidine absorption (12). Preterm infants have developing neurologic systems and immature drug clearance, potentially increasing their risk for adverse consequences and lowering the chlorhexidine concentration at which these consequences may occur. To date, all studies reporting lack of adverse outcomes rely on short-term hospital outcomes. The long-term impact of CHG absorption has not been examined.
Our study does suggest that there could be delayed or ongoing, cumulative absorption of CHG from the skin of preterm infants. We observed that most infants with detectable serum chlorhexidine concentrations had their highest measured concentrations 2 to 3 days after exposure. Chlorhexidine is a cationic biguanide that strongly binds to protein in the outermost layer of the skin, which contributes to its effectiveness as an antimicrobial agent (16). Hexachlorophene, another antimicrobial agent that leaves a residue on the skin, has also been shown to have delayed absorption in neonates (17). A study evaluating the absorption of hexachlorophene in preterm infants after a single whole body application of pHisoHex (Sanofi-Adventis, Bridgewater, NJ, USA) on the first day of life demonstrated peak blood levels of hexachlorophene 2 to 4 days after exposure (17). In addition, these levels were found to be much higher than the levels observed in full-term infants who underwent the same application of pHisoHex (17). Whether washing off the CHG after application decreases absorption while maintaining adequate antimicrobial properties is unknown.
Skin irritation is the most common reported adverse event after CHG exposure (13). Preterm infants born less than 34 weeks gestation have immature skin that takes 2 to 3 weeks to mature to that of a term infant (18). This ineffective epidermal barrier leaves these infants susceptible to skin damage and absorption from potentially harmful substances (19). Several cases of skin burns in preterm infants 24 to 26 weeks gestation have been reported after exposure to alcohol-based CHG preparations (20–23). There have also been case reports of skin burns and skin irritation in preterm infants less than 48 hours of age after exposure to aqueous-based CHG solutions (24, 25). In our study, we used a 2% aqueous-CHG cloth and did not find evidence of any CHG-related skin irritation. Neonates in our study were at least 48 hours of age, however, which may have helped avoid any potential skin irritation. It has been suggested that wiping any excess CHG off the skin after application with normal saline may reduce the risk of skin irritation (25), however skin burns have been reported even after cleansing the skin with saline after exposure (22). Given these reports, precaution should be used when using chlorhexidine products in preterm infants, especially in the first few days of life.
Infants in our study were not eligible for exposure to CHG in the first 48 hours of life. This exclusion was due to two concerns: 1) previously documented risk of burns in preterm infants after CHG exposure in this time period, and 2) concern for increased risk of absorption of CHG shortly after birth due to minimal cornification of the skin. It is unclear whether CHG absorption in the first 48 hours of life would be increased or similar to the results in preterm infants after 48 hours of age, although there was no association of increased CHG absorption with younger age at insertion in our study. The use of CHG in the first 48 hours of life in preterm infants, such as for umbilical line placement, needs further safety evaluation.
A few important considerations should be made when interpreting these data. Preliminary results led to recalibration of the laboratory detection assay to a 6-point calibration that resulted in a more analytically sensitive assay with respect to limit of quantification, but with a reduced calibration slope. Therefore, the quantitative results from Group 1 infants may have been overestimated, but residual blood samples were not available to rerun on the recalibrated assay. In addition, this study included a small number of infants that received limited CHG exposure during a specific time period of 2–14 days of age. Because infants were not followed until there was no detectable chlorhexidine in the blood, a true peak concentration cannot be determined and further studies are needed to elucidate the exact pharmacokinetics of CHG.
CHG is detected in the blood of preterm infants receiving CHG skin antisepsis for PICC insertion. The potential risk of CHG in neonates must be carefully balanced by potential benefits. CHG has lifesaving applications for neonates around the world, including umbilical cord care to reduce neonatal mortality (26). As CHG use increases in hospitalized patients, further studies are needed to determine the clinical relevance of CHG absorption in this vulnerable NICU population.
Acknowledgments
This manuscript was supported by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The authors thank Dr. Craig Hendrix, the Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center NICU nursing staffs, and the Johns Hopkins ICTR Navigators.
Footnotes
Financial Disclosures and Conflict of Interest: A.M. receives grant support from Sage Products. All other authors report no disclosures.
Conflict of Interest: A.M. received grant support from Sage Products. All other authors declare no conflict of interest.
References
- 1.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46(2):274–81. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
- 2.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disese Control and Prevention. Pediatrics. 2002;110(5):e51. doi: 10.1542/peds.110.5.e51. [DOI] [PubMed] [Google Scholar]
- 3.Tamma PD, Aucott SW, Milstone AM. Chlorhexidine use in the neonatal intensive care unit: results from a national survey. Infect Control Hosp Epidemiol. 2010;31(8):846–9. doi: 10.1086/655017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration MedWatch [Internet] Silver Spring, MD: U.S. Food and Drug Administration; 2012. [updated 2012 2012 June 12; cited 2012 November 27]; Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/Safety-RelatedDrugLabelingChanges/ucm307387.htm. [Google Scholar]
- 5.Aggett PJ, Cooper LV, Ellis SH, McAinsh J. Percutaneous absorption of chlorhexidine in neonatal cord care. Arch Dis Child. 1981;56(11):878–80. doi: 10.1136/adc.56.11.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowen J, Ellis SH, McAinsh J. Absorption of chlorhexidine from the intact skin of newborn infants. Arch Dis Child. 1979;54(5):379–83. doi: 10.1136/adc.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garland JS, Alex CP, Uhing MR, Peterside IE, Rentz A, Harris MC. Pilot trial to compare tolerance of chlorhexidine gluconate to povidone-iodine antisepsis for central venous catheter placement in neonates. J Perinatol. 2009;29(12):808–13. doi: 10.1038/jp.2009.161. [DOI] [PubMed] [Google Scholar]
- 8.Johnsson J, Seeberg S, Kjellmer I. Blood concentrations of chlorhexidine in neonates undergoing routine cord care with 4% chlorhexidine gluconate solution. Acta Paediatr Scand. 1987;76(4):675–6. doi: 10.1111/j.1651-2227.1987.tb10544.x. [DOI] [PubMed] [Google Scholar]
- 9.Mullany LC, Khatry SK, Sherchand JB, Leclerq SC, Darmstadt GL, Katz J, et al. A Randomized Controlled Trial of the Impact of Chlorhexidine Skin Cleansing on Bacterial Colonization of Hospital-Born Infants in Nepal. Pediatr Infect Dis J. 2008 doi: 10.1097/INF.0b013e31816791a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien CABJ, Speck WT, Carr H. Effect of bathing with a 4 per cent chlorhexidine gluconate solution on neonatal bacterial colonization. J Hosp Infect. 1984;5 (suppl 1):141. [Google Scholar]
- 11.O’Neill JHM, Challop R, Driscoll J, Speck W, Sprunt K. Percutaneous absorption potential of chlorhexidine in neonates. Curr Ther Research. 1982;31(3):485–9. [Google Scholar]
- 12.Mullany LC, Darmstadt GL, Tielsch JM. Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J. 2006;25(8):665–75. doi: 10.1097/01.inf.0000223489.02791.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman AK, Aucott SW, Milstone AM. Safety of chlorhexidine gluconate used for skin antisepsis in the preterm infant. J Perinatol. 2012;32(1):4–9. doi: 10.1038/jp.2011.148. [DOI] [PubMed] [Google Scholar]
- 14.Milstone ABP, Aucott S, Bearer CF, Tang N. Chlorhexidine Reduces L1-Mediated Neurite Outgrowth. Annual Meeting of the Pediatric Academic Society; 2011; Denver, Colorado. 2011. [Google Scholar]
- 15.Davies GE, Francis J, Martin AR, Rose FL, Swain G. 1:6-Di-4′-chlorophenyldiguanidohexane (hibitane); laboratory investigation of a new antibacterial agent of high potency. Br J Pharmacol Chemother. 1954;9(2):192–6. doi: 10.1111/j.1476-5381.1954.tb00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebben JE. Surgical antiseptics. J Am Acad Dermatol. 1983;9(5):759–65. doi: 10.1016/s0190-9622(83)70192-1. [DOI] [PubMed] [Google Scholar]
- 17.Greaves SJ, Ferry DG, McQueen EG, Malcolm DS, Buckfield PM. Serial hexachlorophene blood levels in the premature infant. N Z Med J. 1975;81(537):334–6. [PubMed] [Google Scholar]
- 18.Kalia YN, Nonato LB, Lund CH, Guy RH. Development of skin barrier function in premature infants. J Invest Dermatol. 1998;111(2):320–6. doi: 10.1046/j.1523-1747.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Barker N, Hadgraft J, Rutter N. Skin permeability in the newborn. J Invest Dermatol. 1987;88(4):409–11. doi: 10.1111/1523-1747.ep12469738. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds PR, Banerjee S, Meek JH. Alcohol burns in extremely low birthweight infants: still occurring. Arch Dis Child Fetal Neonatal Ed. 2005;90(1):F10. doi: 10.1136/adc.2004.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins AM, Keogh EJ. Alcohol burns in the neonate. J Paediatr Child Health. 1992;28(4):306–8. doi: 10.1111/j.1440-1754.1992.tb02673.x. [DOI] [PubMed] [Google Scholar]
- 22.Bringue Espuny X, Soria X, Sole E, Garcia J, Marco JJ, Ortega J, et al. Chlorhexidine-methanol burns in two extreme preterm newborns. Pediatr Dermatol. 2010;27(6):676–8. doi: 10.1111/j.1525-1470.2010.01178.x. [DOI] [PubMed] [Google Scholar]
- 23.Mannan K, Chow P, Lissauer T, Godambe S. Mistaken identity of skin cleansing solution leading to extensive chemical burns in an extremely preterm infant. Acta Paediatr. 2007;96(10):1536–7. doi: 10.1111/j.1651-2227.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- 24.Andersen C, Hart J, Vemgal P, Harrison C. Prospective evaluation of a multi-factorial prevention strategy on the impact of nosocomial infection in very-low-birthweight infants. J Hosp Infect. 2005;61(2):162–7. doi: 10.1016/j.jhin.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Lashkari HP, Chow P, Godambe S. Aqueous 2% chlorhexidine-induced chemical burns in an extremely premature infant. Arch Dis Child Fetal Neonatal Ed. 2012;97(1):F64. doi: 10.1136/adc.2011.215145. [DOI] [PubMed] [Google Scholar]
- 26.Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MR, et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet. 2012;379(9820):1022–8. doi: 10.1016/S0140-6736(11)61848-5. [DOI] [PubMed] [Google Scholar]


