Abstract
Heart valves arise from the cardiac endocardial cushions located at the atrioventricular canal (AVC) and cardiac outflow tract (OFT) during development. A subpopulation of cushion endocardial cells undergoes endocardial to mesenchymal transformation (EMT) and generates the cushion mesenchyme, which is then remodeled into the interstitial tissue of the mature valves. The cushion endocardial cells that do not undertake EMT proliferate to elongate valve leaflets. During EMT and the post-EMT valve remodeling, endocardial cells at the cushions highly express nuclear factor in activated T-cell, cytoplasmic 1 (Nfatc1), a transcription factor required for valve formation in mice. In this review, we present the current knowledge of Nfatc1 roles in the ontogeny of heart valves with a focus on the fate decision of the endocardial cells in the processes of EMT and valve remodeling.
Introduction
Heart valves develop from the embryonic endocardial cushions located at the atrioventricular canal (AVC) and cardiac outflow tract (OFT). Mature valves are made of the valve endothelium and interstitial tissue; both derived primarily from the embryonic endocardial cells lining the endocardial cushions during valve development through two essential morphogenic steps: endocardial to mesenchymal transformation (EMT) and valve elongation. EMT is the early step in which some endocardial cells delaminate from the cushion endocardium and invade the cushions to form the mesenchyme of valve primordium (DeLaughter et al., 2011; Lin et al., 2012a; von Gise and Pu, 2012). The mesenchymalized cushions then remodel into the mature valves and the mesenchymal cells become the valve interstitial tissue (Combs and Yutzey, 2009; Hinton and Yutzey, 2011), whereas the endocardial cells at the leading edge of valve primordium proliferate and become the valve leaflets. This remodeling process is prominent during late gestation and continues into the postnatal period (Aikawa et al., 2006).
Therefore, the cushion endocardial cells are the valve progenitors that have two distinct fates necessary for normal valve development. Identifying the mechanisms by which regulate the cushion endocardial fate development may help understanding of the pathogenesis of congenital heart valve disease. Towards this end, we have studied nuclear factor in activated T-cell, cytoplasmic 1 (Nfatc1), a transcription factor required for valve formation in mice (Chang et al., 2004; de la Pompa et al., 1998b; Ranger et al., 1998a). Our results have identified Nfatc1 as a regulator of the cushion endocardial cell fate during valve development in mice (Wu et al., 2011; Zhou et al., 2005). The information suggests that the mutations in NFATC1 may affect the fate development of the valve progenitor cells, resulting in some forms of congenital valve defects in patients.
Heart valve development: Overview
Heart valve development is an evolutionally and spatiotemporally conserved morphogenic process in which subsets of endocardial cells are specified to form the valves. In the early developing mouse heart, valve-like function is first observed at the AVC and OFT between embryonic day (E) 8.5-9.5 where opposite swellings of extracellular matrix form the endocardial cushions and prevent the blood regurgitation (Baldwin et al., 1991; Paff et al., 1965). Subsequently a subpopulation of endocardial cells down regulates its surface endothelial markers, delaminates from the endocardial sheet lining the AVC or OFT at E9.5-10.5 or E10.5-11.5, and invades into the extracellular matrix of the endocardial cushions (Barnett and Desgrosellier, 2003; Eisenberg and Markwald, 1995; Krug et al., 1985; Person et al., 2005; Runyan and Markwald, 1983; Schroeder et al., 2003).
EMT is a temporal process; it stops soon after the endocardial cushions are occupied by the mesenchymal cells derived from the transformed endocardial cells. The mesenchymalized cushions or the primitive valves then begin their post-EMT remodeling after E11.5 that elongates the valve primordia into the thin valve leaflets (Effmann, 1982; Hurle et al., 1980; Tsuda et al., 2001). The remodeling consists of a balanced regional cell proliferation and apoptosis as well as extracellular matrix composition (Armstrong and Bischoff, 2004; Combs and Yutzey, 2009; Hinton and Yutzey, 2011; Hurle et al., 1980; Lin et al., 2012a; Webb et al., 1998). The growth of the endocardial edge of the mesenchymal projections and evacuation of apoptotic cells underneath the proliferating endocardial rim sculpt the primitive valves into a typical excavated shape of leaflets of mitral and tricuspid valve at AVC and the aortic and pulmonary valve at OFT (Hurle, 1980).
It is known that the ventricular and atrial endocardial cells never undergo EMT, even when they are exposed to inductive signals that are capable to trigger the EMT by the cushion endocardial cells (Barnett and Desgrosellier, 2003; Delot, 2003; Eisenberg and Markwald, 1995; Schroeder et al., 2003). Furthermore, among the endocardial cells lining the cushions, only a subset undergoes EMT, the reminder maintain their endothelia phenotype and become highly proliferative during post-EMT valve elongation (Zhou et al., 2005). Therefore, the cushion endocardial cells form the primary valve progenitor cells with dual fates. To form normal heart valves, a tight control of the fate decisions of the cushion endocardial cells must be in place to allocate appropriate contributions to the endothelial lining of the valve primordia as well as provide adequate mesenchymal cells for the valve structural integrity. Using mouse models, we have investigated the mechanisms by which Nfatc1 regulate valve formation and showed that it plays a critical role in the fate determination of the valve progenitor cell population.
Nfatc1 function and Heart Valve Development
Nfatc1 is a master transcriptional activator for cytokine genes in activated lymphoid cells (Rao et al., 1997). The calcineurin pathway controls Nfatc1 activity during T cell activation. Upon T cell receptor engagement, a sustained increase in the intracellular Ca2+ activates calcineurin, which dephosphorylates Nfatc1 protein and promotes its nuclear translocation. In the nucleus, Nfatc1 binds target genes through a consensus sequence, A/TGGTTTT, and often interacts with an AP1 transcription factor. Cyclosporin A blocks the phosphatase activity of calcineurin (Emmel et al., 1989; Shibasaki et al., 1996) and inhibits T cell activation by preventing nuclear translocation of Nfatc1 (Flanagan et al., 1991). Export of Nfatc1 out of the nucleus is also highly regulated. Glycogen synthetase kinase 3 phosphorylates Nfatc1 at the same sites that are dephosphorylated by calcineurin and promotes Nfatc1 nuclear export (Beals et al., 1997). Thus, Nfatc1 transcriptional activity is regulated at the protein level by a balance between its nuclear import and export, depending on the phosphorylation status (Crabtree, 1999; Crabtree and Olson, 2002).
Nfatc1 is also expressed in the developing mouse embryo and interestingly its expression is restricted to the endocardial layer of the heart tube around E8.5 (de la Pompa et al., 1998b; Ranger et al., 1998a). From E9.5 to E11.5 when EMT is progressing in AVC and OFT, Nfatc1 expression is accentuated in the endocardium of OFT and AVC regions and downregulated in the chamber endocardium. Nfatc1 expression is maintained at a high level in the valve endocardial cells or Nfatc1h cells during valve elongation. No Nfatc1 can be seen in the endothelium outside the embryonic heart. Of particular importance, no expression can be detected in the transformed endocardial cells in the OFT and AVC cushions. Thus, expression of the nuclear active form of Nfatc1 occurs at least 24 hours prior to EMT. As found in the immune cells, the calcium/calcineurin pathway also regulates the nuclear translocation of endocardial Nfatc1 (de la Pompa et al., 1998a; Ranger et al., 1998a). Endocardial Nfatc1 activity is thus tightly regulated via both expression and protein modification during valve formation.
While other members of the Nfatc family, such as Nfatc3 and Nfatc4, are expressed in the myocardium of the developing heart (Bushdid et al., 2003; Graef et al., 2001), Nfatc1 is the Nfatc family member restricted to the endocardium of the developing heart. Consistently, Nfatc1 has a non-redundant function for valve development and embryonic survival, since disruption of the Nfatc1 gene results in absence of semilunar valves and underdeveloped atrioventricular valves, and Nfatc1 null embryos die around E13.5 (de la Pompa et al., 1998a; Ranger et al., 1998a) of rapidly progressive heart failure (Phoon et al., 2004). Further studies have indicated that two waves of Nfatc activities are required for valve formation in mice, one in E9.5 myocardium, i.e. Nfatc3 and Nfatc4, for initiation of EMT and the other, Nfatc1, in E11.5 endocardium for valve elongation (Chang et al., 2004).
Nfatc1 Genomic Locus as a Molecular Tool to Study Endocardial Biology during Valve Development
The spatiotemporally regulated Nfatc1 expression in the endocardial cells of the developing hearts and its essential role in valve formation makes Nfatc1 a unique molecular tool to study endocardial cell differentiation and lineage development during valve formation and the valve-specific gene function underlying these cellular and morphogenic processes. We have therefore studied transcriptional regulation of Nfatc1 by in vivo promoter/enhancer deletion studies using mouse transient/stable transgenesis (Zhou et al., 2005). This study has identified a transcriptional enhancer that regulates the sustained expression of Nfatc1 in the endocardial cells at AVC and OFT cushions and the enhancer activities are amplified through an autoregulatory loop, which is different from the autoregulation loop through the Nfatc1 promoter during activation of T cells (Zhou et al., 2002). In the transgenic mouse embryos harboring the enhancer-driven lacZ reporter gene (Nfatc1enlacZ), lacZ expression marks in the forming valves and is restricted to the cushion or valve endocardium (Figure 1). Consistent with the endogenous Nfatc1 expression, the enhancer-directed lacZ expression is not present in the valve mesenchyme and corresponds to the second wave of Nfatc activities in the valve-forming field required for valve elongation (Chang et al., 2004).
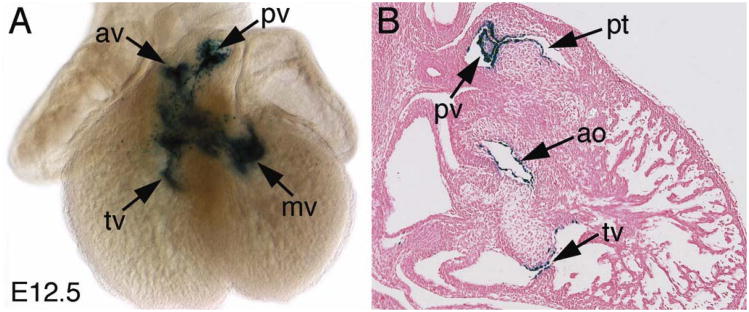
Figure 1. Nfatc1-enhancer activity marks the endocardial cells of forming heart valves.
A. Wholemount X-gal staining of E12.5 heart showing that the enhancer directs lacZ expression in the developing valves.
B. Sectional X-gal staining of E12.5 heart showing that the enhancer-directed lacZ expression is restricted to the endocardium of the primitive valves. Note that no lacZ expression is present within the valve mesenchyme.
Nfatc1 Regulates Endocardial Cell Fate during Valve Development
Nfatc1 autoregulation has been shown involved in cell-fate decisions in T-cell activation/expansion (Ranger et al., 1998b; Zhou et al., 2002), osteoclastogenesis (Asagiri et al., 2005), and hair follicle stem cells (Horsley et al., 2008), suggesting that it may also be functionally involved in the endocardial cell-fate decisions during valve development. To address this question we have utilized the Nfatc1 enhancer to generate a cushion endocardial cell-specific Cre mouse line (Nfatc1enCre) and crossed this transgenic mouse with the Rosa26-lacZ Cre reporter mouse line (R26fslz) to fate map the contribution of Nfatc1-expressing cells in the cushion endocardial cells to valve development (Wu et al., 2011). We have shown in the developing hearts of the Nfatc1enCre;R26fslz embryos that, a subpopulation of cushion endocardial cells identified by the enhancer do not undergo EMT but remain in the endocardium during EMT and post-EMT valve elongation (Figure 2).
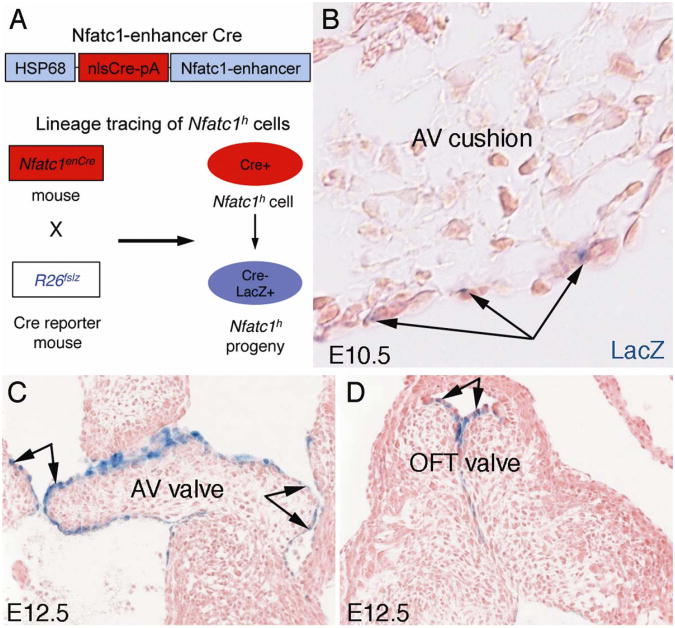
Fig. 2. Fate mapping of endocardial cells during EMT and early valve elongation.
A. A schematic of fate mapping of cushion endocardial cells expressing a high level of Nfatc1 (Nfatc1h) using the Nfatc1-enhancer Cre (Nfatc1enCre) and Rosa26-lacZ Cre reporter (R26fslz) mice.
B. X-gal staining of E10.5Nfatc1enCre;R26fslz heart sections showing that Nfatc1h endocardial cell lineages (arrows) contribute to the cushion endocardium but not mesenchyme during EMT, indicating that Nfatc1h cells do not undergo EMT.
C and D. Showing that Nfatc1h endocardial cells contribute to the leading edge of the growing valve cups during elongation at E12.5. AV, atrioventricular; OFT, outflow tract. (Adapted from Wu et al. Circ. Res. 109;183-192, 2011).
The fate mapping analyses have also revealed that, unlike the atrioventricular valves, the endocardial cushion of the OFT is not a continuous structure composed of a uniform mesenchymal cell population; rather, OFT mesenchyme is composed of two heterogeneous cell populations that form a segmented structure with the cardiac neural crest mesenchyme occupying the distal OFT and the endocardial-derived mesenchyme populating the proximal OFT (Wu et al., 2011). Their interface corresponds to the site for developing semilunar valves in humans (Thompson et al., 1985). In the Nfatc1 null embryos that do not form semilunar valves, this tissue boundary is disrupted by an increased endocardial-derived mesenchyme and a decreased cardiac neural crest-derived mesenchyme (Wu et al., 2011). This observation confirms that the two mesenchymal populations at OFT cushion are distinct populations and an orchestrated interaction between them is required for normal development of the semilunar valves. Functional in vitro collagen gel assays have further revealed a premature loss of cellular adhesiveness and an excessive EMT by the Nfatc1-/- endocardial cells, which likely contributes to the increased cushion mesenchyme seen in the Nfatc1 null embryos (Wu et al., 2011). Indeed, blastocyst complementation analyses have demonstrated an enhanced EMT in vivo by the Nfatc1-/- endocardial cells.
A molecular model for Nfatc1 function in valve development
Not only is Nfatc1 required for the negative regulation of EMT, but also it is necessary for proliferation of the endocardial and mesenchymal cells during post-EMT valve elongation. Consistent with these observations, Nfatc1 and other endothelial markers including VE-Cadherin co-express in the endocardial cell clusters differentiated from the endocardial progenitor cells in vitro. Furthermore, Nfatc1 suppresses expression of Snail1 and Snail2, positive regulators of EMT, thereby maintaining VE-Cadherin expression in vivo (Wu et al., 2011) (Fig. 3A). Subsequent studies have documented that calcineurin/Nfatc1 signaling suppresses apoptosis in the OFT endocardium required for normal valve morphogenesis (Lin et al., 2012b).
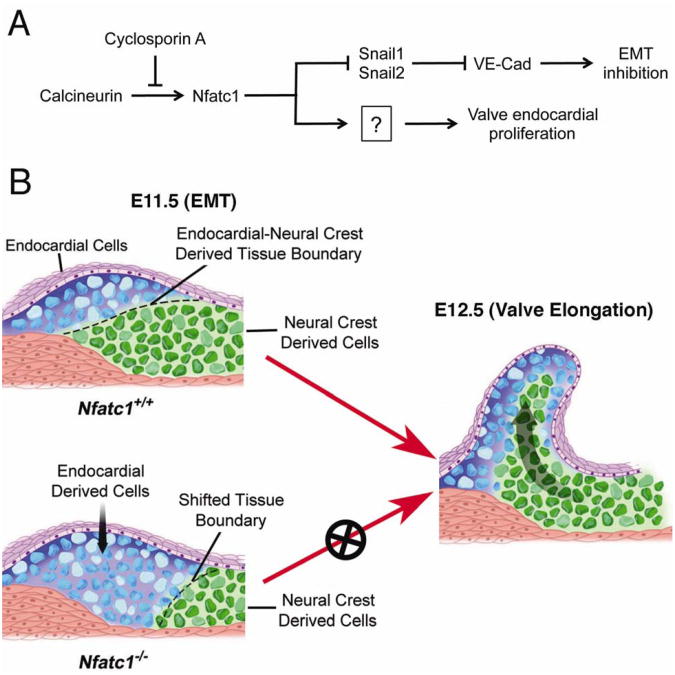
Figure 3. Working model for the role of Nfatc1 in the semilunar valve development.
A. A simplified molecular model for Nfatc1 functions in valve development showing that Nfatc1 nuclear activities are regulated by its upstream activator, calcineurin, which is inhibited cyclosporin. Within the nuclei Nfatc1 suppresses the Snail1 and Snail2, thereby maintaining the valve endocardial cell phenotype during EMT. Nfatc1 also promotes proliferation of endocardial cells required for valve elongation.
B. In wild-type (Nfatc1+/+) embryos at E11.5, autoregulation of Nfatc1 enhancer maintains a high level of Nfatc1 in the cushion endocardium (purple color cells) that determines endocardial cell fate during EMT. Cushion endocardial cells with a high level of Nfatc1 expression do not undergo EMT. The negative regulation of EMT by Nfatc1 is required for the proper formation of endocardial-derived mesenchyme at the proximal OFT (blue colored cells) and its juxtaposition to the cardiac neural crest-derived mesenchyme at the distal OFT (green colored cells), thereby defining the site for semilunar valve formation. While inhibiting EMT, Nfatc1 promotes proliferation of endocardial cells required for valve elongation. In Nfatc1 null (Nfatc1-/-) embryos, this endocardial-neural crest mesenchymal boundary is shifted into the distal OFT by overpopulated endocardial-derived mesenchyme resulted from increased EMT and valve elongation is also disrupted by reduced endocardial proliferation (Adapted from Wu et al. Circ. Res. 109;183-192, 2011).
It is worth to mention that Nfatc1 activities are regulated by it upstream regulator calcineurin phosphatase as well as its co-factors. For example, cooperative interaction between Nfatc1 and Gata5 is required for endocardial gene expression and differentiation of endocardial cell lineages in vitro (Nemer and Nemer, 2002). Indeed, recent studies have revealed that Gata5 is required cell-autonomously within the valve endocardium for normal development and patterning of aortic valves (Laforest et al., 2011). Gata5 interaction with Gata4 or Gata6 is required for normal morphogenesis of cardiac OFT (Laforest and Nemer, 2011).
Normal functions of cardiac neural crest are also required for OFT development. Although early neural crest migration is normal in Nfatc1 null embryos, defects in its late migration and/or expansion are likely present in these embryos. The defects appear affecting a unique neural crest mesenchymal population essential to the semilunar valve elongation and maturation (Jain et al., 2011). Thus, Nfatc1 may also regulate OFT morphogenesis and semilunar valve formation through an additional non-cell autonomous effect on neural crest-derived mesenchymal function. The disruption of normal tissue boundary where semilunar valves develop in Nfatc1 null embryos suggests that the proper spatiotemporal contact of endocardial-derived mesenchyme and neural crest-derived mesenchyme during early OFT morphogenesis is essential for subsequent semilunar valve remodeling (Figure 3B).
Conclusion and the Direction of Future Research
The data from the transgenic mouse studies including fate mapping, blastocyst complementation, and EMT analyses demonstrate that Nfatc1 acts as a ‘molecular switch’ to allocate the endocardial cells to EMT and post-EMT valve elongation, the two morphologic events essential for heart valve formation. This information generated from mouse models suggests that mutations in NFATC1 or in genes that affect the NFATC1 gene regulatory network may underlie some forms of human congenital valve disease.
Genomic alterations in the NFATc1 locus have been identified in patients with CHD (Yehya et al., 2006) and recently, heterozygous mutations in NFATc1 have been identified in a patient with tricuspid atresia (Abdul-Sater et al., 2012). Further research is necessary to determine whether additional point mutations in NFATC1 and its regulatory genes in the NFATC1 signaling pathway (Crabtree and Olson, 2002; Rao, 2009) are associated with congenital heart valve malformations. The next-generation sequencing of exome and whole genome analysis of afflicted large families or in large cohort studies may identify disease-associated genomic lesions that link to NFATC1. Generation of new mouse models carrying the newly identified genomic lesions may then reveal how these lesions interact with NFATC1 mutations that cause human congenital heart valve disease and help understanding the genetic base of this disease.
Acknowledgments
B.Z. is supported by funds from the National Institutes of Health (NIH) and American Heart Association (AHA) (National Scientist Development Award). H.S.B. is supported by funds from the NIH and AHA (National Established Investigator Award).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Sater Z, Yehya A, Beresian J, Salem E, Kamar A, Baydoun S, Shibbani K, Soubra A, Bitar F, Nemer G. Two heterozygous mutations in NFATC1 in a patient with Tricuspid Atresia. PLoS One. 2012;7:e49532. doi: 10.1371/journal.pone.0049532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circulation Research. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. Journal of Experimental Medicine. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HS, Jensen KL, Solursh M. Myogenic cytodifferentiation of the precardiac mesoderm in the rat. Differentiation. 1991;47:163–172. doi: 10.1111/j.1432-0436.1991.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Research Part C, Embryo Today: Reviews. 2003;69:58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Bushdid PB, Osinska H, Waclaw RR, Molkentin JD, Yutzey KE. NFATc3 and NFATc4 are required for cardiac development and mitochondrial function. Circulation Research. 2003;92:1305–1313. doi: 10.1161/01.RES.0000077045.84609.9F. [DOI] [PubMed] [Google Scholar]
- Chang CP, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–663. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circulation Research. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;(109 Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- DeLaughter DM, Saint-Jean L, Baldwin HS, Barnett JV. What chick and mouse models have taught us about the role of the endocardium in congenital heart disease. Birth defects research Part A, Clinical and molecular teratology. 2011;91:511–525. doi: 10.1002/bdra.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delot EC. Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways. Molecular Genetics and Metabolism. 2003;80:27–35. doi: 10.1016/j.ymgme.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Effmann EL. Development of the right and left pulmonary arteries. A microangiographic study in the mouse. Investigative Radiology. 1982;17:529–538. [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circulation Research. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T- cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annual Review of Physiology. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle JM, Colvee E, Blanco AM. Development of mouse semilunar valves. Anatomy & Embryology. 1980;160:83–91. doi: 10.1007/BF00315651. [DOI] [PubMed] [Google Scholar]
- Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. The Journal of Clinical Investigation. 2011;121:422–430. doi: 10.1172/JCI44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug EL, Runyan RB, Markwald RR. Protein extracts from early embryonic hearts initiate cardiac endothelial cytodifferentiation. Developmental Biology. 1985;112:414–426. doi: 10.1016/0012-1606(85)90414-2. [DOI] [PubMed] [Google Scholar]
- Laforest B, Andelfinger G, Nemer M. Loss of Gata5 in mice leads to bicuspid aortic valve. The Journal of Clinical Investigation. 2011;121:2876–2887. doi: 10.1172/JCI44555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforest B, Nemer M. GATA5 interacts with GATA4 and GATA6 in outflow tract development. Developmental Biology. 2011;358:368–378. doi: 10.1016/j.ydbio.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Lin CY, Chen CH, Zhou B, Chang CP. Partitioning the heart: mechanisms of cardiac septation and valve development. Development. 2012a;139:3277–3299. doi: 10.1242/dev.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lin CJ, Chen CH, Chen RM, Zhou B, Chang CP. The secondary heart field is a new site of calcineurin/Nfatc1 signaling for semilunar valve development. Journal of molecular and cellular cardiology. 2012b;52:1096–1102. doi: 10.1016/j.yjmcc.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer G, Nemer M. Cooperative interaction between GATA5 and NF-ATc regulates endothelial-endocardial differentiation of cardiogenic cells. Development. 2002;129:4045–4055. doi: 10.1242/dev.129.17.4045. [DOI] [PubMed] [Google Scholar]
- Paff GH, Boucek RJ, Gutten GS. Ventricular Blood Pressures and Competency of Valves in the Early Embryonic Chick Heart. Anatomical Record. 1965;151:119–123. doi: 10.1002/ar.1091510203. [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. International Review of Cytology. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Phoon CK, Ji RP, Aristizabal O, Worrad DM, Zhou B, Baldwin HS, Turnbull DH. Embryonic heart failure in NFATc1-/- mice: novel mechanistic insights from in utero ultrasound biomicroscopy. Circulation Research. 2004;95:92–99. doi: 10.1161/01.RES.0000133681.99617.28. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998a;392:186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Hodge MR, Gravallese EM, Oukka M, Davidson L, Alt FW, de la Brousse FC, Hoey T, Grusby M, Glimcher LH. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998b;8:125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- Rao A. Signaling to gene expression: calcium, calcineurin and NFAT. Nature Immunology. 2009;10:3–5. doi: 10.1038/ni0109-3. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annual Review of Immunology. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Runyan RB, Markwald RR. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Developmental Biology. 1983;95:108–114. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. Journal of Molecular Medicine. 2003;81:392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Shibasaki F, Price ER, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- Thompson RP, Sumida H, Abercrombie V, Satow Y, Fitzharris TP, Okamoto N. Morphogenesis of human cardiac outflow. Anatomical Record. 1985;213:578–586. doi: 10.1002/ar.1092130414. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Wang H, Timpl R, Chu ML. 2001. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Developmental Dynamics. 1985;222:89–100. doi: 10.1002/dvdy.1172. [DOI] [PubMed] [Google Scholar]
- von Gise A, Pu WT. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circulation Research. 2012;110:1628–1645. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S, Brown NA, Anderson RH. Formation of the atrioventricular septal structures in the normal mouse. Circulation Research. 1998;82:645–656. doi: 10.1161/01.res.82.6.645. [DOI] [PubMed] [Google Scholar]
- Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circulation Research. 2011;109:183–192. doi: 10.1161/CIRCRESAHA.111.245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehya A, Souki R, Bitar F, Nemer G. Differential duplication of an intronic region in the NFATC1 gene in patients with congenital heart disease. Genome. 2006;49:1092–1098. doi: 10.1139/g06-072. [DOI] [PubMed] [Google Scholar]
- Zhou B, Cron RQ, Wu B, Genin A, Wang Z, Liu S, Robson P, Baldwin HS. Regulation of the murine Nfatc1 gene by NFATc2. Journal of Biological Chemistry. 2002;277:10704–10711. doi: 10.1074/jbc.M107068200. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wu B, Tompkins KL, Boyer KL, Grindley JC, Baldwin HS. Characterization of Nfatc1 regulation identifies an enhancer required for gene expression that is specific to pro-valve endocardial cells in the developing heart. Development. 2005;132:1137–1146. doi: 10.1242/dev.01640. [DOI] [PubMed] [Google Scholar]