Abstract
Background
Banked unrelated donor umbilical cord blood (CB) has improved access to hematopoietic stem cell transplantation for patients without a suitably matched donor. In a resource-limited environment, ensuring that the public inventory is enriched with high-quality cord blood units (CBUs) addressing the needs of a diverse group of patients is a priority. Identification of donor characteristics correlating with higher CBU quality could guide operational strategies to increase the yield of banked high-quality CBUs.
Methods
Characteristics of 5267 CBUs donated to the Carolinas Cord Blood Bank, a public bank participating in the National Cord Blood Inventory, were retrospectively analyzed. Eligible CBUs, collected by trained personnel, were processed using standard procedures. Routine quality and potency metrics [post-processing total nucleated cell count (post-TNCC), CD34+, colony-forming units (CFUs)] were correlated with maternal, infant, and collection characteristics.
Results
High-quality CBUs were defined as those with higher post-TNCC (>1.25×109), and CD34+ + CFU in the upper quartile. Factors associated with higher CD34+ or CFU content included a shorter interval from collection to processing (<10 hours), younger gestational age (34–37 weeks; CD34++CFU) Caucasian race, higher birth weight (>3500grams) and larger collection volumes (>80ml).
Conclusions
We describe characteristics identifying high-quality CBUs, which can be used to inform strategies for CBU collection for public banks. Efforts should be made to prioritize collections from larger babies born before 38 weeks of gestation. CBUs should be rapidly transported to the processing laboratory. The lower quality of CBUs from non-Caucasian donors highlights the challenges of building a racially diverse public CB inventory.
Introduction
Umbilical cord blood (CB) is a rich source of hematopoietic stem and progenitor cells for hematopoietic stem cell transplantation (HSCT). Since the first unrelated donor umbilical cord blood transplant (UCBT) in 19931, the use of CB as a donor source for unrelated HSCT has become standard of care for patients without a sufficiently matched related or unrelated adult donor2,3. Advantages of CB donors include ease of procurement, less stringent requirements for human leukocyte antigen (HLA) matching, reduced graft-versus-host disease (GvHD) compared to other stem cell sources and improved access to transplant, especially for racial/ethnic minorities4.
The clinical outcomes after UCBT are influenced by the number of cells available in a single cord blood unit (CBU)5. A typical CB bank inventory contains units with a median postprocessing total nucleated cell count (post-TNCC) of 1.04×109, while the median post-TNCC of units selected for transplantation from the National Marrow Donor Program (NMDP) Be the Match Registry® is 1.76×109 cells6. Effectively, only 8% of the current public inventory meets this criteria6 suggesting that resources allocated to cord blood banking are not being used efficiently. Also, larger patients seeking a CB donor must effectively choose from a limited inventory. Previous studies have shown that potency, as represented by colony-forming units (CFU) and/or CD34+ content of a CBU prior to cryopreservation or after thawing, is correlated with the engraftment potential of an individual CB unit7–9. Efforts to increase the CFU, CD34+ and post-TNCC content of banked CBUs are necessary to increase the overall quality (i.e. racial and ethnic diversity combined with post-TNCC and potency) of CBUs. Additionally, closer HLA matching has been shown to improve outcomes of UCBT3,10,11. Thus, with an increasingly diverse population of patients in need of donors, strategies to bank increasing numbers of racially and ethnically diverse, high-quality CBUs that will have an increased HLA repertoire, are also necessary.
We hypothesized that clinical parameters that are readily available to the obstetrical and collection staff can be used to identify optimal CB donors. If identified, these parameters could be used to guide clinicians on how to prioritize CB collection and processing. In this report, we present the results of an analysis of over 5200 CBUs recently collected and processed by a single public cord blood bank in which we identify, update12–18, and further define characteristics of the mother/infant donor pair and the collection that are associated with higher CBU potency and quality.
Materials and Methods
Study Overview
This is a retrospective study conducted between 2007–2009 by the Carolinas Cord Blood Bank (CCBB), a large public cord blood bank at Duke University Medical Center. CBUs donated by healthy mothers after an uncomplicated pregnancy and after written informed consent were collected at 11 sites and sent to the CCBB for processing, testing and cryopreservation. Correlations between technical parameters routinely measured on a CBU after processing and readily available clinical characteristics of the mother, infant, and collection were examined to determine characteristics that could be used to identify CBUs more likely to yield higher quality CBUs.
Cord Blood Donor Eligibility
Eligible collections included singleton gestations with an estimated gestational age of ≥34 weeks delivered by a mother who was ≥18 years of age at delivery. Collections processed by the CCBB laboratory on Mondays through Thursdays were included in this analysis (n=5267). Extensive maternal medical history and family history were obtained from a maternal donor screening questionnaire and medical records used for donor screening as per CCBB standard operating procedures (SOP). Maternal blood was obtained for donor screening, performed by the American Red Cross Testing Laboratory, in Charlotte, NC, for infectious diseases transmittable through blood. A subset of CBUs included in this analysis (n=606, 11.5%) failed to meet banking specifications (such as maternal history exclusion, positive infectious disease screen, etc.) and were not included in the CCBB public inventory. These CBUs were included in the analysis because the exclusionary factors were found postprocessing and cryopreservation.
Characteristics of the mother, infant and CBU collection
Clinical characteristics that were examined included: maternal age, infant related characteristics such as birth weight, gestational age, gender, race/ethnicity, and collection characteristics such as delivery method, collection volume, time from collection to initiation of processing, and total cell viability after processing. All data was readily available in the CCBB database. This database was initially created by the EMMES Corporation (Rockville, MD) for the COBLT Study4 and has been subsequently upgraded and maintained by EMMES.
The infant donor’s race/ethnicity was based on the mothers’ self-reporting of her own and the baby’s father’s race/ethnicity as part of the maternal questionnaire and determined according to definitions from the United States Office of Management and Budget as used by Health Resources and Services Administration (HRSA)19. Race was classified as Asian, American Indian or Alaska Native, Black or African American, Native Hawaiian or Other Pacific Islanders, and White or Caucasian. Ethnicity considered either Hispanic or Non-Hispanic origin. Assignment to a specific race indicated that both parents were of that race. A “more than one race” category included infants for whom parents were of different races. For purposes of this analysis, we treated Caucasian Hispanic infants (i.e. both parents Caucasian with at least one parent identified as Hispanic/Latino ethnicity) as a separate category. We also analyzed the American Indian or Alaska and Native Hawaiian or Other Pacific Islanders as a single group given the small numbers of patients in these groups.
Cord Blood Collection and Processing
CB collections were performed by trained collection staff using either in-utero or ex-utero collection procedures. At the time of collection, the umbilical cord was stabilized, sterilized with Betadine and alcohol. The umbilical vein was punctured with a 17-gauge needle attached to the collection bag containing 25 or 35 ml of CPD anticoagulant (Sterile Cord Blood Collection Unit (791-08), Pall, Corporation, Medical Subsidiary, Covina, CA). The CB drained by gravity into the collection bag while being gently rocked on a rotating scale to ensure adequate mixing.
At the completion of the collection, the total collection volume (in mls) was measured by weight excluding the anticoagulants. During the study period, a minimum CB collection volume of 40ml was required for transportation to the processing laboratory and to qualify for processing. CBUs not meeting the threshold volume were discarded at the collection site according to the CCBB SOPs. Eligible CBUs were transported to the processing laboratory in a validated shipper by a CCBB courier or via express (overnight) delivery service depending on location of collection site (ranging from 0.25 to over 600 miles from the CCBB). After receipt at the CCBB processing laboratory, trained staff verified appropriate labeling of the CBU based on accompanying documentation and visually inspected CBUs for any signs of leakage or other damage. Those CBUs passing this initial screen were then triaged by collection time and date to ensure that the oldest units were prioritized for processing first. A TNCC was enumerated prior to processing and a minimum 1.0×109 nucleated cells was required to continue to processing. All eligible CBUs were processed using a functionally closed automated system using the Biosafe Sepax system (starting 8/2008; CS-530.2 processing kits, Biosafe, Alexandria, VA) or by manual method using Pall Transfer/Freezing Bag kits (791-02; Pall, Corporation, Medical Subsidiary, Covina, CA) and a refrigerated centrifuge (2007–2008). Both of these processes employ the method of RBC-depletion and plasma reduction. Hydroxyethyl starch at a 1:5 ratio (Hespan, B. Braun Medical, Inc.; Bethlehem, PA) was added prior to centrifugation to assist with the separation of the red blood cells. Following separation of the components, approximately 21 ml of buffy coat enriched product remained. A TNCC was enumerated from the product to determine final cell count recovered. DMSO/Dextran (10% dimethyl sulfoxide in 5% dextran 40, Protide, Lake Zurich, IL) was added as a cryoprotectant and the product was cryopreserved by controlled rate freezing in a 25-mL double-compartment cryopreservation bag (791-05; Pall, Corporation, Medical Subsidiary, Covina, CA). Cryopreserved CBUs were stored under liquid nitrogen.
CBU Characterization
Total Nucleated Cell Enumeration and Viability Assessment
A TNCC was performed on a sample of the collected CB to determine whether an individual CBU contained >1×109 cells and therefore met CCBB criteria for processing. The post-TNCC was also measured after processing to determine if there was adequate recovery and banking criteria were met. The TNCC counts were measured on an automated cell counter (Sysmex K-1000 from 9/07-4/08, Sysmex XE5000 from 4/08 – 7/09; Sysmex America, Inc. Mundelein, IL). Viability was assessed by staining a post-processed CB sample with Trypan blue and scoring viable cells on a hemocytometer after incubating for 5 minutes. The CCBB required the post-processed unit to have ≥90% viability for inclusion in the banking inventory.
CD34+ Enumeration
Enumeration of CD34+ cells was determined after processing by flow cytometry (ProCOUNT, BD Biosciences, San Jose, CA). ProCOUNT reagent (20μL) was added to 12×75 test tubes followed by 50μL of post-processed CB sample. Samples were diluted with PBS/BSA wash (Gibco, Invitrogen, Carlsbad, CA) as needed for a concentration <4×107 cells/ml. Tubes were mixed gently and incubated in the dark at room temperature for 15 minutes. RBCs were further lysed using 450μL of a 1:10 dilution of FACS Lysing Solution (BD Biosciences, San Jose, CA) for samples and incubated in the dark at room temperature for 15 minutes. A two laser, four-color flow cytometer was used to analyze samples. Results were analyzed by CellQuest Pro (BD Biosciences, San Jose, CA) using ProCOUNT gating strategies.
Colony Forming Unit Progenitor Cell Assay
CFU progenitor cells, including Granulocyte Macrophage (CFU-GM), Granulocyte, Erythrocyte, Macrophage and Megakaryocyte (CFU-GEMM), and Burst Forming Unit Erythroid (BFU-E), were enumerated on post-processing samples of CB. Initial samples of 2.5×105 CB cells were removed for testing and diluted in 0.5 ml Iscove’s modified Dulbecco’s Medium (IMDM) plus 2% fetal bovine serum (FBS, StemCell Technologies, Vancouver, BC). Cells were further diluted by adding 5×104 cells in a total of 0.5 ml IMDM plus 2% FBS to achieve a final cell count of 2.5×104/ml when mixed with 1.5ml of MethoCult medium 4434 (StemCell Technologies). Cells were plated in triplicate (0.5 ml/well) at 1.25×104 cells/well in 24-well, tissue culture plates and incubated in a humidified, 37°C, 5% CO2 incubator for 11–14 days. Colony growth was scored in triplicate by trained personnel using an inverted, phase-contrast microscope and reported as the mean colony count per 105 nucleated cells. These numbers were then used to calculate the number of progenitors in the entire graft by correcting for the post-processing TNCC.
Statistical Analysis
Three multivariate logistical regression models were created using the following thresholds: CFU of >34×105 colonies, CD34+ of >3.4×106 cells and post-TNCC of >1.18×109 cells. The thresholds were based on the median post-processing CFU, CD34+ and TNCC for this cohort. As such, any reference to CFU, CD34+ and TNCC content refers to measurements performed after processing unless otherwise indicated. For the purpose of this analysis, we defined pre-term, term and post-term as 34–37 weeks, 38–40 weeks and >40 weeks gestation, respectively. We estimated odds ratios (OR) and 95% confidence intervals for factors associated with higher quality CBUs. Backward selection with a p-value cut-off of <0.05 was used to create the multivariate models. First order interactions were assessed using all factors in the multivariate model. Factors associated with the time to processing from collection were analyzed in a multivariate regression model including day of delivery, time of delivery, collection site (close to CCBB vs. non-local to CCBB), race, infant gender, maternal age and year of processing (Before or After August, 2008 based on the implementation of automated processing). Generalized linear models were used to estimate adjusted means using a Bonferroni correction to describe p-values of the difference in adjusted means. Analyses were conducted using the SAS System version 9.2 (Cary, NC).
Results
We analyzed 5267 CBUs donated by healthy mothers after an uncomplicated pregnancy, labor, and delivery that were processed by the CCBB at Duke from September 2007 through July 2009. Each CBU that met initial volume and cell count criteria was mixed with Hespan, volume and RBC reduced and subsequently assessed for viability and CFUs, CD34+, and post-TNCC content as part of standard testing procedures. Corresponding demographic information and clinical characteristics routinely collected by the bank for each donor CBU were included in this analysis.
Maternal and Infant Donor Demographics
The majority of infants were born by vaginal delivery (59.8%; Table 1) with a median birth weight of 3580g (range, 1800–5724g) with the majority delivering between 38–40 weeks of gestation (82.1%) to mothers with a median age of 29.0 years (range, 18.0–53.0 years). Approximately half of the babies were male (49.4%) with the following infant racial/ethnic representation: Caucasian non-Hispanic (58.8%), Black or African-American (14.4%), Hispanic (12.8%), Asian (2.6%), Native American or Alaska Native (0.1%), and Hawaiian Native or Other Pacific Islander (0.1%) with 10.8% of infants identified as “more than one race”.
Table 1.
Clinical characteristics of maternal and infant donors (n=5267).
| N (%) | |
|---|---|
| Infant gender | |
| Male | 2602 (49.4) |
| Female | 2665 (50.6) |
| Infant race/ethnicity | |
| White/Caucasian (Non-Hispanic) | 3099 (58.8) |
| Black/African-American | 758 (14.4) |
| Hispanic | 675 (12.8) |
| More than one race | 568 (10.8) |
| Asian | 136 (2.6) |
| Other including Native Hawaiian, Pacific Islander or Native American | 31 (0.6) |
| Infant gestational age (weeks)* | |
| 34–37 | 361 (6.9) |
| 38–40 | 4305 (81.7) |
| 41–42 | 573 (10.9) |
| Delivery method | |
| Vaginal | 3149 (59.8) |
| Caesarean | 2188 (40.2) |
| Mean (SD) | Median (range) | |
|---|---|---|
| Birth weight (grams) | 3596 (442) | 3580 (1800–5724) |
| Maternal age (years) | 29.3 (5.6) | 29.0 (18.0–53.0) |
Infant gestational age is unknown for 28 units. The mean and median gestational ages were 39 weeks (SD 1.1; range, 34–42 weeks).
Cord Blood Characteristics
The median volume of CB collected (excluding anticoagulants) was 93.0ml (range, 40.0–286.0ml; Table 2). The median time from collection to the start of processing was 14.1 hours (range, 0.5–47.4 hours). After processing, the median post-TNCC and CD34+ content of the CBUs was 1.18 × 109 nucleated cells (range, 0.63–5.55×109) and 3.21 × 109 CD34+ cells (range, 0.02–9.99×106), respectively (Table 2). Most of the CBUs (99.9%) contained ≥90% viable cells at the conclusion of processing [median 98.5% (range, 84.0–100.0%)]. The median total CFU growth from samples obtained after processing was 34.0 × 105 colonies (range, 0.6–193.3×105).
Table 2.
Characteristics of the processed cord blood units (n=5267) including post-processing cellular parameters.
| Mean (SD) | Median (range) | |
|---|---|---|
| Collection volume (ml) | 97.5 (28.6) | 93.0 (40.0–286.0) |
| Time to processing (hours) | 14.5 (8.9) | 14.1 (0.5–47.4) |
| Total CFU (×105) | 38.3 (22.3) | 34.0 (0.6–193.3) |
| Total CFU per ml collected (×103/ml) | 39.99 (20.94) | 36.60 (0.38–173.13) |
| CD34+ (×106) | 3.66 (2.05) | 3.21 (0.02–9.99) |
| CD34+ per ml collected (×104/ml) | 3.91 (2.21) | 3.44 (0.01–15.32) |
| Post-TNCC (×109) | 1.27 (0.40) | 1.18 (0.63–5.55) |
| Post-TNCC per ml collected (×107/ml) | 13.46 (3.65) | 12.99 (4.20–43.76) |
| Viability (%) | 97.8 (2.40) | 98.5 (84.0–100.0) |
Infant Gestational Age
Infants of younger gestational age were more likely to have CBUs with higher CFU and CD34+ content (both p<0.0001). In multivariate analysis, preterm infants were more likely to have higher CFU as compared to either term or post-term infants (p=0.0182 and p=0.0006, respectively; Table 3, Figure 1A). We observed similar results for CD34+ content (both p<0.0001; Table 4, Figure 1B). While infants ≥38 weeks gestational age had higher post-TNCC as compared to younger infants (Table 5, Figure 1C), this did not correlate with higher potency (i.e., higher CFU and/or CD34+; Figure 1A and B).
Table 3.
Clinical characteristics predictive of CFU >34×105 (median) in univariate and multivariate analysis.
| Characteristics | Univariate | Multivariate |
|---|---|---|
|
| ||
| Odds Ratio (95% CI*), p-value | ||
| Infant gestational age (weeks) | ||
| Overall Effect | p=0.0264 | p=0.0027 |
| 34–37 vs. 38–40 | 1.23 (0.99–1.63), p=0.0567 | 1.32 (1.05–1.65), p=0.0182 |
| 34–37 vs. 41–42 | 1.44 (1.10–1.87), p=0.0071 | 1.63 (1.23–2.16), p=0.0006 |
| 38–40 vs. 41–42 | 1.17 (0.98–1.39), p=0.0859 | 1.24 (1.03–1.49), p=0.0213 |
| Infant Race/Ethnicity+ | ||
| Overall Effect | p=0.0020 | p=0.0076 |
| Caucasian compared to African American | 1.37 (1.17–1.61), p=0.0001 | 1.39 (1.17–1.64), p=0.0001 |
| Hispanic compared to African American | 1.19 (0.97–1.47), p=0.0946 | 1.29 (1.03–1.60), p=0.0233 |
| Infant birth weight (g) | ||
| >3500 | 1.43 (1.28–1.59), p<0.0001 | 1.25 (1.10–1.41), p=0.0003 |
| ≤3500 | 1.00 | 1.00 |
| Infant gender | ||
| Male | 1.18 (1.06–1.32), p=0.0023 | NS |
| Female | 1.00 | |
| Maternal age (years) | ||
| >20 | 1.24 (1.02–1.53), p=0.0381 | NS |
| ≤20 | 1.00 | |
| Collection volume (ml) and delivery type | ||
| ≤80 | ||
| Vaginal | NS | NS |
| Caesarean | ||
| >80 | ||
| Vaginal | 1.18 (1.03–1.34), p=0.0142 | 1.30 (1.13–1.49), p=0.0002 |
| Caesarean | 1.00 | 1.00 |
| Processing time (hours) | ||
| Overall Effect | p<0.0001 | p<0.0001 |
| <10 hours vs. 10–23 hours | 1.29 (1.15–1.46), p<0.0001 | 1.25 (1.11–1.42), p=0.0004 |
| <10 hours vs. ≥24 hours | 1.63 (1.36–1.95), p<0.0001 | 1.64 (1.36–1.98), p<0.0001 |
| 10–23 hours vs. ≥24 hours | 1.26 (1.06–1.50), p=0.0098 | 1.31 (1.09–1.57), p=0.0035 |
CI: Confidence intervals; GA: gestational age; BW: birth weight;
Characteristics that were predictive of higher CFU content in either univariate or multivariate analysis were included. Viability of the cord blood cells was not associated with CFU content and was therefore not included in this table.
All races/ethnicities were compared against each other in both univariate and multivariate analysis. Only the relationships that are statistically significant are shown in the table.
Figure 1.
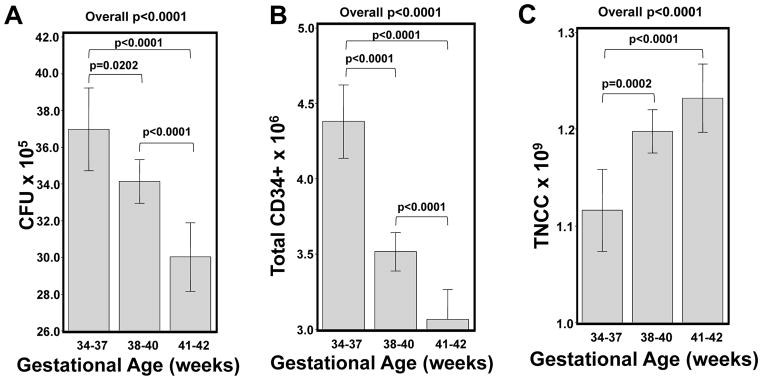
Impact of infant estimated gestational age on the CFU, CD34+ and post-TNCC content. In Panels A–C, the adjusted mean CFU (Panel A), CD34+ (Panel B) and post-TNCC (Panel C) is shown in relationship to infant gestational age after adjusting for infant race/ethnicity, birth weight, gender, collection volume, delivery type and maternal age. Only significant p values are shown. Whisker plots represent the 95% Confidence Intervals.
Table 4.
Clinical characteristics predictive of CD34+ 3.21> ×106 (median) in univariate and multivariate analysis
| Characteristics* | Univariate | Multivariate |
|---|---|---|
|
| ||
| Odds Ratio (95% CI), p-value | ||
| Infant gestational age (weeks) | ||
| Overall Effect | p<0.0001 | p<0.0001 |
| 34–37 vs. 38–40 | 1.89 (1.51–3,20), p<0.0001 | 2.20 (1.74–2.79), p<0.0001 |
| 34–37 vs. 41–42 | 2.44 (1.86–3.20), p<0.0001 | 3.14 (2.35–4.18), p<0.0001 |
| 38–40 vs. 41–42 | 1.29 (1.08–1.53), p=0.0049 | 1.42 (1.18–1.71), p=0.0002 |
| Infant Race/Ethnicity* | ||
| Overall Effect | p=0.0510 | p=0.1172 |
| Caucasian compared to African American | 1.29 (1.10–1.51), p=0.0018 | 1.27 (1.07–1.50), p=0.0057 |
| Hispanic compared to African American | 1.21 (0.98–1.49), p=0.0726 | 1.28 (1.03–1.60), p=0.0259 |
| Infant birth weight (g) | ||
| >3500 | 1.64 (1.46–1.83), p<0.0001 | 1.53 (1.36–1.73), p<0.0001 |
| ≤3500 | 1.00 | 1.00 |
| Infant gender | ||
| Male | 1.36 (1.22–1.51), p<0.0001 | 1.24 (1.11–1.40), p=0.0002 |
| Female | 1.00 | 1.00 |
| Maternal age (years) | ||
| >20 | 1.27 (1.04–1.56), p=0.0217 | NS |
| ≤20 | 1.00 | |
| Collection volume (ml) and delivery type | ||
| ≤80 | ||
| Vaginal | NS | NS |
| Caesarian | ||
| >80 | ||
| Vaginal | NS | 1.19 (1.04–1.37), p=0.0130 |
| Caesarian | 1.00 | |
| Processing time (hours) | ||
| Overall Effect | p<0.0001 | p=0.0004 |
| <10 hours vs. 10–23 hours | 1.16 (1.03–1.30), p=0.0139 | 1.12 (0.99–1.28), p=0.0687 |
| <10 hours vs. ≥24 hours | 1.47 (1.23–1.76), p<0.0001 | 1.46 (1.21–1.76), p<0.0001 |
| 10–23 hours vs. ≥24 hours | 1.27 (1.07–1.51), p=0.0068 | 1.30 (1.08–1.56), p=0.0045 |
| Viability (%) | ||
| 98–100 | NS | NS |
| 90–97 | ||
CI; Confidence Interval; NS: not significant;
Characteristics that were predictive of higher CD34+ content in either univariate or multivariate analysis were included. Viability of the cord blood cells was not associated with CD34+ content and was therefore not included in this table. All races/ethnicities were compared against each other in both univariate and multivariate analysis. Only the relationships that are statistically significant are shown in the table.
Table 5.
Clinical characteristics predictive of Post-TNCC >1.18×109 in univariate and multivariate analysis.
| Characteristics* | Univariate | Multivariate |
|---|---|---|
|
| ||
| Odds Ratio (95% CI), p-value | ||
| Infant gestational age (weeks) | ||
| Overall Effect | p<0.0001 | p<0.0001 |
| 34–37 vs. 38–40 | 0.63 (0.51–0.79), p<0.0001 | 0.62 (0.49–0.79), p=0.0001 |
| 34–37 vs. 41–42 | 0.49 (0.38–0.64), p<0.0001 | 0.51 (0.38–0.68), p<0.0001 |
| 38–40 vs. 41–42 | 0.78 (0.66–0.93), p=0.0060 | 0.82 (0.67–0.99), p=0.0409 |
| Infant race/ethnicity* | ||
| Overall Effect | p<0.0001 | p<0.0001 |
| Caucasian compared to African American | 1.44 (1.25–1.66), p<0.0001 | 1.54 (1.29–1.84), p<0.0001 |
| Caucasian compared to More than One Race | 1.24 (0.87–1.77), p=0.2288 | 1.27 (1.04–1.55), p=0.0193 |
| Caucasian compared to Hispanic | 1.22 (1.03–1.43), p=0.0213 | 1.14 (0.94–1.37), p=0.1752 |
| Asian compared to African American | 1.19 (0.92–1.56) p=0.1914 | 1.59 (1.05–2.40), p=0.0275 |
| Infant birth weight (g) | ||
| >3500 | 1.85 (1.65–2.07), p<0.0001 | 1.37 (1.21–1.56), p<0.0001 |
| ≤3500 | 1.00 | 1.00 |
| Maternal age (years) | ||
| >20 | 1.31 (1.00–1.71), p=0.0480 | NS |
| ≤20 | 1.00 | |
| Collection volume (ml)+ | ||
| ≤80 | ||
| Vaginal | 1.41 (1.05–1.90), p=0.0228 | 1.41 (1.05–1.91), p=0.0242 |
| Caesarian | 1.00 | 1.00 |
| >80 | ||
| Vaginal | 1.18 (1.03–1.35), p=0.0170 | 1.25 (1.08–1.44), p=0.0023 |
| Caesarian | 1.00 | 1.00 |
| Vaginal | ||
| ≤80 | 0.15 (0.13–0.18), p<0.0001 | 0.15 (0.12–0.17), p<0.0001 |
| >80 | 1.00 | 1.00 |
| Caesarian | ||
| ≤80 | 0.12 (0.09–0.16), p<0.0001 | 0.13 (0.10–0.17), p<0.0001 |
| >80 | 1.00 | 1.00 |
| Processing time (hours) | ||
| Overall Effect | p<0.0001 | p=0.0001 |
| <10 hours vs. 10–23 hours | 1.18 (1.05–1.32), p=0.0061 | 1.08 (0.94–1.23), p=0.2674 |
| <10 hours vs. ≥24 hours | 1.60 (1.34–1.91), p<0.0001 | 1.54 (1.26–1.88), p<0.0001 |
| 10–23 hours vs. ≥24 hours | 1.36 (1.14–1.61), p=0.0006 | 1.42 (1.18–1.73), p=0.0003 |
| Viability (%) | ||
| 98–100 | 1.09 (0.97–1.22), p=0.1649 | NS |
| 90–97 | 1.00 | |
CI: Confidence Interval; NS: Not significant;
Infant gender was not significantly associated with TNCC content and therefore was not included in this table.
All races/ethnicities were compared against each other in both univariate and multivariate analysis. Only the relationships that are statistically significant are shown in the table.
Infant Race/Ethnicity
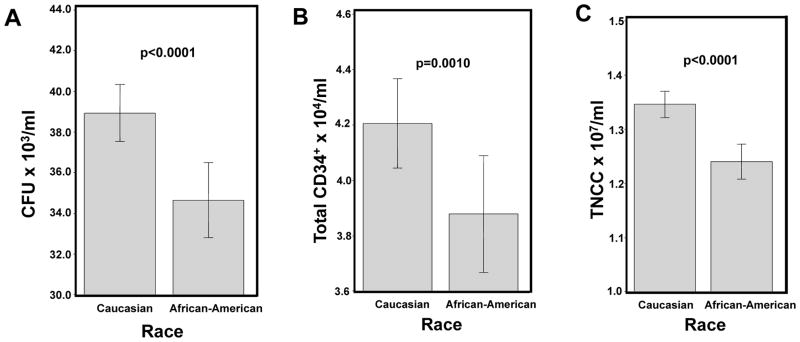
The race/ethnicity of the infant donor was also strongly associated with having higher CBU potency. In multivariate analysis, Caucasian infants were more likely to have higher CFU content [OR 1.39 (95%CI 1.17–1.64), p=0.0001; Table 5], CD34+ [OR 1.27 (95% CI 1.07–1.50), p=0.0057; Table 6) and post-TNCC content [OR 1.54 (95%CI 1.29–1.84). p<0.0001; Table 5] as compared to African American infants. To better understand if there was a biologic basis for this difference, we examined the cell density of the CBUs by race. CBUs from Caucasian infants had significantly higher adjusted mean concentrations (counts per ml of CB) of CFU, CD34+, and post-TNCC (all p<0.0001, Figure 2A–C) as compared to African American infants.
Table 6.
Comparison of significant differences between male and female infants in overall cohort.*
| Male | Female | P value | |
|---|---|---|---|
| Delivery type (Vaginal) | N=1512 (58.1%) | N=1634 (61.4%) | 0.0155 |
|
| |||
| Median (range) | |||
|
| |||
| Birth weight (grams) | 3671 (2227–5473) | 3516 (1800–5724) | <0.0001 |
| Maternal Age (years) | 30 (18–45) | 29 (18–53) | 0.0269 |
| Collection Volume (ml) | 95 (40–286) | 92 (40–284) | <0.0001 |
| Total CFU (×105) | 34.9 (0.6–181.5) | 33.1 (2.3–193.3) | <0.0001 |
| CD34+ (×106) | 3.4121 (0.02–9.99) | 3.0455 (0.252–9.92) | <0.0001 |
There were no differences between male and female infants when comparing gestational age, race/ethnicity or the median TNCC.
Figure 2.
Comparison of the CFU, CD34+, and post-TNCC concentrations for Caucasian and African American infants. In Panels A–C, the adjusted mean CFU per ml (Panel A), CD34+ per ml (Panel B) and post-TNCC per ml (Panel C) is shown in relationship to race for infants of Caucasian and African American race, respectively, after adjusting for infant gestational age, birth weight, gender, collection volume, delivery type and maternal age. Only significant p values are shown. Whisker plots represent the 95% Confidence Intervals.
Infant Birth Weight also correlated with higher quality of the CBUs. In multivariate analysis, heavier babies (>3500g) were more likely to yield CBUs with higher CFU (p=0.0003; Table 3), CD34+ (p<0.0001; Table 4) and post-TNCC content (p<0.0001; Table 5). Each 500g increase in birth weight corresponded to stepwise increases in post-TNCC (p<0.0001; Figure 3C). Furthermore, Infants weighing ≥4500g were 5.37 times (95%CI 3.16–9.15) more likely to have a post-TNCC >1×109 than infants with a birth weight of <3000g (p<0.0001) in univariate analysis. Similar findings were seen for both CFU (Figure 3A) and CD34+ content (Figure 3B).
Figure 3.
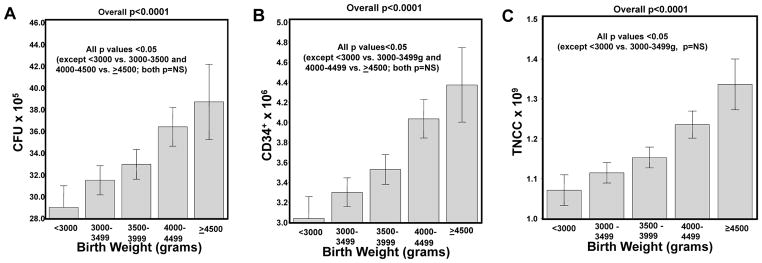
Impact of infant birth weight on the CFU, CD34+ and post-TNCC content. In Panels A–C, the adjusted mean CFU (Panel A), CD34+ (Panel B) and post-TNCC (Panel C) with respect to birth weight is shown after adjusting for infant race/ethnicity, gestational age, gender, collection volume, delivery type and maternal age. Only significant p values are shown. Whisker plots represent the 95% Confidence Intervals.
Infant Gender
When we examined the impact of gender in multivariate analyses, male infants were more likely to have higher total CD34+ content than female infants (p=0.0002). This was related to the fact that male infants had higher median birth weights as compared to female infants (3671g and 3510g, respectively; p<0.0001). Slightly more male infants were born via Caesarean delivery (41.9% males vs. 38.6% for females: p=0.0155) to mothers that were slightly older (30 years old vs. 29 years old, respectively; p=0.0204). Collections from male infants had higher median collection volumes (p=0.0001), CD34+ (p<0.0001) and CFU content (p=0.0013; Table 6). There were no differences between gender with respect to post-TNCC, time to processing or viability.
Maternal Age
In univariate analysis, mothers >20 years old were more likely to donate CBUs with higher CFU (p=0.0381), CD34+ (p=0.0217), and post-TNCC content (p=0.0480; Tables 3–5) compared to younger mothers. This relationship was not significant in multivariate analysis.
Viability
The overall viability as measured by trypan blue exclusion was noted to decrease by small increments as the time from collection to start of processing increased. The median viability for CBUs processed <10 hours and 10–23 hours after collection was 99% and 98%, respectively, as compared to 96% for CBUs processed ≥24 hours after collection (p<0.0001). When we examined the absolute percent of viable cells as related to the post-TNCC content, higher viability (98–100%) was associated with higher post-TNCC content in univariate analysis (Table 5) but not in multivariate analysis. There was no association between cell viability and either the CFU or CD34+ content.
Collection Volume and Delivery Method
CBUs with higher volumes were more likely to have higher potency and quality (all p<0.0001) in univariate analysis. However, when we considered the collection volume in multivariate analysis, an interaction between delivery type (i.e. Caesarean vs. Vaginal delivery) and initial collected volume was observed (Tables 3–5). Specifically, in higher volume CBUs (>80ml), collections from vaginal deliveries were more likely to result in higher CFU (p=0.0002) and CD34+ content (p=0.0130) as compared to collections from babies delivered by Caesarean section. No impact of delivery type on potency was observed for smaller CBUs. Vaginal deliveries with smaller volumes (≤80 ml) were more likely to result in higher post-TNCC (p=0.0242) as compared to those collected after Caesarean section.
Time to Processing
We also examined the effect of time, measured from the start of collection to initiation of processing, on the potency of the processed CBU. For all three parameters, shorter time from collection to processing was associated with increased potency in the processed CBUs (Tables 3–5; Figure 4). When processing was initiated <10 hours from the time of collection, the CBUs were more likely to have higher CFU (p=0.0004; Figure 4A) and a trend to higher CD34+ (p=0.0687; Figure 4B) as compared to CBUs processed 10–23 hours after collection. Furthermore, there were significant differences in overall CBU quality with increases in CFU (p=0.0035), CD34+ (p=0.0045) and post-TNCC content (p=0.0003), in CBUs processed from 10–23 vs. ≥24 hours after collection (Figure 4). Comparing CBUs processed <10 hours to those processed ≥24 hours after collection, the CFU, CD34+ and post-TNCC content (all p<0.0001; Figure 4A–C) were significantly higher in the CBUs that were processed closer to collection. Factors influencing time from collection to processing included distance of the collection site from the processing laboratory, prioritization protocols in the processing laboratory, local courier pickup schedules and processing staff availability (data not shown).
Figure 4.

Impact of time to processing on CFU, CD34+ and post-TNCC content. In Panels A–C, the adjusted mean CFU (Panel A), CD34+ (Panel B) and post-TNCC (Panel C) by time to processing is presented after adjusting for infant race/ethnicity, gender, gestational age, birth weight, collection volume, delivery type, and maternal age. Only significant p values are shown. Whisker plots represent the 95% Confidence Intervals.
Association between Cord Blood Potency (CFU and CD34+) and post-TNCC
Many public CB banks currently use 0.9×109 total nucleated cells as the minimum post-TNCC for inclusion of a CBU in their inventory. This also represents the current post-TNCC threshold used to qualify CBUs for the National Cord Blood Inventory. A recent analysis of the NMDP inventory showed that 68.5% of the units listed had post-TNCCs <1.25, 15.5% between 1.25–1.49, 8.0% between 1.50–1.74 and 7.9% ≥1.75 × 109, respectively. However, the median post-TNCC of units selected by transplant centers for transplantation to their patients was 1.76×109 cells6. We asked whether the practice of selecting higher post-TNCC containing units guarantees use of a unit with high potency (Figure 5). In this analysis, 11.6% of the units (n=616) had post-TNCCs >1.75×109 (Figure 5, Regions A and C). Interestingly, defining optimal potency in CBUs as those with CFU and CD34+ values in the highest quartile, ~25% of high cell count units had lower potency (Figure 5, Region C). Conversely, in the group of CBUs with intermediate post-TNCCs (1.25–1.75×109), 39% had CFU or CD34+ values in the upper quartile (Figure 5, Region B). These CBUs with intermediate post-TNCC and high CFU and/or CD34+ content are likely to perform as well after transplant as the CBUs with higher post-TNCC. Using this approach, 20.7% of units are high quality with high or intermediate (>1.25×109) post-TNCC and high potency (CFU and CD34+ >75th percentile) (Figure 5, Region A+B).
Figure 5.
Considering the potency (CFU content) along with post-TNCC in donor selection. The relationship between post-TNCC and the CFU content (i.e. potency) is shown for the study cohort (n= 5267 CBUs). The vertical lines represent (from left to right): the minimum post-TNCC required for banking (0.9×109), an intermediate post-TNCC (1.25 × 109), and the median post-TNCC of CBUs selected for transplantation (1.75×109)6. Quartiles for the CFU content are also represented [upper quartile: red circle, second quartile: green triangle, third quartile +: gold, and lower quartile: blue x]. Region A refers to CBUs with post-TNCC>1.75x 109 and CFU in the highest quartile. Region B refers to post-TNCC 1.25–1.75×109 with CFU in the highest quartile. Region C refers to CBUs with post-TNCC >1.75×109 and CFU in the lower three quartiles.
Discussion
In this large retrospective study, we examined the clinical characteristics of maternal and infant donors and the collected CBU to identify factors that predict the quality and potency of CBUs collected and processed by a large public CB bank. Characteristics that positively influenced the potency of the CBUs included younger gestational age, Caucasian race/ethnicity of the infant, shorter time to processing, higher birth weight and larger collection volumes. Collection staff and obstetricians could use these parameters to identify donors most likely to yield bankable, high quality CBUs.
The potency of an individual CBU correlates with its potential for engraftment after transplantation. Banks strive to store CBUs with high potency in their inventories. Practically, potency is a measure of the overall health of a CBU and reflects its ability to withstand the stresses produced during processing, cryopreservation and thaw. We previously showed that of all of the parameters measured on CBUs, CFU content, post processing and/or post thaw, best correlate with potency9,20,21 as compared to either CD34+ or post-TNCC content. In a single center retrospective study of 435 single unit UCBTs after myeloablative conditioning, higher CFU dosing was the only pre-cryopreservation graft characteristic predictive of neutrophil (p=0.0024) and platelet engraftment (p=0.0063) in multivariate analysis9. Likewise, post-thaw CFU content was the best predictor of neutrophil and platelet engraftment (both p<0.0001)9. Other reports have shown CD34+ cell content to be a stronger predictor of engraftment as compared to post-TNCC7,8. Thus, for purposes of this analysis, we prioritized CFU content as the best measure of potency followed by CD34+, and lastly, post-TNCC, since CBUs are conventionally selected based on the post-processed TNCC dose (adjusted for patient weight) that would be delivered to a patient. There is, however, a drawback to this approach, because potency measures are not available at the time a unit is processed and cryopreserved, post-TNCC is the only measure available in “real time” and is the parameter utilized to make the decisions “to process or not to process” or “to cryopreserve or not to cryopreserve”. Correlations of post-TNCC with CD34+ and CFU will help banks set thresholds for prioritization protocols for CBU manufacturing.
Using CFU as the best biomarker of potency, shorter processing time, younger gestational age, and race/ethnicity were all highly predictive of CBUs with high potency. Similar trends were seen for CD34+ content. Of note, younger gestational age was associated with lower post-TNCC, due to the smaller size of the baby and resulting smaller CBU collection, but the collections from these younger babies were enriched for both CFUs and CD34+ cell content reflecting higher potency overall. Samples of CB from preterm infants have been shown in vitro to have higher concentration of hematopoietic progenitor cells (i.e. CFU) in addition to increased proliferative potential as compared to term infants22. Previous studies have also found that CBUs derived from donors of younger gestational age have higher CFU12 and/or CD34+ content14,16,17,23,24. These relationships are probably due to the mobilizing signals produced by placental tissue during fetal development.
Our results support previous reports demonstrating a correlation between increased birth weight and CD34+ or post-TNCC4,12,15,16,24,25 and extend this correlation to higher CFU content12,25 content. However, at term, there is a divergence between increasing post-TNCC and baby weight and progenitor cell content (CFU and CD34+); progenitor cell content decreases in post-term infants. Using a “real-world” scenario, if two infants are being delivered simultaneously and only one collection can be performed, the collector might have to decide whether to collect from a larger post-term baby at 42 weeks gestation and a smaller baby at 37 weeks. Based on the results of this analysis, we would recommend prioritizing the younger infant’s collection recognizing that, while it is likely to have a slightly lower post-TNCC, it is more likely to have higher CFUs and CD34+ cells indicative of higher potency.
Whether the mother’s age impacts the quality of the donated CBU is a question that remains unresolved. A recent report26 described increased post-TNCC in units collected from 30–34 years old as compared to 20–24 year old mothers. Another report17 described higher CD34+ content in CBUs from younger mothers. However, other studies have not observed any impact of maternal age on post-TNCC content4,12. While we observed that CBUs donated by mothers <20 years old (comprising 7.6% of the overall cohort) were more likely to have lower post-TNCC, CD34+ and CFU content in univariate analysis, this observation did not remain significant when adjusting for other characteristics. Thus, our findings do not support focusing efforts on collecting from mothers <20 years old.
This analysis also clearly demonstrated that, after adjusting for other variables, the donor’s race/ethnicity is strongly associated with the quality and potency of CBUs. Most notably, Caucasian infants were more likely to have CBUs with higher potency as compared to African American infants. Furthermore, we observed that, despite similar collection volumes, CBUs from Caucasian infants had higher numbers per ml of CFU, CD34+ and post-TNCC cells as compared to African American infants. These results confirm and extend other studies showing the relationship between race and post-TNCC, CD34+ or CFU cell content13,14,27,28. Our finding of lower potency of CBUs from African American donors complicates the path to achieving the stated goals of increasing and maintaining the ethnic and racial diversity of high quality CBU in the national donor registry. Therefore, if donor/recipient matching by race is determined to be associated with superior clinical outcomes in transplantation, it will be critical to enhance recruitment of African American donors and increase resources to collect higher numbers of CBUs from this population.
The variables that are collection-related and that can be modified to increase CBU quality include increasing collection volumes and shortening time to processing. Larger volume CBUs were more likely to have higher potency overall compared to smaller CBUs regardless of delivery type. Thus, the collection staff should be encouraged to use careful technique and take the time to maximize the volume of CB that can be extracted. In the laboratory, strategies to prioritize the processing of recently collected CBUs should be developed. Currently, many banks receive collections from distant sites, and, therefore, delays in processing due to travel may exist. Results of the COBLT study indicated that viability, post-TNCC, and CD34+ content remain stable at room temperature for >48 hours4 leading to the rule that cryopreservation of a processed CBU must begin within 48 hours of collection. However, in this study, we found small but significant losses of CFU, CD34+ cells and TNCC that occur in CB processed ≥24 hours after delivery as compared to those processed <10 hours from collection. Our findings are similar to Pope et al29 who recently correlated decreasing viability with increased time from collection to freeze. These findings indicate that delays in processing could impact the yield of bankable CBUs. These results should inform banks designing algorithms and criteria for prioritization of CBUs in their processing laboratories. For example, in our own bank, this data prompted change in our algorithm, originally solely based on time from collection, to prioritizing processing larger (>150ml for Caucasian; >120ml for non-Caucasians) units received in the lab <24 hours from collection ahead of older units. Practically, this also indicates that banks should consider opening collection sites that are close to their processing laboratories and that courier schedules should be adjusted to transport collected CBUs back to the processing laboratory as quickly as possible. Regionalization of the public banking system should also be considered as an option in which publically donated CBUs would be referred to the geographically closest bank.
Taken at face value, our data indicates that collections from larger, Caucasian, early term babies that are processed within 10 hours of collection yield the highest quality CBUs for public banking. However, if we were to follow this formula to the letter, we would exclude collections from racial and ethnically diverse donors, which would result in a less diverse pool of HLA phenotypes in the inventory. Whether a racially matched CBU will result in better clinical outcomes presumably because of closer HLA-matching has yet to be determined. However, results with adult donors indicate that closer HLA-matching combined with adequate cell content will result in superior clinical outcomes11. Larger datasets of clinical outcomes of UCBT will be necessary to definitively answer this question but if racial matching yields superior post-transplant outcomes, collection of large numbers of non-Caucasian CBUs must occur to add high-quality minority CBUs to the inventory.
Data from the NMDP registry demonstrates that CBUs with higher post-TNCC (median 1.76 × 109) are preferentially selected by transplant centers for transplantation6. We have shown that 75% of these units also have high potency (Figure 5, Region A). However, using the post-TNCC to select a donor unit, transplant physicians would inadvertently select a less potent CBU 25% of the time. It is possible that this discrepancy could contribute to engraftment delay in some cases. We also observed that approximately 40% of units with intermediate (1.25–1.75×109) post-TNCC have potency in the highest quartile (Figure 5, Region B). Therefore, if transplant physicians were to consider the CFU and/or CD34+ content and select more units with intermediate post-TNCC when appropriate, utilization of the existing inventory of donor CBUs would be enhanced (Figure 5, Region A+B).
We have identified several parameters related to donor characteristics, collection procedures, transportation to the processing laboratory and the actual processing procedures which could be optimized to increase the yield of high potency CBU from the pool of prospective donors. Implementing new procedures that incorporate collection priorities and processing protocols that optimize these parameters may increase this yield, but competes with the goal of rapidly expanding both the number and diversity of the national CBU inventory. Studies to objectively determine the role of race matching in cord blood transplantation should be conducted to help inform this discussion. Thus, the observations made in this report should be used to guide decisions about bank organization, location of collection sites, and prioritization of units in the processing lab to work towards creating an inventory with the highest potency for clinical applications.
Acknowledgments
The authors thank the generous donors and their families for the donating their cord blood for public use and the collection and processing staff of the Carolinas Cord Blood Bank at Duke and the Duke Stem Cell Laboratory for their dedication, hard-work and commitment to the patients that are ultimately served.
Funding: This project was supported by Grant Number K23HL104575 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
References
- 1.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, Ciocci G, Carrier C, Stevens CE, Rubinstein P. Placental Blood as a Source of Hematopoietic Stem Cells for Transplantation into Unrelated Recipients. N Engl J Med. 1996;335:157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lowe LA, Wall D, Kapoor N, Guinan EC, Feig SA, Wagner EL, Kernan NA on behalf of the CSC. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–27. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eapen M, Rubinstein P, Zhang M-J, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM, Wagner JE. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 4.Kurtzberg J, Cairo MS, Fraser JK, Baxter-Lowe L, Cohen G, Carter SL, Kernan NA. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–55. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, Rosenfield RE, Stevens CE. Outcomes among 562 Recipients of Placental-Blood Transplants from Unrelated Donors. N Engl J Med. 1998;339:1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 6.Bart T, Boo M, Balabanova S, Fischer Y, Nicoloso G, Foeken L, Oudshoorn M, Passweg J, Tichelli A, Kindler V, Kurtzberg J, Price T, Regan D, Shpall EJ, Schwabe R. Selection and Sustainability: Impact on selection of the cord blood units from the United States and Swiss registries on the cost of banking operations. Transfus Med Hemother. 2012 doi: 10.1159/000345690. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NKC, Davies SM. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, de Lima M, Cairo MS, Furst S, Rio B, Dalley C, Carreras E, Harousseau J-L, Mohty M, Taveira D, Dreger P, Sureda A, Gluckman E, Rocha V. Analysis of Risk Factors for Outcomes After Unrelated Cord Blood Transplantation in Adults With Lymphoid Malignancies: A Study by the Eurocord-Netcord and Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2009;27:256–63. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 9.Page KM, Zhang L, Mendizabal A, Wease S, Carter S, Gentry T, Balber AE, Kurtzberg J. Total Colony-Forming Units Are a Strong, Independent Predictor of Neutrophil and Platelet Engraftment after Unrelated Umbilical Cord Blood Transplantation: A Single-Center Analysis of 435 Cord Blood Transplants. Biol Blood Marrow Transplant. 2011;17:1362–74. doi: 10.1016/j.bbmt.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115:1843–9. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballen KK, Klein JP, Pedersen TL, Bhatla D, Duerst R, Kurtzberg J, Lazarus HM, LeMaistre CF, McCarthy P, Mehta P, Palmer J, Setterholm M, Wingard JR, Joffe S, Parsons SK, Switzer GE, Lee SJ, Rizzo JD, Majhail NS. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18:903–12. doi: 10.1016/j.bbmt.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballen KK, Wilson M, Wuu J, Ceredona AM, Hsieh C, Stewart FM, Popovsky MA, Quesenberry PJ. Bigger is better: maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplant. 2001;27:7–14. doi: 10.1038/sj.bmt.1702729. [DOI] [PubMed] [Google Scholar]
- 13.Ballen KK, Kurtzberg J, Lane TA, Lindgren BR, Miller JP, Nagan D, Newman B, Rupp N, Haley NR. Racial diversity with high nucleated cell counts and CD34 counts achieved in a national network of cord blood banks. Biol Blood Marrow Transplant. 2004;10:269–75. doi: 10.1016/j.bbmt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Cairo MS, Wagner EL, Fraser J, Cohen G, Van De Ven C, Carter SL, Kernan NA, Kurtzberg J. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion. 2005;45:856–66. doi: 10.1111/j.1537-2995.2005.04429.x. [DOI] [PubMed] [Google Scholar]
- 15.Chandra T, Afreen S, Kumar A, Singh U, Gupta A. Does umbilical cord blood-derived CD34+ cell concentration depend on the weight and sex of a full-term infant? J Pediatr Hematol Oncol. 2012;34:184–7. doi: 10.1097/MPH.0b013e318249adb6. [DOI] [PubMed] [Google Scholar]
- 16.Jan RH, Wen SH, Shyr MH, Chiang BL. Impact of maternal and neonatal factors on CD34+ cell count, total nucleated cells, and volume of cord blood. Pediatr Transplant. 2008;12:868–73. doi: 10.1111/j.1399-3046.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa R, Watanabe T, Kawano Y, Kanai S, Suzuya H, Kaneko M, Watanabe H, Okamoto Y, Kuroda Y, Nakayama T the Chugoku-Shikoku Cord Blood B. Analysis of maternal and neonatal factors that influence the nucleated and CD34+ cell yield for cord blood banking. Transfusion. 2004;44:262–7. doi: 10.1111/j.1537-2995.2004.00645.x. [DOI] [PubMed] [Google Scholar]
- 18.Solves P, Larrea L, Soler MA, Mirabet V. Relationship between gestational age and cord blood quality. Transfusion. 2001;41:302–4. doi: 10.1046/j.1537-2995.2001.41020302.x. [DOI] [PubMed] [Google Scholar]
- 19.Humes K, Jones NA, Ramirez RR. US Department of Commerce EaSA, editor. Overview of Race and Hispanic Origin: 2010. US Census Bureau; Mar, 2011. [Google Scholar]
- 20.Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K, Lakshminarayanan S, Allison J, Wood S, Semmel D, Escolar ML, Martin PL, Carter S, Kurtzberg J. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–89. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page KM, Zhang L, Mendizabal A, Wease S, Carter S, Shoulars K, Gentry T, Balber AE, Kurtzberg J. The Cord Blood Apgar: a novel scoring system to optimize selection of banked cord blood grafts for transplantation (CME) Transfusion. 2012;52:272–83. doi: 10.1111/j.1537-2995.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luzo AC, Duarte AS, Salles TS, Queiroz ML, Lorand-Metze I, Costa FF, Saad ST. Early proliferation of umbilical cord blood cells from premature neonates. Vox Sang. 2007;93:145–53. doi: 10.1111/j.1423-0410.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 23.Gasparoni A, Ciardelli L, Avanzini MA, Bonfichi M, di Mario M, Piazzi G, Martinotti L, Vanelli L, Rondini G, Chirico G. Immunophenotypic changes of fetal cord blood hematopoietic progenitor cells during gestation. Pediatr Res. 2000;47:825–9. doi: 10.1203/00006450-200006000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Wen SH, Zhao WL, Lin PY, Yang KL. Associations among birth weight, placental weight, gestational period and product quality indicators of umbilical cord blood units. Transfus Apher Sci. 2012;46:39–45. doi: 10.1016/j.transci.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Mancinelli F, Tamburini A, Spagnoli A, Malerba C, Suppo G, Lasorella R, de Fabritiis P, Calugi A. Optimizing umbilical cord blood collection: impact of obstetric factors versus quality of cord blood units. Transplant Proc. 2006;38:1174–6. doi: 10.1016/j.transproceed.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 26.Omori A, Hirai M, Chiba T, Takahashi K, Yamaguchi S, Takahashi TA, Kashiwakura I. Quality-assessments of characteristics of placental/umbilical cord blood associated with maternal age- and parity-related factor. Transfus Apher Sci. 2012;46:7–13. doi: 10.1016/j.transci.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Askari S, Miller J, Chrysler G, McCullough J. Impact of donor- and collection-related variables on product quality in ex utero cord blood banking. Transfusion. 2005;45:189–94. doi: 10.1111/j.1537-2995.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- 28.George TJ, Sugrue MW, George SN, Wingard JR. Factors associated with parameters of engraftment potential of umbilical cord blood. Transfusion. 2006;46:1803–12. doi: 10.1111/j.1537-2995.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- 29.Pope B, Mitsakos K, Bilgin A, Hokin B, Grant R. Predicting overall viability of cord blood harvests. Transfusion. 2012;52:1079–85. doi: 10.1111/j.1537-2995.2011.03386.x. [DOI] [PubMed] [Google Scholar]