Abstract
Healthy skeletal muscle has a remarkable capacity for regeneration. Even at a mature age, muscle tissue can undergo a robust rebuilding process that involves the formation of new muscle cells and extracellular matrix and the re-establishment of vascular and neural networks. Understanding and reverse-engineering components of this process is essential for our ability to restore loss of muscle mass and function in cases where the natural ability of muscle for self-repair is exhausted or impaired. In this article, we will describe current approaches to restore the function of diseased or injured muscle through combined use of myogenic stem cells, biomaterials, and functional tissue-engineered muscle. Furthermore, we will discuss possibilities for expanding the future use of human cell sources towards the development of cell-based clinical therapies and in vitro models of human muscle disease.
Introduction
Skeletal muscle is the most abundant tissue in the human body, comprising nearly 45% of the total body weight. Muscle tissue consists of aligned bundles of multinucleated, striated, and contractile muscle cells, termed myofibers. The contractile function of the myofibers is supported by a network of nerves, blood vessels, and extracellular matrix (ECM) proteins and carbohydrates. In response to common acute injury, such as exercise-induced tears or lacerations, skeletal muscle shows a remarkable capacity for regeneration. Even at a mature state, muscle tissue has the ability to undergo daily renewal [1]. The cellular and molecular mechanisms underlying the process of muscle regeneration have been studied extensively, and the key players have been identified; most predominantly, the resident muscle stem cell, termed the satellite cell.
Despite significant capacity for regeneration, large muscle injuries and chronic degenerative diseases, such as muscular dystrophy, create non-homeostatic environments in which the muscle loss becomes irreversible. In such cases, reparative fibrosis overpowers formation of new muscle, leaving excess scar tissue that eventually yields a sub-innervated, malfunctioning muscle. Furthermore, with aging and severe congenital disorders, the loss of muscle mass and function is additionally exacerbated by the reduced self-renewing capacity of satellite cells.
In this review, current bioengineering strategies to rebuild or replace dysfunctional muscle will be discussed. The process of natural regeneration will be first outlined as it provides the mechanistic foundation used by researchers to develop therapeutics. We will then describe studies utilizing isolated myogenic stem and precursor cells in conjunction with engineered biomaterials, co-delivery of growth factors, genetic engineering, and other strategies to generate functional muscle both in vitro and in vivo. Finally, we will summarize important considerations as researchers transition to use of optimal human cell sources and methods for cell-based therapies and design of high-fidelity in vitro models of human muscle disease.
Skeletal Muscle Regeneration
Muscle regenerative response involves synchronized action of resident and circulating myogenic cells, local non-myocytes, and inflammatory cells that rapidly yield production of new muscle [2], provisional ECM, and supporting vascular network. Specifically, upon muscle injury, inflammatory cells infiltrate the site of damage, acting to clear necrotic debris and amplify the immune response through secretion of pro-inflammatory cytokines [3]. The release of TNFα [4] and IL-6 [5], among other factors, yields activation of local satellite cells (SCs) which eventually generate new muscle cells, a process known as myogenesis. SCs, normally quiescent in adult muscle, reside beneath the basal lamina surrounding myofibers and are marked by expression of the paired-box transcription factor, Pax7 [2]. Once activated, Pax7+ SCs gain expression of myogenic regulatory factor MyoD, proliferate, and either commit to myogenic fate yielding a population of muscle precursor cells termed myoblasts, or self-renew and ultimately return to quiescence. When committed to myogenesis, myoblasts continue to proliferate and differentiate, gain expression of the mature muscle marker myogenin, and lose Pax7 expression. Committed myoblasts either undergo primary fusion, in which they fuse to one another to form new multinucleated myofibers, or secondary fusion, in which they fuse to pre-existing, damaged myofibers to restore or even augment their function [2]. Concurrent with myogenesis, residing fibroblasts proliferate and migrate to the injury site to transiently form a supportive ECM in response to the release of the pro-fibrotic transforming growth factor-β (TGF-β) [6]. Simultaneously, increased expression of angiogenic factors (e.g. VEGF, angiopoeitin-1/2) yields the formation of new capillaries along with the formation of new myofibers ensuring the maintenance of blood supply [7, 8]. These three components of regenerative process, myogenesis, fibrosis, and angiogenesis, act to provide a proper environment for the muscle regrowth and maturation and restore compromised contractile function.
The robust regenerative ability of skeletal muscle can be diminished in severe injuries where massive muscle loss yields a reparative, stabilizing fibrotic response that proceeds more rapidly than the growth of new muscle [9]. In this case, dense fibrotic tissue can inhibit neuronal and vascular ingrowth or oxygen diffusion to leave distal muscle denervated and ischemic [10]. Furthermore, regenerative capacity of muscle stem cells can be reduced or exhausted by repetitive activations caused by chronic degenerative disease. One of the most severe examples is Duchenne muscular dystrophy (DMD), a genetic disorder in which patients lack a force-bearing protein on the muscle cell membrane which renders the myofibers susceptible to damage and necrosis. In such diseases, the muscle continuously cycles between injury and regeneration, to eventually undergo severe fibrosis and fat accumulation [11]. The constant activation of the muscle stem cells results in decline of their regenerative capacity, evidenced by shortening of telomere length with age [12].
Muscle Repair Strategies
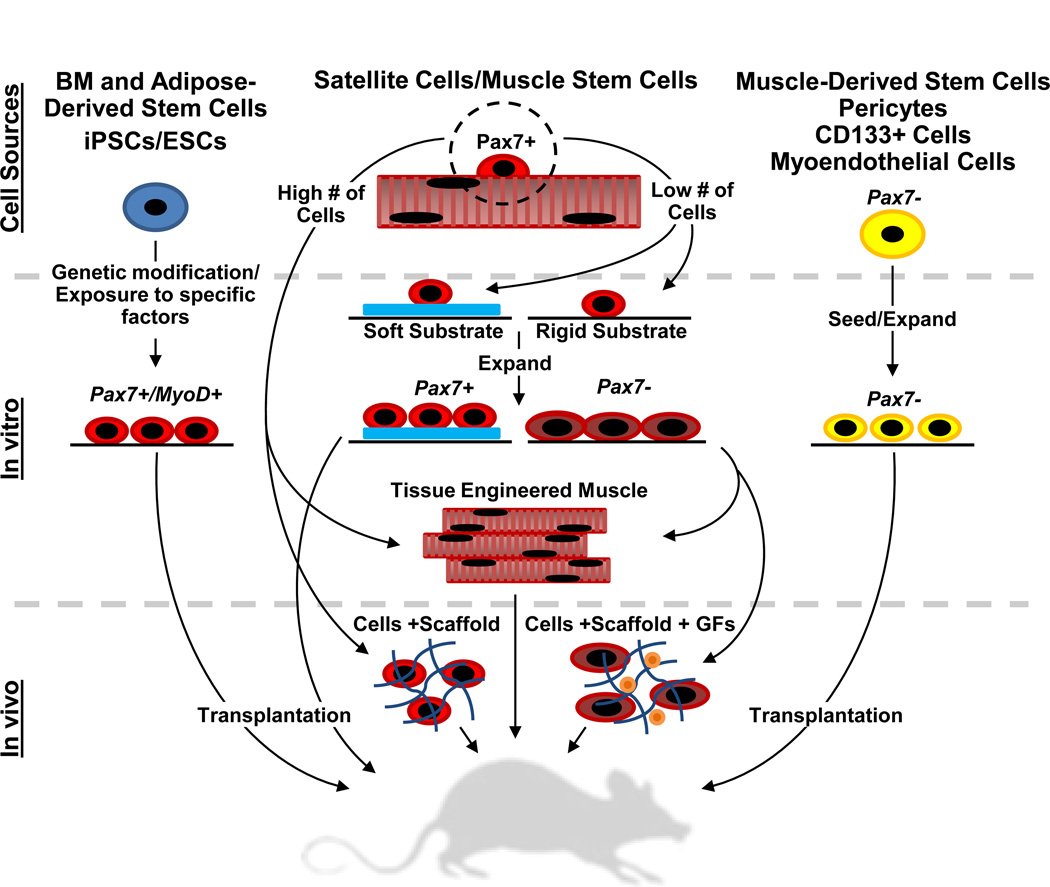
Various cell- and material-based approaches have been explored in animal and clinical studies to aid in the replacement of injured or diseased skeletal muscle or restoration of its regenerative capacity (Figure 1). For example, co-delivery of growth factors and engineered matrices with different types of stem or progenitor cells have been utilized to augment cell viability, vascularization, and myogenesis upon implantation. Tissue-engineered muscle constructs designed in vitro with appropriate structural and mechanical properties have been implanted to promote rapid muscle repair. Different methods to accelerate angiogenic and neurogenic responses have been developed to support integration and function of regenerating muscle.
Figure 1.
Current strategies for regenerative skeletal muscle repair. The flow diagram shows examples of cell-based approaches to regenerate or replace muscle tissue; from the cell sources, in vitro manipulation, and in vivo transplantation of cells or implantation of tissue constructs. GF, growth factor. BM, bone marrow. iPSC/ESC, induced pluripotent/embryonic stem cell.
Specifically, satellite cells have been a highly investigated cell source for transplantation studies due to their requisite roles in the regenerative process. The SCs can be isolated from single muscle fibers and expanded in vitro; however, expansion causes them to commit to a myoblast (Pax7−) fate. Early studies that implanted expanded myoblast populations to damaged muscle observed high cell mortality followed by loss of regenerative capacity [13, 14]. In contrast, implanted freshly isolated (Pax7+) satellite cells were able to fuse to existing muscle, home to the satellite cell niche [14–16], rescue muscle function, and provide continued capacity for repair, emphasizing the importance of maintained ‘stemness’ for the process of muscle repair and regeneration. In the case of larger muscle ablation, satellite cell transplantation will need to be able to fully recover muscle mass, suggesting the need for an engineered scaffold to provide structural support for volumetric tissue reconstruction.
Ideally, engineered scaffolds should provide control over initial distribution of implanted myogenic cells in the injury site, enhance cell concentration and retention, present cues for accelerated myogenesis, angiogenesis, and neurogenesis, and progressively degrade to allow regenerating myofibers to occupy a physiologically-high tissue volume fraction [17, 18]. Isolated mouse satellite cells have been previously delivered allogeneically using various synthetic and naturally-derived scaffolds, including micro-patterned poly(glycolic acid) [19], collagen [20] and hyaluronic acid hydrogels [15]. While use of engineered scaffolds with Pax7+ SCs yielded functional muscle recovery and replenishment of the endogenous SC pools [15], significant numbers of SCs needed for human muscle therapy may be difficult to obtain from standard muscle biopsy or surgical samples [21]. Alternatively, scaffold-mediated sustained release of myogenic insulin-like growth factor-1 (IGF-1) has been used to enhance implanted myoblast survival and participation in muscle repair [21]. While promising, it is unlikely that already committed myoblasts can fill satellite cell niche and contribute continued regenerative response.
Compared to use of undifferentiated myogenic cells (alone or with scaffolds), implantation of biomimetic tissue-engineered muscle may offer several unique advantages including the ability to: 1) precisely design patient-specific muscle architecture, 2) significantly reduce mechanical overload at the injury site by using already functional tissue, 3) attain specific mechanical or metabolic properties by in vitro preconditioning, and 4) generate native-like SC niche within engineered muscle for better protection from harsh injury environment [22]. Methods for in vitro engineering of 3-dimensional muscle tissues containing dense aligned myofibers have involved casting myogenic cells in cylindrically-molded collagen- and fibrin-based hydrogels [23–25], the self-assembly of myoblasts into scaffold-free ‘myooids’ [26], or culturing muscle cells within collagen scaffolds with oriented pores [27]. The use of soft lithography and fibrin-based hydrogels, as shown by our group, can further facilitate design of custom muscle architectures [28] [29]. The application of biophysical cues such as passive or cyclic stretch has been shown to facilitate alignment and fusion of myoblasts [30, 31] as well as promote myogenesis, myofiber hypertrophy, contractile function [23], and the secretion of vascular endothelial growth factor (VEGF) which in turn can yield enhanced vascularization in vivo [32]. Electrical stimulation can also enhance myogenesis and maturation of myofibers [20, 33]. Similarly, biochemical factors such as IGF-1 and TGF-β have been shown to promote differentiation, hypertrophy, and contractile force production in engineered muscle [34–37]. While applying biophysical and biochemical stimuli can promote functional myogenesis in vitro, the resulting myofiber diameter and force generating capacity of engineered muscle (even for high cell densities) remain far inferior to those of the adult muscle. The underlying mechanisms may be lack of vascular and neurohumoral support and/or appropriate active or passive loading conditions.
In addition to potential roles in muscle differentiation, vascularization and innervation of implanted or regenerated muscle are critical for its survival and ability to co-function with the surrounding muscle. Main approaches currently employed to promote vascularization of implanted muscle grafts include: 1) genetic modification of donor cells to express angiogenic factors [38] or controlled release of these factors from polymer scaffolds [21], 2) "prevascularization" of engineered tissue constructs prior to transplantation by co-culture of muscle and endothelial cells or vascular progenitors in vitro [39], and 3) growing muscle cells around blood vessels (e.g. arterio-venous loop or femoral artery) in vivo [40, 41]. Recently, increased organization of capillary structures obtained by extended co-culture of myoblasts, fibroblasts, and endothelial cells has been shown to yield accelerated vascularization, perfusion, and functional maturation of engineered muscle post-implantation [39]. Nevertheless, contractile stresses generated by the explanted engineered muscle in this study remained significantly lower than those of native tissue. In addition to vascularization, engineered muscle implants need to undergo successful innervation which is expected to further promote implant survival, differentiation, and hypertrophy by supply of neurotrophic factors and basal muscle tone. Previously, engineered muscle tissue were co-cultured with neuronal cells in vitro [42, 43] or implanted around nerves in vivo [44] to yield improved contractile function [43, 44]; however, these strategies may be impractical to implement in clinical setting. Alternatively, the use of soluble neurotrophic and synaptogenic factors during cell culture may promote formation of neuromuscular junction structures and improve function of engineered muscle, as previously shown for recombinant miniagrin [45].
While potentially promising, the above approaches need to be validated in highly variable conditions encountered in different types of muscle injury and disease. Importantly, the methods successful in animal models need to be successfully translated to human cells and therapies.
Transition to Human Therapy
Understandably, candidate methodologies to treat and study muscle repair have been predominantly developed using animal (mostly rodent) cells and models. Still, primary or stem cell-derived human muscle cells remain the most relevant resource for the development of cell-based clinical therapies and generation of accurate in vitro models of human muscle disease. Research using human cells, however, faces difficult challenges with identifying clinically-applicable cell sources and developing methods for in vitro engineering of functional human muscle tissues.
Currently, successful functional recovery of the injured muscle and restoration of stem cell pool in rodents can be achieved by transplantation of freshly isolated Pax7+ satellite cells with robust regenerative potential [14–16]. In human muscle, the satellite cells appear to have similar roles in regeneration, but as in rodent models, only comprise about 4% of all adult muscle nuclei [46, 47]. The low stem cell density coupled with the limited availability of muscle biopsies presents a daunting obstacle to use of freshly isolated satellite cells for human myogenic repair. Use of bioengineering techniques to generate niche-like environments in vitro by modifying physical (e.g. stiffness) and/or chemical properties of the culture substrate may allow expansion of satellite cells while preserving their "stemness", as shown by Gilbert et al. who accomplished this in murine cells by utilizing laminin-coated polyethylene glycol substrate with muscle-like stiffness [48]. Further discovery of underlying mechanisms could lead to design of small molecule drugs that could improve this strategy, which is yet to be applied to human muscle stem cells.
Besides the satellite cell, other cell types have been identified for potential use in human muscle repair. Ideally, these cells should be: 1) easy to isolate, 2) readily expandable in vitro, 3) engraft and improve muscle function in vivo, and 4) able to integrate within the local muscle stem cell pool. Human myoendothelial cells (co-expressing myogenic and endothelial cell markers) [49] and vessel-associated pericytes [50] are two expandable cell types able to regenerate muscle and fill the satellite cell compartment upon transplantation into mice, but are still hard to isolate, similar to satellite cells. Bloodderived CD133+ cells are easily accessible and upon systemic delivery can contribute to muscle regeneration and fill stem cell niche [51], yet, may be difficult to expand in vitro [52] and harder to engraft than their muscle-derived counterparts [51]. Human Pax7+ myogenic cells derived from adult bone marrow stromal cells through Notch1 intracellular domain gene transfer [53] and, more recently, from induced pluripotent stem cells (iPSCs) by conditional lentiviral expression of Pax7 [54], have been shown to engraft, fill the satellite cell niche upon implantation, and aid in subsequent rounds of regeneration in diseased mice. However, these results are achieved only by viral methods and in immunocompromised setting, and derived myogenic cells are not yet tested for their ability to rebuild large volumes of muscle tissue or recapitulate human muscle function in vitro. Nevertheless, the ability to derive large numbers of myogenic cells starting from highly proliferative iPSCs opens doors for the application of powerful gene-editing techniques (e.g. TALE and Zn finger nucleases [55]) to correct congenital muscle disorders by use of autologous cell therapy. Furthermore, use of iPSC technology or genetic engineering to create immortalized myoblast lines from patient biopsies [56] is expected to foster development of more predictive human muscle disease assays for screening of candidate drug, gene, and cell therapies.
With the identification of appropriate myogenic cell sources, tissue engineering of human functional muscle can further provide models for quantitative studies of muscle injury, disease, and drug action in vitro, and enable development of new regenerative therapies for volumetric muscle loss. Human crossstriated myofibers have been generated from isolated myoblasts using 2D substrates with optimized stiffness [57] or cell-adhesion properties [58]. Furthermore, isolated human myoblasts have been embedded in collagen- [23] or fibrin-based [59] hydrogels to form 3D muscle tissues in which they exhibited accelerated time course of differentiation compared to 2D culture [59]. However, in contrast to use of rodent cells, there are no reports that engineered human muscle can produce active contractile response to electrical stimulation, a hallmark of normal muscle excitability and function. Similarly, despite capacity to fuse, human multinucleated myofibers usually do not spontaneously contract, a limitation that could be overcome by co-culture with human motoneurons in a serum-free environment [60]. Taken together, field of human muscle tissue engineering is still in infancy and lacks the ability to yield muscle tissues with normal function and phenotype necessary for both regenerative therapies and efficient drug development. Reasons for inferior functionality of human vs. rodent engineered muscle are not obvious, and may relate to slower developmental clock of human cells, a requisite need for neuromuscular connections, or other unknown factors.
Future Challenges
Future use of bioengineered skeletal muscle mimetics as a reliable in vitro model of human physiological function or disease, drug development tool, or therapeutic approach will require the existence of robust methodologies to support or replicate native muscle biology, structure, and function. First, it will be necessary to identify and/or derive an ideal human myogenic cell source with the ability to replicate the potency of self-renewing muscle stem cells. Aforementioned human iPSC-derived Pax7+ muscle stem cells [54] currently stand as the best option that, when combined with powerful genome editing techniques [61], may also aid the treatment and modeling of congenital muscle diseases; however more robust and non-viral methods for generating such a cell pool must be established. Furthermore, the ideal cell source should enable the in vitro formation of functional engineered muscle with homeostatic and self-regenerating capacity characteristic on native muscle. In particular, one of the largest challenges facing the field will be the ability to engineer fully mature replicas of adult muscle tissues as the predictive and therapeutically relevant models of muscle disease and function. Finally, to potentially develop a complex, physiologically-accurate model of skeletal muscle or a tissue graft with increased capacity for survival and functional integration in vivo, it will be essential to engineer and incorporate vascular, neuronal and other structural components (fascia, tendons) of natural muscle. Overall, significant challenges still remain before researchers can truly and robustly recreate human skeletal muscle function and structural complexity in vitro or significantly promote repair of large muscle loss in vivo. Overcoming these challenges will be an important step towards successful understanding and treatment of various muscle pathologies.
Conclusions
In this review, we have described normal regenerative response of healthy skeletal muscle and outlined current biological and engineering strategies aimed at employing biomimetic conditions to restore or augment muscle repair when the natural response is inadequate or malfunctioning. These strategies involve: 1) refining techniques to obtain easily expandable, robustly myogenic, and genetically modifiable stem cell sources, 2) providing structural support, and biophysical and biochemical cues to promote functional myogenesis in vitro and in vivo, and 3) stimulating neurovascular ingrowth to facilitate the exogenous and endogenous muscle repair. We anticipate that the ongoing merger of cell biology with genetic and tissue engineering techniques will lead to more sophisticated and widespread use of human cells and model systems. Establishing robust and reproducible models of human skeletal muscle function and disease in vitro will be critical for gaining invaluable knowledge as researchers seek to develop safe and efficient solutions for treatment of muscle disorders.
Highlights.
Overview of the natural process of skeletal muscle regeneration.
Discussion of current muscle repair strategies.
Development of human engineered muscle models and therapies.
Acknowledgements
We are grateful to Dr. Weining Bian for assistance with manuscript preparation. This work was supported by the National Science Foundation’s Graduate Research Fellowship to M.J. and grants R01AR055226 from National Institute of Arthritis and Musculoskeletal and Skin Diseases and UH2TR000505 from the NIH Common Fund for the Microphysiological Systems Initiative to N.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark Juhas, Email: mark.juhas@duke.edu.
Nenad Bursac, Email: nbursac@duke.edu.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
•• of outstanding interest
- 1.Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 3.McClung JM, Davis JM, Carson JA. Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp Physiol. 2007;92:219–232. doi: 10.1113/expphysiol.2006.035071. [DOI] [PubMed] [Google Scholar]
- 4.Chen SE, Gerken E, Zhang Y, Zhan M, Mohan RK, Li AS, Reid MB, Li YP. Role of TNF-{alpha} signaling in regeneration of cardiotoxin-injured muscle. Am J Physiol Cell Physiol. 2005;289:C1179–C1187. doi: 10.1152/ajpcell.00062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Shanti N, Saini A, Faulkner SH, Stewart CE. Beneficial synergistic interactions of TNF-alpha and IL-6 in C2 skeletal myoblasts--potential cross-talk with IGF system. Growth Factors. 2008;26:61–73. doi: 10.1080/08977190802025024. [DOI] [PubMed] [Google Scholar]
- 6.Zhou XD, Xiong MM, Tan FK, Guo XJ, Arnett FC. SPARC, an upstream regulator of connective tissue growth factor in response to transforming growth factor beta stimulation. Arthritis Rheum. 2006;54:3885–3889. doi: 10.1002/art.22249. [DOI] [PubMed] [Google Scholar]
- 7.Scholz D, Thomas S, Sass S, Podzuweit T. Angiogenesis and myogenesis as two facets of inflammatory post-ischemic tissue regeneration. Mol Cell Biochem. 2003;246:57–67. [PubMed] [Google Scholar]
- 8.Wagatsuma A. Endogenous expression of angiogenesis-related factors in response to muscle injury. Mol Cell Biochem. 2007;298:151–159. doi: 10.1007/s11010-006-9361-x. [DOI] [PubMed] [Google Scholar]
- 9. Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–774. doi: 10.1007/s00441-011-1185-7. • Comprehensive review of muscle regeneration with focus of contribution of inflammatory cells
- 10.Nguyen F, Guigand L, Goubault-Leroux I, Wyers M, Cherel Y. Microvessel density in muscles of dogs with golden retriever muscular dystrophy. Neuromuscul Disord. 2005;15:154–163. doi: 10.1016/j.nmd.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet. 2002;11:263–272. doi: 10.1093/hmg/11.3.263. [DOI] [PubMed] [Google Scholar]
- 12.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 1999;144:1113–1121. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 15. Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, Reggiani C, Vitiello L, Elvassore N, De Coppi P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25:2296–2304. doi: 10.1096/fj.10-174755. • In situ repair using a novel biomaterial and muscle stem cells resulting in the formation of neural and vascular networks as well as the reconstitution of the satellite cell niche
- 16.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32:8905–8914. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi CA, Pozzobon M, De Coppi P. Advances in musculoskeletal tissue engineering: moving towards therapy. Organogenesis. 2010;6:167–172. doi: 10.4161/org.6.3.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boldrin L, Elvassore N, Malerba A, Flaibani M, Cimetta E, Piccoli M, Baroni MD, Gazzola MV, Messina C, Gamba P, et al. Satellite cells delivered by micro-patterned scaffolds: a new strategy for cell transplantation in muscle diseases. Tissue Eng. 2007;13:253–262. doi: 10.1089/ten.2006.0093. [DOI] [PubMed] [Google Scholar]
- 20.Serena E, Flaibani M, Carnio S, Boldrin L, Vitiello L, De Coppi P, Elvassore N. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol Res. 2008;30:207–214. doi: 10.1179/174313208X281109. [DOI] [PubMed] [Google Scholar]
- 21.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian W, Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng Med Biol Mag. 2008;27:109–113. doi: 10.1109/MEMB.2008.928460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1557–C1565. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- 24.Rhim C, Lowell DA, Reedy MC, Slentz DH, Zhang SJ, Kraus WE, Truskey GA. Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve. 2007;36:71–80. doi: 10.1002/mus.20788. [DOI] [PubMed] [Google Scholar]
- 25.Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32:3575–3583. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YC, Dennis RG, Larkin L, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol. 2005;98:706–713. doi: 10.1152/japplphysiol.00273.2004. Epub 2004 Oct 2008. [DOI] [PubMed] [Google Scholar]
- 27.Kroehne V, Heschel I, Schugner F, Lasrich D, Bartsch JW, Jockusch H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J Cell Mol Med. 2008;12:1582–1838. doi: 10.1111/j.1582-4934.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bian W, Bursac N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials. 2009;30:1401–1412. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian W, Liau B, Badie N, Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc. 2009;4:1522–1534. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheema U, Yang SY, Mudera V, Goldspink GG, Brown RA. 3-D in vitro model of early skeletal muscle development. Cell Motil Cytoskeleton. 2003;54:226–236. doi: 10.1002/cm.10095. [DOI] [PubMed] [Google Scholar]
- 31.Vandenburgh HH, Hatfaludy S, Karlisch P, Shansky J. Skeletal muscle growth is stimulated by intermittent stretch-relaxation in tissue culture. Am J Physiol. 1989;256:C674–C682. doi: 10.1152/ajpcell.1989.256.3.C674. [DOI] [PubMed] [Google Scholar]
- 32.van der Schaft DW, van Spreeuwel AC, van Assen HC, Baaijens FP. Mechanoregulation of vascularization in aligned tissue-engineered muscle: a role for vascular endothelial growth factor. Tissue Eng Part A. 2011;17:2857–2865. doi: 10.1089/ten.TEA.2011.0214. [DOI] [PubMed] [Google Scholar]
- 33.Langelaan ML, Boonen KJ, Rosaria-Chak KY, van der Schaft DW, Post MJ, Baaijens FP. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J Tissue Eng Regen Med. 2011;5:529–539. doi: 10.1002/term.345. [DOI] [PubMed] [Google Scholar]
- 34.Gawlitta D, Boonen KJ, Oomens CW, Baaijens FP, Bouten CV. The influence of serum-free culture conditions on skeletal muscle differentiation in a tissue-engineered model. Tissue Eng Part A. 2008;14:161–171. doi: 10.1089/ten.a.2007.0095. [DOI] [PubMed] [Google Scholar]
- 35.Takano K, Watanabe-Takano H, Suetsugu S, Kurita S, Tsujita K, Kimura S, Karatsu T, Takenawa T, Endo T. Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science. 2010;330:1536–1540. doi: 10.1126/science.1197767. [DOI] [PubMed] [Google Scholar]
- 36.Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, Valentini R, Crawford G. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve. 2008;37:438–447. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- 37.Weist MR, Wellington MS, Bermudez JE, Kostrominova TY, Mendias CL, Arruda EM, Larkin LM. TGF-beta1 enhances contractility in engineered skeletal muscle. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koffler J, Kaufman-Francis K, Yulia S, Dana E, Daria AP, Landesberg A, Levenberg S. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci U S A. 2011;108:14789–14794. doi: 10.1073/pnas.1017825108. • Demonstration that long-term tri-culture of C2C12 mouse myoblasts, human umbilical vein endothelial cells, and human foreskin fibroblasts to pre-form vascular structures in vitro promotes survival, maturation and integration of engineered muscle in vivo
- 40.Borschel GH, Dow DE, Dennis RG, Brown DL. Tissue-engineered axially vascularized contractile skeletal muscle. Plast Reconstr Surg. 2006;117:2235–2242. doi: 10.1097/01.prs.0000224295.54073.49. [DOI] [PubMed] [Google Scholar]
- 41.Messina A, Bortolotto SK, Cassell OC, Kelly J, Abberton KM, Morrison WA. Generation of a vascularized organoid using skeletal muscle as the inductive source. Faseb J. 2005;19:1570–1572. doi: 10.1096/fj.04-3241fje. [DOI] [PubMed] [Google Scholar]
- 42.Bach AD, Beier JP, Stark GB. Expression of Trisk 51, agrin and nicotinic-acetycholine receptor epsilon-subunit during muscle development in a novel three-dimensional muscle-neuronal co-culture system. Cell Tissue Res. 2003;314:263–274. doi: 10.1007/s00441-003-0757-6. [DOI] [PubMed] [Google Scholar]
- 43.Larkin LM, Van der Meulen JH, Dennis RG, Kennedy JB. Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim. 2006;42:75–82. doi: 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- 44.Dhawan V, Lytle IF, Dow DE, Huang YC, Brown DL. Neurotization improves contractile forces of tissue-engineered skeletal muscle. Tissue Eng. 2007;13:2813–2821. doi: 10.1089/ten.2007.0003. [DOI] [PubMed] [Google Scholar]
- 45. Bian W, Bursac N. Soluble miniagrin enhances contractile function of engineered skeletal muscle. FASEB J. 2012;26:955–965. doi: 10.1096/fj.11-187575. • Demonstration that transient application of synaptogenic factor agrin improves contractile function and acetylcholine receptor clustering in neonatal rat engineered muscle in vitro
- 46.Boldrin L, Muntoni F, Morgan JE. Are human and mouse satellite cells really the same? J Histochem Cytochem. 2010;58:941–955. doi: 10.1369/jhc.2010.956201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmalbruch H, Hellhammer U. The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec. 1977;189:169–175. doi: 10.1002/ar.1091890204. [DOI] [PubMed] [Google Scholar]
- 48. Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. •• Demonstration that modifications in substrate stifness may allow the expansion of adult muscle stem cells without the loss of their regenerative capacity
- 49.Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 50.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 51.Benchaouir R, Meregalli M, Farini A, D'Antona G, Belicchi M, Goyenvalle A, Battistelli M, Bresolin N, Bottinelli R, Garcia L, et al. Restoration of human dystrophin following transplantation of exon-skipping- engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell. 2007;1:646–657. doi: 10.1016/j.stem.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Farini A, Razini P, Erratico S, Torrente Y, Meregalli M. Cell based therapy for Duchenne muscular dystrophy. J Cell Physiol. 2009;221:526–534. doi: 10.1002/jcp.21895. [DOI] [PubMed] [Google Scholar]
- 53.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 54. Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RC. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. •• Demonstration that conditional expression of Pax7 in human ES/iPS cells enables derivation of myogenic precursors which engraft efficiently in nude and mdx mice and seed the satellite cell compartment
- 55.Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mamchaoui K, Trollet C, Bigot A, Negroni E, Chaouch S, Wolff A, Kandalla PK, Marie S, Di Santo J, St Guily JL, et al. Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skelet Muscle. 2011;1:34. doi: 10.1186/2044-5040-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serena E, Zatti S, Reghelin E, Pasut A, Cimetta E, Elvassore N. Soft substrates drive optimal differentiation of human healthy and dystrophic myotubes. Integr Biol (Camb) 2010;2:193–201. doi: 10.1039/b921401a. [DOI] [PubMed] [Google Scholar]
- 58.Sengupta D, Gilbert PM, Johnson KJ, Blau HM, Heilshorn SC. Protein-engineered biomaterials to generate human skeletal muscle mimics. Adv Healthc Mater. 2012;1:785–789. doi: 10.1002/adhm.201200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiron S, Tomczak C, Duperray A, Laine J, Bonne G, Eder A, Hansen A, Eschenhagen T, Verdier C, Coirault C. Complex interactions between human myoblasts and the surrounding 3D fibrin-based matrix. PLoS One. 2012;7:e36173. doi: 10.1371/journal.pone.0036173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32:9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. • Demonstration of functional nueromuscular synapses in co-culture of human myotubes and human motoneurons resulting in spontaneous myotube contractions
- 61.Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]