Abstract
While activation of the Notch pathway is observed in many human cancers, it is unknown whether elevated Notch1 expression is sufficient to initiate tumorigenesis in most tissues. To test the oncogenic potential of Notch1 in solid tumors, we expressed an activated form of NOTCH1 (N1ICD) in the developing mouse brain. N1ICD;hGFAP-cre mice were viable but developed severe ataxia and seizures, and died by weaning age. Analysis of transgenic embryonic brains revealed that N1ICD expression induced p53-dependent apoptosis. When apoptosis was blocked by genetic deletion of p53, 30~40% of N1ICD;GFAP-cre;p53+/− and N1ICD;GFAP-cre;p53−/− mice developed spontaneous medulloblastomas. Interestingly, Notch1-induced medulloblastomas most closely resembled the sonic hedgehog (SHH) subgroup of human medulloblastoma at the molecular level. Surprisingly, N1ICD-induced tumors do not maintain high levels of the Notch pathway gene expression, except for Notch2, demonstrating that initiating oncogenic events may not be decipherable by analyzing growing tumors in some cases. In summary, this study demonstrates that Notch1 has an oncogenic potential in the brain when combined with other oncogenic hits, such as p53 loss, and provides a novel mouse model of medulloblastoma.
Introduction
The Notch pathway is a highly conserved signaling pathway that is essential for normal development, and is implicated in both somatic stem cell homeostasis and human cancer (1–4). The canonical Notch pathway mediates cell-cell communication among adjacent cells that are in direct contact with one another, through membrane-bound ligand-receptor interactions (juxtacrine signaling). When a ligand (DELTA and JAGGED family members) binds to NOTCH receptors (NOTCH1 - 4 in mammals), NOTCH is cleaved through highly controlled step-wise processes, and the intracellular domain of NOTCH (NICD) is released from the membrane and enters the nucleus to form a transcriptional complex. In the nucleus, NICD displaces repressive co-factors bound to RBPJ and recruits MAML to turn on expression of downstream target genes, including Hes1, Hes5, Hey1, and Hey2 (5).
A growing body of evidence implicates the Notch pathway in human cancers. For example, >50% of T-ALL (T-cell Acute Lymphoblastic Leukemia) patients have activating mutations in the Notch1 gene (6, 7), indicating that Notch1 has oncogenic potential in the hematopoietic system. While activating mutations in Notch genes have not been widely reported in solid cancers, Notch pathway activity level is high in many solid cancers, including brain cancers (2, 3, 8).
Medulloblastoma is the most common form of pediatric brain tumor and is considered a classical example of a developmental cancer. They are thought to arise from abnormal proliferation of EGL progenitors in the cerebellum (9), although there is evidence that neural stem cells (NSCs) can also be transformed to give rise to medulloblastomas (10, 11). Genetically, two key developmental signaling pathways, WNT and SHH pathways, have been implicated in heritable forms of medulloblastoma, Turcot’s syndrome, and Gorlin syndrome, respectively (12, 13). Although the Notch pathway is not genetically linked to medulloblastomas, several lines of evidence implicate the Notch pathway in medulloblastoma pathogenesis. First, NOTCH2 RNA level is reported to be higher in medulloblastomas than normal fetal brain, and NOTCH2 and HES1 expression levels significantly correlate with shorter survival time among medulloblastoma patients (14). Second, ~15% of PNET and medulloblastomas contained amplification of the genomic region containing the NOTCH2 gene (14), and recent sequencing studies of medulloblastoma genome identified mutations within the NOTCH3 and NOTCH4 genes (15, 16). Third, Notch signaling is necessary to maintain cancer stem cells in brain tumors: inhibition of the Notch pathway leads to reduced self-renewal, tumorigenic capacity, and radio-resistance of medulloblastoma and glioma stem cells (17–20). Together, these studies suggest that aberrant activation of the Notch pathway may lead to tumor formation in the brain; however, the oncogenic potential of Notch pathway activation has not yet been demonstrated experimentally (21–25).
In this study, we provide in vivo evidence that aberrant Notch pathway activation can be an initiating oncogenic event in the brain. We used a transgenic approach to express a constitutively activated form of Notch1 (N1ICD) in the developing mouse brain, and show that while N1ICD expression alone is not sufficient to cause brain tumor formation, N1ICD expression accompanied by loss of single or both copies of p53 results in medulloblastoma formation.
Results
Ectopic N1ICD expression results in abnormal brain development
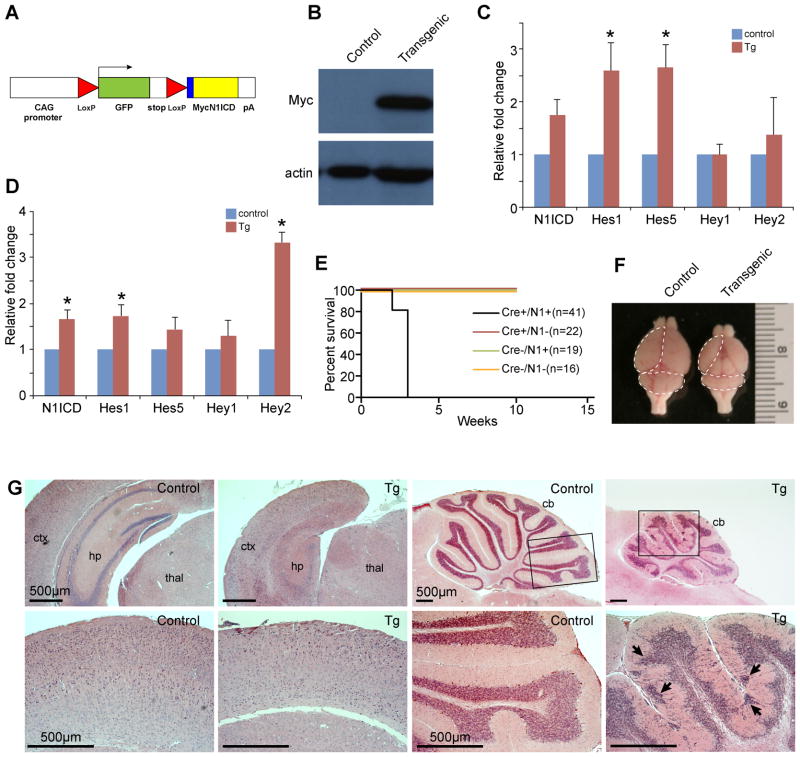
To determine whether Notch1 can act as an oncogene in the brain, we conditionally expressed a constitutively activate form of Notch1, N1ICD, in the developing mouse brain using a Cre-lox system (Fig. 1A). When N1ICD mice were crossed to hGFAP-cre mice, the double transgenic (N1ICD;hGFAP-cre) embryos showed high expression of the transgene in the developing brain (Fig. 1 B). Realtime RT-PCR analysis of N1ICD;hGFAP-cre and control littermate cortices and cerebella at E15.5 confirmed elevated level of N1ICD and Notch pathway target genes, Hes1, Hes5, Hey1, and Hey2 at the RNA level in transgenic brains (Fig. 1C, D). N1ICD;hGFAP-cre mice were viable but developed severe ataxia and seizures by weaning age, and all mice died by 4 weeks of age (Fig. 1E). Gross morphological examination of brains from postnatal day 20 control and N1ICD;hGFAP-cre littermates showed severely hypoplastic brains in transgenic mice compared to controls (Fig. 1F). Histological analysis showed that transgenic brains were characterized by severely hypoplastic hippocampus lacking dentate gyrus, thinner and disorganized cortical layers with reduced number of neurons, and grossly hypoplastic cerebellum with obvious heterotopia of granule cells (Fig. 1G).
Figure 1. Ectopic N1ICD expression in the developing brain results in ataxia, seizures, and early lethality.
(A) Schematic of the N1ICD transgenic allele. The transgene consists of N-terminal fusion of Myc epitope tag to aa1810-2556 of the mouse N1ICD lacking the RAM domain. (B) Immunoblot analysis showing Myc-N1ICD expression in N1ICD;hGFAP-cre embryo brains. (C) Realtime RT-PCR analysis of control and N1ICD;hGFAP-cre littermate cortices at E15.5. p-values = 0.0774 (N1ICD), 0.0495 (Hes1), 0.0218 (Hes5), 0.9741 (Hey1), 0.6529 (Hey2). (D) Realtime RT-PCR analysis of control and N1ICD;hGFAP-cre littermate cerebella at E15.5. p-values = 0.033 (N1ICD), 0.0397 (Hes1), 0.1663 (Hes5), 0.4509 (Hey1), 0.0004 (Hey2). (E) Survival curve for N1ICD;hGFAP-cre transgenic mice, compared to all other control littermates. (F) Gross image of control and N1ICD;hGFAP-cre mice brains at p20. Dotted lines mark the cortex and cerebellum. (G) Hematoxylin and eosin staining of coronal sections of forebrain at low magnification showing hypoplastic and disorganized neocortex and hippocampus, lacking dentate gyrus. Lower panels show a higher magnification of the cortex. Sagittal views of control and N1ICD;hGFAP-cre cerebellum showing hypoplasia and heterotopia in the transgenic brain. Boxed areas in the in the upper panels of the cerebella are shown in higher magnification below. Abbreviations: ctx: cortex, hp: hippocampus, thal: thalamus, CB: cerebellum. Scale bars=500 μm. Error bars show standard error mean. * denotes p<0.05.
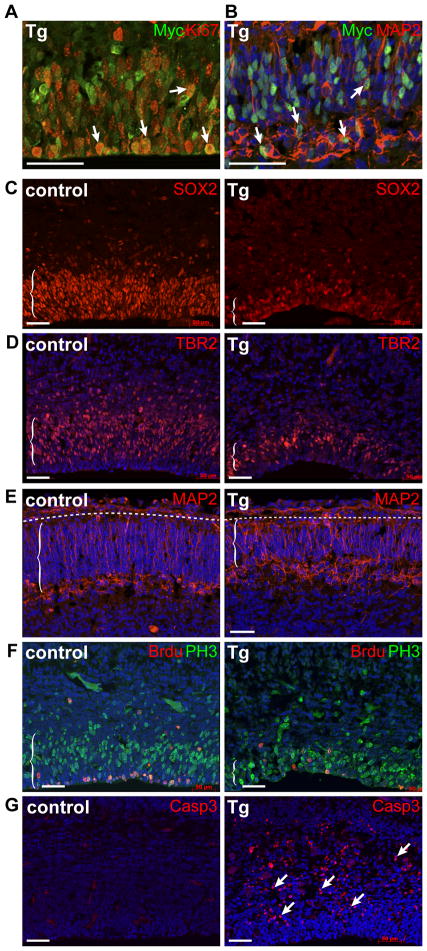
To identify the developmental origin of these brain defects, we examined N1ICD;hGFAP-cre and control littermate brains at E15.5, three days after the Cre recombinase expression and the peak of neurogenesis in the cerebral cortex. N1ICD was robustly expressed in proliferating and differentiating cells of the cortex, as indicated by the Myc-tag expression in KIi67+ proliferating cells (Fig. 2A) and MAP2+ differentiating neurons (Fig. 2B). Cell-type specific analyses with antibodies marking NSCs (SOX2, PAX6), intermediate progenitors (TBR2, Neurogenin2), and neurons (MAP2, NeuN, β-3-tubulin) showed greatly reduced numbers of all three cell types in transgenic cortices (Fig. 2C, D, E and not shown). In addition, analyses with markers for cellular proliferation (phospho-Histone 3, BrdU) showed reduced proliferation (Fig. 2F) and a marker for apoptosis (cleaved caspase3) showed concurrent increase in the number of apoptotic cells (Fig. 2G).
Figure 2. N1ICD expression increases apoptosis and reduces the number of proliferating cells and neurons in the developing cortex.
Immunofluorescence analyses with markers for: (A) the transgene, Myc, and proliferating cells (Ki67), (B) the transgene and differentiating neurons (MAP2), (C) neural stem cells (SOX2), (D) intermediate progenitors (TBR2), (E) neurons (MAP2), and (F) proliferation (PH3: M-phase, BrdU 1hour labeling: S-phase), (G) apoptosis (cleaved caspase 3). All images show coronal sections of cortices from E15.5 control and N1ICD;hGFAP-cre littermates. Arrows point to positively stained cells. Dotted lines in (E) mark the pial surface interface. Scale bar = 50μm.
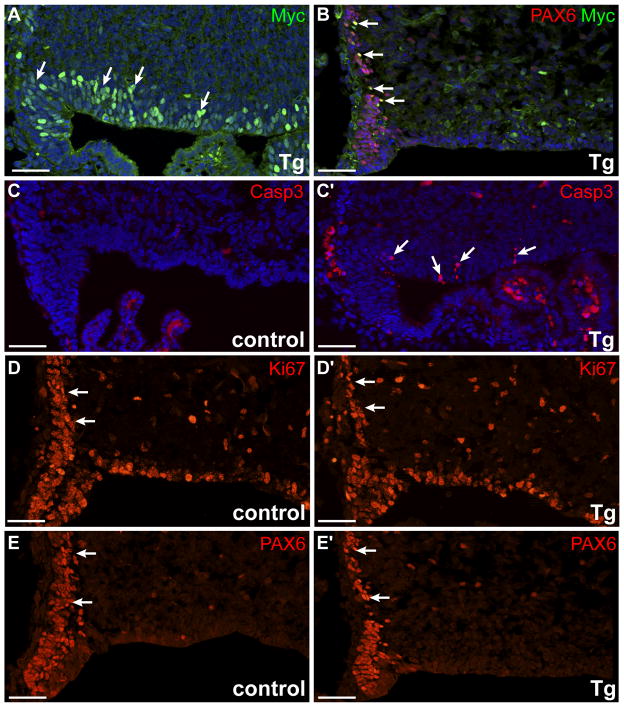
Similar to cortex, Myc+ cells were observed in the neuroepithelium and in PAX6+ EGL progenitor cells in the developing cerebellum (Fig. 3A, B). At E15.5, increased apoptosis and reduced proliferation resulted in decreased numbers of PAX6+ neural precursor cells in the transgenic cerebellum, compared to littermate control (Fig. 3C, D, E). Together, these results indicate that hypoplasia observed in N1ICD;hGFAP-cre brain regions arise from both increased cell death and early loss of proliferating stem and progenitor cells.
Figure 3. Ectopic expression of N1ICD induces apoptosis and reduces the number of neural precursors in the developing cerebellum.
Immunofluorescence analyses of E14.5 cerebella with antibodies against: (A) the transgene (Myc) in the neuroepithelium, (B) Myc and PAX6 in early EGL precursors, (C) cleaved capase 3, (D) proliferating cells (Ki67), and (E) neural precursor marker PAX6 at matching levels. All images show sagittal sections of rhombic lip region in control and N1ICD;hGFAP-cre littermates. Arrows point to positively stained cells. Scale bar = 50 μm.
N1ICD expression activates the p53 pathway in vivo
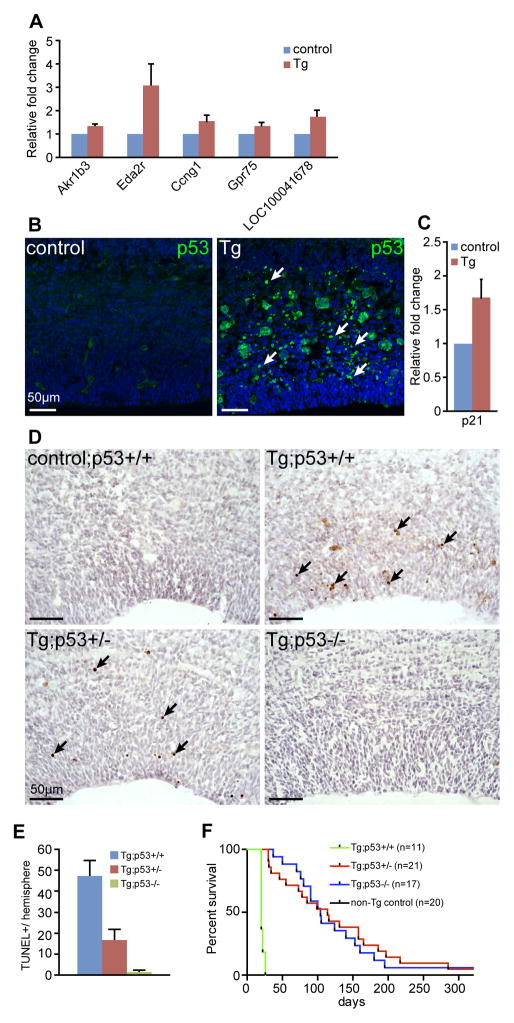
To understand the molecular basis for these brain phenotypes, we performed a transcriptome analysis of control and N1ICD:hGFAP-cre littermate cortices from E13.5 embryos, one day after the Cre recombinase is activated in the developing cortex. Only five genes (Akr1b3, Eda2r, Ccng1, Gpr75, and LOC100041678) were identified to show statistically significant expression changes (q<0.05, relative fold change (RFC)> +/− 2). The small number of differentially expressed genes observed is likely due to the variable severity of phenotypes in individual transgenic samples and the brevity of transgene expression at the time of analysis, which was designed to capture the earliest gene expression changes. Realtime RT-PCR analyses on independent sets of samples confirmed increased expression of Akr1b3, Eda2r, Ccng1 and Gpr75 in N1ICD;hGFAP-cre cortices (Fig. 4A). Interestingly, Ccng1 is involved in G2/M arrest in response to DNA damage, and subsequent cellular proliferation (26); further, Ccng1 and Eda2r are transcriptional targets of the p53 tumor suppressor gene (27, 28). These results suggest that ectopic expression of N1ICD might induce genotoxic stress that activates the p53 pathway.
Figure 4. p53 is essential for N1ICD-induced apoptosis.
(A) Realtime RT-PCR analysis of genes up-regulated in N1ICD;hGFAP-cre cortices at E13.5. (B) immunofluorescence analysis with an antibody against p53 protein, showing coronal sections through E15.5 control and N1ICD;hGFAP-cre cortices. Arrows point to p53+ cells. (C) Realtime RT-PCR analysis of a p53 target gene, Cdkn1a/p21, in control and littermate N1ICD;hGFAP-cre cortices. (D) TUNEL staining of control and transgenic (Tg: N1ICD;GFAP-cre) cortices at E15.5 in p53 wildtype, heterozygous, and homozygous backgrounds. Arrows point to TUNEL+ cells. (E) Quantitation of TUNLE+ cells in each hemisphere. >8 hemisphere sections were counted for each genotype. (F) Kaplan-Meier survival curves of N1ICD;GFAP-cre;p53 wildtype, heterozygous, and homozygous mice. Scale bar = 50μm.
To test this hypothesis, we examined p53 activation and its target gene expression in control and N1ICD;hGFAP-cre transgenic brains at E15.5. p53 protein level, assessed by immunofluorescence analysis, was elevated in transgenic cortices compared to control littermates (Fig. 4B), indicating activation of the p53 pathway. We also detected increased expression of p21, a downstream effector of p53 that mediates DNA damage response and apoptosis (Fig. 4C). Together, these results suggest that the observed increase in apoptosis in N1ICD;hGFAP-cre brains is mediated through the p53 pathway.
Loss of p53 rescues N1ICD-induced apoptosis and premature gliogenesis
To genetically test whether the increased apoptosis in N1ICD;hGFAP brains was indeed induced by the p53 pathway, we analyzed N1ICD;hGFAP-cre;p53+/− and N1ICD;hGFAP-cre;p53−/− brains at E15.5. Apoptosis was blocked in brains of N1ICD;hGFAP-cre;p53−/− mice as anticipated. Interestingly, the number of apoptotic cells was also greatly reduced in N1ICD;hGFAP-cre;p53+/− brains, as indicated by significant reductions in the number of TUNEL+ or cleaved Caspase3+ cells (Fig. 4D, E, Supplementary Fig. 1).
To determine whether blocking p53-mediated apoptosis is sufficient to rescue the neurological phenotype and premature death of N1ICD;hGFAP-cre mice (Fig. 1E), we generated postnatal N1ICD;hGFAP-cre;p53+/− and N1ICD;hGFAP-cre;p53−/− mice and aged them. Consistent with apoptosis marker analyses, loss of one copy of p53 was sufficient to rescue early lethality to an extent similar to that by loss of both copies of p53 (Fig. 4F). Gross analysis of wean age (P20) brains showed observable rescue in brain sizes in p53+/− and p53−/− transgenic brains, particularly in the cortex (Supplementary Fig. 2).
Although loss of p53 rescued the size of N1ICD transgenic brains to a large extent, >50% of N1ICD;hGFAP-cre;p53−/− and N1ICD;hGFAP-cre;p53+/− mice still died by 4 months of age with neurological phenotypes (Fig. 4F). Since previous studies have reported that ectopic N1ICD expression in the developing nervous system induces precocious expression of GFAP, a marker for astrocytes (25, 29), we tested whether premature gliogenesis is responsible for the neurological phenotype in p53 mutant transgenic mice. Analysis of GFAP expression in transgenic mice with 0,1, or 2 copies of p53 showed that loss of p53 expression also blocked precocious GFAP expression in both N1ICD;hGFAP-cre;p53−/− and N1ICD;hGFAP-cre;p53+/− mice (Supplementary Fig. 3). This observation suggests that premature gliogenesis is not likely to be the major cause of death or neural phenotypes in N1ICD;hGFAP-cre;p53+/− and N1ICD;hGFAP-cre;p53−/− mice. Furthermore, it suggests that aberrant activation of GFAP expression by N1ICD expression in vivo requires a p53.
N1ICD;GFAP-cre;p53−/− and N1ICD;GFAP-cre;p53+/− mice develop medulloblastomas
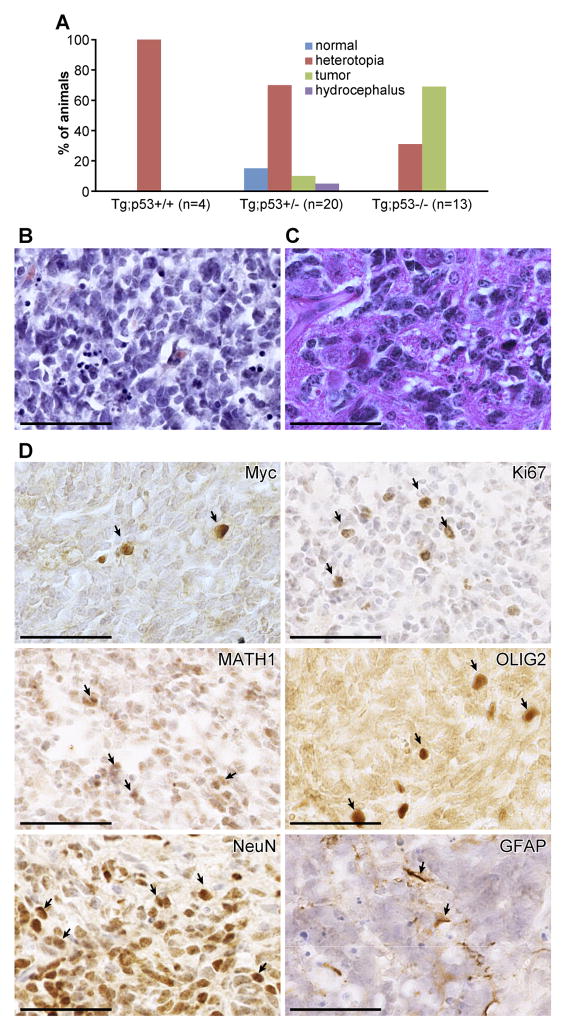
To further investigate the cause of death of N1ICD;hGFAP-cre;p53+/− and N1ICD;hGFAP-cre;p53−/− mice, we analyzed weaning-age brains of N1-ICD;hGFAP-cre transgenic mice with 0, 1, or 2 copies of the p53 gene. We observed heterotopia in a subset of N1ICD;hGFAP-cre transgenic brains, regardless of the p53 status (Fig. 5A). Notably, we also observed medulloblastomas in transgenic mice lacking one or both copies of p53 (Fig. 5A). Histologically, 11 of 12 tumors analyzed showed classical medulloblastoma histology, composed primarily of small EGL progenitor-like cells (Fig. 5B, Supplementary Fig 4). One mouse showed different tumor histology, resembling a large-cell anaplastic medulloblastoma (Fig. 4C). Immunohistochemical analyses showed that N1ICD-induced tumors are highly proliferative (Ki67+), with most cells expressing the early neural stem/progenitor marker OLIG2 (Fig. 4D). Many tumor cells expressed an EGL progenitor marker, ATOH1/MATH1, and a neuronal marker, NeuN (Fig. 4D), but not the glia marker GFAP, confirming that these tumors are medulloblastomas. Interestingly, only a small number of tumor cells continued to express the transgene, as indicated by Myc antibody staining (Fig. 4D). Interestingly, no other types of brain tumors were observed in the N1ICD;hGFAP-cre;p53−/− and N1ICD;hGFAP-cre;p53+/− mice, even though other regions of the brain contained a large number of apoptotic cells in N1ICD;hGFAP-cre embryos (Fig 2G and not shown).
Figure 5. N1ICD transgenic mice with reduced p53 dose develop spontaneous medulloblastomas.
(A) Distribution of mice with normal brain, hydrocephalus, heterotopia, and medulloblastomas among N1ICD;hGFAP-Cre;p53 wildtype, heterozygous, and homozygous mice. (B, C) Hematoxylin and eosin staining of medulloblastomas that form in N1ICD;GFAP-Cre;p53+/− and N1ICD;GFAP-Cre;p53−/− mice. (D) Immunohistochemical analyses of N1ICD medulloblastomas with markers for the transgene (Myc), proliferation (Ki67), EGL-progenitors (ATOH1/MATH1), neural stem/progenitors (OLIG2), neurons (NeuN), and glia (GFAP). Scale bar = 50μm.
N1ICD-induced medulloblastomas most closely resemble the SHH subgroup of human medulloblastomas
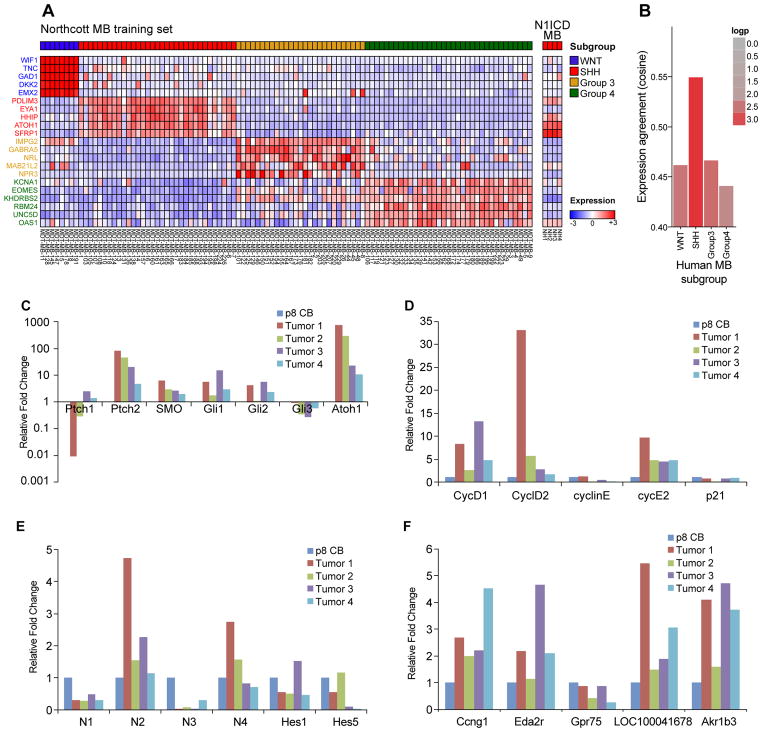
Currently, human medulloblastomas are categorized into four molecular subgroups: WNT, Sonic Hedgehog (SHH), Group 3 and Group 4 (30). Elevated Notch pathway activity has been observed in a subset of human medulloblastomas but is not associated with any given molecular subgroup (31). To determine whether N1ICD-induced tumors model a particular molecular subgroups of human medulloblastomas, we performed a transcriptome analysis of N1ICD-induced tumors and assessed the expression of signature genes of the four human medulloblastoma molecular subgroups. Surprisingly, N1ICD-induced medulloblastomas most closely matched the gene signature associated with the SHH-subgroup of human medulloblastomas (Fig. 6A,B).
Figure 6. N1ICD-induced medulloblastomas most closely model the SHH-subgroup of human medulloblastomas.
(A) Microarray analysis of N1ICD-induced medulloblastomas, compared against signature genes (Northcott MB training set) that distinguish the 4 molecular subgroups in human medulloblastomas. (B) Expression agreement analysis of N1ICD-induced tumors against the human medulloblastoma subgroups shows the highest correlation to the SHH-subtype. (C–E). Reatime RT-PCR analysis on RNAs isolated from four independent N1ICD-induced medulloblastomas. Expression levels are normalized against wildtype cerebellum at postnatal day 8. (C) SHH pathway genes, (D) cell cycle regulators, (E) Notch pathway genes, (F) genes downstream of N1ICD expression in N1ICD;hGFAP-cre cortices.
To confirm the SHH subgroup categorization of N1ICD-induced medulloblastomas, we performed realtime RT-PCR analyses, comparing the tumors to wildtype postnatal day 8 cerebella of C57BL/6J (B6) mice. As shown in Figure 6C, the SHH pathway genes Gli1, Gli2, and Ptch2 were highly expressed in N1ICD medulloblastomas. Consistent with microarray data (Fig. 6A) and the antibody staining (Fig. 5D), Atoh1/Math1, a marker for EGL progenitors, was also significantly increased in N1ICD tumors at the RNA level (Fig. 6C). In addition, we observed significantly increased expression of cell cycle regulators, Cyclins D1, D2, and E2 in N1ICD tumors (Fig. 6D). These results are consistent with the histological analysis showing that N1ICD tumors are composed of highly proliferative cells resembling EGL progenitors and the SHH subgroup designation. Surprisingly, most of the Notch pathway genes were not highly expressed in the bulk tumor cells (Fig. 6E). In particular, N1ICD level was not significantly up-regulated at the bulk tumor level, consistent with the immunohistochemical analysis that only a small number of cells continue to express the transgene in the tumor (Fig. 5D). On the other hand, Notch2, normally expressed in EGL progenitor cells and shown to promote their proliferation (32), was higher expressed in tumors, supporting the idea that bulk tumor cells are mostly composed of EGL-progenitor like cells. Interestingly, genes that were identified to be downstream of N1ICD expression in N1ICD;hGFAP-cre cerebral cortices at E13.5 (Ccng1, Eda2r, Akr1b3, LOC1000041678) were also highly elevated in the tumors (Fig. 6F), suggesting that these genes were also activated in the developing cerebellum upon N1ICD expression and maintained in the tumor. Together, these marker analyses suggest that most N1ICD medulloblastomas are composed of EGL progenitor-like cells that have high levels of Atoh1, Ptch2, Gli1 and Gli2 expression, and that N1ICD induced tumor may model a subset of SHH subgroup of human medulloblastomas.
DISCUSSION
Notch pathway activation is observed in many human cancers, including brain cancer (2, 3). In addition, it has been shown to be necessary for maintaining brain cancer stem cells from gliomas and medulloblastomas in vitro and in vivo (17–19). To directly test whether Notch1 has the potential in brain tumor initiation, we generated a mouse model in which activated Notch1 is expressed in the developing brain. Similar to over-expression of Notch2-ICD in the brain (23), N1ICD over-expression alone was not sufficient to induce brain tumors. However, 10~60% of N1ICD;hGFAP-cre;p53+/− and N1ICD;hGFAP-cre;p53−/− mice developed medulloblastomas (Fig. 5A). Hence, this study demonstrates that elevated Notch1 activity, when combined with cooperating oncogenic events, can be an initiating oncogenic hit in the mammalian brain.
Interestingly, while we observed p53-mediated apoptosis in all regions of the brain in N1ICD;hGFAP-cre mice, we did not observe any brain tumor types other than medulloblastomas in N1ICD;hGFAP-cre;p53+/− and N1ICD;hGFAP-cre;p53−/− mice. Previous analyses of mice with mutations in the DNA-damage-repair pathway genes also reported similar occurrences of medulloblastomas (and not of other brain tumors) on p53 mutant backgrounds (33–36). This is consistent with our observation that the major cellular response to N1ICD expression is a DNA damage response (Fig. 4). Together, these results suggest that cerebellar cells may be more sensitive to DNA damage-induced cellular transformation in comparison to cells in other brain regions. Alternatively, there may be a selection against other types of tumors, such as gliomas, that would cause embryonic lethality if they developed in utero, or that would have formed in older mice. However, the latter possibility is unlikely, as we did not observe other types of brain tumor in any of the older N1ICD;hGFAP-Cre;p53+/− and N1ICD;hGFAP-Cre;p53−/− mice we examined, although we did observe nasal epithelial tumors in some mice.
High-level Notch pathway activity, in contrast to Wnt or SHH pathway activity, does not identify a specific subgroup of human medulloblastomas (31). However, N1ICD-induced medulloblastomas express high levels of the SHH-pathway genes, categorizing the tumors as models for the SHH subgroup of human medulloblastomas (Fig. 6). Interestingly, previous studies have shown that Notch pathway activity is dispensable for SHH-induced medulloblastoma initiation or maintenance (37, 38). While it remains to be determined whether Notch pathway cooperates with other oncogenic pathways, our study clearly indicates that Notch activation is a strong inducer of medulloblastomas in p53 mutant backgrounds. Notably, p53 mutations are observed in 12–14% of WNT and SHH subgroups of human medulloblastomas (39), suggesting that this model is physiologically relevant. Our observation that p53 functions downstream of N1ICD to modulates Notch1 target gene expression, such as GFAP (Supplementary Fig. 3), suggests that p53 interaction with the Notch pathway in vivo may be more complicated than is currently known.
In the future, it will be important to further elucidate the molecular mechanism of cellular transformation induced by Notch1 activation. Consistent with earlier studies performed on bone marrow cells (40, 41), N-terminal truncation (deletion of the RAM domain) of the N1ICD protein analyzed in this study did not affect cellular transformation or target gene expression. In support of this conclusion, we observed similar increase in apoptosis and reduced numbers of proliferating stem/progenitor cells in the brain of another allele of N1ICD transgenic mouse that expressed human N1ICD that contains the RAM domain (Supplementary Fig 5, 6, (25)). Interestingly, our analysis of yet another allele of N1ICD transgenic mouse in which N1ICD lacking the PEST domain was knocked into the ROSA locus showed increased neural stem cell expansion but not increased apoptosis or tumorigenesis (24). Together, results of these studies show that Notch pathway activation in vivo results in drastically different biological outcome depending on the cellular context and the domains and/or dose of N1ICD expression. Comparisons of these different alleles of N1ICD transgenic mice will be useful in elucidating the molecular mechanism of Notch1 function in stem cell maintenance and cellular transformation.
In summary, we demonstrate that Notch1 can be an oncogene in the brain and that elevation of Notch pathway activation in p53-deficient mice generates a novel mouse model of medulloblastoma. Interestingly, these tumors most closely resemble the SHH-subgroup of human medulloblastomas and show high levels of the SHH pathway activity. Unexpectedly while we observe high levels of Notch2 expression in these tumors, consistent with normal Notch2 expression in EGL progenitor cells (32), we did not detect high-level expression of other Notch pathway components. This observations suggests a hit-and-run or some other mechanism of oncogenesis by N1ICD that does not require sustained expression of N1ICD in growing tumor cells, such as non-cell-autonomous effect of p53 recently reported (42). It also suggests the possibility that Notch pathway may play a significant role in tumor initiation (but not maintenance) in other tissues in which the Notch pathway plays a critical role in stem/progenitor cell proliferation and survival. Finally, our results suggest that a molecular signature of the initiating oncogenic event is not necessarily preserved in established tumors and that analysis of dominant maintenance pathway in growing, bulk tumor cells may not be informative in elucidating early oncogenic events in some tumors.
MATERIALS AND METHODS
Animals
All mice were treated according to the guidelines of The Jackson Laboratory and The Jackson Laboratory Animal Care and Usage Committee approved all procedures. We analyzed two different founder transgenic N1ICD lines (N1ICD-43 and N1ICD-29) that were generated in Jeong Yoon’s laboratory at MMCRI (43), and observed the same phenotype in both lines. In these mice, intracellular domain of mouse Notch1 minus the RAM domain (aa 1810-2531) is conditionally expressed upon Cre excision (Supplementary Fig 6). p53 (B6.129S2-Trp53tm1Tyj/J: JR#2101), GFAP-cre (JAX FVB-Tg(GFAP-cre)25Mes/J: JR#4600 ), and ACTB-Ntoch1 (C57BL/6J-Tg(ACTB-NOTCH1)1Shn/J; human N1ICD: JR#6481) mice were obtained from The Jackson Laboratory Repository. All analyses were performed by comparing littermates to minimize variability and in a minimum of three independent sets of samples.
Immunohistochemistry/immunofluorescence
Standard immunohistochemistry and immunofluorescence protocols were used with antibodies listed in the Supplementary Methods section on either frozen and/or paraffin sections.
Microarray analysis
Affymetrix ST1.0 chips were used with 3 independent sets of samples from control and N1ICD;hGFAP-cre E13.5 cortices from littermates or from 4 independent N1ICD;hGFAP-cre;p53 tumors. For analytical details, please see Supplementary Methods.
Realtime RT-PCR
To measure relative RNA levels of control and transgenic cortices or tissues, total RNA was isolated using Trizol. Genomic DNA was removed by treating RNA samples with DNAse following the recommended protocol from Ambion (TURBO DNA-free™, Ambion). cDNAs were generated using the iScript kit from BioRad, and primers specific for each target gene (see Supplementary Methods) were used with SYBR green reaction mix from BioRad. All reactions were run in triplicates, and 18S levels were used to normalize samples to one another. Relative fold change compared to wildtype control is presented in all figures.
Supplementary Material
Acknowledgments
We thank Jane Johnson for providing the MATH1 antibody, Jesse Hammer for assistance with figures, Gene Expression Service at JAX for Affymetrix analysis, Stephen Sampson for manuscript editing, Patsy Nishina and Kevin Mills for critical reading of the manuscript, and members of the Yun lab for their support. This work was supported in part by the Maine Cancer Foundation and TJL Cancer Center Support grant CA034196 to KY. SLA is an investigator of the Howard Hughes Medical Institute.
Footnotes
There is not conflict of interest to report by any of the authors.
References
- 1.Allenspach EJ, Maillard I, Aster JC, Pear WS. Notch signaling in cancer. Cancer biology & therapy. 2002;1:466–76. doi: 10.4161/cbt.1.5.159. [DOI] [PubMed] [Google Scholar]
- 2.Koch U, Radtke F. Notch signaling in solid tumors. Curr Top Dev Biol. 2010;92:411–55. doi: 10.1016/S0070-2153(10)92013-9. [DOI] [PubMed] [Google Scholar]
- 3.Pierfelice TJ, Schreck KC, Eberhart CG, Gaiano N. Notch, neural stem cells, and brain tumors. Cold Spring Harb Symp Quant Biol. 2008;73:367–75. doi: 10.1101/sqb.2008.73.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierfelice T, Alberi L, Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron. 2011;69:840–55. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 5.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–71. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 8.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–63. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 9.Behesti H, Marino S. Cerebellar granule cells: insights into proliferation, differentiation, and role in medulloblastoma pathogenesis. Int J Biochem Cell Biol. 2009;41:435–45. doi: 10.1016/j.biocel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–45. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–34. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332:839–47. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 14.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer research. 2004;64:7787–93. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 15.Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012 doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–5. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 18.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019–29. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang L, Fan X, Chaudhry A, Wang M, Gaiano N, Eberhart CG. Notch3 signaling initiates choroid plexus tumor formation. Oncogene. 2006;25:487–91. doi: 10.1038/sj.onc.1209074. [DOI] [PubMed] [Google Scholar]
- 22.Pierfelice TJ, Schreck KC, Dang L, Asnaghi L, Gaiano N, Eberhart CG. Notch3 activation promotes invasive glioma formation in a tissue site-specific manner. Cancer research. 2011;71:1115–25. doi: 10.1158/0008-5472.CAN-10-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchorz JS, Tome M, Cloetta D, Sivasankaran B, Grzmil M, Huber RM, et al. Constitutive Notch2 signaling in neural stem cells promotes tumorigenic features and astroglial lineage entry. Cell Death Dis. 2012;3:e325. doi: 10.1038/cddis.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K. Genome-Wide Analysis of N1ICD/RBPJ Targets in vivo Reveals Direct Transcriptional Regulation of Wnt, SHH, and Hippo Pathway Effectors by Notch1. Stem Cells. 2012 doi: 10.1002/stem.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Kimura SH, Ikawa M, Ito A, Okabe M, Nojima H. Cyclin G1 is involved in G2/M arrest in response to DNA damage and in growth control after damage recovery. Oncogene. 2001;20:3290–300. doi: 10.1038/sj.onc.1204270. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto K, Beach D. Cyclin G is a transcriptional target of the p53 tumor suppressor protein. The EMBO journal. 1994;13:4816–22. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brosh R, Sarig R, Natan EB, Molchadsky A, Madar S, Bornstein C, et al. p53-dependent transcriptional regulation of EDA2R and its involvement in chemotherapy-induced hair loss. FEBS letters. 2010;584:2473–7. doi: 10.1016/j.febslet.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 29.Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 30.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta neuropathologica. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS ONE. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–68. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, McKinnon PJ. DNA ligase IV suppresses medulloblastoma formation. Cancer research. 2002;62:6395–9. [PubMed] [Google Scholar]
- 34.Tong WM, Ohgaki H, Huang H, Granier C, Kleihues P, Wang ZQ. Null mutation of DNA strand break-binding molecule poly(ADP-ribose) polymerase causes medulloblastomas in p53(−/−) mice. The American journal of pathology. 2003;162:343–52. doi: 10.1016/S0002-9440(10)63825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frappart PO, Lee Y, Russell HR, Chalhoub N, Wang YD, Orii KE, et al. Recurrent genomic alterations characterize medulloblastoma arising from DNA double-strand break repair deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1880–5. doi: 10.1073/pnas.0806882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan CT, Kaushal D, Murphy M, Zhang Y, Datta A, Chen C, et al. XRCC4 suppresses medulloblastomas with recurrent translocations in p53-deficient mice. Proc Natl Acad Sci U S A. 2006;103:7378–83. doi: 10.1073/pnas.0601938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Julian E, Dave RK, Robson JP, Hallahan AR, Wainwright BJ. Canonical Notch signaling is not required for the growth of Hedgehog pathway-induced medulloblastoma. Oncogene. 2010;29:3465–76. doi: 10.1038/onc.2010.101. [DOI] [PubMed] [Google Scholar]
- 38.Hatton BA, Villavicencio EH, Pritchard J, LeBlanc M, Hansen S, Ulrich M, et al. Notch signaling is not essential in sonic hedgehog-activated medulloblastoma. Oncogene. 2010;29:3865–72. doi: 10.1038/onc.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, et al. Medulloblastomics: the end of the beginning. Nature reviews Cancer. 2012;12:818–34. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aster JC, Xu L, Karnell FG, Patriub V, Pui JC, Pear WS. Essential roles for ankyrin repeat and transactivation domains in induction of T-cell leukemia by notch1. Molecular and cellular biology. 2000;20:7505–15. doi: 10.1128/mcb.20.20.7505-7515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aster JC, Robertson ES, Hasserjian RP, Turner JR, Kieff E, Sklar J. Oncogenic forms of NOTCH1 lacking either the primary binding site for RBP-Jkappa or nuclear localization sequences retain the ability to associate with RBP-Jkappa and activate transcription. The Journal of biological chemistry. 1997;272:11336–43. doi: 10.1074/jbc.272.17.11336. [DOI] [PubMed] [Google Scholar]
- 42.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, et al. Non-Cell-Autonomous Tumor Suppression by p53. Cell. 2013;153:449–60. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatesh DA, Park KS, Harrington A, Miceli-Libby L, Yoon JK, Liaw L. Cardiovascular and hematopoietic defects associated with Notch1 activation in embryonic Tie2-expressing populations. Circulation research. 2008;103:423–31. doi: 10.1161/CIRCRESAHA.108.177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.