Abstract
The current research used event-related potentials (ERPs) to investigate neurophysiological responses to intact and disrupted actions embedded within an event in children and adults. Responses were recorded as children (24-month-olds) and adults observed a relatively novel event composed of three actions. In one condition pauses were inserted at intact boundaries (i.e., at the endpoint of each action), whereas in the other condition they were inserted at breakpoints that disrupted the action (i.e., in the middle of each action). Evoked responses revealed differences across conditions in both groups; disrupted actions elicited a prolonged negative slow wave from 100 to 700 ms in children, whereas adults demonstrated two distinct negative peaks between 50–150 and 250–350 ms. These findings contribute the first electrophysiological evidence that children readily detect disruptions to ongoing events by the end of the second year, even with limited exposure to the event itself. Furthermore, they suggest that adults rely on two distinct mechanisms when processing novel events. Results are discussed in relation to the role of perceptual and conceptual levels of analysis in the development of action processing.
Keywords: Event-related potentials (ERPs), Development, Novel events, Action understanding, Event segmentation, Spatial learning
Introduction
From the very beginning, human action unfolds around infants and young children. The temporal and hierarchical organization of these actions is a crucial component of event meaning. Take, for example, the busy mother who is simultaneously preparing for work and getting her children ready for school. The mother’s dressing and collecting of her documents is distinct from dressing her children and preparing breakfast. Each event is composed of action units that, in sum, distinguish it from simultaneously occurring events as well as from events that have occurred in the past or may occur in the future. The ability to detect relevant action units within the event stream may serve as a foundation for forming logical conclusions about observed behaviors, planning and executing appropriate responses within a dynamic environment, and developing an understanding of what people do and why they do it. To date, however, we have very little evidence as to how children process unfamiliar events, and we know even less about the development of neural mechanisms that subserve this ability. The current article contributes to the field by bringing neurophysiological evidence to bear on the question of whether observed human action is processed meaningfully from early in life.
Multiple sources of information operating in tandem facilitate adults’ ability to process (Raisig, Welke, Hagendorf, & van der Meer, 2010; Zacks & Swallow, 2007) and evaluate the rationality of actions (Jastorff, Clavagnier, Gergely, & Orban, 2011) with bottom-up perceptual cues complementing top-down conceptual knowledge (Zacks & Tversky, 2001). Whereas a substantial body of literature suggests that children also exhibit at least some ability to evaluate the rationality of observed actions within the first and second years (Biró, Csibra, & Gergely, 2007; Király, Jovanovic, Prinz, Aschersleben, & Gergely, 2003; Klossek & Dickinson, 2012), greater uncertainty surrounds the mechanisms that subserve this developing capacity. According to the “teleological” stance, 12-month-olds evaluate action as it pertains to the intended goal and situational constraints (Gergely & Csibra, 2003); adults rely on the same trichotomy (i.e., action, goal, and constraints) but extend their representations by attributing mental intentional states to the agent. Alternatively, it has been suggested that infants’ ability to evaluate others’ actions may reflect a statistical learning mechanism (Cicchino, Aslin, & Rakison, 2011; Kirkham, Slemmer, & Johnson, 2002; Olofson & Baldwin, 2011). In one recent study, adults rapidly modified their expectations, whereas 9-month-olds continued to predict an inefficient action based on frequency of exposure (Paulus et al., 2011). Whether and to what extent action processing in children is similarly governed by perceptual versus conceptual processes, and whether the two systems are both activated in response to relatively novel events, is currently unknown.
To address this issue, the current study used event-related potentials (ERPs, i.e., electrical activity time-locked to the presentation of a stimulus and recorded at the scalp) to measure neural responses as children and adults observed a relatively novel event1 composed of three actions in which an agent manipulated objects. For the purpose of this article, we consider an “event” to be a set of interrelated agents, actions, and objects situated in a specific time and space. Importantly, in the current research, there are temporal contingencies between actions within the larger event such that the completion of each action leads logically and proximally to the beginning of the next action. This is consonant with usage in developmental literature focusing on event perception (Baillargeon & Wang, 2002; Johnson, Amso, Frank, & Shuwairi, 2008), event segmentation (Baldwin, Baird, Saylor, & Clark, 2001; Friend & Pace, 2011), and event memory (Bauer et al., 2006; Carver, Bauer, & Nelson, 2000). The event was relatively novel in the sense that participants had not previously observed this particular sequence of actions involving this unique combination of objects. Thus, we could be sure that participants had not established any a priori expectations about the event. We emphasize novelty to contrast with previous research that has focused on familiar everyday events such as eating and washing the dishes (Baldwin et al., 2001; Meints, Plunkett, & Harris, 2008; Reid et al., 2009). The increased processing of unexpected variations in familiar events suggests early sensitivity to violations of rationality. In the current study, we asked whether children evince similar sensitivity in unfamiliar events.
Several questions regarding the development of early rationality emerge from the literature on action perception and comprehension in young children. Foremost is how observers construct accurate representations of events, especially when they are initially encountered. Developing the ability to perceive the hierarchical organization of an event may lay the groundwork for more sophisticated action interpretation (Reid, Hoehl, Landt, & Striano, 2008). Converging behavioral and neural evidence has demonstrated that adults rapidly and automatically process events at multiple levels, moving seamlessly between fine- and coarse-grained representations (Newtson, 1973; Newtson & Enquist, 1976; Zacks & Tversky, 2001). Fine- and coarse-grained representations, in turn, correspond to distinct levels of analysis. For example, even an everyday event such as making a sandwich can be divided in myriad ways. Segments of action such as opening the refrigerator and selecting the ingredients can be further disassembled at fine-grained breakpoints that tend to coincide with perceptual changes in velocity or trajectory (e.g., grabbing the handle, swinging the door open, bending down to peruse the shelves). Linking these segments together at a coarse-grained level allows the event to be characterized conceptually in terms of goals (e.g., she is preparing lunch) and intentions (e.g., because she is hungry). Although these processes have been studied extensively in adults, much less is known about their development in young children.
The current article explores the neurocognitive mechanisms that subserve children’s developing capacity for action processing, with specific interest in the role of perceptual and conceptual levels of analysis. Here, we define perceptual levels as those based on observable features of dynamic motion (e.g., trajectory and velocity), and we define conceptual levels as those based on non-observable features related to psychological attributes abstracted from the event context (e.g., goals and intentions). It is important to emphasize that these levels of analysis are not mutually exclusive; event representations constructed from observable perceptual features may overlap in many ways with those constructed from conceptual inferences. Moreover, the rationality of many actions can be readily evaluated from information that is perceptually available. For example, take the case of a woman reaching for a glass of water. We can determine that she extends her arm across the shortest distance with acceleration early in the reach and deceleration as she approaches the glass using perceptual cues to velocity, trajectory, and spatial location. Conceptually, we can infer that she is thirsty or that she needs to put out a fire, depending on the context. What if she fails to reach the glass or pauses inexplicably in her reach? These are deviations in the expected course of the event that can be detected perceptually but may be understood perhaps only by conceptual inference. As this example illustrates, multiple levels of analysis contribute to understanding observed human action. Whether young children spontaneously process novel events at both perceptual and conceptual levels remains a pressing question in the literature on how children develop an understanding of rational actions.
Behavioral evidence suggests that infants develop early and robust sensitivity to perceptual information, rooted in their proclivity to attend to spatial and temporal properties of motion within dynamic events. For example, 14- and 17-month-olds were able to discriminate both path (i.e., the trajectory of a figure) and manner (i.e., the way in which an action was performed) in a nonlinguistic animated motion event (Pulverman, Golinkoff, Hirsh-Pasek, & Sootsman Buresh, 2008). The ability to parse continuous action into discrete units may arise within a similar time frame. For example, infants have been shown to individuate different actions from a heterogeneous sequence (Sharon & Wynn, 1998; Wynn, 1996), recognize a familiar pattern of motion embedded within a more complex event (Hespos, Saylor, & Grossman, 2010), and develop explicit memory for a novel multi-step event within the first and second years (Bauer et al., 2006; Carver et al., 2000). By 24 months of age, infants are able to spontaneously segment a sequence of three relatively novel actions and isolate the specific actions at coarse action boundaries (Friend & Pace, 2011). Discriminating action steps using perceptual information may be a precursor for efficient action perception.
Do infants also represent the conceptual structure of an event or, alternatively, can early rationality be explained primarily in terms of surface-level features such as patterns of motion in human action? Behavioral evidence suggests that infants begin to interpret observed action in terms of goals by the end of the first year (Csibra, Bíró, Koós, & Gergely, 2003; Csibra & Gergely, 2007; Csibra, Gergely, Bíró, Koós, & Brockbank, 1999; Lakusta, Wagner, Hearn, & Landau, 2007). In one study, Baldwin et al. (2001) demonstrated that 10- and 11-month-olds segment events at intentional boundaries by attending to patterns of spatial and temporal motion that reflect the agent’s intentions. Infants were habituated to videos of everyday events (e.g., washing dishes). At test, they showed renewed interest in these videos when pauses were inserted at intervals that interrupted ongoing activity, but not when pauses were inserted at natural action boundaries. These findings indicate that infants can detect disruptions within familiar events. The authors interpreted these results to suggest that infants are sensitive to the abundance and redundancy of cues at multiple levels and that they use this information to make sense of event structure and interpret others’ actions. However, because fine- and coarse-grained boundaries frequently coincide in time and space (Zacks & Swallow, 2007), it is not clear whether participants used bottom-up perceptual properties of motion-in-action or top-down conceptual inferences about the actor’s intentions to parse ongoing action.
The method of ERPs is particularly suited to explore bottom-up versus top-down processing in children’s action perception for two principal reasons. First, it affords a noninvasive online measure of the way in which a stimulus is processed without the requirement of a behavioral response (Männel, 2008). Second, previous research involving adults has suggested that perceptual and conceptual processes may be dissociable on the basis of ERP measurements that differ in latency and amplitude (Federmeier & Kutas, 2001). This methodology is guided theoretically by an interest in identifying, at the neurophysiological level, the mechanisms that support toddlers’ analysis of events and the actions of which they are composed. To this end, differences in the amplitude of components between conditions can be used to infer the extent to which neural activity is generated in response to disrupted actions, relative to intact actions, within a novel event (Luck, 2005). Differences in latency from stimulus onset reflect relative processing speed; in general, earlier components are associated with perceptual processing, whereas later components are associated with conceptual processing (Thierry, 2005). Finally, scalp distribution provides gross spatial information about the underlying neural networks that may be involved in processing and evaluating observed human action. Differences in neurophysiological responses to intact and disrupted actions permit exploration of whether novel events are processed at both perceptual and conceptual levels by 24 months of age.
Two previous studies have explored the neural correlates of everyday event processing in children. Reid, Csibra, Belsky, and Johnson (2007) identified increased gamma-band oscillations in response to incomplete versus completed familiar action (e.g., pouring) at 8 months of age, suggesting that infants detected the interruption of observed goal-directed activity. In another collaboration, Reid et al. (2009) investigated electrophysiological responses in adults and infants (7- and 9-month-olds) to anticipated and unanticipated outcomes. Participants observed an everyday event (e.g., eating) in which three images were presented onscreen consecutively. The first image displayed the general context of the action (e.g., a man holds a bagel), the second image displayed the action initiation (e.g., the man opens his mouth and moves the bagel closer), and the third image displayed either an anticipated conclusion (e.g., the man eats the bagel) or an unanticipated conclusion to the sequence (e.g., the man puts the bagel to his ear). ERPs revealed differential processing of anticipated versus unanticipated final images by 9 months of age, suggesting that infants develop early sensitivity to incongruous conclusions in familiar contexts and that this information influences expectations about outcomes. Until now, however, there has been no neurophysiological investigation into the way in which children process novel events.
Of interest in this study was whether the ability to detect event disruptions is realized in response to unfamiliar events. To address this question, we presented children (24-month-olds) and adults with a relatively novel three-action event in which pauses were inserted at intact action boundaries (i.e., at the endpoint of each action) or disrupted breakpoints within the action (i.e., in the middle of each action), and we measured the neurophysiological response using ERPs. Components within two distinct time windows are of interest. First, early-latency components identified within approximately the first 100 to 200 ms are thought to reflect brain activity governed by perceptual analyses of the input in adults and children (Fonaryova Key, Dove, & Maguire, 2005; Hillyard, Teder-Sälejärvi, & Münte, 1998; Holcomb, Coffey, & Neville, 1992; Kutas & Federmeier, 2011; Thierry, 2005). This early time window is expected to capture components that may reflect sensitivity to perceptual disruptions within the novel event (e.g., violations of the expected spatial and temporal pattern).
Second, mid-latency components identified after approximately 200 ms are thought to reflect conceptual processing of event meaning using goal-related or intentional information. In adults, previous research on how meaning is gleaned from nonlinguistic events has identified larger negative deflections between 250 and 500 ms to images that were incongruent with the event context than to those that were congruent (Bach, Gunter, Knoblich, Prinz, & Friederici, 2009; Hamm, Johnson, & Kirk, 2002; Sitnikova, Kuperberg, & Holcomb, 2003; West & Holcomb, 2002). Peaks within the early portion of this time window tend to display a fronto-central maximum at approximately 325 ms and are thought to reflect modality-specific conceptual processing that is particular to pictorial stimuli and is not based on linguistic mediation (N300) (Federmeier & Kutas, 2001; Hamm et al., 2002; McPherson & Holcomb, 1999; West & Holcomb, 2002). Peaks within the later portion of this time window tend to display more widespread negativities maximal at approximately 450 ms (N400) (Barrett & Rugg, 1990; Federmeier & Kutas, 2001; see Kutas & Federmeier, 2011, for a review) and are thought to reflect a “unification” process in which meaning is extracted from the observed event and integrated with stored multimodal (i.e., linguistic and nonlinguistic) event knowledge (Hagoort, Baggio, & Willems, 2009; Kutas & Federmeier, 2011; West & Holcomb, 2002). Relative to adults, these mid-latency components have a tendency to occur at a delay of approximately 50 to 100 ms in young children (Friedrich & Friederici, 2005; Männel, 2008).
The hypothesis that young children and adults are sensitive to both perceptual and conceptual information within nonlinguistic motion events led to several predictions. For adults, we expected a clearly discernible peak in response to the disrupted actions relative to intact actions, reflecting the detection of perceptual discrepancies between conditions within approximately 200 ms of stimulus onset. For 24-month-olds, we expected that disrupted actions would generate significant differences between conditions within this early time window (~0–200 ms). The presence of an early-latency effect in children’s waveforms would be consistent with the interpretation that toddlers initially process new events at a perceptual level, perhaps relying on spatial and temporal information.
If young children and adults attach meaning to novel events, we would expect enhanced activation of mid-latency components in response to disrupted actions within our second window of interest. For both children and adults, we expected disrupted actions to elicit enhanced processing (i.e., greater negative amplitudes) within the mid-latency window (~200–500 ms in adults, ~300–600 ms in children). It is as yet unknown whether event disruptions elicit effects characteristic of the modality-specific N300 component or the modality-independent N400 component. A main effect of condition within this mid-latency window would be consistent with the interpretation that young children and adults activate conceptual processing to evaluate observed human action even in the context of novel events.
Methods
Participants
A sample of 20 adult undergraduates (15 women and 5 men) received course credit for their participation. These numbers reflect the gender distribution of our participant pool. An additional 11 participants were not analyzed due to excessive noise or motion artifact (n = 4) or to equipment failure or experimental error (n = 7). In addition, 14 24-month-old children (6 girls and 8 boys) were also tested (mean age = 23.2 months, range = 22.9–26.7). An additional 20 toddlers were tested but were excluded due to fussiness (n = 2), equipment failure or experimenter error (n = 11), or excessive noise or motion artifact (n = 7). All toddlers were recruited from a city in Southern California on the U.S. West Coast and were born full-term (37–41 weeks). For inclusion in the grand average, adults were required to have at least 20 artifact-free trials in each condition and toddlers were required to have at least 15.
Materials
Toddlers were shown a video of a female agent performing a sequence of three relatively novel actions involving unfamiliar objects. The three actions were constructed into an activity station to facilitate a single fluid event following Friend and Pace (2011). In the first action, the agent placed a clown in a truck and pushed a button, causing the truck to move along a track. Next, she removed the clown from the truck, placed it in a basket, and hoisted it up to the top of a frame using a pulley. In the final action, she placed the clown into a car and pushed the car down a curved ramp. The activity station was organized so that each action led logically and proximally to the next action. For example, in Action 1, the truck moved along a track until it stopped below the pulley; in Action 2, hoisting the clown with the pulley brought it to the top of the platform above the curved ramp where the car for Action 3 awaited. The duration of the completed event was 13 s; the video was looped 12 times so that the entire video lasted for approximately 2 min 36 s (2:36). All participants included in the sample attended to the video for at least 50% of the duration, as noted by the experimenter with a stopwatch (M = 2:05, range = 1:19–2:30), and were required to watch at least one full loop including the three actions. The video phase served to introduce toddlers to the uninterrupted event.
This particular event was selected for three reasons. First, it is a relatively novel yet sensible sequence of three actions. This allowed us to control exposure to the event to assess children’s perception of actions that had not been encountered previously and to determine whether children’s ability to process observed human action is grounded in event familiarity or can be applied to relatively novel contexts. Second, action processing in adults is guided by both perceptual and conceptual information (Baldwin, Andersson, Saffran, & Meyer, 2008; Tversky, Zacks, & Hard, 2008; Zacks & Swallow, 2007). The use of a relatively novel event provided means for exploring the way in which toddlers and adults use multiple sources of information to process unfamiliar events. Finally, in previous research (Friend & Pace, 2011), toddlers spontaneously segmented an unfamiliar event into three discrete actions at coarse-grained boundaries (i.e., Actions 1, 2, and 3) in the absence of communicative intent demarcating action boundaries. The current study allowed us to extend these findings to explore the neurocognitive mechanisms underlying the process of event representation.
For ERP testing, adult and toddler stimuli were identical. The event was filmed using a Canon ZR45MC Hi-8 digital camcorder, and the video was digitized using iMovie into 260 still frames at 20 frames per second (fps). Stimuli consisted of a sequence of 30 still images selected by the authors that captured the defining structure of the event. Thus, each action (Action 1, 2, or 3) was represented by 10 still frames. Presentation of still frames in lieu of video has been used into study gesture (Sheehan, Namy, & Mills, 2007; Stürmer, Aschersleben, & Prinz, 2000), sign language (Neville et al., 1997), action perception (Kourtzi & Kanwisher, 2000), event representation (Reid et al., 2009), and the effect of visually depicted actions (e.g., punching, applauding) on language comprehension (Knoeferle, Urbach, & Kutas, 2011). Static frames were chosen because they provide a clear onset for time-locking the ERP stimuli and research indicates that they are processed in ways similar to dynamic events (Bach et al., 2009).
ERP design and procedure
Participants were seated in a dimly lit room approximately 30 inches away from a computer monitor. Adult participants were instructed to pay attention for the duration of the experiment and to blink when they saw the blank screen between trials; they were not required to make any overt responses or to perform a task during observation. This paradigm was selected for four reasons. First, previous research has shown similar neural activation during both passive observation (i.e., no overt response required) and active observation (i.e., participants pressed a button to indicate action boundaries) (Zacks & Swallow, 2007). Second, the presentation of stimuli was of short enough duration (4 min) that adults could attend without external reminders. Third, we wanted to facilitate comparison with children’s ERP responses. Fourth, in some cases, overt responses have been shown to obscure effects in the 300- to 500-ms latency window (Kutas & Hillyard, 1989; Kutas & Van Petten, 1994; Picton et al., 2000). Toddlers viewed the stimuli while seated on their parent’s lap. In child-directed speech, the experimenter instructed toddlers to pay attention while they watched a movie.
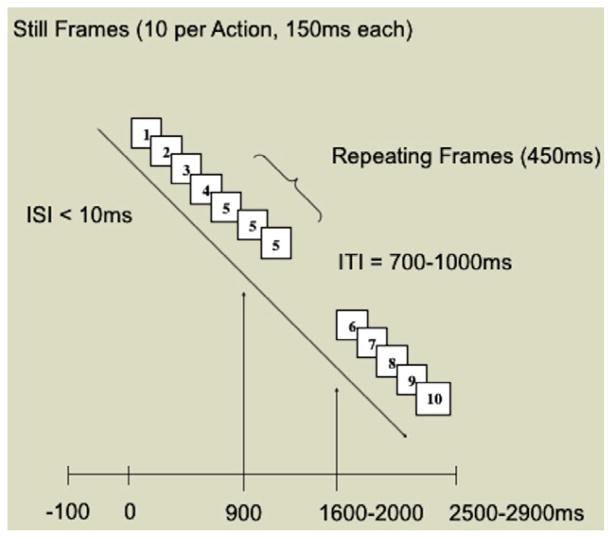
Presentation of the visual stimuli lasted for approximately 4.5 min and consisted of a single block of 100 trials. Each trial consisted of the presentation of the still frames representing Actions 1, 2, and 3. Frames remained onscreen for 150 ms each and were presented in rapid succession (<10 ms interstimulus interval [ISI]) to preserve temporal dependencies within the event. There were two within-participants conditions: Intact and Disrupted. In the Intact condition, the final frame of each action was repeated three times, creating a 450-ms “pause” that marked the naturally occurring boundary between actions (Fig. 1). In the Disrupted condition, the frame that interrupted the action of both the agent (i.e., experimenter) and the object (i.e., clown) was repeated to create a 450-ms “pause” that interrupted the action at an unnatural location (Fig. 2).
Fig. 1.
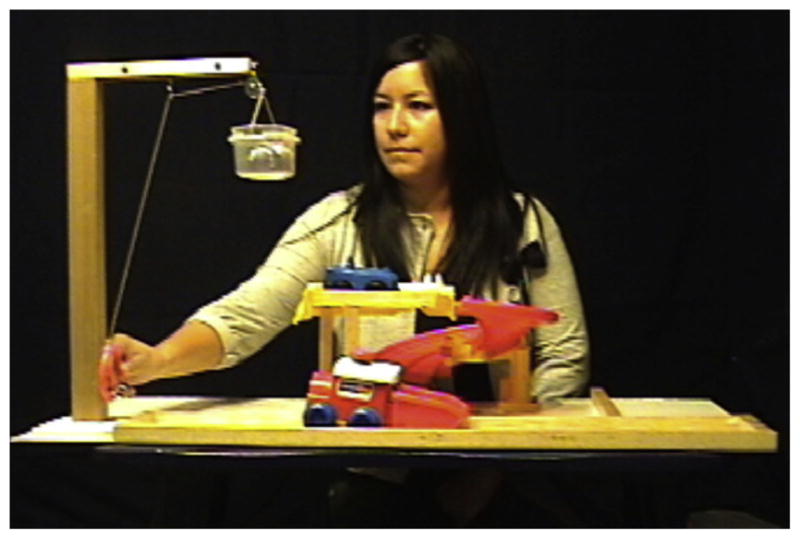
Still frame depicting the completion of Action 2 in the Intact condition.
Fig. 2.
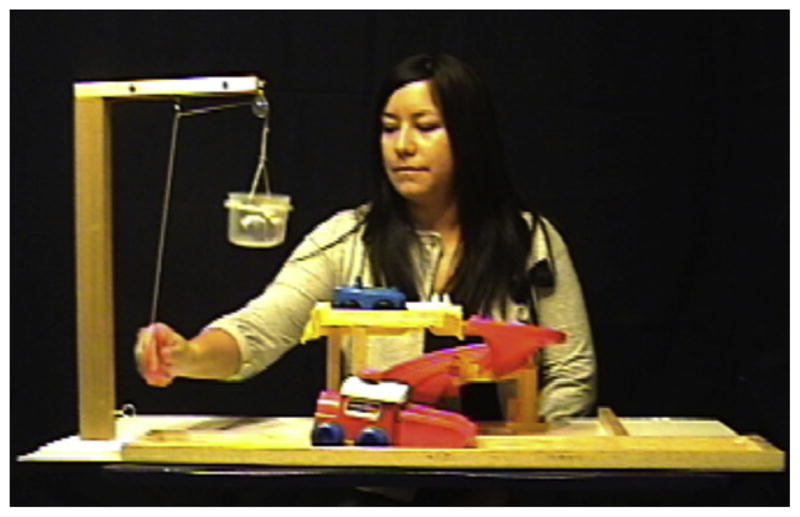
Still frame depicting the interruption of Action 2 in the Disrupted condition.
Controls were implemented to ensure that any effect of condition would be attributable solely to the position of the temporal violation either at the natural action boundary or in the midst of the action. First, identical frames occurred in both conditions; the only difference was the location of the simulated pause. Second, each still frame appeared an equal number of times in both conditions for a total of 100 trials. Third, conditions were randomized so that no more than two presentations of either condition occurred consecutively. The ERP recording epoch was time-locked to the onset of the final presentation of the repeated frame, and continuous electroencephalogram (EEG) was recorded for an epoch of 700 ms after each pause. There was a randomized intertrial interval (ITI) of 700 to 1100 ms between each consecutive presentation to avoid expectancy effects due to the repetition of stimuli (Picton et al., 2000) (Fig. 3).
Fig. 3.
Timing of stimulus presentation for disrupted actions. ISI, interstimulus interval; ITI, intertrial interval.
ERP recording and analyses
Adults and children wore a standard fitted cap (Electrocap International, Eaton, OH, USA) with electrodes placed according to the international 10–20 system. Continuous EEG was recorded with a NeuroScan 4.4 System (Compumedics, Charlotte, NC, USA) with a reference electrode at Cz and re-referenced offline to the average activity at left and right mastoids. ERPs were recorded at 33 scalp locations using silver/silver chloride (Ag/AgCl) electrodes at standard sites (Pz, Fz, O1, O2, P3, P4, T3, T4, T5, T6, C3, C4, Cz, F3, F4, F7, F8, A1, and A2) and additional sites (CPz, FCz, CP5, CP6, CP1, CP2, FC1, FC2, FC5, FC6, FP1, FP2, AF7, and AF8). Electrode resistance was kept under 10,000 Ohms. Continuous EEG was amplified with a lowpass filter (70 Hz), a directly coupled highpass filter (DC), and a notch filter (60 Hz). The signal was digitized at a rate of 500 samples per second via an analog-to-digital converter. Eye movement artifacts and blinks were monitored via horizontal electrooculogram (EOG) placed at the outer canthi of each eye and vertical EOG placed above and below the left eye.
All trials were scanned offline for artifacts; trials containing eye blinks—characterized by amplitude greater than 150 mV resolving within 150 ms over frontal and EOG electrodes—were excluded from the data. In addition, trials in which the amplitude of the activity deviated more than 200 mV from the baseline were excluded from further analyses. Brain activity for both the Intact and Disrupted conditions was averaged across multiple stimulus presentations. After artifact rejection, adult participants provided an average of 32 trials in the Intact condition (SD = 6.06, range = 20–43) and 32 trials in the Disrupted condition (SD = 6.63, range = 21–42); toddlers provided 26 trials in the Intact condition (SD = 6.07, range = 17–35) and 28 trials in the Disrupted condition (SD = 5.76, range = 18–36). There were no significant differences in the number of trials analyzed between groups, t(33) = 1.096, p = .281, and no significant differences in the number of trials analyzed by condition in children, t(13) = 1.660, p = .121, or adults, t(19) = .100, p = .921. The ERP data were then averaged across conditions at electrodes of interest, creating grand average waveforms. To compare the effects elicited by the different conditions (Intact and Disrupted), average ERPs were quantified by calculating the peak amplitudes within time windows of interest for adults and mean amplitudes within consecutive time windows of 100 ms duration for children.
Selection of specific electrodes within our regions of interest was guided primarily by the only previous study that has compared ERP components associated with action processing in young children and adults (Reid et al., 2009). Findings from this research identified activation at broad scalp locations, including frontal, central, and parietal sites. Mean amplitudes for children were calculated for consecutive time windows of 100 ms duration between 100 and 700 ms following stimulus onset because no clear peaks were identified, as is standard in the developmental literature (Friedrich & Friederici, 2005). For adults, peak amplitudes were calculated for time windows with discernible peaks (50–150 and 250–350 ms), and mean amplitudes were calculated for additional windows of interest (350–500 and 500–700 ms).
Planned analyses were guided by previous ERP research that has identified components associated with nonlinguistic action processing (Reid et al., 2009; Sitnikova, Holcomb, Kiyonaga, & Kuperberg, 2008; West & Holcomb, 2002). The resulting data in each time window were analyzed via two separate analyses of variance (ANOVAs) for repeated measures to examine significant differences occurring between hemispheres and at the midline. For lateral sites, three-way ANOVAs with condition (Intact or Disrupted), hemisphere (left or right), and region (frontal [F3/F4], central [C3/C4], parietal [P3/P4], or occipital [O1/O2]) as within-participants factors were conducted for each group separately. For midline sites, two-way ANOVAs with condition (Intact or Disrupted) and region (frontal [Fz], central [Cz], or parietal [Pz]) as within-participants factors were performed. Greenhouse–Geisser adjustments to degrees of freedom were applied to correct for violation of the assumption of sphericity; the reported numbers reflect the original degrees of freedom with the corrected p values. Paired-samples t tests were conducted to follow up significant effects; reported p values reflect Bonferroni adjustments for multiple comparisons.
Results
Adult results
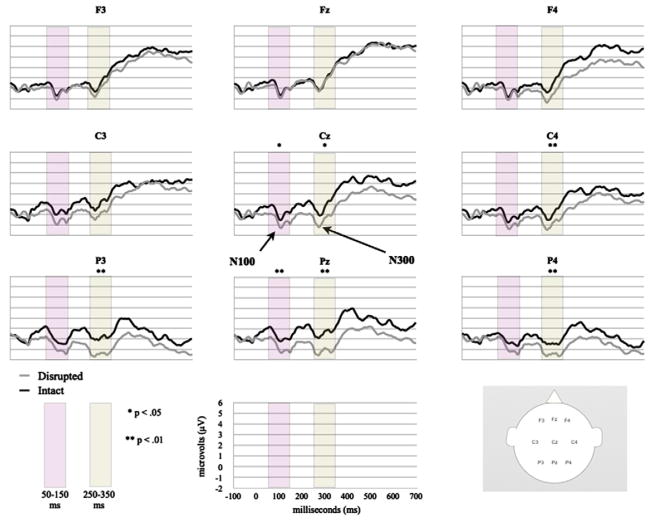
Fig. 4 displays the adult grand average ERP waveforms (N = 20) for the Intact and Disrupted conditions at our electrodes of interest. The waveforms revealed differential processing of intact and disrupted events at central and parietal sites. No significant effects were found at occipital leads, so this region was excluded from additional analyses (p > .05 for all time windows). Waveforms for disrupted events were more negative than those for intact events. The ERPs elicited by intact actions displayed two small negative peaks at approximately 120 and 286 ms, followed by an extended positivity. The ERPs elicited by disrupted actions displayed enhanced peak negativities at approximately 110 and 287 ms, followed by a long slow positivity similar to that observed for intact events.
Fig. 4.
Grand average ERPs for adults (N = 20) in response to intact and disrupted events at frontal, central, and parietal sites.
The 50- to 150-ms window
A clear negative-going peak was observed during this time window (50–150 ms) in adult waveforms. A repeated measures ANOVA at midline sites revealed a main effect of condition, F(1,19) = 5.118, p = .036. The amplitude of this component showed greater negativity in response to disrupted actions relative to intact actions. A Region × Condition interaction was also observed, F(2,38) = 4.713, p = .015. Planned comparisons indicate that this effect was driven by significant differences at central and parietal midline sites: Cz, t(19) = 2.192, p = .041, and Pz, t(19) = 3.218, p = .005. There were no significant effects at lateral electrode sites within this time window.
The 250- to 350-ms window
During this time window (250–350 ms), a second negative-going peak was observed in adult waveforms. Disrupted actions elicited greater negative peaks than intact actions at the midline. Midline analyses revealed a main effect of region, F(2,38) = 5.879, p = .011, and a main effect of condition, F(1,19) = 6.090, p = .023, with a Condition × Region interaction, F(2,38) = 6.897, p = .011. Planned comparisons revealed that amplitudes were consistently more negative in response to disrupted actions when compared with intact actions and that central and parietal midline sites continued to drive this effect: Cz, t(19) = 2.550, p = .020, and Pz, t(19) = 4.184, p = .001. In addition, analyses of lateral sites revealed a main effect of condition, F(1,19) = 7.235, p = .015. Pairwise comparisons showed that this effect was driven by central and parietal sites in the left and right hemispheres: C4, t(19) = 2.293, p = .033; P3, t(19) = 3.242, p = .004; and P4, t(19) = 2.086, p = .050.
The 350- to 500-ms and 500- to 700-ms windows
Analyses of mean amplitudes from 350 to 500 ms revealed a significant effect of region at midline sites, F(2,38) = 9.410, p = .003. In addition, a main effect of condition and a Region × Condition interaction approached conventional significance, F(1,19) = 4.164, p = .055, and F(2,38) = 3.788, p = .053. Pairwise comparisons indicated that this marginal effect was driven by significant differences at Cz, t(19) = 2.140, p = .046, and Pz, t(19) = 3.296, p = .004. Analyses of mean amplitudes from 500 to 700 ms revealed a main effect of region at midline sites, F(2,38) = 12.918, p = .001. Post hoc estimated marginal means indicated that amplitudes were greater in the frontal and central regions as compared with the parietal regions across conditions during this time window. There were no significant effects at lateral sites in the frontal, central, parietal, or occipital region (F3/F4, C3/C4, P3/P4, or O1/O2) during either time window.
Child results
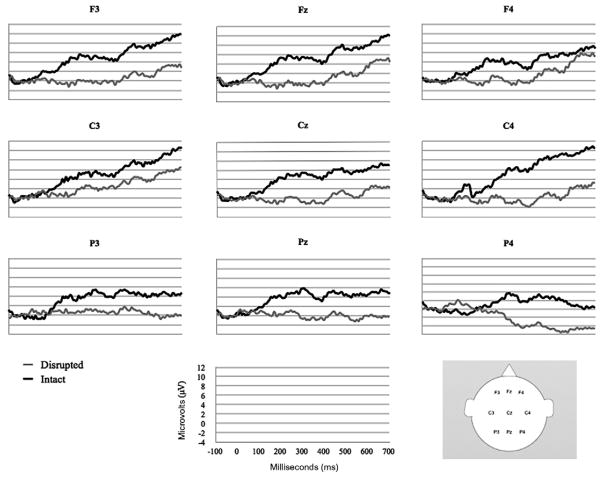
Fig. 5 displays the grand average ERP waveforms of 24-month-olds (N = 14) in response to the Intact and Disrupted conditions. The waveforms revealed a prolonged negativity in response to disrupted versus intact actions distributed broadly across midline and lateral sites. Waveforms for disrupted actions were more negative than those for intact actions throughout the duration of the recording epoch, with our windows of interest differing primarily in the distribution of activation across sites. To preserve this dynamic aspect of the child waveform data, we report effects across the entire recording epoch with a particular focus on characterizing changes in the distribution of activity over time.
Fig. 5.
Grand average ERPs for children (N = 14) in response to intact and disrupted events at frontal, central, and parietal sites.
Repeated measures ANOVAs revealed significant effects of condition at midline sites (Fz, Cz, and Pz) from 100 to 700 ms and at lateral sites (F3/F4, C3/C4, and P3/P4) from 300 to 600 ms (Table 1). No significant effects were found at occipital leads, so this region was excluded from additional analyses (p > .05 for all time windows). Pairwise comparisons revealed that differential activation at midline sites began centrally from 100 to 200 ms (Cz, p = .019), spread frontally from 200 to 300 ms (Fz, p < .034), recruited parietal regions from 300 to 400 ms (Fz, p = .023; Cz, p = .004; and Pz, p = .044), and shifted to primarily fronto-central activation from 400 to 700 ms (400–500 ms: Fz, p = .002, and Cz, p = .007; 500–600 ms: Fz, p = .002, and Cz, p = .019; 600–700 ms: Fz, p = .044, and Cz, p = .031) (Table 2).
Table 1.
Condition effects (F statistics) in 24-month-olds (N = 14).
| Epoch (ms) | Midline: condition F(1,13) | Lateral sites: condition F(1,13) |
|---|---|---|
| 100–200 | 5.035* | |
| 200–300 | 8.061* | |
| 300–400 | 9.946** | 10.078** |
| 400–500 | 10.010** | 7.132* |
| 500–600 | 6.783* | 5.749* |
| 600–700 | 5.314* |
p < .05.
p < .01.
Table 2.
Pairwise comparisons (t statistics) for mean amplitudes in response to intact versus disrupted events at frontal, central, and parietal electrodes in children (N = 14).
| Epoch (ms) | F3 | Fz | F4 | C3 | Cz | C4 | P3 | Pz | P4 |
|---|---|---|---|---|---|---|---|---|---|
| 100–200 | 2.671* | ||||||||
| 200–300 | 2.363* | 2.956* | |||||||
| 300–400 | 3.071** | 2.573* | 3.470** | 2.234* | 3.269** | ||||
| 400–500 | 3.941** | 3.752** | 3.172** | 2.690* | |||||
| 500–600 | 4.171** | 3.796** | 2.685* | 2.369* | |||||
| 600–700 | 2.227* | 2.424* |
p < .05.
p < .01.
Subsequent analyses of electrodes at lateral sites revealed that significant differences between the Intact and Disrupted conditions were located at frontal (F3/F4, p = .042) and parietal (P3/P4, p = .015) brain regions from 300 to 600 ms, but only approached conventional significance at central sites (C3/C4, p = .068). Follow-up comparisons between specific lateral electrodes revealed activation at primarily left frontal and right parietal electrodes within this time window (Table 2). This pattern suggests that differences were consistent in broadly distributed midline brain regions from 100 to 700 ms, with recruitment of additional frontal and parietal regions from 300 to 600 ms.
Discussion
The main goal of the current research was to characterize the neurophysiological activity associated with children’s and adults’ processing of a relatively novel event. In particular, the experiment was designed to explore whether the novel event elicited distinct components within the waveforms that index perceptual and conceptual levels of action processing in both adults and children. ERPs were recorded as adults and children (24-month-olds) viewed a relatively novel event in which interruptions either coincided with the completion of coarse actions or disrupted the flow of the event stream at unexpected points within each action. We hypothesized that adults would show enhanced neural responses to disrupted action in early-latency windows (~0–200 ms) associated with detection of perceptual (i.e., spatial and temporal) information in the event stream as well as responses in mid-latency windows (~200–500 ms) associated with conceptual processing of event meaning. To the extent that children process novel events in the same way as adults, we expected children to show increased activation in response to disrupted action in roughly the same early- and mid-latency windows (~0–200 and 300–600 ms).
Characterization of adult ERPs
In adult ERPs, disrupted actions elicited more negative waveforms than intact actions within a relatively novel event. Importantly, average waveforms contained two distinct peaks that were more negative for disrupted actions than for intact actions. A significant effect of condition was evident in an early negative deflection that peaked at approximately 110 ms after stimulus onset in response to disrupted actions. This effect was most prominent at central and parietal midline leads, with no significant differences at frontal or lateral sites. A second negativity peaking at approximately 286 ms in response to disrupted actions was also present. This effect had a more widespread spatial distribution; whereas the effect was still focused around central and parietal sites, it extended to central and parietal sites in the left and right hemispheres (C4 and P3/P4) rather than remaining restricted to the mid-line. In sum, the unique spatio-temporal patterns of activation for these two negative peaks suggest that they may, in fact, reflect distinct neural mechanisms associated with different levels of action processing.
Given its early latency, the first negative deflection is consistent with enhanced visual attention to disrupted actions (Hillyard et al., 1998; Mangun, 1995). The observed component shares important characteristics with the visual N100 reported in previous literature (Fonaryova Key et al., 2005). In particular, the N100 has been shown to reflect selective attention to basic stimulus features, pattern recognition, or the operation of a general visual discrimination mechanism (Gomez, Clark, Fan, Luck, & Hillyard, 1994; Luck et al., 1994; Vogel & Luck, 2000). Thus, the observed early negativity in the current study suggests that disrupted actions recruited additional visual attention.
This interpretation is consistent with behavioral and neural evidence for adults’ reliance on low-level movement features during the perceptual analysis of observed human action (Baldwin, Loucks, & Sabbagh, 2008; Kourtzi & Kanwisher, 2000; Shipley & Maguire, 2008; Tversky et al., 2008). In particular, information regarding velocity and acceleration tends to be associated with adults’ ability to represent action boundaries (Zacks, 2004) and predict upcoming actions (Stadler, Springer, Parkinson, & Prinz, 2012; Stapel, Hunnius, & Bekkering, 2012). Thus, it is likely that adults relied on kinematic information (e.g., the geometric trajectory of the agent’s reach or the relative speed of the moving objects) in part as a mechanism for discriminating disrupted actions from intact actions. It would be helpful in future research to determine the relative importance of distinct kinematic cues in evaluating the rationality of action.
Adults’ neurophysiological responses within the 250- to 350-ms time window share important characteristics with N300 and N400 effects observed in previous ERP studies on event processing. Temporally, peaks were maximal in response to disrupted actions at approximately 286 ms, a time course that is more consistent with the N300 reflecting modality-dependent conceptual processing of nonverbal stimuli rather than the modality-independent N400 (West & Holcomb, 2002). The latency of this component suggests that the time course of cognitive processing is similar for familiar and unfamiliar events in adults. Spatially, scalp distribution differed from what is characteristic of the N300; negativities for pictorial and video stimuli are typically distributed over more anterior electrode sites (Barrett & Rugg, 1990; Hamm et al., 2002; Holcomb & McPherson, 1994; McPherson & Holcomb, 1999), yet adult responses in our study were more consistent with posterior scalp topography elicited by verbal stimuli (Friederici, Pfeifer, & Hahne, 1993; Kuperberg et al., 2003; Kutas & Van Petten, 1994).
One possible explanation for the topographical differences found between adult ERPs in this study and the N300 in previous research centers on the minimal task demands on our participants. In the current research, adults were instructed to pay attention for the duration of the stimuli presentation but were not required to make any overt responses. This left ample time to process the event linguistically and to activate posterior networks involved in semantic processing (e.g., “She puts the clown in the truck, then pushes the button so that it moves along a track…”). This sort of covert linguistic activation in which stored lexical knowledge related to event meaning is accessed during nonlinguistic tasks has been shown to influence ERP topography (Federmeier & Kutas, 2001; Nigam, Hoffman, & Simons, 1992).
Characterization of ERPs in children
The general pattern evident in ERPs of 24-month-olds was similar to that observed in adults; actions that were disrupted elicited waveforms that were more negative through the duration of the recording epoch than actions that were intact. Furthermore, in children as in adults, differences between conditions reached significance within the early-latency time window associated with perceptual processing. These data provide insight into the neurophysiological time course of action processing; by 24 months of age, children were able to detect differences between intact and disrupted actions within the first 200 ms even when the observed event was relatively novel. Temporally, the onset of this early activation is comparable to the N100 effect observed in adults, suggesting that children may have detected differences between intact and disrupted actions via perceptual mechanisms. This interpretation is consistent with behavioral findings that suggest children use their knowledge of low-level movement features such as an object’s trajectory (Hespos et al., 2010) or an agent’s manner of motion (e.g., push vs. pull) to help them extract salient action information (Loucks & Baldwin, 2006; Olofson & Baldwin, 2011). It is possible that these results, in part, reflect young children’s ability to track regularities between various motion components via a statistical learning mechanism (Kirkham et al., 2002) and apply this information as they process human action (Cicchino et al., 2011).
Between 300 and 600 ms, differences between intact and disrupted waveforms reached significance in children’s ERPs at frontal and parietal sites within the left and right hemispheres. These data suggest that additional neural regions were recruited within the time window associated with conceptual processing (Duncan et al., 2009; Kutas & Federmeier, 2011). Differences between conditions in this mid-latency window share important characteristics with N300- and N400-like effects observed in response to nonlinguistic stimuli in children. Specifically, previous research with developmental populations has identified activation across the scalp at anterior as well as posterior brain regions in the generation of the N300 (Coch, Maron, Wolf, & Holcomb, 2002) and N400 (Cummings, Ceponiene, Dick, Saygin, & Townsend, 2008; Friedrich & Friederici, 2004; Reid et al., 2009). Furthermore, the slightly delayed onset and prolonged duration of the effect in the current study is typical in young children’s ERPs when compared with adult data (Friedrich & Friederici, 2005; Webb, Long, & Nelson, 2005). The spatial distribution and extended duration of children’s responses in this time window may reflect more diffusely allocated resources and extended processing time in response to disrupted actions.
In general, these findings are consistent with an interpretation that children also use goal-related information to process visual events. For example, children may use an agent’s intended target object or action outcome in constructing event representations (Lakusta et al., 2007; Sootsman Buresh & Woodward, 2007). However, this interpretation must be treated with caution. The absence of a distinct peak within the mid-latency window, a fairly common phenomenon in young children’s ERPs (Männel, 2008; Nelson & Luciana, 1998), prevents us from ruling out carryover effects from the previous window of interest. Specifically, the difference between conditions from 300 to 600 ms may be driven by an early negativity maintained throughout the duration of the recording epoch. For this reason, we cannot conclusively reject the possibility that perceptual-level features drove children’s action processing.
Conclusion
Events are frequently disrupted—dinner preparation is halted to answer a phone call, a conversation is interrupted mid-sentence by a knock on the door, a game of hockey in the street is paused to let a car pass. The ability to use bottom-up perceptual information to detect violations within the temporal structure of events is crucial for identifying unexpected occurrences. Equally important is the ability to use top-down conceptual information to interpret anomalies within an event to enable accurate predictions and make appropriate adjustments in behavioral responses. The main goal of the current research was to characterize the neurophysiological activity associated with children’s and adults’ processing of a relatively novel event using event-related potentials. Findings lend new insight into the brain mechanisms underlying the way in which children and adults interpret observed human action.
In adults, we obtained evidence for both a perceptual mechanism during the initial stages of action processing and activation of a modality-dependent conceptual processing mechanism involved in interpreting nonlinguistic event information. These findings suggest that adults used perceptual information such as the truck’s trajectory of motion, the pulley’s change in location, and the car’s velocity to detect disruptions within each action as well as conceptual inferences regarding the agent’s intended outcome to interpret the event as a whole. These results are consistent with empirical evidence showing that adults process familiar events at multiple levels simultaneously (Hard, Recchia, & Tver-sky, 2011; Zacks, Kumar, Abrams, & Mehta, 2009; Zacks & Tversky, 2001) and represent the first data to extend this finding to novel events. Thus, it is likely that the joint operation of perceptual and conceptual mechanisms facilitates adults’ ability to evaluate the rationality of observed human action.
There are two possible interpretations of the pattern observed in children’s ERPs. First, the lack of clear peaks and prolonged duration of the effect may reflect spreading activation characteristic of a single extended processing mechanism. This interpretation supports a developmental account in which morphological differences between children’s and adults’ waveforms reflect changes in the organization of brain regions recruited during the processing of novel events. Alternatively, it is possible that the distinct temporal and spatial activation from 100 to 700 ms at the midline and from 300 to 600 ms at lateral sites may reflect the emergence of two independent processes with unique functional significance. It is possible that these data represent a first step toward identifying how perceptual and conceptual mechanisms develop in conjunction to guide the detection of relevant units of action within the event stream.
Although the current research is suggestive of multiple levels of action processing in adults, follow-up studies are needed to clarify the role of perceptual and conceptual information in evaluating the rationality of observed human action throughout development. Importantly, our stimuli consisted of a single event composed of three relatively novel actions. How these findings extend to activities that are more or less familiar to the observer remains to be seen. For example, an accomplished fencer may detect subtle boundaries, such as an attaque au fer, at a conceptual level reflecting top-down knowledge of the sport, whereas a novice may rely more heavily on perceptual cues to motion. In addition, the actions in our event were presented in only one sequential order. What about activities that have different constraints imposed by hierarchical structure? For example, the steps involved in baking a cake are dependent on sequential order; you must mix the batter before putting it in the oven. Shopping for groceries, on the other hand, can be accomplished in any number of autonomous steps; it does not matter whether the bananas are selected before or after the milk. We can predict that disruptions within a sequence of dependent actions would be more informative than those within a series of autonomous actions.
These findings contribute to the debate on early rationality in several ways. First, this study indicates that young children, like adults, can discriminate intact actions from disrupted actions even if they are relatively novel. This is consistent with the teleological perspective in that young children seem to be able to evaluate a wide range of events, including unfamiliar human actions (Csibra & Gergely, 2007; Király et al., 2003). However, differences between children’s and adults’ ERP waveforms suggest that the mechanisms involved in action processing may not be fully functional during early childhood. Thus, it is possible that children’s ERPs reflect, in part, sensitivity to low-level disruptions of movement features rather than rational evaluation of the event as a whole. At the most basic level, complex human action can be described in terms of movement patterns along paths through space. This type of information would be of great importance early in development. On a daily basis, children are immersed in unfamiliar event contexts for which they have no prior knowledge to support comprehension or interpretation. Sensitivity to properties of physical movement may support children’s initial action perception without the need to rely on acquired world knowledge. In the context of our novel event, using movement features—such as disruption of the truck’s trajectory at peak velocity—to detect disruptions in the expected pattern may have been sufficient for processing the observed actions. Indeed, neurophysiological responses early in the recording epoch are consistent with this interpretation, and additional research is needed to specify the parameters that permit individuals to evaluate the rationality of a novel action solely on the basis of perceptual information.
Here, we are also interested in when and how motor percepts (or actions in our terminology) come to fit together as the conceptual wholes (events) that are meaningfully encoded. Neurophysiological responses observed later in the recording epoch are consistent with the interpretation that participants were viewing (and perhaps rationally evaluating) the event as more than merely a pattern of dynamic motion. This finding is supported by recent imaging evidence for the activation of partially non-overlapping neural networks during adults’ subjective segmentation of everyday actions such as ironing (which can be processed based on motion and prior action knowledge), as compared with movement such as tai chi (which can be processed based on motion alone) (Schubotz, Korb, Schiffer, Stadler, & von Cramon, 2012). Common activation of motor areas in response to both movement and event-relevant action suggests that motion provides a bottom-up cue to boundary detection across a variety of dynamic contexts. Importantly, activation in additional regions in response to event-relevant actions (but not movement) suggests that conceptual knowledge may provide supplementary information about actions in a meaningful context. Thus, the ability to understand rational human action may depend not only on the expected pattern of dynamic movement but also on the ability to make appropriate inferences based on information that might not be readily observable within the event. Clarifying the level at which children process novel events is key to characterizing the development of early rationality in action understanding.
Exactly when and how children develop the ability to integrate both perceptual and conceptual information into their evaluation of rational action remains an empirical question. This research represents a starting point for characterizing how children and adults process novel events. Generating meaning may rely on the integration of cues from multiple levels of action processing.
Acknowledgments
Preparation of this article was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, R01HD068458) and National Institutes of Health (NIH) Training Grants T32 DC7361-03 and T32 DC000041. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH. Funding was also provided by a Norman Anderson Graduate Research grant to Amy Pace from the UCSD Department of Psychology.
Footnotes
Note that, in this context, “event” refers to a general category of observed human action. We are not referring to the events to which EEG is time-locked during ERP collection.
References
- Bach P, Gunter TC, Knoblich G, Prinz W, Friederici AD. N400-like negativities in action perception reflect the activation of two components of an action representation. Social Neuroscience. 2009;4:212–232. doi: 10.1080/17470910802362546. [DOI] [PubMed] [Google Scholar]
- Baillargeon R, Wang S. Event categorization in infancy. Trends in Cognitive Sciences. 2002;6:85–93. doi: 10.1016/s1364-6613(00)01836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D, Andersson A, Saffran J, Meyer M. Segmenting dynamic human action via statistical structure. Cognition. 2008;106:1382–1407. doi: 10.1016/j.cognition.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Baldwin DA, Baird JA, Saylor MM, Clark MA. Infants parse dynamic action. Child Development. 2001;72:708–717. doi: 10.1111/1467-8624.00310. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Loucks J, Sabbagh MA. Pragmatics of human action. In: Shipley TF, Zacks JM, editors. Understanding events: From perception to action. New York: Oxford University Press; 2008. pp. 96–129. [Google Scholar]
- Barrett SE, Rugg MD. Event-related potentials and the semantic matching of pictures. Brain and Cognition. 1990;14:201–212. doi: 10.1016/0278-2626(90)90029-n. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Lukowski AF, Haight JC, Waters JM, et al. Electrophysiological indexes of encoding and behavioral indexes of recall: Examining relations and developmental change late in the first year of life. Developmental Neuropsychology. 2006;29:293–320. doi: 10.1207/s15326942dn2902_2. [DOI] [PubMed] [Google Scholar]
- Biró S, Csibra G, Gergely G. The role of behavioral cues in understanding goal-directed actions in infancy. Progress in Brain Research. 2007;164:303–322. doi: 10.1016/S0079-6123(07)64017-5. [DOI] [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Developmental Science. 2000;3:234–246. [Google Scholar]
- Cicchino JB, Aslin RN, Rakison DH. Correspondences between what infants see and know about causal and self-propelled motion. Cognition. 2011;118:171–192. doi: 10.1016/j.cognition.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D, Maron L, Wolf M, Holcomb PJ. Word and picture processing in children: An event-related potential study. Developmental Neuropsychology. 2002;22:373–406. doi: 10.1207/S15326942dn2201_3. [DOI] [PubMed] [Google Scholar]
- Csibra G, Bíró S, Koós O, Gergely G. One-year-old infants use teleological representations of actions productively. Cognitive Science. 2003;27:111–133. [Google Scholar]
- Csibra G, Gergely G. “Obsessed with goals”: Functions and mechanisms of teleological interpretation of actions in humans. Acta Psychologica. 2007;124:60–78. doi: 10.1016/j.actpsy.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Csibra G, Gergely G, Bíró S, Koós O, Brockbank M. Goal attribution without agency cues: The perception of “pure reason” in infancy. Cognition. 1999;72:237–267. doi: 10.1016/s0010-0277(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Cummings A, Ceponiene R, Dick F, Saygin AP, Townsend J. A developmental ERP study of verbal and non-verbal semantic processing. Brain Research. 2008;1208:137–149. doi: 10.1016/j.brainres.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Näätänen R, et al. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clinical Neurophysiology. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M. Meaning and modality: Influences of context, semantic memory organization, and perceptual predictability on picture processing. Cognition. 2001;27:202–224. [PubMed] [Google Scholar]
- Fonaryova Key AP, Dove GO, Maguire MJ. Linking brainwaves to the brain: An ERP primer. Developmental Neuropsychology. 2005;27:183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Friederici AD. N400-like semantic incongruity effect in 19-month-olds: Processing known words in picture contexts. Journal of Cognitive Neuroscience. 2004;16:1465–1477. doi: 10.1162/0898929042304705. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Friederici AD. Semantic sentence processing reflected in the event-related potentials of one- and two-year-old children. Developmental Neuroscience. 2005;16:16–19. doi: 10.1097/01.wnr.0000185013.98821.62. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Pfeifer E, Hahne A. Event-related brain potentials during natural speech processing: Effects of semantic, morphological, and syntactic violations. Cognitive Brain Research. 1993;1:183–192. doi: 10.1016/0926-6410(93)90026-2. [DOI] [PubMed] [Google Scholar]
- Friend M, Pace A. Beyond event segmentation: Spatial- and social-cognitive processes in verb-to-action mapping. Developmental Psychology. 2011;47:867–876. doi: 10.1037/a0021107. [DOI] [PubMed] [Google Scholar]
- Gergely G, Csibra G. Teleological reasoning in infancy: The naive theory of rational action. Trends in Cognitive Sciences. 2003;7:287–292. doi: 10.1016/s1364-6613(03)00128-1. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Clark VP, Fan S, Luck SJ, Hillyard SA. Sources of attention-sensitive visual event-related potentials. Brain Topography. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Baggio G, Willems RM. Semantic unification. In: Gazzaniga MS, editor. The cognitive neurosciences. 4. Cambridge, MA: MIT Press; 2009. pp. 819–836. [Google Scholar]
- Hamm JP, Johnson BW, Kirk IJ. Comparison of the N300 and N400 ERPs to picture stimuli in congruent and incongruent contexts. Clinical Neurophysiology. 2002;113:1339–1350. doi: 10.1016/s1388-2457(02)00161-x. [DOI] [PubMed] [Google Scholar]
- Hard BM, Recchia G, Tversky B. The shape of action. Journal of Experimental Psychology. 2011;140:586–604. doi: 10.1037/a0024310. [DOI] [PubMed] [Google Scholar]
- Hespos SJ, Saylor MM, Grossman SR. Infants’ ability to parse continuous actions. Developmental Psychology. 2010;45:575–585. doi: 10.1037/a0014145. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Teder-Sälejärvi WA, Münte TF. Temporal dynamics of early perceptual processing. Current Opinion in Neurobiology. 1998;8:202–210. doi: 10.1016/s0959-4388(98)80141-4. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Coffey SA, Neville HJ. Visual and auditory sentence processing: A developmental analysis using event-related brain potentials. Developmental Neuropsychology. 1992;8:203–241. [Google Scholar]
- Holcomb PJ, McPherson WB. Event-related brain potentials reflect semantic priming in an object decision task. Brain and Cognition. 1994;24:259–276. doi: 10.1006/brcg.1994.1014. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Clavagnier S, Gergely G, Orban GA. Neural mechanisms of understanding rational actions: Middle temporal gyrus activation by contextual violation. Cerebral Cortex. 2011;21:318–329. doi: 10.1093/cercor/bhq098. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Amso D, Frank M, Shuwairi S. Perceptual development in infancy as the foundation of event perception. In: Shipley TF, Zacks JM, editors. Understanding events: From perception to action. New York: Oxford University Press; 2008. pp. 436–464. [Google Scholar]
- Király I, Jovanovic B, Prinz W, Aschersleben G, Gergely G. The early origins of goal attribution in infancy. Consciousness and Cognition. 2003;12:752–769. doi: 10.1016/s1053-8100(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Slemmer JA, Johnson SP. Visual statistical learning in infancy: Evidence for a domain general learning mechanism. Cognition. 2002;83:B35–B42. doi: 10.1016/s0010-0277(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Klossek UMH, Dickinson A. Rational action selection in 1½- to 3-year-olds following an extended training experience. Journal of Experimental Child Psychology. 2012;111:197–211. doi: 10.1016/j.jecp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Knoeferle P, Urbach TP, Kutas M. Comprehending how visual context influences incremental sentence processing: Insights from ERPs and picture–sentence verification. Society. 2011;48:495–506. doi: 10.1111/j.1469-8986.2010.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Activation in human MT/MST by static images with implied motion. Journal of Cognitive Neuroscience. 2000;12:48–55. doi: 10.1162/08989290051137594. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Holcomb PJ, Sitnikova T, Greve D, Dale AM, Caplan D. Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. Journal of Cognitive Neuroscience. 2003;15:272–293. doi: 10.1162/089892903321208204. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP) Annual Review of Psychology. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. An electrophysiological probe of incidental semantic association. Journal of Cognitive Neuroscience. 1989;1:38–49. doi: 10.1162/jocn.1989.1.1.38. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten CK. Psycholinguistics electrified: Event-related brain potential investigations. In: Gernsbacher MA, editor. Handbook of psycholinguistics. San Diego: Academic Press; 1994. pp. 83–143. [Google Scholar]
- Lakusta L, Wagner L, Hearn KO, Landau B. Conceptual foundations of spatial language: Evidence for a goal bias in infants. Language Learning and Development. 2007;3:179–197. [Google Scholar]
- Loucks JT, Baldwin D. When is a grasp a grasp? Characterizing some basic components of human action processing. In: Hirsh-Pasek K, Golinkoff RM, editors. Action meets word: How children learn verbs. New York: Oxford University Press; 2006. pp. 228–261. [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Männel C. The method of event-related brain potentials in the study of cognitive processes: A tutorial. In: Friederici A, Thierry G, editors. Early language development: Bridging brain and behaviour. Amsterdam: John Benjamins; 2008. pp. 1–22. [Google Scholar]
- McPherson WB, Holcomb PJ. An electrophysiological investigation of semantic priming with pictures of real objects. Psychophysiology. 1999;36(1):53–65. doi: 10.1017/s0048577299971196. http://dx.doi.org/10.1017/S0048577299971196. [DOI] [PubMed] [Google Scholar]
- Meints K, Plunkett K, Harris PL. Eating apples and houseplants: Typicality constraints on thematic roles in early verb learning. Language and Cognitive Processes. 2008;23:434–463. [Google Scholar]
- Nelson CA, Luciana M. Electrophysiological studies II: Evoked potentials and event-related potentials. In: Coffey CE, Brumback RA, editors. Textbook of pediatric neuropsychiatry. Washington, DC: American Psychiatric Press; 1998. pp. 331–356. [Google Scholar]
- Neville HJ, Coffey SA, Lawson DS, Fischer A, Emmorey K, Bellugi U. Neural systems mediating American sign language: Effects of sensory experience and age of acquisition. Brain and Language. 1997;57:285–308. doi: 10.1006/brln.1997.1739. [DOI] [PubMed] [Google Scholar]
- Newtson D. Attribution and the unit of perception of ongoing behavior. Journal of Personality and Social Psychology. 1973;28:28–38. [Google Scholar]
- Newtson D, Enquist G. The perceptual organization of ongoing behavior. Journal of Experimental Psychology. 1976;12:436–450. [Google Scholar]
- Nigam A, Hoffman JE, Simons RF. N400 to semantically anomalous pictures and words. Journal of Cognitive Neuroscience. 1992;4:15–22. doi: 10.1162/jocn.1992.4.1.15. [DOI] [PubMed] [Google Scholar]
- Olofson EL, Baldwin D. Infants recognize similar goals across dissimilar actions involving object manipulation. Cognition. 2011;118:258–264. doi: 10.1016/j.cognition.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Paulus M, Hunnius S, van Wijngaarden C, Vrins S, van Rooij I, Bekkering H. The role of frequency information and teleological reasoning in infants’ and adults’ action prediction. Developmental Psychology. 2011;47:976–983. doi: 10.1037/a0023785. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pulverman R, Golinkoff RM, Hirsh-Pasek K, Sootsman Buresh J. Infants discriminate manners and paths in non-linguistic dynamic events. Cognition. 2008;108:825–830. doi: 10.1016/j.cognition.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisig S, Welke T, Hagendorf H, van der Meer E. I spy with my little eye: Detection of temporal violations in event sequences and the pupillary response. International Journal of Psychophysiology. 2010;76:1–8. doi: 10.1016/j.ijpsycho.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Reid VM, Csibra G, Belsky J, Johnson MH. Neural correlates of the perception of goal-directed action in infants. Acta Psychologica. 2007;124:129–138. doi: 10.1016/j.actpsy.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Reid VM, Hoehl S, Grigutsch M, Groendahl A, Parise E, Striano T. The neural correlates of infant and adult goal prediction: Evidence for semantic processing systems. Developmental Psychology. 2009;45:620–629. doi: 10.1037/a0015209. [DOI] [PubMed] [Google Scholar]
- Reid VM, Hoehl S, Landt J, Striano T. Human infants dissociate structural and dynamic information in biological motion: Evidence from neural systems. Social Cognitive and Affective Neuroscience. 2008;3:161–167. doi: 10.1093/scan/nsn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, Korb FM, Schiffer AM, Stadler W, von Cramon DY. The fraction of an action is more than a movement: Neural signatures of event segmentation in fMRI. NeuroImage. 2012;61:1195–1205. doi: 10.1016/j.neuroimage.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Sharon T, Wynn K. Individuation of actions from continuous motion. Psychological Science. 1998;9:357–362. [Google Scholar]
- Sheehan EA, Namy LL, Mills DL. Developmental changes in neural activity to familiar words and gestures. Brain and Language. 2007;101:246–259. doi: 10.1016/j.bandl.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Shipley TF, Maguire MJ. Geometric information for event segmentation. In: Shipley TF, Zacks JM, editors. Understanding events: From perception to action. New York, NY US: Oxford University Press. ; 2008. pp. 415–435. http://dx.doi.org/10.1093/acprof:oso/9780195188370.003.0018. [Google Scholar]
- Sitnikova T, Holcomb PJ, Kiyonaga KA, Kuperberg GR. Two neurocognitive mechanisms of semantic integration during the comprehension of visual real-world events. Journal of Cognitive Neuroscience. 2008;20:2037–2057. doi: 10.1162/jocn.2008.20143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnikova T, Kuperberg G, Holcomb PJ. Semantic integration in videos of real-world events: An electrophysiological investigation. Psychophysiology. 2003;40:160–164. doi: 10.1111/1469-8986.00016. [DOI] [PubMed] [Google Scholar]
- Sootsman Buresh J, Woodward AL. Infants track action goals within and across agents. Cognition. 2007;104:287–314. doi: 10.1016/j.cognition.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Stadler W, Springer A, Parkinson J, Prinz W. Movement kinematics affect action prediction: Comparing human to non-human point-light actions. Psychological Research/Psychologische Forschung. 2012;76(4):395–406. doi: 10.1007/s00426-012-0431-2. [DOI] [PubMed] [Google Scholar]
- Stapel JC, Hunnius S, Bekkering H. Online prediction of others’ actions: The contribution of the target object, action context, and movement kinematics. Psychological Research. 2012;76:434–445. doi: 10.1007/s00426-012-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer B, Aschersleben G, Prinz W. Correspondence effects with manual gestures and postures: A study of imitation. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1746–1759. doi: 10.1037//0096-1523.26.6.1746. [DOI] [PubMed] [Google Scholar]
- Thierry G. The use of event-related potentials in the study of early cognitive development. Infant and Child Development. 2005;14:85–94. [Google Scholar]
- Tversky B, Zacks JM, Hard BM. The structure of experience. In: Shipley TF, Zacks JM, editors. Understanding events: From perception to action. New York: Oxford University Press; 2008. pp. 436–464. [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8:605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- West CW, Holcomb PJ. Event-related potentials during discourse-level semantic integration of complex pictures. Cognitive Brain Research. 2002;13:363–375. doi: 10.1016/s0926-6410(01)00129-x. [DOI] [PubMed] [Google Scholar]
- Wynn K. Infants’ individuation and enumeration of actions. Psychological Science. 1996;7:164–169. [Google Scholar]
- Zacks JM. Using movement and intentions to understand simple events. Cognitive Science. 2004;28(6):979–1008. http://dx.doi.org/10.1016/j.cogsci.2004.06.003. [Google Scholar]
- Zacks JM, Kumar S, Abrams R, Mehta R. Using movement and intentions to understand human activity. Cognition. 2009;112:201–216. doi: 10.1016/j.cognition.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Swallow KM. Event segmentation. Current Directions in Psychological Science. 2007;16:80–84. doi: 10.1111/j.1467-8721.2007.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Tversky B. Event structure in perception and conception. Psychological Bulletin. 2001;127:3–21. doi: 10.1037/0033-2909.127.1.3. [DOI] [PubMed] [Google Scholar]