Abstract
Implicit learning is a process of acquiring knowledge that occurs without conscious awareness of learning, whereas explicit learning involves the use of overt strategies. To date, research related to implicit learning following stroke has been largely restricted to the motor domain and has rarely addressed implications for language. The present study investigated implicit and explicit learning of an auditory word sequence in 10 individuals with stroke-induced agrammatic aphasia and 18 healthy age-matched participants using an adaptation of the Serial Reaction Time task. Individuals with aphasia showed significant learning under implicit, but not explicit, conditions, whereas age-matched participants learned under both conditions. These results suggest significant implicit learning ability in agrammatic aphasia. Furthermore, results of an auditory sentence span task indicated working memory deficits in individuals with agrammatic aphasia, which are discussed in relation to explicit and implicit learning processes.
Keywords: Aphasia, Implicit learning, Serial Reaction Time, Working memory, Procedural memory
Introduction
Extracting patterns from complex stimuli in the environment is an essential aspect of learning and may occur without conscious awareness. The concept of implicit learning was originally expounded in Arthur Reber’s seminal study relating how people learn to respond to the structural relations of an artificial grammar without using explicit strategies or acquiring verbalizable knowledge about the grammar (Reber 1967). Reber suggested that this incidental, inductive learning process is intrinsic to language learning. However, few studies have investigated implicit learning in relation to language learning following stroke. The present study used an adaptation of an extensively studied implicit learning paradigm (i.e., the Serial Reaction Time (SRT) task) to examine learning processes in individuals with stroke-induced agrammatic aphasia.
Explicit and implicit learning tasks are closely associated with declarative and nondeclarative memory systems, respectively. Declarative memory refers to the conscious recall of facts and events, whereas nondeclarative (implicit) memory underlies non-conscious learning processes such as priming, classical conditioning, and procedural memory for skills and habits (Squire and Zola 1996). Although a large body of research has focused on these memory systems, only recently have studies begun to address the role of declarative and procedural memory in language processing and in aphasia. In the framework of Ullman’s declarative/procedural model, a frontal/basal ganglia network in the brain that underlies procedural memory also subserves the grammatical rules of language, whereas temporal lobe structures associated with declarative memory subserve the mental lexicon containing word-specific knowledge (Ullman 2001, 2004). Broca’s aphasia, the prototypical nonfluent aphasia syndrome, is associated with damage to the left inferior frontal gyrus, sometimes extending to underlying white matter, the insula, and the basal ganglia (Damasio 2008). Considering the site of these lesions, the declarative/procedural model suggests that grammatical deficits characteristic of nonfluent aphasia are associated with damage to the frontal/basal ganglia procedural memory system (Ullman et al. 2005). Agrammatic symptoms often observed in nonfluent aphasia include grammatical encoding deficits (Lee and Thompson 2010), greater difficulty producing verbs than nouns (Kim and Thompson 2000; Zingeser and Berndt 1990), and difficulty producing and comprehending sentences, particularly syntactically complex sentences with noncanonical word order (e.g., Caplan and Hanna 1998; Cho and Thompson 2010; Grodzinsky 1986; Linebarger et al. 1983; Meyer et al. 2012; Schwartz et al. 1980).
In addition to long-term memory systems, working memory also plays an important role in language processing. Working memory involves processing as well as storage functions, which can be assessed by a variety of tasks, including sentence span tasks in which a person is required to process written or spoken sentences while retaining the final word of each sentence for later recall (Daneman and Carpenter 1980; Tompkins et al. 1994). Studies using the sentence span and other standard working memory tasks show that many aphasic individuals have verbal working memory impairments, which are highly interrelated with language comprehension abilities and overall aphasia severity (Caspari et al. 1998; Friedmann and Gvion 2003; Sung et al. 2009). However, little is known about how working memory deficits impact learning in aphasia. In healthy young adults, there is evidence that working memory capacity may have different effects on explicit versus implicit learning processes. Individuals with high working memory spans exhibit larger learning effects than those with low working memory spans when performing a visuomotor task under explicit learning conditions, but the two groups do not differ when performing the same task under implicit learning conditions (Unsworth and Engle 2005).
To date, evidence regarding implicit learning in individuals with language disorders is sparse and has yielded mixed results. Weak or absent learning effects in implicit learning paradigms have been demonstrated in specific language impairment (Evans et al. 2009; Tomblin et al. 2007) and dyslexia (Menghini et al. 2006; Vicari et al. 2003, 2005), although contradictory results have also been found in dyslexia (Kelly et al. 2002; Rüsseler et al. 2006). In individuals with aphasia, research suggests intact visuomotor implicit learning, but impairments in implicitly learning a sequence of phonemes and in the implicit acquisition of an artificial grammar composed of visual symbols (Christiansen et al. 2010; Goschke et al. 2001).
The Serial Reaction Time (SRT) task is a useful paradigm for the investigation of learning processes because it may be performed implicitly (i.e., by extracting an underlying sequential structure through repetitive exposure without conscious awareness) or explicitly (i.e., by using declarative knowledge of the underlying structure). In the SRT task, visual stimuli appear on a screen in one of four locations in a repeating sequence of locations. Participants, who are typically unaware of the existence of a sequence, respond by pressing keys corresponding to the location of each stimulus (Nissen and Bullemer 1987). Learning of the visuomotor sequence is indicated by response times, which decrease over trials of sequenced stimuli and increase when the sequence is replaced by a random order of stimuli.
In healthy children and adults, providing explicit knowledge of a sequence prior to beginning the SRT task results in larger learning effects (Boyd and Winstein 2004, 2006; Vicari et al. 2003) and engages different neural systems (Rüsseler et al. 2003) compared to performing the same task under implicit conditions. Presumably, under explicit conditions, participants use an overt strategy to learn the composition of the sequence and consciously anticipate each successive response, resulting in faster reaction times for sequenced stimuli and a greater disruption when the stimuli switch to a random order. However, in adults with brain damage due to stroke, the provision of explicit knowledge prior to administering SRT tasks has been shown to interfere with motor learning rather than enhance it (Boyd and Winstein 2004, 2006).
Although almost all studies of implicit learning in people who have suffered a stroke have used visuomotor tasks (e.g., Boyd and Winstein 2004, 2006; Exner et al. 2001; Gomez-Beldarrain et al. 1998; Orrell et al. 2007), a novel adaptation of the SRT paradigm permits the investigation of perceptual sequence learning independent of motor response patterns. In this task, which Goschke and colleagues term the Serial Search Task (SST), participants view four letters on a screen, hear a repeating sequence of the letters/phonemes, and respond by pressing the keys corresponding to the letters’ locations (Goschke et al. 2001). Like the original SRT task, participants exhibit implicit learning when reaction times decrease for sequenced stimuli and increase when the stimuli are presented randomly. Unlike the SRT task, however, only the auditory stimuli repeat in a sequence. The locations of the visual stimuli, and therefore the motor responses as well, are random throughout the entire experiment. Studies that have used the SST with phoneme and word sequences demonstrate implicit learning of auditory sequences in healthy young and older adults (Goschke et al. 2001; Dennis et al. 2006). However, Goschke et al. (2001) showed that individuals with Broca’s aphasia were unable to learn the phoneme sequence of the SST, although they showed intact motor sequence learning, suggesting domain-specific implicit learning deficits.
The purpose of the present study was to use the SST to investigate learning processes in individuals with agrammatic aphasia and healthy age-matched adults. Based on the results of Goschke et al. (2001), it was hypothesized that individuals with agrammatism would be impaired in implicit learning of an auditory word sequence. The study also included an explicit learning condition to investigate whether explicit knowledge of the sequence would aid performance on the task, as in healthy adults, or interfere with performance, as in previous studies of individuals with brain lesions (Boyd and Winstein 2004, 2006). Additionally, because working memory may play an important role in certain learning tasks, a sentence span task was administered to evaluate participants’ working memory capacity.
Method
Participants
Participants included 10 individuals with stroke-induced agrammatic aphasia (7 male; age 33–74, M = 55) and 18 healthy controls (9 male; age 46–74, M = 61). Aphasic and control participants did not significantly differ in age (t (26) = 0.61, p = .55) or years of education (t (26) = 0.82, p = .43). All participants were native English speakers with normal or corrected-to-normal vision and hearing and provided written informed consent approved by the Institutional Review Board of Northwestern University. Control participants had no history of neurological impairment or learning or language disability. Aphasic participants were more than 1 year post-stroke (2–20 years post, M = 7.6, SD = 6.6) and exhibited symptoms consistent with agrammatism, as indicated by low fluency scores in the Western Aphasia Battery-Revised (WAB-R; Kertesz 2007), greater impairment of noncanonical as compared to canonical sentence structures in the Northwestern Assessment of Verbs and Sentences (NAVS; Thompson 2011), relatively intact noun naming in the Boston Naming Test (BNT; Kaplan et al. 1983), and low mean length of utterance and percent grammatical sentences in narrative sample analyses. Narrative speech samples were collected by showing participants a wordless picture book depicting the Cinderella story and then asking them to tell the story in their own words. Audio recordings of the speech samples were transcribed and analyzed for words per minute, mean length of utterance, noun to verb ratio, and the percentage of sentences that were grammatically correct. Participants’ working memory was assessed using a listening sentence span (as detailed in Procedures below). Diagnostic data for aphasic participants are reported in Tables 1 and 2.
Table 1.
Language testing data
| Assessment | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Aphasia Battery-Revised | |||||||||||
| Aphasia quotient | 77.6 | 89.6 | 65 | 71.7 | 80 | 87.8 | 58.9 | 83 | 74.3 | 78 | 76.6 |
| Fluency | 5 | 5 | 4 | 4 | 5 | 6 | 2 | 5 | 5 | 5 | 4.6 |
| Information content | 9 | 10 | 8 | 9 | 9 | 9 | 7 | 9 | 8 | 10 | 8.8 |
| Auditory verbal comprehension | 7.8 | 10 | 7.5 | 8.45 | 8.4 | 9.6 | 6.95 | 9.8 | 7.45 | 7.9 | 8.4 |
| Repetition (words, phrases, and sentences) | 9.4 | 10 | 6.2 | 5.1 | 8.6 | 10 | 7.6 | 8.8 | 8.4 | 7.6 | 8.2 |
| Naming and word finding | 7.6 | 9.8 | 6.8 | 9.3 | 9 | 9.3 | 5.9 | 8.9 | 8.3 | 8.5 | 8.3 |
| Boston Naming Test | |||||||||||
| Spontaneously given correct responses | 57 | 54 | 33 | 49 | 53 | 58 | 31 | 60 | 40 | 38 | 47.3 |
| Northwestern Assessment of Verbs and Sentences | |||||||||||
| Verb naming | |||||||||||
| 1 argument verbs | 80 | 100 | 100 | 100 | 100 | 100 | 80 | 100 | 60 | 100 | 92.0 |
| 2 argument verbs | 90 | 100 | 80 | 100 | 100 | 100 | 40 | 90 | 90 | 100 | 89.0 |
| 3 argument verbs | 71 | 71 | 71 | 100 | 86 | 86 | 71 | 71 | 71 | 71 | 76.9 |
| Verb comprehension | 100 | 100 | 100 | 100 | 100 | 100 | 95 | 100 | 100 | 100 | 99.5 |
| Argument Structure Production | |||||||||||
| 1 argument verbs | 100 | 100 | 80 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.0 |
| 2 argument verbs | 100 | 100 | 60 | 100 | 100 | 100 | 93 | 100 | 100 | 100 | 95.3 |
| 3 argument verbs | 67 | 92 | 42 | 58 | 50 | 92 | 42 | 83 | 83 | 83 | 69.2 |
| Sentence Production Priming Test | |||||||||||
| Canonical | 53 | 100 | N/A | 33 | 87 | 100 | N/A | 93 | N/A | N/A | 77.8 |
| Non canonical | 20 | 100 | N/A | 0 | 53 | 87 | N/A | 67 | N/A | N/A | 54.4 |
| Sentence Comprehension Test | |||||||||||
| Canonical | 53 | 100 | N/A | 87 | 93 | 100 | 60 | 87 | N/A | 60 | 80.0 |
| Non canonical | 67 | 87 | N/A | 80 | 93 | 100 | 60 | 73 | N/A | 73 | 79.2 |
Agrammatic participants’ language testing data. N/A = not available (subtest discontinued by participant)
Table 2.
Narrative sample data
| Narrative measure | P1 | P2 | P3 | P4 | P5 | P6 | P8 | P9 | P10 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean length of utterance | 6.07 | 8.07 | 4.35 | 4.80 | 8.04 | 7.68 | 5.91 | 6.15 | 10.39 | 6.83 |
| Words per minute | 33.06 | 58.19 | 35.73 | 73.48 | 57.72 | 40.06 | 55.68 | 26.55 | 81.65 | 51.35 |
| Percent grammatical sentences | 64 | 82 | 0 | 26 | 54 | 79 | 60 | 74 | 48 | 54.11 |
| Noun:verb ratio | 1.05 | 0.98 | 4.00 | 1.48 | 1.02 | 1.17 | 1.00 | 1.50 | 0.90 | 1.46 |
Agrammatic participant’s narrative speech data (speech data for P7 consisted of unintelligible utterances and therefore were not analyzable)
The learning and working memory tasks used in the present study require auditory comprehension of single words and simple sentences. Therefore, only individuals with relatively intact auditory comprehension were included, as indicated by scores on the WAB-R measure of Auditory Verbal Comprehension (M = 8.4/10, SD = 1.0), including the word recognition subtest of the measure (M = 56.4/60, SD = 5.5).
Stimuli
Because each participant completed the SST twice, once for the implicit condition and once for the explicit condition, two different sets of the stimuli were created (Set A and Set B). Eight monosyllabic concrete nouns were selected. Four nouns were assigned to Set A (cake, knife, desk, shoe) and four to Set B (pie, fork, chair, dress). The words were matched in spoken frequency of occurrence for Set A (range 12–34; M = 24) and Set B (range 10–35; M = 22) according to the CELEX database (Baayen et al. 1993). The two sets also consisted of items from the same four semantic categories (i.e., dessert, utensil, furniture, clothing). All words were recorded by the same female native English speaker and normalized to 72 dB SPL. Recorded words ranged from 627 to 639 ms in length (M = 634; SD = 4).
The four words of Set A were assigned to the underlying 8-item sequence used by Goschke et al. (2001) (ACDBDCAB), yielding the following word sequence: shoe desk cake knife cake desk shoe knife. The four words of Set B were assigned to a second sequence of equal length (ABDCBCAD), yielding the word sequence dress fork pie chair fork chair dress pie. Both sequences were ambiguous, in that each word could be followed by one of two different words, depending on the position within the sequence.
Eight black and white line drawings were selected to correspond with the eight nouns. The drawings were obtained from a standardized set of pictures (Snodgrass and Vanderwart 1980) and from Clip Art images. To ensure that these drawings elicited the corresponding words, ten healthy young adults were asked to name the pictures, and all responded with the intended noun names. Additionally, all aphasic participants were asked to point to each of the eight picture stimuli as they were named aloud by the experimenter, prior to beginning the experimental tasks. All participants correctly identified the picture stimuli.
The stimulus sets were presented horizontally on the computer monitor (see Fig. 1), with the arrangement of the four pictures varying on each trial. All stimuli were presented using Superlab software with a Cedrus RB-420 response pad for data collection.
Fig. 1.
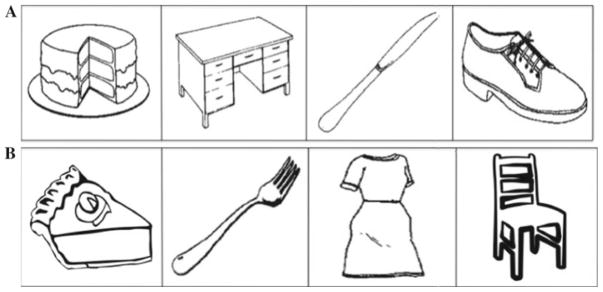
Example of the visual display in Set A (top) and Set B (bottom)
Procedure
Overview of Learning Sessions
Session 1 consisted of the SST under implicit learning conditions. Either Set A or Set B of the stimuli was selected for Session 1, with the order counterbalanced across participants. Participants were not informed of the existence of a sequence within the stimuli, but only that the study examined response times over many trials of practice. After the SST was completed, a test of explicit knowledge of the sequence was administered (see below).
The implicit and explicit learning conditions could not be counterbalanced because providing knowledge of the existence of a sequence in the explicit session precludes subsequent implicit learning with the same paradigm. However, to minimize fatigue effects, the second (explicit) learning session took place 1–3 days after Session 1. In Session 2, participants followed the same procedures as in Session 1 to complete the SST and explicit knowledge task. The only difference in Session 2 was that participants were informed of the existence of a repeating word sequence that would occur for most of the experiment, and listened to the 8-item word sequence once, before beginning the SST. To verify comprehension of the instructions, participants were allowed to briefly view the sequence depicted in both pictures and words as they answered a question about the pattern. (E.g., “If you hear the word desk and then the word cake, which word would you hear next?”) To avoid transfer of knowledge from the previous session, participants were presented with the stimulus set that was not used in Session 1; therefore the underlying sequence as well as the surface stimuli were different from the implicit learning session. Additionally, Session 2 concluded with the listening sentence span task (see below).
Serial Search Task (SST)
Participants were instructed to locate the picture depicting each word that was spoken and press the button on the response pad that corresponded to that picture’s location, as quickly and accurately as they could. Ten random practice trials familiarized participants with the task prior to beginning the sequenced blocks of the experiment.
On each experimental trial, the four pictures were presented horizontally on the computer monitor, with each of the four locations corresponding with a button on the response pad. The pictures were displayed for 500 ms before one of the four words was presented over the speakers. The trial ended with the participant’s button press response, and a blank screen appeared for 500 ms before the beginning of the next trial (see Fig. 2). The four pictures were randomly assigned to the four locations on each trial, with the condition that no specific arrangement occurred on two consecutive trials. In sequenced blocks of the experiment, the auditory stimuli were presented in the order of the underlying 8-item sequence. Therefore, each repetition of the sequence required 8 trials. Accuracy and reaction times (measured from the onset of the spoken word) were recorded on each trial.
Fig. 2.
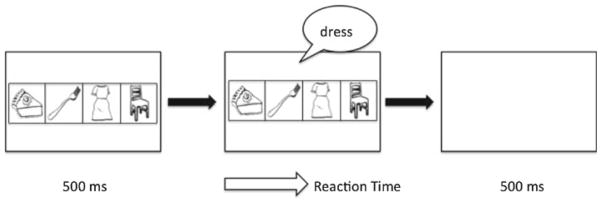
Schematic of one trial of the SST
The SST was organized in 8 blocks, although to participants it appeared as one continuous task. Blocks 1–6 each consisted of ten repetitions of the 8-item sequence of spoken words (i.e., 80 trials per block). Block 7 consisted of 80 trials with the four words occurring in a pseudorandom order, with the conditions that the words occurred with equal frequency and no word was repeated on two consecutive trials. Block 8 returned to sequenced trials with ten repetitions of the sequence. Participants were allowed short breaks after Block 2 and after Block 5.
Assessment of Explicit Knowledge
After completing the implicit SST, participants were informed that the spoken words had followed a sequence during most of the experiment. To evaluate any explicit knowledge of the sequence that may have been acquired during the task, the word prediction test was administered after the SST in both the implicit and explicit learning sessions. In this test, participants were instructed to listen to three-word fragments of the sequence and try to identify the next word in the pattern. The trials included all possible 3-word fragments of the 8-item sequence, in a randomized order. On each trial, the fragment of the sequence was presented over speakers, with 1 s of silence separating each word (e.g., cake … knife … cake). After the three spoken words, the four pictures were displayed on the computer monitor, and the participant was asked to select the picture corresponding with the word that came next in the sequence. Scores on the word prediction test consisted of the percentage of correct responses.
This task was similar to the sequence recognition or generation tasks that are typically administered following the SRT task (e.g., Goschke et al. 2001; Vakil et al. 2000). Chance performance on such tasks indicates that participants did not gain declarative knowledge of the sequence. Assuming the participants knew only that no word was repeated on two consecutive trials, chance performance on this particular test was 33 %.
Additionally, after completion of the SST in both the implicit and explicit learning sessions, participants were given the opportunity to self-report the word sequence, or any portion of it that they remembered. The responses to these queries were quantified according to the highest sequence length that was correctly recalled, ranging from 2 words in the correct order to the entire 8-word pattern.
Listening Sentence Span
Each participant completed a listening span working memory task modeled after Daneman and Carpenter’s (1980) sentence span test. Sentence stimuli for the listening span task consisted of simple active sentences, each eight words long (e.g., The students entered the classroom after the bell). The sentence-final words had one or two syllables (M = 1.42, SD = 0.50) and were designed to be frequently occurring in the English language (log lemma frequency 0.60–2.64; M = 1.47, SD = 0.46) according to the CELEX database (Baayen et al. 1993) and concrete (474–637; M = 596, SD = 28) and imageable (494–637; M = 590, SD = 36) according to the MRC database (http://www.psy.uwa.edu.au/mrcdatabase). All sentences were recorded by a male native English speaker and normalized to 72 dB SPL.
Participants were instructed to listen to sets of sentences and hold the last word of each sentence in memory until asked to recall the words, in any order, from that set. The number of sentences presented in each trial varied from two to six. The test included three trials at each of these five levels, administered in a randomized order, so that at the beginning of each trial the participant could not predict how many sentences would be presented. Practice trials were administered to ensure comprehension of the task prior to beginning the 15 test trials.
On each trial of the task, the series of sentences was presented over computer speakers, followed by a visual question mark on the monitor prompting the participant to recall aloud the final words of the sentences, which the experimenter recorded on a score sheet. To ensure that participants processed the sentences in addition to recalling the final words, a yes/no comprehension question regarding the last sentence in the series was presented over the speakers (e.g., Did the students enter the classroom after the bell?). The participant responded aloud, and the experimenter recorded the answer. The working memory score consisted of the number of words correctly recalled on all trials, out of the maximum total of 60 words.
Data Analyses
Accuracy and reaction time (RT) were recorded on each trial of the SST. RTs for incorrect trials were excluded from analyses. To eliminate outliers from the RT data, the mean and standard deviation of RTs were calculated for each block (i.e., 80 trials) of the task, and any RTs above or below three standard deviations of the mean in each block were excluded from further analyses. Outliers constituted 1.0 % of the data in the control group and 1.7 % in the aphasia group.
Due to large differences in absolute RTs between aphasic and control participants, the RT data were standardized using z-scores for data analyses directly comparing the two groups. The overall means and standard deviations of RTs were calculated for each participant’s implicit learning session and for each participant’s explicit learning session. The RT value for each trial was then converted to a z-score, indicating how many standard deviations the value fell above or below the participant’s mean RT for that learning session. Finally, the average of the z-scores was calculated for each of the eight blocks of the task.
To examine performance during the first six (sequenced) training blocks in the SST, the mean z-scores were entered into 2 × 6 ANOVAs with Group (aphasia, control) as a between-subjects factor and Block (Blocks 1–6) as a within-subjects factor, with the Greenhouse-Geisser correction used when necessary. However, any change in RTs across sequenced blocks may be attributed to general task practice, separate from or in addition to sequence-specific learning. The critical test of sequence learning is the comparison between the pseudorandom Block 7 and the immediately preceding sequenced Block 6. Thus, the mean z-scores for these two blocks were entered into 2 × 2 (Block) ANOVAs for the implicit and explicit learning tasks.
Results
Implicit Learning
Errors on the SST under implicit learning conditions constituted 7.3 % of trials in the aphasic group and 1.3 % of trials in the control group. Repeated measures ANOVAs using an alpha level of .05 indicated that there were no significant differences in the number of errors between blocks in either group.
In the age-matched control group, reaction times (RT) during the SST under implicit learning conditions decreased over sequenced blocks and increased from a mean of 895 ms in Block 6 to 942 ms in the pseudorandom Block 7, indicating an implicit learning effect for the auditory word sequence. For aphasic participants, the overall average RT (M = 2, 205, SD = 947) was significantly higher than for age-matched controls (M = 918, SD = 153; t (9) = 4.14, p < .01). Importantly, however, RTs in the aphasic group increased from a mean of 2,169 ms in Block 6 to 2,278 ms in the pseudorandom Block 7. Paired-samples t tests indicated that the difference in RT between the pseudorandom Block 7 and the immediately preceding sequenced Block 6 was significant for both healthy control participants (t (17) = 3.04, p < .01) and aphasic individuals (t (9) = 2.98, p = .016).
Figure 3 displays mean z-scores across the blocks of the implicit SST for the aphasia and control groups. The 2 (Group) × 6 (Blocks 1–6) ANOVA with these z-scores as the dependent variable revealed a significant main effect of Block (F(2.5, 64.7) = 3.43, p = .03), indicating the decrease in RTs over the six sequenced training blocks. There was no main effect of Group (F(1, 26) = 0.02, p = .90) or Group × Block interaction (F(2.5, 64.7) = 1.40, p = .25). The 2 (Group) × 2 (Blocks 6 and 7) ANOVA revealed a significant main effect of Block (F(1, 26) = 10.61, p < .01), indicating significantly higher RT in the pseudorandom Block 7 compared to the immediately preceding sequenced Block 6. There was no main effect of Group (F(1, 26) = 0.15, p = .70) or Group × Block interaction (F(1, 26) = 0.43, p = .52) for these two blocks.
Fig. 3.
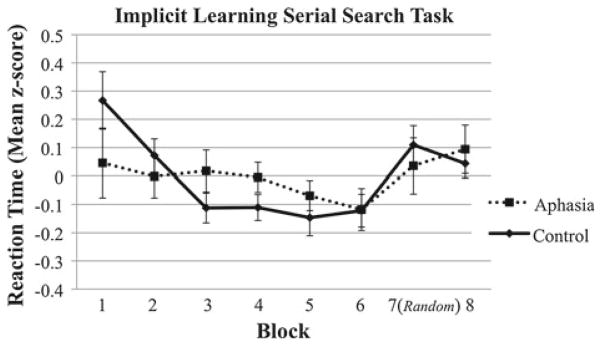
Standardized reaction time values across the 8 blocks of the SST under implicit learning conditions in the aphasic and control groups. Bars represent one standard error above and below the mean
Results of the word prediction test of explicit knowledge administered following the implicit learning task showed accuracy ranging from 25 to 62.5 % (M = 44.4, SD = 14.4) for the age-matched control group and from 25 to 37.5 % (M = 34.4, SD = 8.8) for the aphasic group. These scores were not significantly different from chance performance (33 %) in the aphasic group (one-sample t test: t (7) = 0.44, p = .67), but were greater than chance in the control group (one-sample t test: t (17) = 3.38, p < .01). After performing the implicit SST, only 5 of the 18 control participants and 1 of 8 aphasic participants1 self-reported a portion of the sequence that they had heard during the task, with the length of correct sequence fragments recalled ranging from two to four words.
To ensure that the implicit learning effects observed in the SST were not the result of explicit knowledge of the sequence, paired-samples t tests were also performed excluding the participants who were able to report any fragment of the word sequence. When excluding these six participants, the RT increase between Block 6 and Block 7 was significant in both the control (t (12) = 3.16, p < .01) and aphasic (t (8) = 3.10, p = .015) groups.
Explicit Learning
Errors on the SST under explicit learning conditions constituted 8.0 % of trials in the aphasic group and 1.9 % of trials in the control group. Repeated measures ANOVAs using an alpha level of .05 indicated that there were no significant differences in the number of errors between blocks in either group. Additionally, the number of days (1–3) that elapsed between the implicit and explicit learning sessions did not significantly differ between the two groups (t (26) = 1.11, p = .28).
In the age-matched control group, RTs during the SST under explicit learning conditions decreased over sequenced blocks and increased from a mean of 745 ms in Block 6 to 928 ms in Block 7. A paired-samples t test confirmed that the difference in RT between Block 6 and Block 7 was significant (t (17) = 4.19, p < .01). As in the implicit learning task, the overall average RT for aphasic individuals (M = 2, 090, SD = 823) was significantly higher than for age-matched controls (M = 827, SD = 161) during the explicit learning task (t (9) = 4.70, p < .01). In contrast to the implicit learning session, however, when performing the SST under explicit conditions, aphasic individuals exhibited a relatively small increase from 2,032 ms in Block 6 to 2,078 ms in Block 7. This difference was not statistically significant (t (9) = 0.71, p = .50).
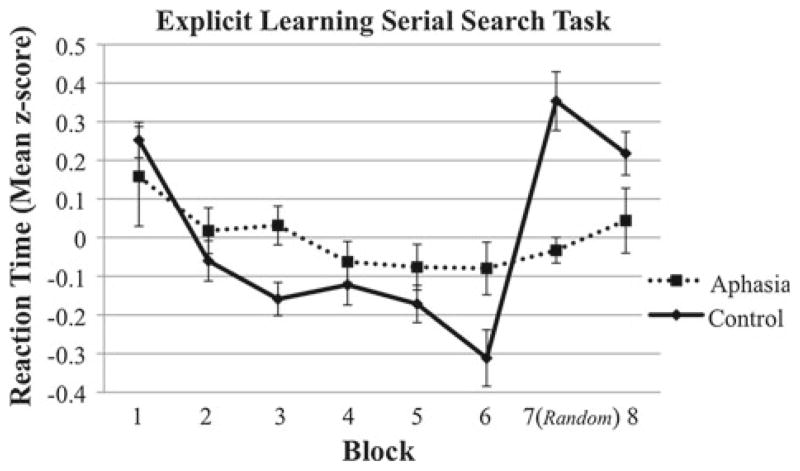
Figure 4 displays the mean z-scores for the two groups during the SST under explicit conditions. The 2 (Group) × 6 (Blocks 1–6) ANOVA with these z-scores as the dependent variable revealed significant main effects of Group (F(1, 26) = 10.90, p < .01) and Block (F(3.5, 91.2) = 8.48, p < .01). These effects indicate that RTs decreased over the six sequenced training blocks, with overall lower standardized RTs in the control group than in the aphasic group. There was no Group × Block interaction (F(3.5, 91.2) = 1.46, p = .23). The 2 (Group) × 2 (Blocks 6 and 7) ANOVA revealed a significant main effect of Block (F(1, 26) = 14.84, p < .01). However, this main effect was almost entirely attributable to the control group, which increased from −0.31 in Block 6 to 0.35 in Block 7, whereas the aphasic group only slightly increased from −0.08 to −0.03. This difference between the groups was indicated by the significant Group × Block interaction (F(1, 26) = 11.20, p < .01). There was no significant main effect of Group (F(1, 26) = 1.82, p = .19).
Fig. 4.
Standardized reaction time values across the 8 blocks of the SST under explicit learning conditions in the aphasic and control groups. Bars represent one standard error above and below the mean
Results of the word prediction test administered following the explicit learning task showed accuracy ranging from 25 to 100 % (M = 47, SD = 19) for the age-matched control group, with the mean percent accuracy significantly greater than the chance level of 33 % (t (17) = 3.09, p < .01). Additionally, 14 of the 18 control participants reported a part of the sequence, with the length of correct fragments ranging from 2 words to the entire 8-word sequence. In contrast, aphasic individuals did not exhibit significant declarative knowledge of the sequence following the explicit learning task. Results of the word prediction test showed accuracy ranging from 12.5 to 75 % (M = 29.2, SD = 20.7) for aphasic participants.2 The mean percent accuracy was not significantly different from chance performance (t (8) = 0.56, p = .59). Only one aphasic individual reported any portion of the sequence, recalling four words in the correct order.
Working Memory
All participants correctly answered at least 11 out of 15 yes/no comprehension questions in the listening span task (M = 13, SD = 1), and the number of correct comprehension answers did not significantly differ between the aphasic and control groups (t (25) = 1.3, p = .21). However, working memory scores in the control group ranged from 25 to 54 words recalled out of the total possible 60 words (M = 46.2, SD = 7.6), whereas scores in the aphasic group ranged from 14 to 38 (M = 25.3, SD = 7.7).3 The difference in scores between the two groups was statistically significant (t (25) = 6.68, p < .01).
Language Deficits and Task Performance
Although all agrammatic participants demonstrated relatively intact auditory comprehension (see Table 1), impairments in auditory comprehension or lexical retrieval could potentially influence performance on the learning and working memory tasks. To investigate these possible effects, agrammatic participants were divided into lower and higher auditory comprehension subgroups based on the group median WAB-R Auditory Verbal Comprehension score (lower group: M = 7.52, SD = 0.37; higher group: M = 9.25, SD = 0.77). Independent-samples t tests with an alpha level of .05 indicated no significant differences between the two subgroups in the RT difference between Block 6 and Block 7 during implicit or explicit learning sessions. However, a significant difference was found between the two subgroups in the listening span score (t (6.68) = 2.98, p = .02), indicating better performance on the listening span among participants with higher auditory comprehension scores.
Similarly, agrammatic participants were divided into lower and higher noun naming ability subgroups based on the group median Boston Naming Test score (lower group: M = 38.2, SD = 7.0; higher group: M = 56.4, SD = 2.9). Independent-samples t tests indicated no significant differences between the two subgroups in the RT differences between Block 6 and Block 7 or in listening span scores.
Discussion
Results of the present study demonstrate significant implicit learning ability in individuals with agrammatic aphasia, contrary to the original hypothesis. Although not consciously aware of the presence of an auditory word sequence, both healthy adults and individuals with aphasia responded significantly more slowly when the sequence was switched to a random word order during the SST. This difference in performance during the randomly ordered block indicates that participants had implicitly learned the word sequence.
These results differ from those of Goschke et al. (2001), who did not find significant implicit learning in individuals with Broca’s aphasia in a SST with an auditory phoneme sequence. It is possible that the sample size of five aphasic participants in Goschke et al. (2001) did not have sufficient power to detect an implicit learning effect in the phoneme version of the task, although the same group of participants showed intact learning of a visuomotor version of the task. Alternatively, differences in stimuli may have contributed to the divergent findings of the two studies. Stimuli for the SST in Goschke et al. (2001) consisted of four auditory phonemes and their corresponding visual letter representations (i.e., A B C D), which may have been particularly difficult to process for individuals with aphasia, many of whom have difficulty with phoneme discrimination and perceiving place of articulation in consonants (e.g., Blumstein et al. 1984; Miceli et al. 1980). In contrast, the present study used frequently occurring, concrete words in the auditory sequences, which were associated with easily identifiable visual images during the SST. These stimuli may have been easier to process, enabling the detection of a significant implicit learning effect in individuals with aphasia.
The present results also have important implications with regard to the procedural memory system. If individuals with agrammatic aphasia have damage to the neural system underlying procedural memory, as Ullman’s declarative/procedural model suggests, then they would be expected to be impaired in implicitly learning SRT tasks, which are strongly associated with the procedural memory system. This prediction is contrary to the findings of the present study and the results of the visuomotor SRT task in Goschke et al. (2001). Notably, the evidence for procedural memory impairment in nonfluent aphasia is largely based on deficits in processing regular English past tense verbs, which are argued to rely on procedural memory, as compared to irregular verbs, which are argued to rely on declarative memory (Ullman et al. 2005). However, many studies do not find a dissociation between regular and irregular verbs in nonfluent aphasia (see Faroqi-Shah 2007).
Additionally, the declarative/procedural model’s predictions regarding nonfluent aphasia are based on presumed damage to frontal and basal ganglia brain regions in this population. Extensive evidence suggests that the basal ganglia in particular support procedural learning, in which habits and stimulus-response associations are acquired incrementally (see Packard and Knowlton 2002). However, not all individuals with agrammatism have lesions that extend to the basal ganglia. Future research examining individual differences in both memory abilities and the extent of damage to the frontal/basal ganglia system could shed light on the neural substrates of memory in individuals with aphasia.
Interestingly, agrammatic individuals in the present study did not learn the auditory word sequence under explicit conditions, when they were consciously aware of the presence of a sequence while performing the task. In contrast, healthy age-matched participants exhibited a large, significant increase in reaction times during the random block of the SST, indicating robust sequence learning under explicit conditions. Additionally, tests of declarative knowledge administered after the explicit learning task indicated that individuals with aphasia did not gain explicit knowledge of the word order that was repeated during the task, whereas healthy controls scored significantly above chance on the word prediction test.
Impaired sequence learning under explicit conditions is in line with previous studies of learning after stroke. Boyd and Winstein (2004, 2006) found that providing explicit information regarding the sequences in SRT and continuous tracking tasks aided motor learning in healthy participants but interfered with learning in stroke patients. The authors suggested that providing explicit information may place demands on working memory that are unmanageable for some stroke patients, and thereby disrupt learning when patients try to use an overt strategy for the task. In contrast, studies of learning under implicit conditions do not find significant associations with working memory (Feldman et al. 1995; Unsworth and Engle 2005; Yang et al. 2011).
The difference in working memory demands between implicit and explicit learning tasks is also relevant to the present study. Under implicit learning conditions, participants performed the task without conscious awareness of a sequence. In contrast, under explicit learning conditions, the SST involved maintaining an eight-word sequence, or at least some portion of the sequence, in working memory while performing the task. The aphasic participants in this study demonstrated deficits in working memory and yet exhibited implicit learning comparable to healthy age-matched adults. Unlike the control group, however, the aphasic participants did not demonstrate significant learning under explicit conditions. These results suggest that individuals with aphasia may be impaired in applying explicit instruction in learning tasks that involve high working memory demands.
An important issue in the investigation of learning and memory in aphasia is that participants’ language deficits can potentially interfere with performance on tasks designed to evaluate learning and memory. The present study did not find associations between performance on the learning tasks and the auditory comprehension or naming abilities of participants with aphasia. However, agrammatic participants with greater auditory comprehension deficits performed significantly worse on the listening span working memory task compared to participants with less impaired auditory comprehension. These results are consistent with previous research indicating that language comprehension and working memory are closely related in aphasia (Friedmann and Gvion 2003; Sung et al. 2009). However, poor performance on the listening span task may be attributed to the demands that the task placed on auditory comprehension, in addition to working memory. Further research is needed to clarify the relationship between working memory and linguistic deficits in aphasia. For example, Christensen and Wright (2010) varied the linguistic demands of an n-back working memory task and found that individuals with aphasia performed worse than healthy adults on both verbal and nonverbal versions of the task, which indicates that aphasic individuals’ poor performance on working memory tasks is not solely the result of language deficits. Additionally, future research should investigate how long-term and working memory abilities are impaired in different subtypes of aphasia, and how individual differences such as time post-onset affect memory in people with aphasia.
Conclusions
Performance on an adaptation of the Serial Reaction Time task indicates significant implicit sequence learning ability in individuals with agrammatic aphasia. Further work is needed to examine individual differences in implicit learning in this population, as well as in fluent aphasia. Future research should also examine implicit learning of more complex linguistic stimuli that more closely approximate natural language. Individuals with intact implicit learning ability may greatly benefit from treatment approaches that promote implicit language learning. The present study also suggests that individuals with agrammatic aphasia may have difficulty in deriving benefits from explicit instruction when a learning task involves high working memory demands. Although numerous studies have reported working memory deficits in aphasia, further research is needed to examine the implications of memory impairments for implicit and explicit approaches to relearning language after stroke. Both implicit and explicit learning processes have the potential to be utilized in language treatment, and future research in this area may help engage individuals with aphasia in learning strategies that will be most beneficial for them.
Acknowledgments
We would like to thank the individuals who participated in this study and the family members of the aphasic participants. This study was supported by the National Institutes of Health grant T32DC009399.
Footnotes
Two aphasic individuals declined to participate in the word prediction test.
One aphasic individual declined to participate in the word prediction test.
One aphasic individual was unable to perform the task accurately.
Contributor Information
Julia Schuchard, Email: schuchard@.northwestern.edu, Department of Communication Sciences and Disorders, Northwestern University, 2240 Campus Drive, Room 3-380, Evanston, IL 60208, USA.
Cynthia K. Thompson, Department of Communication Sciences and Disorders, Northwestern University, 2240 Campus Drive, Room 3-380, Evanston, IL 60208, USA. Department of Neurology, Northwestern University, Evanston, IL, USA
References
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX lexical database (Release 1) [CD-ROM] Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1993. [Distributor] [Google Scholar]
- Blumstein SE, Tartter VC, Nigro G, Statlender S. Acoustic cues for the perception of place of articulation in aphasia. Brain and Language. 1984;22(1):128–149. doi: 10.1016/0093-934x(84)90083-x. [DOI] [PubMed] [Google Scholar]
- Boyd L, Winstein C. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learning & Memory. 2004;11(4):388–396. doi: 10.1101/lm.80104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd L, Winstein C. Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. Journal of Neurologic Physical Therapy. 2006;30(2):46–57. doi: 10.1097/01.npt.0000282566.48050.9b. [DOI] [PubMed] [Google Scholar]
- Caplan D, Hanna J. Sentence production by aphasic patients in a constrainted task. Brain and Language. 1998;63:184–218. doi: 10.1006/brln.1998.1930. [DOI] [PubMed] [Google Scholar]
- Caspari I, Parkinson SR, LaPointe LL, Katz RC. Working memory and aphasia. Brain and Cognition. 1998;37(2):205–223. doi: 10.1006/brcg.1997.0970. [DOI] [PubMed] [Google Scholar]
- Cho S, Thompson CK. What goes wrong during passive sentence production in agrammatic aphasia: An eyetracking study. Aphasiology. 2010;24(12):1576–1592. doi: 10.1080/02687031003714442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SC, Wright HH. Verbal and non-verbal working memory in aphasia: What three n-back tasks reveal. Aphasiology. 2010;24(6–8):752–762. [Google Scholar]
- Christiansen M, Kelly L, Shillcock R, Greenfield K. Impaired artificial grammar learning in agrammatism. Cognition. 2010;116:382–393. doi: 10.1016/j.cognition.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Damasio H. Neural basis of language disorders. In: Chapey R, editor. Language intervention strategies in adult aphasia. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 20–41. [Google Scholar]
- Daneman M, Carpenter P. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19(4):450–466. [Google Scholar]
- Dennis N, Howard J, Howard D. Implicit sequence learning without motor sequencing in young and old adults. Experimental Brain Research. 2006;175(1):153–164. doi: 10.1007/s00221-006-0534-3. [DOI] [PubMed] [Google Scholar]
- Evans J, Saffran J, Robe-Torres K. Statistical learning in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2009;52(2):321. doi: 10.1044/1092-4388(2009/07-0189). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner C, Weniger G, Irle E. Implicit and explicit memory after focal thalamic lesions. Neurology. 2001;57(11):2054. doi: 10.1212/wnl.57.11.2054. [DOI] [PubMed] [Google Scholar]
- Faroqi-Shah Y. Are regular and irregular verbs dissociated in non-fluent aphasia?: A meta-analysis. Brain Research Bulletin. 2007;74(1–3):1–13. doi: 10.1016/j.brainresbull.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Feldman J, Kerr B, Streissguth AP. Correlational analyses of procedural and declarative learning performance. Intelligence. 1995;20(1):87–114. [Google Scholar]
- Friedmann N, Gvion A. Sentence comprehension and working memory limitation in aphasia: A dissociation between semantic-syntactic and phonological reactivation. Brain and Language. 2003;86(1):23–39. doi: 10.1016/s0093-934x(02)00530-8. [DOI] [PubMed] [Google Scholar]
- Gomez-Beldarrain M, Garcia-Monco J, Rubio B, Pascual-Leone A. Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Experimental Brain Research. 1998;120(1):25–30. doi: 10.1007/s002210050374. [DOI] [PubMed] [Google Scholar]
- Goschke T, Friederici A, Kotz S, Van Kampen A. Procedural learning in Broca’s aphasia: Dissociation between the implicit acquisition of spatio-motor and phoneme sequences. Journal of Cognitive Neuroscience. 2001;13(3):370–388. doi: 10.1162/08989290151137412. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y. Language deficits and the theory of syntax. Brain and Language. 1986;27:135–159. doi: 10.1016/0093-934x(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kelly S, Griffiths S, Frith U. Evidence for implicit sequence learning in dyslexia. Dyslexia. 2002;8(1):43–52. doi: 10.1002/dys.208. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery-revised. San Antonio, TX: PsychCorp; 2007. [Google Scholar]
- Kim M, Thompson CK. Patterns of comprehension and production of nouns and verbs in agrammatism: Implications for lexical organization. Brain and Language. 2000;74:1–25. doi: 10.1006/brln.2000.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Thompson CK. Real-time production of arguments and adjuncts in normal and agrammatic speakers. Language and Cognitive Processes. 2010;22(9):1993–2011. doi: 10.1080/01690965.2010.496237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linebarger MC, Schwartz MF, Saffran EM. Sensitivity to grammatical structure in so-called agrammatic aphasics. Cognition. 1983;13:361–392. doi: 10.1016/0010-0277(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg G, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: an fMRI study. NeuroImage. 2006;33(4):1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Mack JE, Thompson CK. Tracking passive sentence comprehension in agrammatic aphasia. Journal of Neurolinguistics. 2012;25:31–43. doi: 10.1016/j.jneuroling.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, Gainotti G, Caltagirone C, Masullo C. Some aspects of phonological impairment in aphasia. Brain and Language. 1980;11(1):159–169. doi: 10.1016/0093-934x(80)90117-0. [DOI] [PubMed] [Google Scholar]
- Nissen M, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19(1):1–32. [Google Scholar]
- Orrell A, Eves F, Masters R, Macmahon K. Implicit sequence learning processes after unilateral stroke. Neuropsychological Rehabilitation. 2007;17(3):335–354. doi: 10.1080/09602010600832788. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25(1):563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Reber A. Implicit learning of artificial grammars. Journal of Verbal Learning and Verbal Behavior. 1967;6(6):855–863. [Google Scholar]
- Rüsseler J, Gerth I, Münte T. Implicit learning is intact in adult developmental dyslexic readers: Evidence from the serial reaction time task and artificial grammar learning. Journal of Clinical and Experimental Neuropsychology. 2006;28(5):808–827. doi: 10.1080/13803390591001007. [DOI] [PubMed] [Google Scholar]
- Rüsseler J, Hennighausen E, Münte TF, Rösler F. Differences in incidental and intentional learning of sensorimotor sequences as revealed by event-related brain potentials. Cognitive Brain Research. 2003;15(2):116–126. doi: 10.1016/s0926-6410(02)00145-3. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Saffran EM, Marin OS. The word order problem in agrammatism. I: Comprehension. Brain and Language. 1980;10(2):249–262. doi: 10.1016/0093-934x(80)90055-3. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Squire L, Zola S. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences. 1996;93(24):13515. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JE, McNeil MR, Pratt SR, Dickey MW, Hula WD, Szuminsky NJ, et al. Verbal working memory and its relationship to sentence-level reading and listening comprehension in persons with aphasia. Aphasiology. 2009;23(7–8):1040–1052. [Google Scholar]
- Thompson CK. Northwestern assessment of verbs and sentences. IL: Evanston; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin J, Mainela-Arnold E, Zhang X. Procedural learning in adolescents with and without specific language impairment. Language Learning and Development. 2007;3(4):269–293. [Google Scholar]
- Tompkins CA, Bloise CG, Timko ML, Baumgaertner A. Working memory and inference revision in brain-damaged and normally aging adults. Journal of Speech and Hearing Research. 1994;37(4):896–912. doi: 10.1044/jshr.3704.896. [DOI] [PubMed] [Google Scholar]
- Ullman M. A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience. 2001;2(10):717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Ullman M. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92(1–2):231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman M, Pancheva R, Love T, Yee E, Swinney D, Hickok G. Neural correlates of lexicon and grammar: Evidence from the production, reading, and judgment of inflection in aphasia. Brain and Language. 2005;93(2):185–238. doi: 10.1016/j.bandl.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle R. Individual differences in working memory capacity and learning: Evidence from the serial reaction time task. Memory & Cognition. 2005;33(2):213. doi: 10.3758/bf03195310. [DOI] [PubMed] [Google Scholar]
- Vakil E, Kahan S, Huberman M, Osimani A. Motor and non-motor sequence learning in patients with basal ganglia lesions: The case of serial reaction time (SRT) Neuropsychologia. 2000;38(1):1–10. doi: 10.1016/s0028-3932(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Vicari S, Finzi A, Menghini D, Marotta L, Baldi S, Petrosini L. Do children with developmental dyslexia have an implicit learning deficit? British Medical Journal. 2005;76(10):1392. doi: 10.1136/jnnp.2004.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Marotta L, Menghini D, Molinari M, Petrosini L. Implicit learning deficit in children with developmental dyslexia. Neuropsychologia. 2003;41(1):108–114. doi: 10.1016/s0028-3932(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Yang J, Clark P, Swick K, Watkins H, Li P. The role of working memory in explicit and implicit artificial grammar learning; Annapolis, MD. Poster session presented at Society for the Neurobiology of Language.2011. [Google Scholar]
- Zingeser LB, Berndt RS. Retrieval of nouns and verbs in agrammatism and anomia. Brain and Language. 1990;39:14–32. doi: 10.1016/0093-934x(90)90002-x. [DOI] [PubMed] [Google Scholar]