Abstract
Background
A growing body of research suggests that the intrauterine environment influences fetal neurodevelopment by altering the functional placental epigenome. A number of miRNAs are expressed in the placenta, may be sensitive to dysregulation by environmental exposures, and are associated with adverse pregnancy outcomes. Our study aimed to identify relationships between placental miRNA expression and newborn neurobehavior.
Methods
We examined the association between the expression of miR-16, miR-21, miR-93, miR-135b, miR-146a, and miR-182 in total RNA from the placentas of 86 term infants as measured by quantitative real-time PCR and newborn neurobehavioral outcomes as assessed using the NICU Network Neurobehavioral Scales (NNNS).
Results
Bivariate analysis revealed that placental miR-16 expression is negatively associated with attention score (p=0.006), while expression of miR-146a and miR-182 are both positively associated with quality of movement score (p=0.016 and p=0.016, respectively). Controlling for potential confounders, high miR-16 expression is significantly associated with reduced attention score (p=0.04), and high miR-146a expression and high miR-182 expression are significantly associated with increased quality of movement score (p=0.04 and p=0.01, respectively).
Conclusions
These results suggest that placental miRNA expression is associated with early neurobehavioral outcomes and miRNAs in the placenta may contribute to the developmental origins of infant neurobehavior.
Introduction
The period of fetal development is a critical period during which changes in the intrauterine environment can have significant consequences for future health. The early focus of the field of fetal programming was on associations between prenatal exposures and metabolic diseases and related disorders, such as coronary heart disease, obesity, and diabetes (1). More recently, research has investigated influences of the prenatal environment on neurobehavioral outcomes, such as schizophrenia, depression, inhibitory control, and attention-deficit/hyperactivity disorder (2).
As a highly active regulator of metabolic and endocrine status in utero, the placenta plays an important role in modulating the fetal environment and in providing appropriate hormones and peptides needed for fetal growth and development. Because of its role in modulating the influence of prenatal exposures and its major role in production of important neuropeptides like corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH), the placenta has been considered by some to be the ‘third brain’ (3). In this role, the placenta links the developed maternal brain and developing fetal brain to the influence of environmental insults (4), some of which may have life-altering effects on the newborn.
The importance of epigenetic regulation has gained prominence in investigations of molecular mechanisms by which the prenatal environment can influence the placenta (5). One important mode of epigenetic regulation is expression of microRNAs (miRNA), small non-coding RNAs which are involved in post-transcriptional gene regulation. Through post-transcriptional gene regulation and tissue-specific expression and function, miRNAs have been shown to regulate a number of key cell processes, including invasion, migration, proliferation, and death (5,6) and are critical regulators of embryonic development. miRNAs have been shown to be expressed in the placenta (7), and dysregulated placental miRNA expression has been associated with prenatal exposures (8,9), pregnancy outcomes (7,10,11), and fetal growth (12). miRNAs have also been shown to be associated with a variety of neurobehavioral outcomes, including pathways which may lead to neurobehavioral dysfunction (13) or addiction (14).
Given the growing body of literature suggesting that the functional epigenome of the placenta may be associated with infant neurobehavioral outcomes (15,16) and that placental miRNA expression may be both dysregulated by the intrauterine environment (8) and also associated with fetal outcome (12,17), we hypothesized that placental miRNA expression would be associated with infant neurobehavioral outcomes as assessed by the NICU Network Neurobehavioral Scales (NNNS) (18).
Results
Eighty-six human placentas were analyzed for the expression of six candidate miRNAs previously shown to be expressed in the placenta, associated with in utero exposures (8) and fetal outcomes (12), and involved in regulating cell processes: miR-16, miR-21, miR-93, miR-135b, miR-146a, and miR-182. Demographic information is described in Table 1. Clinical characteristics and NNNS summary scores are presented in Table 2. Based on the a priori design of the cohort, the study population is overrepresented by small for gestational age (SGA) and large for gestational age (LGA) infants. The mean birth weight percentile calculated using the method of Fenton (19) was 44.2%. Table 2 also provides descriptive statistics of the summary scores for infant attention, arousal, excitability, hypertonicity, stress/abstinence, and quality of movement as measured by the NNNS. As the size of the study population was modest compared to the number of domains evaluated, we focused our examinations on these 6 NNNS summary scores. This is consistent with prior reports which have noted that these summary scores were the most reflective of underlying at-risk profiles and are most sensitive to various influences of the perinatal environment (20).
Table 1.
Demographics of the study population (n=86)
| Gender | |
| Male (n, %) | 35 (41%) |
| Female (n, %) | 51 (59%) |
| Maternal Insurance | |
| Private (n, %) | 50 (58%) |
| Not Private (n, %) | 36 (42%) |
| Maternal Smoking During Pregnancy | |
| Yes (n, %) | 5 (6%) |
| No (n, %) | 80 (93%) |
| Maternal Age in Years (Mean, SD) | 29.1 (6.09) |
| Delivery Method | |
| Vaginal (n, %) | 54 (63%) |
| C-section (n, %) | 31 (36%) |
| Birthweight percentile in % (Mean, SD) | 44.2 (35.99) |
| Percent maternal weight gained during pregnancy in % (Mean, SD) | 20 (10.0) |
Table 2.
Clinical characteristics and NNNS summary scores
| Variable | n | Mean | Std. Dev. | Median | Min | Max |
|---|---|---|---|---|---|---|
| Birth weight percentile | 86 | 44.2 | 35.99 | 42.5 | 0 | 99 |
| Maternal Age (yrs) | 86 | 29.1 | 6.09 | 29.5 | 18 | 40 |
| Attention | 86 | 3.8 | 1.13 | 3.64 | 1.57 | 6.17 |
| Quality of Movement | 86 | 4.05 | 0.68 | 4.17 | 2.17 | 5.33 |
| Stress Abstinence | 86 | 0.22 | 0.07 | 0.22 | 0.08 | 0.41 |
| Arousal | 86 | 4.11 | 0.8 | 4.07 | 2 | 6 |
| Hypertonicity | 86 | 0.29 | 0.68 | 0 | 0 | 3 |
| Excitability | 86 | 4.58 | 2.99 | 4.5 | 0 | 13 |
NNNS = NICU Network Neurobehavioral Scales (18)
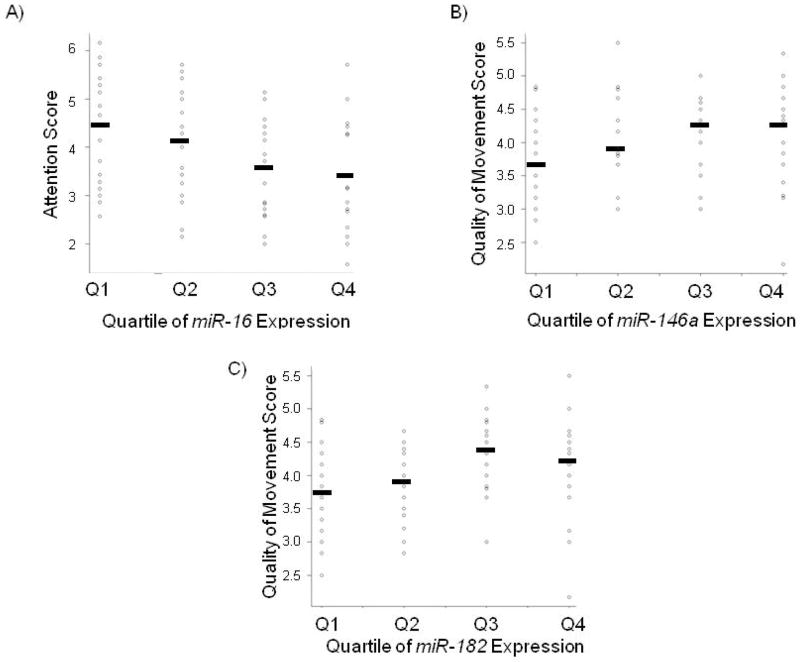
Supplemental Table S1 describes the expression of the 6 candidate miRNA in all of the 86 samples, based on quantitative RT-PCR (qRT-PCR) and absolute quantification from a standard curve. As observed previously (12), placental miRNA data was not normally distributed. Therefore, the miRNA expression profiles were split into quartiles (Q1=lowest quartile, 0–25%; Q2=lower-middle quartile, 26–50%; Q3=upper-middle quartile, 51–75%; Q4=highest quartile, 76–100%). NNNS summary scores were plotted by quartile of miRNA expression, and Kruskal-Wallis tests were used to assess differences in all six NNNS summary scores across quartiles of miRNA expression. Supplemental Table S2 presents the complete results of Kruskal-Wallis tests for differences of all six NNNS summary scores of interest across all six miRNA measured. Analyses revealed that attention score significantly differed across quartiles of placental miR-16 expression (p=0.046), quality of movement score significantly differed across quartiles of miR-146a expression (p=0.022), and quality of movement score significantly differed across quartiles of miR-182 expression (p=0.027). Figure 1A–C shows the distribution of infant NNNS score (y-axis) by primary term human placenta miRNA expression quartiles (x-axis) for these significant results.
Figure 1.
Distribution of infant NNNS score (y-axis) by primary term human placenta miRNA expression quartiles (x-axis). Black bar indicates mean NNNS score within each quartile. (A) Placental miR-16 expression is negatively associated with attention score (p=0.046), (B) Placental miR-146a expression is positively associated with quality of movement score (p=0.022), and (C) Placental miR-182 expression is positively associated with quality of movement score (p=0.027)
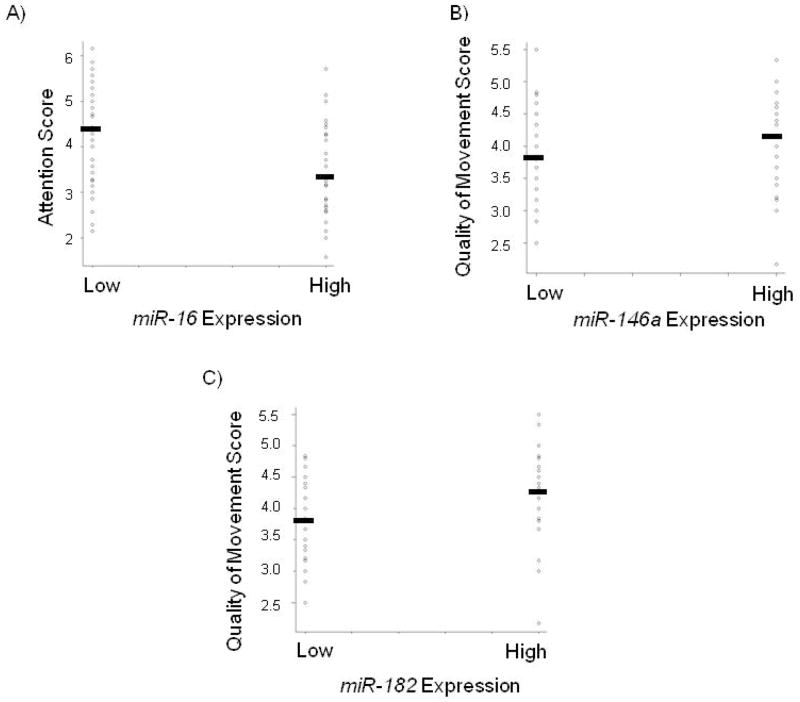
Since the highest quartiles of miR-16, miR-146a, and miR-182 expression were associated with differential attention score or quality of movement score, respectively, we focused on the association between high expression (>median vs. ≤median) of the miRNA and respective NNNS summary score. Tests revealed that high miR-16 expression was negatively associated with attention score (p=0.006), high miR-146a expression was positively associated with quality of movement score (p=0.016), and high miR-182 expression was positively associated with quality of movement score (p=0.016). These relationships are shown graphically in Figure 2A–C. Multivariable linear regression models were then used to examine associations between high miRNA expression and NNNS summary scores, controlling for potential confounders. The results of these models are summarized in Table 3 and include effect sizes (β values) and statistical significance. After controlling for confounders, the linear regression analysis showed that high miR-16 expression was negatively associated with attention score (β= −0.58, p=0.04), high miR-146a expression was positively associated with quality of movement score (β= 0.32, p=0.04), and high miR-182 expression was positively associated with quality of movement score (β= 0.39, p=0.01).
Figure 2.
Distribution of infant NNNS score (y-axis) by high-low miRNA expression groups (x-axis). Black bar indicates mean NNNS score within each group (A) Placental miR-16 expression is negatively associated with attention score (p=0.006), (B) Placental miR-146a expression is positively associated with quality of movement score (p=0.016), and (C) Placental miR-182 expression is positively associated with quality of movement score (p=0.016).
Discussion
miRNA expression has been associated with a variety of disease-associated or developmentally-associated neurobehavioral outcomes (13,14), and epigenetic mechanisms in the placenta have been associated with infant neurobehavioral outcomes (15,16). However, to our knowledge, our analysis is one of the first to report associations between placental miRNA expression and infant neurobehavioral measures as assessed by the NNNS. Specifically, we have identified the association of increased miR-16 expression with decreased infant attention score, increased miR-146a expression with increased infant quality of movement score, and increased miR-182 expression with increased quality of movement score.
The attention summary score scale describes an infant’s response to auditory and visual stimulation and ability to maintain a quiet awake state (21). Infants with high attention summary scores exhibit good turning and following during orientation tests, showing a level of sustained alertness throughout. Low attention summary scores are reflective of minimal turning and following and briefer periods of alertness during the presentation of orientation items, indicative of an overall poor quality of response. Furthermore, a low attention score has been described to reflect a low threshold for stimulation and has been frequently seen in infants who have been described as physiologically unstable (21). Our linear regression models, after controlling for potential confounders, suggested that high placental miR-16 expression was associated with a significant decrease in attention score of 0.58 units, reflective of an overall poor quality of response and reduced infant alertness.
The quality of movement summary score is an overall measure of motor control, including descriptions of an infant’s smoothness, maturity, modulation of movement of the arms and legs, and startles and tremors (21). High scores suggesting good quality of movement are descriptive of infants who exhibit smooth movement with a low degree of jitteriness, tremors and startles and relatively average amounts of spontaneous and elicited motor activity (21). Low scores suggesting an overall poor quality of movement describe infants who are predominantly jittery with a low degree of smoother movement of their arms and legs and with a great degree of tremors, startles, and a high overall level of activity (21). Our linear regression models controlling for potential confounders suggested that high placental miR-146a expression was associated with a significant increase in quality of movement score of 0.32 units, and high placental miR-182 expression was associated with a significant increase in quality of movement score of 0.39 units. Data suggesting that high miR-146a and high miR-182 expression are positively associated with higher quality of movement scores may suggest that these two miRNA may play a role in the pathway regulating fetal nerve and muscle development, with higher placental miR-146a and miR-182 expression associated with better quality of movement score. We have previously demonstrated that exposure to cigarette smoking in utero leads to downregulation of miR-146a (8). Taken together with these findings, our results suggest that tobacco smoke exposure may elicit some of its negative effects such as those reported on newborn muscle tone (22) and overall neurobehavior through alteration of miRNA expression.
Previous groups have used a variety of both in silico target prediction strategies coupled with gold-standard approaches to empirically-validate miRNA targets (5,8,12,17), thereby attempting to investigate the mechanisms by which such miRNA may regulate key cell and developmental processes. While previous work has suggested expression of miR-16 in the placenta is associated with maternal cigarette smoking during pregnancy (8) and fetal growth (12), we are unaware of any reported findings investigating placental miR-16 expression and infant neurobehavior, more specifically infant attention. Recently, miR-16 was shown to regulate serotonin transporter (SERT) levels. Studies have demonstrated that miR-16 is expressed at relatively higher levels in noradrenergic than in serotonergic cells, with reduction of miR-16 expression in noradrenergic neurons leading to de novo SERT expression (23). Dysregulated signaling through the serotonin pathway associated with prenatal cocaine exposure and prenatal stress has been reported to increase behavioral disturbances in attention, emotional behavior and stress response, and other outcomes (24). We have recently shown that intrauterine exposure to as modest of an alteration as an alteration in maternal mood is associated with increased placental SERT expression (25). Given that miR-16 targets SERT mRNA, increased miR-16 expression may lead to decreased levels of SERT, thereby dysregulating signaling through the serotonin pathway which may lead to disrupted attention. This may partially explain the negative association we found between miR-16 expression in the placenta and infant attention score. Future research in both human cohorts and animal models is needed to more definitively test this hypothesis.
Previously reported target prediction analysis (8), coupled with empirical validation (26), has demonstrated TRAF6 as a target of miR-146a. This suggests that miR-146a can influence signaling through the NFκβ pathway. Previous work has reported that TRAF6 deficiency can result in exencephaly and plays a role in promoting programmed cell death (PCD) in the developing central nervous system (CNS) (27). Future work using model systems to more comprehensively investigate how dysregulated placental miR-146a expression may influence the developing CNS will be important in furthering our understanding of quality of movement in infants.
Differential expression of miR-182 in the placentas of patients with preeclampsia has been described (7). One of the targets of miR-182 is FOXO1(28), a transcription factor important for regulating genes in pathways regulating cell cycle checkpoints, apoptosis, and metabolism. Using a previously described in silico target prediction strategy (8), we further predicted targets for miR-182 to include cAMP-dependent protein kinase catalytic subunit beta (PRKACB), protocadherin 8 (PCDH8), cortactin (CTTN), and endothelial PAS domain protein 1 (EPAS1). Table 4 summarizes these findings. PRKACB is a member of the Ser/Thr protein kinase family and a catalytic subunit of protein kinase A (PKA), which has been previously shown to interact with the ryanodine receptor 2 (29) and the low affinity nerve growth factor receptor (30). Previous work has suggested that PKA is involved in dopamine signaling in cells and furthermore, that PKA inhibition blocks the acquisition but not expression of amphetamine-produced conditioning in amphetamine-dosed rats (31). If PRKACB is a target of miR-182, increased miR-182 expression might lead to decreased levels of PRKACB which may have consequences for a number of cellular processes, including dysregulated activation of the reward system in neurons in the nucleus accumbens. In the placenta, crosstalk between the PKA and MAPK signaling pathways can mediate placental leptin expression, thereby having an effect on the proliferation and survival of trophoblast cells (32). PCDH8 is the human ortholog of PAPC, an important mediator during vertebrate embryogenesis (33,34). CTTN is present in many cell types and when activated, plays an important role in promoting cell migration and in lamellipodia and invadopodia formation (35–37). Initial reports have investigated EPAS1 levels in the placenta associated with preeclampsia and hypoxia (38), finding that EPAS1 was upregulated in preeclamptic placentas. Future work to empirically validate PRKACB, PCDH8, CTTN, and EPAS1 as targets of miR-182 will be necessary to better elucidate the mechanisms altered by dysregulated placental miR-182 expression.
Table 4.
Predicted mRNA targets of miR-182
| miRNA | Predicted Target mRNA |
|---|---|
| miR-182 | PRKACB |
| PCDH8 | |
| CTTN | |
| EPAS1 |
Targets for miR-182 were predicted using a modified target prediction strategy (8). Three target prediction algorithms, miRanda (August 2010 release, http://www.microrna.org/microrna/home.do), PicTar (as cited in ref. (40)), http://pictar.mdc-berlin.de/cgi-bin/new_PicTar_vertebrate.cgi), and TargetScan 6.1 (www.targetscan.org/), were used to predict targets for miR-182. In order to be considered a predicted target, the target must have appeared in the top 100 targets in all three prediction algorithms.
A growing body of literature is also describing how the placenta and placental gene expression is crucial for proper growth and neurodevelopment of the fetus. Many of the genes implicated as predicted and/or empirically-validated targets of miR-16, miR-146a, and miR-182 are involved in placental invasion into the maternal decidua as well as ensuring proper functioning of the placenta during development. Improper invasion or anchoring of the placenta has been hypothesized to increase risk for hypoxia, which, along with other prenatal stressors, can lead to both intrauterine growth restriction as well as changes to the hippocampus in the infant (39). Our work also suggests that the miRNA may be affecting key neuro-active peptide and hormone production by the placenta. These alterations may be additional mode(s) through which the environment can impact infant neurobehavioral development.
Our work is limited by an inability to definitively test causality. The associations we have observed may be further tested using model systems. Another limitation of this study is the relatively moderate sample size for examining the number of neurobehavioral outcomes as determined by the NNNS assessment. To date, our follow-up of the infants studied is limited. Therefore, more extensive associations between miRNA expression and long-term neurobehavioral outcomes remain to be elucidated (15). Additionally, our samples were limited to term placentas, which may not accurately represent the changes in placental miRNA expression throughout the various stages in pregnancy. Due to this limitation, we cannot properly speculate about associations between placental miRNA expression and neurobehavioral outcomes in premature infants. Future directions also include performing our analysis in a larger set of mother-infant pairs to examine more complex interactions between placental miRNA expression and infant neurobehavioral outcomes.
In summary, this study reveals associations between placental miRNA expression and infant neurobehavioral outcomes, as measured by the NNNS. The predictive nature of these early neurobehavioral measures has been validated and thus warrants further follow-up of these infants to more definitively determine the association of placental miRNA expression with longer-term neurobehavioral outcomes. Future studies focused on enhancing our understanding of the influence of the prenatal environment on neurobehavioral outcomes through epigenetic mechanisms will be important in elucidating the developmental origins of health and neurobehavior.
Patients and Methods
Study population
Study subjects are part of the ongoing Rhode Island Child Health Study (RICHS) in which mother-infant pairs are enrolled following delivery at Women and Infants Hospital of Rhode Island (12,15,16). In brief, term infants born small for gestational age (SGA, lowest 10th percentile), or large for gestational age (LGA, highest 10th percentile) based on birth weight and gestational age and calculated using the Fenton growth chart (19) are selected. Appropriate for gestational age (AGA) infants matched on gender, gestational age (+/− 3 days), and maternal age (+/− 2 years) are also enrolled. Only viable, singleton infants are included. Additional exclusion criteria include congenital or chromosomal abnormality of the infant, maternal age <18 years or >40 years or a life-threatening complication of the mother. Data from maternal inpatient medical records from delivery were collected by structured chart review. Mothers were subjected to an interviewer-administered questionnaire including questions regarding exposure histories, lifestyle, and demographics. All subjects provided written informed consent for participation under appropriate protocols approved by the Institutional Review Boards for Women and Infants’ Hospital of Rhode Island and Brown University.
Neurobehavioral assessment
Certified psychometrists, blinded to the study hypothesis, administered the NNNS during the newborn inpatient stay and prior to discharge. The NNNS assessments, as well as established summary scores, have been described previously (18,20). This analysis examined 86 subjects enrolled between September 2009 and September 2010 having complete NNNS data, placental miRNA expression profiling, and demographics information.
Placenta sample collection and RNA extraction
The placenta sample collection and RNA extraction has been described previously (12,16). For each patient, 12 samples of placenta tissue, 3 from each of 4 quadrants (totaling approximately 1 g of tissue) were excised from the placenta within 2 hours of delivery. All samples were taken from the maternal side of the placenta, 2 cm from the umbilical cord insertion site, free of maternal decidua. Immediately after sample collection, the samples were placed in RNAlater (Ambion/Life Technologies, Carlsbad, CA) and stored at 4°C. At least 72 hours later, placenta samples were removed from RNAlater, blotted dry, snap-frozen in liquid nitrogen, and homogenized using a mortar and pestle, to create a single homogenized sample, thereby reducing variability introduced by sampling site. Samples are stored at −80°C until needed for further examination. RNA was extracted from the homogenized samples using the miRvana miRNA Isolation Kit (Ambion/Life Technologies, Carlsbad, CA) and manufacturer protocols as described previously (8,12).
Quantitative RT-PCR (qRT-PCR) for mature miRNA
Absolute quantitation of the expression of mature miRNA was measured using TaqMan miRNA Assays (Applied Biosystems, Valencia, CA) on an Applied Biosystems 7900HT Real-Time PCR system and analyzed with 7900HT System Software (Applied Biosystems, Valencia, CA) as described previously (12). All reactions were run in triplicate on 384-well plates, and RNU-44 was used as an internal control to assess that starting concentrations were standardized. For the 86 samples, the mean Ct value of RNU-44 was 24.72 and the standard deviation was 0.62, suggesting highly standardized starting concentrations, and allowing use of the absolute quantification in subsequent analyses. No-RT controls were run to assure samples were free of genomic DNA contamination.
Statistical analysis
As miRNA expression profiles were not normally distributed as reported previously (12), bivariate associations between NNNS Summary Scores and quartiles of miRNA expression were assessed using non-parametric Kruskal-Wallis tests. Kruskal-Wallis tests were used to assess differences in all six NNNS summary scores across quartiles of miRNA expression, and p<0.05 was used to indicate a significant difference in respective NNNS summary score across quartiles of respective miRNA expression. We focused on 6 summary scores shown to be most sensitive based on our preliminary studies, specifically attention, quality of movement, arousal, excitability, hypertonicity, and stress abstinence (20). Linear regression models were used to investigate associations between NNNS Summary Scores and miRNA expression while controlling for confounders which may influence this association. Final models of infant attention scores and quality of movement scores and miRNA expression included infant gender, maternal age, maternal insurance, relative maternal weight gained during pregnancy, maternal smoking during pregnancy, delivery method, and birth weight percentile, thereby controlling for potential confounders. All statistical analyses were performed in R (R Version 2.12.0, The R Foundation for Statistical Computing).
in silico miRNA target prediction
Targets for miRNAs of interest were predicted using a modified target prediction strategy described previously (8). Three target prediction algorithms accessible online, miRanda (August 2010 release), PicTar (as cited in ref. (40)), and TargetScan 6.1, were used to predict targets for miRNAs of interest. In order to be considered a predicted target, the target must have appeared in the top 100 targets in all three prediction algorithms.
Supplementary Material
Table 3.
Linear Regression Models for the Association Between miRNA Expression and NNNS Score
| Attention Score | Quality of Movement Score | Quality of Movement Score | ||||
|---|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| miR-16 Expression | ||||||
| Low | Reference | -- | -- | -- | -- | |
| High | −0.58 | 0.04 | -- | -- | -- | -- |
| miR-146a Expression | ||||||
| Low | -- | -- | Reference | -- | -- | |
| High | -- | -- | 0.32 | 0.04 | -- | -- |
| miR-182 Expression | ||||||
| Low | -- | -- | -- | -- | Reference | |
| High | -- | -- | -- | -- | 0.39 | 0.01 |
| Infant Gender | ||||||
| Female | Reference | Reference | Reference | |||
| Male | −0.17 | 0.54 | 0.23 | 0.16 | 0.26 | 0.11 |
| Maternal Age in years | −0.01 | 0.57 | 0.01 | 0.62 | 0.01 | 0.55 |
| Maternal Insurance | ||||||
| Public | Reference | Reference | Reference | |||
| Private | 0.52 | 0.07 | 0.14 | 0.40 | 0.20 | 0.24 |
| Relative Pregnancy Weight Gain | −0.75 | 0.56 | 0.38 | 0.62 | 0.46 | 0.55 |
| Maternal Smoking During Pregnancy | ||||||
| No | Reference | Reference | Reference | |||
| Yes | −0.12 | 0.83 | −0.26 | 0.42 | −0.22 | 0.50 |
| Delivery Method | ||||||
| Caesarian-section | Reference | Reference | Reference | |||
| Vaginal | 0.09 | 0.73 | 0.11 | 0.48 | 0.11 | 0.50 |
| Birthweight Percentile in Percent | 0.003 | 0.30 | 0.0006 | 0.77 | 0.0008 | 0.69 |
Acknowledgments
The authors wish to thank Gilda Ferro and Joyce Lee for their work in recruitment of subjects into the study and the staff of the Brown Center for the Study of Children at Risk for their efforts.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
Author Contributions
The authors of the manuscript contributed to the work in the following ways. Conceived and designed the experiments: CJM MAM. Performed the experiments: MAM. Analyzed the data: MAM CJM. Wrote the manuscript: MAM CJM JFP BML VSK.
Financial Disclosure: This work was supported by National Institutes of Health grants from the National Center for Research Resources (P20 RR018728), the National Institute of Mental Health (R01 MH094609), and the National Institute of Alcohol Abuse and Alcoholism 2 T32 AA 07459-26 (MAM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–8. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 2.Van den Bergh BR. Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Dev Med Child Neurol. 2011;53 (Suppl 4):19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- 3.Yen SS. The placenta as the third brain. J Reprod Med. 1994;39:277–80. [PubMed] [Google Scholar]
- 4.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 5.Maccani MA, Marsit CJ. Epigenetics in the placenta. Am J Reprod Immunol. 2009;62:78–89. doi: 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–8. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Pineles BL, Romero R, Montenegro D, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196:261 e1–6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5:583–9. doi: 10.4161/epi.5.7.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avissar-Whiting M, Veiga KR, Uhl KM, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;29:401–6. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482–90. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 11.Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31:781–4. doi: 10.1016/j.placenta.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS One. 2011;6:e21210. doi: 10.1371/journal.pone.0021210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocerha J, Faghihi MA, Lopez-Toledano MA, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A. 2009;106:3507–12. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li MD, van der Vaart AD. MicroRNAs in addiction: adaptation’s middlemen? Mol Psychiatry. 2011;16:1159–68. doi: 10.1038/mp.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsit CJ, Lambertini L, Maccani MA, et al. Placenta-Imprinted Gene Expression Association of Infant Neurobehavior. J Pediatr. 2011 doi: 10.1016/j.jpeds.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-Beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One. 2012;7:e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maccani MA, Marsit CJ. Exposure and fetal growth-associated miRNA alterations in the human placenta. Clinical Epigenetics. 2011;2:401–4. doi: 10.1007/s13148-011-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641–67. [PubMed] [Google Scholar]
- 19.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Bann C, Lester B, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125:e90–8. doi: 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boukydis CF, Bigsby R, Lester BM. Clinical use of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:679–89. [PubMed] [Google Scholar]
- 22.Stroud LR, Paster RL, Goodwin MS, et al. Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics. 2009;123:e842–8. doi: 10.1542/peds.2008-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–41. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 24.Williams SK, Lauder JM, Johns JM. Prenatal Cocaine Disrupts Serotonin Signaling-Dependent Behaviors: Implications for Sex Differences, Early Stress and Prenatal SSRI Exposure. Curr Neuropharmacol. 2011;9:478–511. doi: 10.2174/157015911796557957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponder KL, Salisbury A, McGonnigal B, Laliberte A, Lester B, Padbury JF. Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: implications for fetal programming. Dev Psychobiol. 2011;53:711–23. doi: 10.1002/dev.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou J, Wang P, Lin L, et al. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–8. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 27.Lomaga MA, Henderson JT, Elia AJ, et al. Tumor necrosis factor receptor-associated factor 6 (TRAF6) deficiency results in exencephaly and is required for apoptosis within the developing CNS. J Neurosci. 2000;20:7384–93. doi: 10.1523/JNEUROSCI.20-19-07384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–16. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12. 6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 30.Higuchi H, Yamashita T, Yoshikawa H, Tohyama M. PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. EMBO J. 2003;22:1790–800. doi: 10.1093/emboj/cdg177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdjikov TV, Giles AC, Swain SN, Beninger RJ. Nucleus accumbens PKA inhibition blocks acquisition but enhances expression of amphetamine-produced conditioned activity in rats. Psychopharmacology (Berl) 2007;190:65–72. doi: 10.1007/s00213-006-0590-1. [DOI] [PubMed] [Google Scholar]
- 32.Maymo JL, Perez AP, Gambino Y, Calvo JC, Sanchez-Margalet V, Varone CL. Review: Leptin gene expression in the placenta--regulation of a key hormone in trophoblast proliferation and survival. Placenta. 2011;32 (Suppl 2):S146–53. doi: 10.1016/j.placenta.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125:4681–90. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- 34.Rhee J, Takahashi Y, Saga Y, Wilson-Rawls J, Rawls A. The protocadherin papc is involved in the organization of the epithelium along the segmental border during mouse somitogenesis. Dev Biol. 2003;254:248–61. doi: 10.1016/s0012-1606(02)00085-4. [DOI] [PubMed] [Google Scholar]
- 35.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–34. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 36.Weaver AM, Karginov AV, Kinley AW, et al. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–4. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 37.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvenpaa J, Vuoristo JT, Savolainen ER, Ukkola O, Vaskivuo T, Ryynanen M. Altered expression of angiogenesis-related placental genes in pre-eclampsia associated with intrauterine growth restriction. Gynecol Endocrinol. 2007;23:351–5. doi: 10.1080/09513590701350291. [DOI] [PubMed] [Google Scholar]
- 39.Lodygensky GA, Seghier ML, Warfield SK, et al. Intrauterine growth restriction affects the preterm infant’s hippocampus. Pediatr Res. 2008;63:438–43. doi: 10.1203/PDR.0b013e318165c005. [DOI] [PubMed] [Google Scholar]
- 40.Lall S, Grun D, Krek A, et al. A genome-wide map of conserved microRNA targets in C. elegans. Curr Biol. 2006;16:460–71. doi: 10.1016/j.cub.2006.01.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.