Abstract
Purpose
Vagus nerve stimulation (VNS) therapy is a procedure to control seizure frequency in patients with medically intractable epilepsy. However, there is no data on efficacy in the subset of these patients with brain tumors. The purpose of this study is to evaluate the efficacy of VNS therapy in patients with brain tumor-associated medically intractable epilepsy.
Methods
Data from the VNS therapy Patient Outcome Registry, maintained by the manufacturer of the device, Cyberonics Inc. (Houston, TX, USA), was queried to characterize the response of patients in whom a brain tumor was listed as the etiology of epilepsy. A case–control analysis was implemented and patient outcome was measured by Engel classification, median seizure response and responder rate (≥50% seizure reduction) using t-tests and chi-squared tests.
Results
In 107 patients with an epilepsy etiology related to a brain tumor, seizure reduction was 45% at 3 months and 79% at 24 months with a responder rate of 48% at 3 months and 79% at 24 months. There was no statistical difference in seizure reduction compared with 326 case–control patients from the registry without brain tumors. There was no significant difference in anti-epileptic drug (AED) usage from baseline to 24 months post implant in either group.
Conclusions
VNS therapy is equally effective in patients who suffer seizures secondary to brain tumors as in patients without history of a brain tumor. VNS therapy is a viable treatment option for patients with brain tumor associated medically intractable epilepsy, assuming cytoreductive and other adjuvant therapies have been fully explored.
Keywords: Vagus nerve stimulation, Medically intractable epilepsy, Seizures, Brain tumors
1. Introduction
Approximately 25% of epilepsy patients remain refractory to antiepileptic drugs (AEDs).1,2 An alternative treatment option is vagal nerve stimulation (VNS).3 VNS therapy (Cyberonics Inc., Houston, TX, USA) is used as an adjunctive therapy in reducing the frequency of partial onset seizures in medically refractory patients.4
Numerous studies have supported VNS therapy for medically intractable epilepsy.5–9 However, patient characteristics predictive of responsiveness to VNS therapy remain unknown. Studies have yet to consistently find association between VNS therapy responsiveness and patient features.10,11
VNS therapy has not been prospectively studied in patients with partial seizures caused by the presence of a brain tumor. Approximately 30–50% of brain tumor patients will have a seizure at some point12 and approximately 30% of these will become intractable.13 The treatment of choice for these tumors is surgical resection, a procedure capable of achieving seizure freedom in 30–60% of cases.14–17 However, there exists a subset of tumor patients in whom removal of the tumor does not control seizures either due to incomplete tumor resection or establishment of an independent seizure focus.12,18,19 Only a portion of these patients may be candidates for epilepsy surgery.19,20 In this group, seizure control can be extremely important for the quality of life.21–23
There are no reports establishing the efficacy of VNS for tumor-related epilepsy and hence little data to serve physicians. The aim of this study is to evaluate the efficacy of VNS therapy for seizure control in patients with brain tumor-associated intractable epilepsy.
2. Methods
The study leveraged data from the VNS therapy Patient Outcome Registry maintained by the manufacturer of the device, Cyberonics Inc. Data were prospectively and voluntarily provided by 1285 prescribing physicians from 978 centers (911 in the U.S. and Canada and 67 international) at patients’ pre-operative baselines and intervals post-VNS implantation. Neurologists sent case report forms (CRFs) based on patient medical history to Cyberonics. At baseline, a patient history and implant form was submitted to collect information on patient demographics, epilepsy etiology and syndrome, medical history, baseline seizure types and monthly frequencies, current AEDs, and quality of life assessments. At each follow-up visit of 3, 6, 12, and 24 months post-implantation, information was collected on seizure types, seizure frequency (overall and by seizure type), current AEDs, and quality of life assessments. An independent auditing agency has previously authenticated the integrity of the systems for collecting and processing the registry data.24 Active data collection ceased in 2003, and the registry was queried in March 2012 to characterize the response of patients with intractable epilepsy associated with a history of brain tumors.
Patients with brain tumors were initially identified from the CRFs as having any history of a brain tumor. The listed epilepsy etiologies and brain tumor information for these patients were reviewed, and only patients with an etiology related to the brain tumor remained in the study (e.g. “brain tumor”, “astrocytoma”, “meningioma”, “oligodendroglioma”, etc.) while patients with possibly unrelated epilepsy etiologies were removed (e.g. “tuberous sclerosis”, “no information”, “hamartoma”, “brain injury”, etc.). Follow-up visits of 3, 6, 12 and 24 months were examined, and the response of each VNS therapy patient was calculated as the percent decrease of overall seizure frequency by comparing the seizure frequency reported by the treating neurologist at each follow-up visit (via a 1 month average) to the pre-operative baseline recording (via a 3 month average). The patients were classified according to the Engel classification scheme.25 Patients were designated as a “responder” if they had at least a 50% reduction in seizures from their baseline frequency.
It was desired to compare these results against VNS therapy patients without brain tumor history as the efficacy of VNS therapy for all patients with partial onset seizures is well established.4,5 A case–control study design was utilized that matched two non-brain tumor patients for each brain tumor patient at each available follow-up visit. Patients were excluded from the study if they had no specified etiology or if there were no data for any follow-up visits. A sub-analysis of brain tumor patients who had undergone resection surgery prior to receiving VNS was also performed with seizure response outcomes reported only at 12 months due to small sample size.
Two controls without brain tumors from the same VNS therapy Patient Outcome Registry were selected for each case with brain tumors, without any consideration of the response variables based on the following criteria to control potentially confounding variables: match of follow-up period, match of gender, age of implant within 8 years, age of diagnosis within 8 years, and baseline seizure frequency within 60%. For the 9% of brain tumor patients with a missing age of diagnosis, the two controls were selected without restriction on age of diagnosis, but the age of implant was still included; no other variables had any missing data. If at least two non-brain tumor controls met all of the above criteria, the two that most closely matched the seizure type profile of the case were selected, with exact matches made where possible. If two appropriate controls were not found, then the brain tumor case was not included. This methodology allowed for close matches at each follow-up visit period where the outcome analysis was to be performed, and the case–control selection was considered acceptable if all potential confounders had a non-significant two-sided p-value > 0.05 for unique patients included in the study. Patient gender, race, age at diagnosis, age at implantation, pre-operative duration of epilepsy, baseline seizure frequency, baseline seizure type, and number of AEDs were compared for patients with brain tumors versus patients without non-brain tumors. Student’s t-tests were used to compare means of continuous variables, unless the data were non-normal (i.e. baseline seizure frequency), in which case the Wilcoxon Rank-Sum test was used to compare medians; chi-squared tests were used for categorical variables.
Patient outcome was measured by Engel classification, median seizure response and responder rate (i.e. percentage of patients with at least 50% seizure reduction from baseline). The Cochran–Mantel–Haenszel statistic for row means scores was used to test whether there was a relationship between brain tumor status and Engel class, controlling for the four follow-up time points in all instances. An odds ratio with 95% confidence interval (CI) was calculated to measure the overall likelihood of achieving clinical response (≥50% reduction in seizure frequency) between patients with and without brain tumors, utilizing the Breslow–Day test for homogeneity of the odds ratios at the different follow-up times. 95% CIs were calculated for median seizure response using distribution-free limits and for responder rate using score confidence limits. The Wilcoxon Rank-Sum test and two-proportion z-test were used to compare median percent decrease in seizure frequency and responder rates, respectively, between patients with and without brain tumors at each follow-up visit. Power analysis was performed at 12 and 24 months to detect a 20% difference in responder rates. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
3. Results
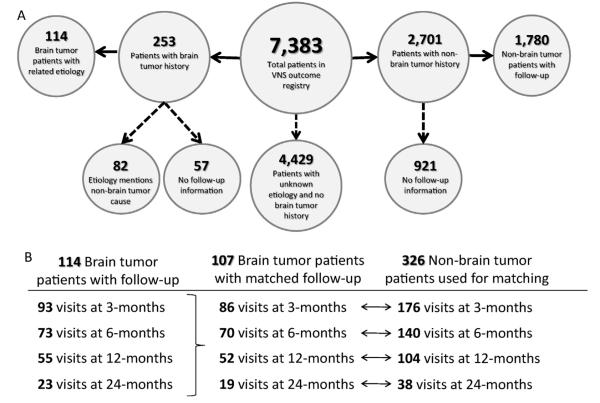
The response rates of VNS therapy patients with an epilepsy etiology related to their brain tumor history and VNS therapy patients without a history of any brain tumor were calculated from 7383 total individuals in the VNS therapy Patient Outcome Registry. Individuals without a specified etiology or brain tumor history were excluded from the study, as well as those without any data on follow-up visits (Fig. 1A). In total, the registry contained 244 follow-up visits for 114 brain tumor patients and 3846 follow-up visits for 1780 non-brain tumor patients.
Fig. 1.
Study design summary. Data pertaining to patients with and without brain tumor history were extracted from 7383 total individuals in the VNS therapy Patient Outcome Registry (A). Individuals without a specified etiology or brain tumor history were excluded from the study, as well as those without any data on follow-up visits. Solid lines indicate included patients; dashed lines indicate excluded patients. Of the 114 brain tumor patients with a known epilepsy etiology related to the tumor, 107 patients had at least one follow-up visit matched to two non-brain tumor patient follow-up visits (B).
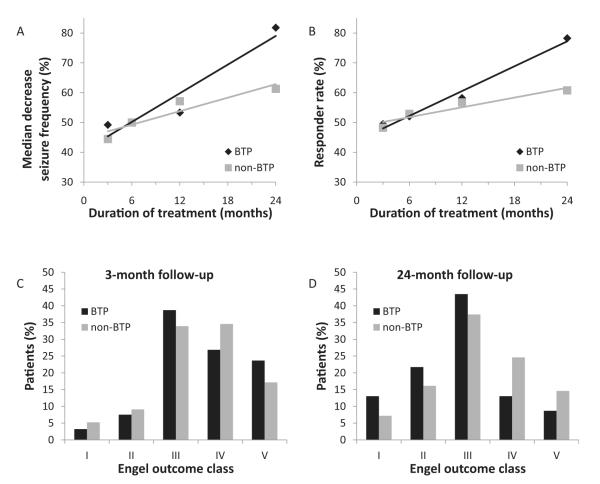
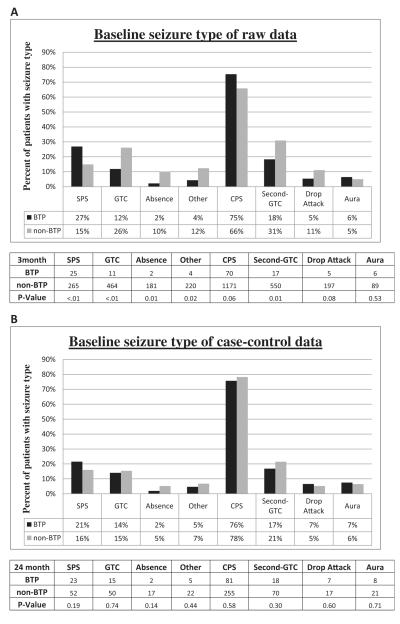
Initial analysis of the overall groups showed similarly improving response rates over time for both groups (Fig. 2). The median seizure reduction in brain tumor patients increased from 49% (CI: 33%, 58%) at 3 months to 82% (CI: 68%, 94%) at 24 months, marginally better than the results of non-brain tumor patients of 44% (CI: 40%, 50%) at 3 months and 61% (CI: 54%, 67%) at 24 months. Likewise, the clinical response rate of brain tumor patients increased from 49% (CI: 40%, 59%) at 3 months to 78% (CI: 58%, 90%) at 24 months, with a similar increase in non-brain tumor patients of 48% (CI: 46%, 51%) at 3 months to 61% (CI: 56%, 65%) at 24 months. However, comparing potential confounders showed significant differences between the two overall groups in age of implant, age of diagnosis, and baseline seizure types (Table 1A and Fig. 4A). Therefore the raw responses could not be directly compared between the two groups. As a result, a case–control study was utilized to match each available follow-up visit for all brain tumor patients with two similar follow-up visits from non-brain tumor patients matched for age of implant, age of diagnosis, and baseline seizure rate. There was no matching for the response variables. Additional variables (i.e. gender, race, and baseline seizure frequency) that were not significantly different between the two groups were included in the matching control process as they are potentially relevant covariates or relate directly to the response calculations. A total of 17 follow up visits for patients with brain tumors were excluded from the study due to insufficient matches with follow-up visits for patients without brain tumors; the excluded visits numbered 7, 3, 3 and 4 at months 3, 6, 12 and 24, respectively. The remaining 227 follow-up patient visits for 107 brain tumor patients and 454 follow-up visits for 326 non-brain tumor patients were used for the remainder of the analysis (Fig. 1B). After case–control selection, the baseline demographics of all identified potential confounders were no longer significantly different, indicating a successful control selection (Table 1B). As many patients presented with multiple seizure types, each seizure type was analyzed separately to show that no difference existed between the groups after matching (Fig. 4B).
Fig. 2.
Raw data depicting seizure outcomes after VNS therapy in brain tumor patients (BTP) versus non-BTP. Median percent decrease in seizure frequency (A) and responder rates (B) are shown for BTP and non-BTP patients after VNS therapy at 3, 6, 12, and 24 months. Engel outcomes are shown at 3 months (C) and 24 months (D) after VNS therapy. For (A–D), statistical analysis is deferred given unequal samples (see Table 1). N = 93, 73, 55, and 12 for BTP and 1509, 1004, 943, and 390 for non-BTP at 3, 6, 12, and 24 months, respectively.
Table 1.
Demographics of BTP versus non-BTP
| Variable | BTP patients (% or mean ± SD) | Non-BTP patients (% or mean ± SD) | P-value* |
|---|---|---|---|
| A. Raw patient data | |||
| Gender | 51% male | 55% male | 0.38 |
| 49% female | 45% male | ||
| Race | 83% White; 3% Black; 7% Hispanic; 7% other | 83% White; 5% Black; 7% Hispanic; 5% other | 0.58 |
| Age of implant | 34.4 ± 13.8 years | 26.9 ± 15.5 years | <0.001** |
| Age of diagnosis | 14.2 ± 14.8 years | 7.1 ± 10.0 years | <0.001** |
| Pre-operative duration of epilepsy | 20.2 ± 12.8 years | 19.8 ± 13.5 years | 0.79 |
| Baseline seizure frequencya | 25.0 ± 16.0 per month | 28.0 ± 24.0 per month | 0.06 |
| Baseline AEDs | 2.28 ± 0.81 | 2.49 ± 0.90 | 0.01** |
| B. Case–control data | |||
| Gender | 51% male | 51% male | 0.93 |
| 49% male | 49% male | ||
| Race | 83% White; 2% Black; 8% Hispanic; 7% other | 83% White; 5% Black; 6% Hispanic; 6% other | 0.50 |
| Age of implant | 33.2 ± 13.2 years | 32.2 ± 12.7 years | 0.52 |
| Age of diagnosis | 12.8 ± 13.6 years | 10.2 ± 11.5 years | 0.06 |
| Pre-operative duration of epilepsy | 20.4 ± 12.4 years | 22.2 ± 12.2 years | 0.21 |
| Baseline seizure frequencya | 20.0 ± 15.0 per month | 15.9 ± 12.1 per month | 0.27 |
| Baseline AEDs | 2.27 ± 0.79 | 2.35 ± 0.86 | 0.37 |
For A: N = 114 brain tumor patients (BTP) and 1780 non-brain tumor patients (non-BTP). For B: N = 107 (BTP) and 326 (non-BTP).
For baseline seizure frequency, median and MAD (median absolute deviation) are reported instead of mean and standard deviation.
P-value reflects Student’s t-test to compare the means of continuous variables, unless data were non-normal, in which case the Wilcoxon Rank-Sum test was used to compare medians (i.e. baseline seizure frequency); chi-squared test were used for categorical variables.
Statistically significant value (P < 0.05).
Fig. 4.
Baseline seizure types. Baseline seizure types are shown for BTP versus non-BTP using raw data (A) and following case–control selections (B). Many patients presented with multiple seizure types, so each seizure type was analyzed separately using chi-square tests to show that no difference existed between the groups after matching. *Statistically significant value (P < 0.05). CPS, complex partial seizure; GTC, generalized tonic-clonic seizure; second-GTC, secondarily generalized tonic-clonic seizure; SPS, simple partial seizure.
The number of AEDs at baseline was not part of the case–control selection, and analysis shows that the difference in the group means was not significant after the matching was performed (P = 0.37) (Table 1). The average number of AEDs that the patients was taking also were not significantly different between the groups at 24 months after VNS therapy (P = 0.65). For patients in both groups followed at least two years, the change in average number of AEDs from baseline to the 24 month follow-up visit was insignificant (from 2.00 to 1.79 for brain tumor patients, P = 0.46, and from 2.05 to 1.92 for non-brain tumor patients, P = 0.54).
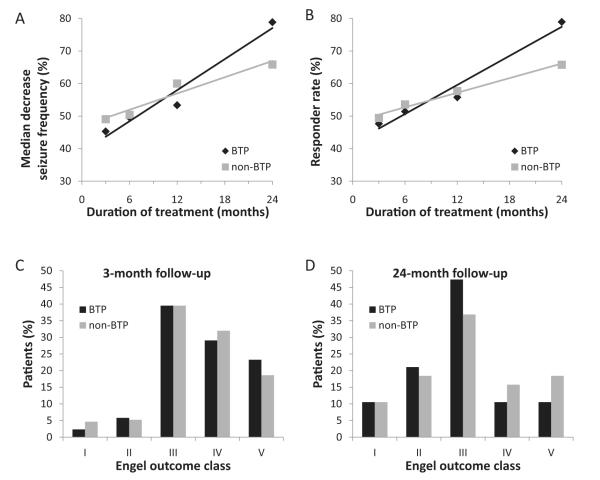
Once the case–control study design was applied, the two groups’ seizure responses were similar to the raw analysis, prior to the case–control design, with improvement over time but no significant differences between the two groups in any response measure at any of the four time points (Fig. 3). The median seizure reduction in brain tumor patients increased from 45% (CI: 33%, 53%) at 3 months to 79% (CI: 68%, 94%) at 24 months (both statistically different from no improvement, P < 0.001), but the results were not statistically different from non-brain tumor patients at the same post-operative follow-up time points (P = 0.45 and P = 0.21, respectively). Likewise, the rate of clinical response of brain tumor patients increased from 48% (CI: 37%, 58%) at 3 months to 79% (CI: 57%, 91%) at 24 months without a significant difference when compared to non-brain tumor patients (P = 0.79 and P = 0.31, respectively). The odds ratio for brain tumor patients to have a clinical response to VNS therapy versus their non-brain tumor counterparts was 0.97 with a non-significant confidence interval of 0.70–1.34. The Breslow–Day Test (P = 0.75) indicated that the odds ratio is the same across the four follow-up visits.
Fig. 3.
Case–control design data depicting seizure outcomes after VNS therapy in BTP versus non-BTP. Median percent decrease in seizure frequency (A) and responder rates (B) are shown for BTP and non-BTP patients after VNS therapy at 3, 6, 12, and 24 months. Engel outcomes are shown at 3 months (C) and 24 months (D) after VNS therapy. Statistical significance (P < 0.05) was not reached at any time point between BTP and non-BTP for any seizure outcome measure. For A and B, statistical analysis reflects Wilcoxon Rank-Sum test and two-proportion z-test, respectively. For (C and D), statistical analysis reflects Cochran–Mantel–Haenszel statistic for row means scores. N = 86, 70, 52, and 19 for BTP and 172, 140, 104, and 38 for non-BTP at 3, 6, 12, and 24 months, respectively.
Furthermore, there is no association between the patient’s brain tumor status and Engel classification response when controlling for the four follow-up durations (P = 0.86, Cochran–Mantel–Haenszel statistic).
With 52 brain tumor patients at 12 months the power was 66% for detecting a 20% difference in responder rate, and with 19 brain tumor patients at 24 months the power for detecting the same difference was 27%.
The sub-analysis of brain tumor patients who underwent resection surgery, as reported in the registry, prior to receiving VNS Therapy included 23 of the 52 matched brain tumor patients at 12 months, and this subset experienced a median seizure reduction of 54% (CI: 33%, 83%) and a responder rate of 61% (CI: 41%, 78%) one year after receiving VNS.
4. Discussion
VNS therapy has been used since the 1990s as a treatment for pharmacoresistant partial onset epilepsy.4,5 Elliott et al. reported a 61.0% mean seizure reduction in 18 patients with either a tumor, cavernoma, or arteriovenous malformation; however, there is a lack of studies exclusively on the subgroup of VNS therapy patients with a brain tumor.26 As can be derived from the number of patients with a history of brain tumor enrolled in the Cyberonics registry, VNS therapy has been implemented in a limited fashion by clinicians, indicating an implicit assumption on their part of efficacy in this particular subgroup. Nevertheless, there is likely a large group of patients with epilepsy secondary to brain tumors that is not offered VNS. Reasons for limited use may be the lack of published evidence of efficacy in this subgroup, lack of knowledge of VNS therapy in neuro-oncologists who often treat patients with brain tumor associated epilepsy, suspicion that tumor progression will undermine therapeutic efficacy, or belief that a potentially limited life-expectancy may reduce the indication for another surgical procedure, albeit a safe outpatient one.
A median seizure reduction of close to 50% indicates clear efficacy, similar to the efficacy in non-tumor related epilepsy for VNS therapy in brain tumor-associated epilepsy. One characteristic of long term VNS therapy is an increase in seizure control over time.9 Our study shows a similar long term increase in median seizure reduction and clinical responder rate in brain tumor patients. The seizure response outcomes in the sub-analysis of brain tumor patients who reported a prior resection surgery were statistically equivalent to those patients without a report of prior resection surgery.
Although VNS may be effective in this subpopulation, it is critical to appreciate that the best treatment for brain tumor associated epilepsy is complete resection of the mass lesion. Gross total lesionectomy has been shown to have seizure-freedom rates as high as 79%.27,28 In certain circumstances, an additional “epilepsy” surgery with either implanted subdural electrodes or resection of additional epileptogenic tissue is often warranted and can lead to increases in rates of seizure-freedom.27,29 Nevertheless, there is a significant proportion of patients in whom a portion of the tumor is unresectable or adjacent tissue has been rendered epileptogenic. Some patients may elect a lower risk procedure. In addition, there are patients with prolonged remission of their tumor who still have intractable epilepsy. If patients are not amenable to further open surgery, and adequate trials of AEDs have been implemented with seizure persistence, VNS therapy should be offered as a potential therapeutic option.
Another important caveat is the histology of the tumor. The most common cause of increased seizure frequency and intractability in patients with tumors is tumor progression. Patients with anaplastic or malignant tumors, in whom tumor progression may be imminent, are not likely to respond as effectively to VNS, although our study did not analyze this variable, as tumor progression data was not available in the registry. Seizure reduction through adjuvant oncologic therapy such as chemotherapy, immunotherapy, and radiation therapy may lead to more effective seizure reduction through tumor control. Likewise, patients with limited life expectancy may not want to undergo further surgery, although VNS implantation is an outpatient, low morbidity procedure.30 Though frequent MRI scans required to follow patients with tumors may seem to be a barrier to VNS implantation, certain MRI scans are conditionally safe as long as particular precautions are made, including turning the device “off” before the scan and then turning it back “on” after the scan.31,32 In addition, the position of the generator with respect to the MRI scanner may reduce the need to turn the device “off”.33
There are several limitations to this study. The first is that a registry is voluntary. The sample is not all patients with a stimulator but rather patients whom treating physicians have entered into the registry; therefore, there is the potential for sample bias. Secondly, only patients with a medical history of brain tumor and an etiology reportedly related to the tumor were included, but the authors are unable to verify the clinical context in which VNS was performed for these brain tumor patients. The third limitation is that the histology of the tumor, progression of the tumor, use of repeat tumor resection and additional adjuvant therapy is not specified. Therefore, benign and malignant tumors of all histologies are represented in this database without discrimination. Lack of efficacy from tumor progression in more malignant histologies would not be indicated, nor would false efficacy from additional tumor resection or adjuvant therapy that might lead to increased seizure control. Overall, it is likely that more benign histologies will lead to greater response rates since tumors will progress more slowly, if at all. However, the most benign pathologies, such as ganglioglioma or dysembryoplastic neuroepithelial tumor (DNET) are often cured with lesionectomy alone and do not likely represent a high proportion of intractable cases in this database.34
It is also important to consider AED use when considering the efficacy of VNS therapy. Decreasing AED use can increase quality of life by eliminating or reducing side effects. Reductions in AEDs can lead to decreased interactions with chemotherapy drugs, as AED interactions can reduce the efficacy of anticancer drugs by lowering drug concentrations via induction of the cytochrome p450 system.35 This study shows a slight decrease in AEDs in both brain tumor and non-brain tumor patients with VNS therapy. Of the 19 brain tumor patients followed at least two years, 3 were using at least one fewer AED at the end of the study (2 completely off AEDs), 14 were using the same number of AEDs, and the remaining 2 added at least one AED. Prior studies of AED reductions from VNS therapy in non-brain tumor patients have reported varied changes in AED use over time.36,37 For example, Uthman et al. reported an increase in mean number of AEDs over extended time on VNS while Tatum et al. found a significant decrease in AEDs in 42.9% of patients.38 These results suggest that when discussing VNS with patients who have seizures and brain tumors, perhaps physicians should be less confident in predicting decreased AED burdens, and more confident in predicting seizure rate reductions.
5. Conclusions
Our study supports the idea that VNS therapy is equally effective in patients who suffer seizures secondary to brain tumors as in patients without history of a brain tumor. VNS therapy should be a viable treatment option for patients with brain tumor associated medically intractable epilepsy, assuming cytoreductive and other adjuvant therapies have been adequately explored.
Acknowledgments
Funding This work was supported the Clinical and Translational Science Center at Weill-Cornell Medical College [UL1RR024996]. This grant is funded through the National Institutes of Health and therefore its publication must comply with the National Institutes of Health Public Access Policy.
Footnotes
Conflict of interest Authors MS Gordon and KH Hassnain are salaried employees of Cyberonics Inc., Houston, TX, USA. Cyberonics Inc. is the manufacturer of the VNS device. MS Gordon also owns stock in the company. The remaining authors have no conflicts of interest.
References
- 1.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B, et al. Two-year remission and subsequent relapse in children with newly diagnosed epilepsy. Epilepsia. 2001;42:1553–62. doi: 10.1046/j.1528-1157.2001.21101.x. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–90. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- 3.Benbadis SR, Tatum WO, Vale FL. When drugs don’t work: an algorithmic approach to medically intractable epilepsy. Neurology. 2000;55:1780–4. doi: 10.1212/wnl.55.12.1780. [DOI] [PubMed] [Google Scholar]
- 4.Baaj AA, Benbadis SR, Tatum WO, Vale FL. Trends in the use of vagus nerve stimulation for epilepsy: analysis of a nationwide database. Neurosurgical Focus. 2008;25:E10. doi: 10.3171/FOC/2008/25/9/E10. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Menachem E. Vagus nerve stimulation for treatment of seizures? Archives of Neurology. 1998;55:231–2. doi: 10.1001/archneur.55.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 8.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01–E05. Neurology. 1999;53:1731–5. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 10.Labar D. Vagus nerve stimulation for 1 year in 269 patients on unchanged antiepileptic drugs. Seizure. 2004;13:392–8. doi: 10.1016/j.seizure.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. Journal of Neurosurgery. 2011;115:1248–55. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 12.You G, Sha Z, Jiang T. The pathogenesis of tumor-related epilepsy and its implications for clinical treatment. Seizure. 2012;21:153–9. doi: 10.1016/j.seizure.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Pierzchala K. Pharmacoresistant epilepsy—epidemiology and current studies. Neurologia i Neurochirurgia Polska. 2010;44:285–90. doi: 10.1016/s0028-3843(14)60043-8. [DOI] [PubMed] [Google Scholar]
- 14.Awad IA, Rosenfeld J, Ahl J, Hahn JF, Luders H. Intractable epilepsy and structural lesions of the brain: mapping, resection strategies, and seizure outcome. Epilepsia. 1991;32:179–86. doi: 10.1111/j.1528-1157.1991.tb05242.x. [DOI] [PubMed] [Google Scholar]
- 15.Cascino GD. Epilepsy and brain tumors: implications for treatment. Epilepsia. 1990;31(Suppl. 3):S37–44. doi: 10.1111/j.1528-1157.1990.tb05858.x. [DOI] [PubMed] [Google Scholar]
- 16.Cascino GD, Kelly PJ, Hirschorn KA, Marsh WR, Sharbrough FW. Stereotactic resection of intra-axial cerebral lesions in partial epilepsy. Mayo Clinic Proceedings. 1990;65:1053–60. doi: 10.1016/s0025-6196(12)62716-5. [DOI] [PubMed] [Google Scholar]
- 17.Goldring S, Rich KM, Picker S. Experience with gliomas in patients presenting with a chronic seizure disorder. Clinical Neurosurgery. 1986;33:15–42. [PubMed] [Google Scholar]
- 18.Kerrigan S, Grant R. Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database of Systematic Reviews. 2011:CD008586. doi: 10.1002/14651858.CD008586.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Groot M, Reijneveld JC, Aronica E, Heimans JJ. Epilepsy in patients with a brain tumour: focal epilepsy requires focused treatment. Brain. 2012;135(Pt 4):1002–16. doi: 10.1093/brain/awr310. http://dx.doi.org/10.1093/brain/awr310. Epub 2011 Dec 13. [DOI] [PubMed] [Google Scholar]
- 20.Englot DJ, Berger MS, Chang EF, Garcia PA. Characteristics and treatment of seizures in patients with high-grade glioma: a review. Neurosurgery Clinics of North America. 2012;23:227–35. doi: 10.1016/j.nec.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Chavel SM, Westerveld M, Spencer S. Long-term outcome of vagus nerve stimulation for refractory partial epilepsy. Epilepsy Behav. 2003;4(3):302–9. doi: 10.1016/s1525-5050(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 22.Dodrill CB, Morris GL. Effects of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy Behav. 2001;2(1):46–53. doi: 10.1006/ebeh.2000.0148. [DOI] [PubMed] [Google Scholar]
- 23.Cramer JA. Exploration of changes in health-related quality of life after 3 months of vagus nerve stimulation. Epilepsy Behav. 2001;2(5):460–5. doi: 10.1006/ebeh.2001.0248. [DOI] [PubMed] [Google Scholar]
- 24.Amar AP, Apuzzo ML, Liu CY. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery. 2004;55:1086–93. doi: 10.1227/01.neu.0000141073.08427.76. [DOI] [PubMed] [Google Scholar]
- 25.Engel JJ, Van Ness P, Rasmussen T. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of epilepsies. Raven Press; New York: 1993. pp. 609–21. [Google Scholar]
- 26.Elliott RE, Morsi A, Kalhorn SP, Marcus J, Sellin J, Kang M, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy and Behavior. 2011;20:57–63. doi: 10.1016/j.yebeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70:921–8. doi: 10.1227/NEU.0b013e31823c3a30. [discussion 928] [DOI] [PubMed] [Google Scholar]
- 28.Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012;53:51–7. doi: 10.1111/j.1528-1167.2011.03269.x. [DOI] [PubMed] [Google Scholar]
- 29.Ghareeb F, Duffau H. Intractable epilepsy in paralimbic Word Health Organization Grade II gliomas: should the hippocampus be resected when not invaded by the tumor? Journal of Neurosurgery. 2012;116:6–1234. doi: 10.3171/2012.1.JNS112120. [DOI] [PubMed] [Google Scholar]
- 30.Landy HJ, Ramsay RE, Slater J, Casiano RR, Morgan R. Vagus nerve stimulation for complex partial seizures: surgical technique, safety, and efficacy. Journal of Neurosurgery. 1993;78:26–31. doi: 10.3171/jns.1993.78.1.0026. [DOI] [PubMed] [Google Scholar]
- 31.Sucholeiki R, Alsaadi TM, Morris GL, 3rd, Ulmer JL, Biswal B, Mueller WM. fMRI in patients implanted with a vagal nerve stimulator. Seizure. 2002;11:157–62. doi: 10.1053/seiz.2001.0601. [DOI] [PubMed] [Google Scholar]
- 32.Physician’s manual, V.T.A.M.G. MRI with the VNS therapy system. Cyberonics. 2011 26-0006-4200/5 (U.S.) [Google Scholar]
- 33.Maniker A, Liu WC, Marks D, Moser K, Kalnin A. Positioning of vagal nerve stimulators: technical note. Surgical Neurology (United States) 2000:178–81. doi: 10.1016/s0090-3019(99)00176-7. [DOI] [PubMed] [Google Scholar]
- 34.Ozlen F, Gunduz A, Asan Z, Tanriverdi T, Ozkara C, Yeni N, et al. Dysembryoplastic neuroepithelial tumors and gangliogliomas: clinical results of 52 patients. Acta Neurochirurgica. 2010;152:1661–71. doi: 10.1007/s00701-010-0696-4. [DOI] [PubMed] [Google Scholar]
- 35.Yap KY, Chui WK, Chan A. Drug interactions between chemotherapeutic regimens and antiepileptics. Clinical Therapeutics. 2008;30:1385–407. doi: 10.1016/j.clinthera.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Elliott RE, Morsi A, Tanweer O, Grobelny B, Geller E, Carlson C, et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS > 10 years. Epilepsy and Behavior. 2011;20:478–83. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 37.Uthman BM, Reichl AM, Dean JC, Eisenschenk S, Gilmore R, Reid S, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124–6. doi: 10.1212/01.wnl.0000138499.87068.c0. [DOI] [PubMed] [Google Scholar]
- 38.Tatum WO, Johnson KD, Goff S, Ferreira JA, Vale FL. Vagus nerve stimulation and drug reduction. Neurology. 2001;56:561–3. doi: 10.1212/wnl.56.4.561. [DOI] [PubMed] [Google Scholar]