Abstract
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder that is associated with negative cardiovascular consequences and adverse events from excessive daytime sleepiness. Insomnia is the inability to initiate or maintain sleep accompanied by daytime dysfunction. OSA and insomnia co-occur at a high rate, and such patients appear to have distinct clinical features of both disorders. Although empirically supported treatments are now available for OSA and insomnia independently, there are currently no standards or guidelines for how to combine or initiate these treatments for patients who suffer from both sleep disorders. Our goal was to review the literature on current diagnostic considerations, clinical features, pathophysiology, and treatment approaches for patients with OSA and comorbid insomnia. In particular, the potential benefits and challenges of using a multidisciplinary treatment model are discussed, including a research strategy that could inform implementation of pulmonary and behavioral sleep medicine treatments. The research, clinical, and policy implications of treating both OSA and insomnia are discussed with the hope that further activity will establish standards or guidelines for patients with OSA and insomnia.
Keywords: Insomnia, Obstructive Sleep Apnea, Comorbidity, Multidisciplinary Approach, Behavioral Sleep Medicine
Sleep disturbances are an important public health problem with over 70 diagnosable sleep disorders listed in the ICSD-2 (American Academy of Sleep Medicine, 2005). Two of the most prevalent sleep disorders are Obstructive Sleep Apnea (OSA) and insomnia (Ram, Seirawan, Kumar, & Clark, 2010). Much is known about the clinical features of these two disorders and there are effective treatments available for each disorder. Moreover, OSA and insomnia commonly co-occur in patients who present to sleep disorders clinics. However, much less is known about the characteristics and optimum treatment of this comorbid condition. Our primary objective was to review the current literature on the diagnostic considerations, clinical features, pathophysiology, and treatment approaches for patients with OSA and comorbid insomnia and to highlight the potential benefits of a multidisciplinary treatment model for this comorbid condition. A secondary objective was to discuss challenges in treatment implementation for this population and to present a research strategy for investigating treatment pathways. We conclude by discussing areas for further research and clinical considerations that could optimize care of patients with comorbid sleep disorders.
Diagnostic considerations, clinical features, and pathophysiology
OSA is a sleep-related breathing disorder characterized by repetitive narrowing or collapsing of the upper airway during sleep that causes sleep fragmentation and oxygen desaturation. Sleep apnea is highly prevalent in the middle aged to older adults, with estimates varying from 2-4% (Young et al., 1993) to 10-20% in a recent report from the Agency for Healthcare Research and Quality (Balk et al., 2011). The consequences of untreated OSA are well documented and include cardiovascular conditions, such as hypertension, myocardial infarction, and stroke, and adverse events related to excessive daytime sleepiness, such as motor vehicle accidents, diminished quality of life (Young, Peppard, & Gottlieb, 2002). In OSA, the sleep disturbance is primarily sleep fragmentation, or repetitive arousals from sleep. In contrast, insomnia is the inability to fall asleep or maintain sleep causing prolonged periods of involuntary wakefulness during the intended sleep episode. Approximately one-third to one-half of adults experience symptoms of insomnia while 10-18% report chronic insomnia (Jansson-Frojmark & Linton, 2008; LeBlanc et al., 2009; Ohayon, 2002). Insomnia has significant consequences on the individual's mood and daytime functioning and carries a large economic burden, costing an estimated $63 billion in lost work performance (Kessler et al., 2011).
Although difficulty initiating and maintaining sleep can be a complaint or symptom of another disorder, it is now recognized that insomnia can still be diagnosed in the presence of another disorder. The National Institutes of Health recommended that the term “comorbid insomnia” be used instead of the previous term “secondary insomnia” (National Institutes of Health State of the Science Conference Statement, 2005). Furthermore, the proposed revisions for the upcoming DSM-V recognize this paradigm shift by suggesting the term “insomnia disorder” whenever criteria for insomnia are met with additional specifiers to document the occurrence of another medical, psychiatric, or sleep disorder (American Psychiatric Association, 2012). The rationale behind this proposed view is based upon recent evidence that attribution of the causes of insomnia (i.e., primary versus secondary) cannot be reliably made by clinicians (Edinger et al., 2011).
This paradigm shift has important diagnostic implications for sleep disorders because a complaint of insomnia is listed as a criterion for OSA (ICSD-2). Studies examining the co-occurrence of insomnia in OSA have found that between 22% and 54.9% of those with OSA report comorbid insomnia. Conversely, studies that have examined the presence of OSA among those with insomnia have found that 29% to 67% of those with insomnia also have OSA (Chung, 2005; Gooneratne et al., 2006; Guilleminault, Palombini, Poyares, & Chowdhuri, 2002; Krakow et al., 2001; Krell & Kapur, 2005; Lichstein, Riedel, Lester, & Aguillard, 1999; Ong, Gress, San Pedro-Salcedo, & Manber, 2009; Smith, Sullivan, Hopkins, & Douglas, 2004). Previously, the co-occurrence of OSA and insomnia was termed “sleep apnea plus” (Cherniack, 2005). However, this conceptualization seems to prioritize the apnea and minimize the impact of the insomnia on the comorbid condition. In contrast, studies seeking to characterize patients with both OSA and insomnia have yielded evidence that the insomnia features are more prominent that the OSA features. Yang and colleagues (2011) compared the psychological profiles of individuals with OSA only, insomnia only, and both OSA and insomnia. They found that those with both OSA and insomnia had elevations in maladaptive sleep cognitions, elevated sleep-related anxiety, maladaptive sleep-related behaviors, and anxiety and depression that more closely resembled the features of those with insomnia only rather than those with OSA only. Examining subtypes of insomnia, Chung (2005) found that patients with sleep onset insomnia were less likely to report daytime sleepiness and were more likely to have lower apnea-hypopnea indices (AHI), which was taken as evidence to suggest that OSA patients with sleep onset-insomnia were more likely to be hyperaroused, a presumed cause of insomnia (Bonnet & Arand, 2010). Rather than “sleep apnea plus”, it appears that an argument can be made to call this co-occurrence “insomnia plus.”
The co-occurrence of OSA and insomnia is also associated with significant morbidity. Patients with both OSA and insomnia report more psychiatric symptoms, chronic pain, and higher rates of comorbid sleep disorders (Krell & Kapur, 2005; Smith et al., 2004). In a study on older adults, Gooneratne and colleagues (2006) found that having both OSA and insomnia was associated with significantly greater daytime impairment and longer reaction times on a psychomotor vigilance task when compared to those with neither disorder or just one disorder (OSA or insomnia). Both forms of sleep disturbance have been associated with sympathetic activation and might lead to cardiovascular consequences (Luyster, Buysse, & Strollo, 2011). OSA is a risk factor for hypertension (Nieto et al., 2000) , coronary artery disease (Shahar et al., 2001) , heart failure (Shahar et al., 2001) , Type II diabetes (Pamidi, Aronsohn, & Tasali, 2010), and stroke (Arzt, Young, Finn, Skatrud, & Bradley, 2005). Insomnia increases the risk of developing hypertension (Fernandez-Mendoza et al., 2012; Vgontzas et al., 2009) and insomnia with short sleep duration has been associated with Type 2 diabetes (Vgontzas et al., 2009). Unfortunately, studies have only examined insomnia and OSA separately, so it is not known if the comorbidity of the two sleep disorders increases the risk of cardiovascular consequences (Luyster et al., 2011).
Given that research on patients with both OSA and insomnia is only beginning to emerge, the pathophysiology of this comorbid condition has yet to be established. Several review papers have speculated on possible etiologies, focusing attention on the order of development of the two sleep disorders and the phenotype of the insomnia. With regards to the order of development, one hypothesized pathway suggests that the OSA develops first and the repeated respiratory events serve as a precipitating factor that, if left untreated, becomes a perpetuating factor for insomnia (Al-Jawder & Bahammam, 2011; Luyster et al., 2011). An alternative hypothesis holds that the prolonged awakenings and sleep loss from an insomnia disorder could have a negative impact on the tone of the pharyngeal muscles, thus leading to the development or exacerbation of apneas and hypopneas (Al-Jawder & Bahammam, 2011; Luyster et al., 2011; Wickwire & Collop, 2011). The second etiological factor involves the insomnia phenotype (i.e., difficulty falling asleep or staying asleep). Given that OSA is a disorder that occurs after sleep onset, it would appear more likely that sleep maintenance insomnia would be causally connected with OSA. Indeed, in a study by Chung (2005), the most common insomnia complaint was frequent awakenings during the night and the least common complaint was difficulty falling asleep. Thus, it has been hypothesized that sleep maintenance insomnia has causal links with OSA, but sleep-onset insomnia might be an independent co-occurring sleep disorder (Beneto et al., 2009; Chung et al., 2005). Finally, Beneto and colleagues (2009) proposed that the hypothalamic-pituitary-adrenal (HPA) axis is a link between OSA and insomnia via metabolic and endocrine involvement. These hypotheses remain to be tested and at present, it would appear that patients with OSA and insomnia might be heterogeneous with regards to pathophysiology and clinical features.
Taken together, the existing literature reveals that insomnia and OSA co-occur at a high rate, and patients with both sleep disorders are at higher risk for daytime impairment, psychological distress, and possibly more cardiovascular consequences. Moreover, these patients appear to exhibit distinct clinical features, although the pathophysiology remains unknown. These findings have important clinical implications for the assessment and treatment of this comorbid condition.
In search of a treatment model for OSA and insomnia
Given that individuals who suffer from both OSA and insomnia might have symptoms and complaints associated with both OSA and insomnia, optimal treatment should target both disorders. Fortunately, there are effective treatments for each of these sleep disorders (see Table 1). The current recommendations for first-line treatment of OSA is positive airway pressure (PAP), which includes continuous positive airway pressure (CPAP) and bi-level positive airway pressure (Bi-PAP), and it is the most widely-used and accepted treatment for OSA (Epstein et al., 2009; Kushida, Littner et al., 2006; Loube et al., 1999). PAP is a device that maintains a consistent pressure of airflow that serves as a pneumatic splint and prevents collapsing of the upper airway during sleep. Although PAP has demonstrated efficacy for improving sleep symptoms and daytime sleepiness across several clinical trials (Balk et al., 2011; Ballester et al., 1999; Barbe et al., 2001; Bardwell, Ancoli-Israel, Berry, & Dimsdale, 2001; Engleman et al., 1996; Engleman et al., 1999; Engleman et al., 2002; Kushida, Morgenthaler et al., 2006), adherence to nightly use is variable with studies reporting adherence rates between 28% and 77% with regular use in about 40-50% of patients (Balk et al., 2011; Engleman, Martin, & Douglas, 1994; Kribbs et al., 1993; Krieger, 1992; McArdle et al., 1999; Meurice et al., 1994; Reeves-Hoche, Meck, & Zwillich, 1994; Sanders, Gruendl, & Rogers, 1986; Wolkove, Baltzan, Kamel, Dabrusin, & Palayew, 2008). The average use per night is between 4.5 and 5.6 hours. Furthermore, it has been reported that the presence of insomnia can have a negative impact on PAP compliance. Wickwire et al (2010) found that problems with sleep-maintenance predicted poor adherence to PAP, defined as minutes of PAP used per night as well as regular use of PAP. Therefore, the presence of insomnia might adversely affect treatment of OSA.
Table 1.
Standard Treatments for OSA and Insomnia
| OSA | Insomnia | |
|---|---|---|
| Standard Treatments | PAP1 | CBT Stimulus control Relaxation therapy Hypnotic Medications |
| Guideline Treatments | Positional Therapy Weight loss Oral appliance2 ENT surgery |
Paradoxical intention Sleep Restriction Biofeedback |
PAP is standard treatment for moderate to severe OSA
Oral Appliance is recommended as a guideline treatment for: a) mild to moderate OSA and patient preference, b) patients who are non-responsive to treatment, or c) patients who fail CPAP and other behavioral measures.
For insomnia, both behavioral treatments and hypnotic medications have demonstrated treatment efficacy (see Table 1). Considerable evidence supports the efficacy and effectiveness of cognitive-behavior therapy for insomnia (CBTI) and is recommended as a first line treatment for both primary and comorbid insomnia (Morgenthaler et al., 2006; Stepanski, 2005). CBTI consists of a multicomponent treatment package that includes behavioral strategies (sleep restriction, stimulus control, and sleep hygiene) and cognitive strategies (e.g., cognitive restructuring). Hypnotics such as zolpidem, eszcopliclone, and zaleplon also have demonstrated efficacy for insomnia but are associated with adverse effects that include residual daytime effects (Buscemi et al., 2007) and drug dependency (Schuckit, Smith, Kramer, Danko, & Volpe, 2002).
Traditional treatment models consists of treating only the OSA (i.e., with CPAP) or treating only the insomnia (i.e., with CBTI or hypnotics) and consider adjunctive or concurrent treatment only if the first line treatment fails. However, the literature reviewed suggests that the optimal treatment plan should target both OSA and insomnia. Unfortunately, there are no clear guidelines for how to proceed with this plan. Therefore, an emerging clinical need is the establishment or implementation of a treatment model for patients with OSA and insomnia.
Multidisciplinary approaches
Multidisciplinary treatment teams containing biopsychosocial expertise are emerging as a preferred model of treatment in many areas of medicine. For example, a systematic review found that rehabilitation for chronic low back pain using an intensive multidisciplinary, biopsychosocial approach reduces pain and improves physical function (Guzman et al., 2001). A similar multidisciplinary approach appears to be well-suited for comorbid conditions such as OSA and insomnia. Behavioral treatments for insomnia have appeal because they can help target the psychological profile of patients with the comorbid condition was established by previous studies (i.e., hyperarousal, sleep-related anxiety, maladaptive beliefs about sleep). Also, behavioral treatments have proven efficacy and are often preferred over hypnotics (Morin, Gaulier, Barry, & Kowatch, 1992; Vincent & Lionberg, 2001). Moreover, OSA is a medical condition that also impacts psychosocial functioning, such as depressed mood and reduced daytime energy (Borak et al., 1994; Edinger, Carwile, Miller, Hope, & Mayti, 1994; Flemons & Tsai, 1997; Kales et al., 1985), which is not addressed by the use of medical treatment (i.e., PAP) alone. Indeed, studies using multidisciplinary treatment teams have begun to appear in the literature. In an open trial of patients whose primary complaint was insomnia, Krakow and colleagues (2004) treated 17 patients who were diagnosed with insomnia and OSA or Upper Airway Resistance Syndrome using a sequence of CBTI followed by OSA treatment (14 received CPAP, 2 an oral appliance, and 1 a turbinectomy). Only 8 of 17 patients attained clinically significant improvement of insomnia after CBTI, while 15 of the 17 patients reported clinically significant improvements of insomnia after receiving both treatments. Notably, they reported that many patients were not initially interested in or ready for OSA treatment following diagnosis, despite knowledge of the benefits of treatment. Using a crossover design, Guilleminault and colleagues (2008) treated 30 patients with mild OSA and insomnia using CBTI and ENT surgery. They found that optimal outcomes were achieved for patients who received both treatments, with some evidence that initiating OSA treatment prior to CBTI might lead to better outcomes (e.g., resolution of insomnia prior to CBTI).
Rush multidisciplinary model
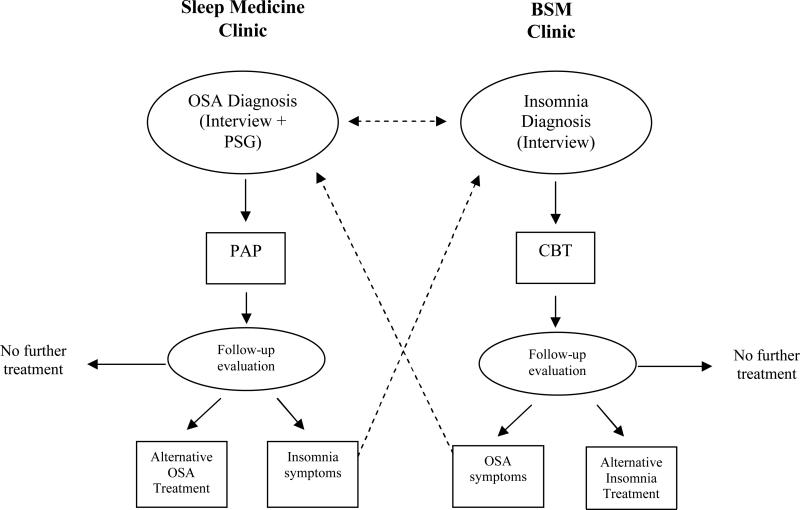
At the Rush Sleep Center, we have employed a multidisciplinary treatment program combining pulmonary sleep medicine with behavioral sleep medicine (BSM). As shown in Figure 1, patients might be initially seen in either the sleep medicine clinic or the BSM clinic. Those who are diagnosed with OSA and insomnia could follow several different treatment pathways, but are typically seen by both a board-certified sleep physician and a BSM-certified (CBSM) psychologist. The physician and psychologist discuss cases, exchanging information on the patient profile, medical and psychiatric history, and patient preferences. Decisions are made on a case-by-case basis regarding which treatment to use (PAP, surgery or oral appliance for OSA and CBTI or medications for insomnia) and when to initiate the treatment. For some patients, the treatment pathway is to begin with PAP, followed by CBTI if the insomnia continues after PAP treatment. For others, PAP and CBTI might begin concurrently. Some patients choose to begin with CBTI and defer PAP treatment until the insomnia is adequately controlled and some with mild OSA might elect to not treat the sleep apnea if the sleep symptoms improve overall following CBTI. Other patients require only PAP and no additional treatment. Progress is discussed by the physician and psychologist who monitor changes in the treatment plan. Using this multidisciplinary approach, the team is in a position to match empirically supported treatments for both OSA and insomnia with patient needs and preferences. It also provides flexibility to allow the treatment team to make adjustments and provide alternatives if the initial treatment plan fails.
Figure 1. Multidisciplinary Treatment Model.
Schematic flow of the multidisciplinary treatment approach at the Rush Sleep Center. Dotted lines represent cross-referrals, which can occur concurrently or sequentially. Follow-up evaluation following PAP is typically 90 days or less. CBT typically consists of weekly visits for a total of 4-8 sessions, with follow-up evaluation occurring at the last session. Alternative OSA treatment includes oral appliance, referral for ENT surgery, or positional training. Alternative insomnia treatment might include a trial of hypnotic medication.
Preliminary data collected on 8 patients who completed both CBTI and CPAP in our multidisciplinary clinic revealed significant (p < .05) baseline to post-treatment reductions in self-reported ratings of fatigue and maladaptive sleep-related cognitions, while total sleep time, as reported in sleep diaries, increased (Ong et al., 2012). These findings are promising and demonstrate the feasibility of our multidisciplinary approach, but the small sample size and lack of a control group limits our ability to draw conclusions regarding efficacy. Moreover, treatment decisions were not standardized and patient preferences sometimes led to variability in the initiation or discontinuation of treatment. Therefore, clinical trials are needed to generate treatment guidelines.
Testing a multidisciplinary treatment model for OSA and insomnia
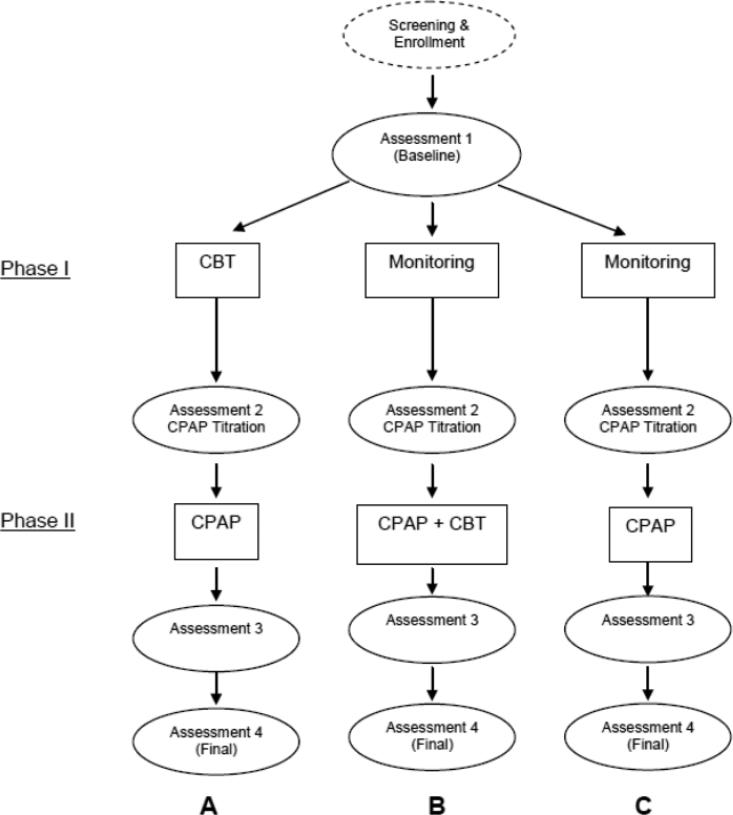
Recently, we received funding to conduct a randomized controlled trial that tests three treatment models for improving CPAP adherence and sleep quality in patients with OSA and insomnia. Each treatment model serves as an arm in this three-arm trial (see Figure 2). Model A consists of a 4-session CBTI during the first 30 days followed by 90 days of CPAP use. Model B consists of completing sleep diaries and monitoring sleep symptoms for 30 days followed by the concurrent initiation of the 4-session CBTI and CPAP. Participants in this condition will receive both CBTI and CPAP for the first 30 days and will then continue to use CPAP for 60 days beyond the termination of CBTI. In Model C, participants will complete sleep diaries and monitor their sleep symptoms for 30 days and then receive CPAP only for 90 days. Outcome measures for CPAP adherence will be the percent of days using CPAP and the average hours of use per night obtained from the machines and the outcome measures for sleep will be the Pittsburgh Sleep Quality Index and the sleep diaries. In addition, benchmarks for clinical significance will be used to document participants who are “regular CPAP users” (CPAP use ≥ 4 hours on ≥ 70% of nights) and who become “good sleepers” (PSQI < 5 and sleep efficiency > 85%) at the final assessment. The primary aim of this study is to compare the multidisciplinary approach used in Models A and B versus the standard approach used in Model C. The secondary aim is to determine if there are benefits associated with the sequence of treatment initiation for CBTI (Model A versus Model B). The overall goal of this project is to identify the optimal treatment model and to determine if there is evidence to support a causal link between insomnia symptoms and CPAP non-adherence.
Figure 2. Study Design.
*Note. CBT = 4 weekly session in 30 days; CPAP titration study and Assessment 2 conducted 30 days after initiation of Phase I. Assessment 3 taken 30 days after initiation of Phase II. Assessment 4 is the final study endpoint conducted 90 days after initiation of Phase II.
Does treatment sequence matter?
A key innovation in the design of this study is the sequence of treatment initiation in each model. In our current multidisciplinary clinic, the most commonly debated clinical decision is the order in which CBTI and CPAP should be initiated. As noted earlier, CPAP is the first line treatment for moderate to severe OSA (Epstein et al., 2009; Kushida, Littner et al., 2006; Loube et al., 1999) and clinical lore has been to apply the same guideline to patients who have OSA and insomnia. However, there is no empirical evidence to support generalizing the same approach to this comorbid population. Instead, some patients might not be ready for OSA treatment prior to insomnia treatment, despite knowledge of the benefits of OSA treatment (Krakow et al., 2004). Given that sleep-maintenance insomnia predicts poor compliance with CPAP (Wickwire et al., 2010), it is possible that treating the insomnia first might improve downstream compliance with CPAP. Anecdotally, we have encountered patients in our clinic who are resistant to undergoing an overnight diagnostic study or a CPAP titration study (used to determine the approach CPAP pressure) because the prolonged awakenings in the laboratory are too distressing. Indeed, increased sleep efficiency from the diagnostic study to the titration study has been found to predict better CPAP adherence (Drake et al., 2003). Therefore, initiating CBTI prior to CPAP might be advantageous.
Alternatively, initiating CBTI and CPAP concurrently might have appeal. Targeting both disorders concurrently might yield faster relief of symptoms than a sequential approach, where patients have to wait until one phase is completed before starting the next phase. Logistically, patients might find it more convenient to initiate both CBTI and CPAP concurrently as there is increased attention and opportunities to intervene during the initial stages of treatment for both the insomnia and OSA. Given the typical patient flow in our current multidisciplinary clinic, it is fairly common to have an overlap between the timing of CBTI and CPAP.
Since the clinic setting is not conducive to making standardized decisions, the research protocol is designed to provide the rigor and structure needed to answer this important clinical question. Moreover, we selected CPAP alone (Model C) as our comparison condition because it is the current standard treatment and this design will allow us to maximize the clinical utility of our findings. If the multidisciplinary models (Model A or B) are found to be superior to the current standard treatment (Model C), the findings would more readily inform practice guidelines for treating patients with both OSA and insomnia. In essence, each treatment model in the study serves as a potential pathway for patients who present to a sleep clinic.
Improving patient care for OSA and comorbid insomnia: Research and clinical implications
As the field of sleep medicine matures with improved methods of assessment and evidence-based treatments, the goal should be to optimize delivery of these services in order to maximize patient outcomes. For patients with comorbid sleep apnea and insomnia, the challenge lies not in discovering new treatments, but uncovering more effective means of diagnosing the comorbid condition and developing appropriate treatment plans. Further research is needed to characterize the clinical profiles for those patients with OSA and comorbid insomnia. Specifically, investigating differences in patients with OSA and sleep onset insomnia versus sleep maintenance insomnia might yield more information regarding the role of insomnia phenotype and the interaction between these two disorders. Also, future studies should include a more comprehensive psychological assessment that includes measures of mood and anxiety. Better characterization can also aid in matching patients to treatments that can more effectively target their profile rather than a “one-size fits all approach” that is the current standard. Within this framework, various treatment models should be tested. In this paper, we focused on a multidisciplinary model using BSM and PAP. However, other models using PAP and hypnotics have examined the impact on PAP adherence and daytime outcomes. In a series of studies, Lettieri and colleagues examined the benefits of hypnotic medication for individuals with OSA (Lettieri, Collen, Eliasson, & Quast, 2009; Lettieri, Quast, Eliasson, & Andrada, 2008; Lettieri, Shah et al., 2009). They found that administration of eszopiclone (3mg) on the titration night improved the quality of the titration study and improved CPAP adherence when compared to placebo (Lettieri, Collen et al., 2009; Lettieri et al., 2008). In another study, they compared eszopiclone with placebo during the first 14 days of CPAP use and found greater CPAP adherence in the eszopiclone group in terms of percent of nights CPAP used (64.4% vs. 42.5%), hours per night (3.57 versus 2.42 hours), and greater decreases in sleepiness and fatigue along with greater improvement on the Functional Outcomes of Sleep Questionnaire (FOSQ) up to 6 months after treatment (Lettieri, Shah et al., 2009). Bradshaw et al (2006), compared zolpidem (10mg) versus placebo concurrent with the first 14 days of initiating CPAP at home, compared to standard treatment (no zolpidem or placebo) and found no differences between the groups on measures of sleepiness, FOSQ, or CPAP adherence. Unfortunately, these studies did not document insomnia so it is unclear if this approach would generalize to those with OSA and comorbid insomnia. Other untested multidisciplinary models include using dental sleep medicine with BSM. For example, oral appliances are often used for those with mild OSA or those whose OSA is position-related (i.e., almost exclusively when lying supine). It might be possible to combine BSM with dental sleep medicine for those with mild OSA and insomnia. Research methods using adaptive treatment designs (Murphy, Lynch, Oslin, McKay, & TenHave, 2007), might be particularly useful to identify a clinical algorithm for sequencing a treatment plan for OSA and insomnia based upon response to treatment.
Clinicians should develop a greater awareness of the comorbidity between OSA and insomnia and increased attention to treating both disorders. Unfortunately, most physicians are trained to treat the OSA and most psychologists are trained to treat the insomnia. Therefore, medical and graduate education in sleep medicine and BSM should include guidelines for evaluating both disorders and considerations for treating these comorbid conditions. Another key challenge is that community-based sleep medicine practices often do not have a BSM practitioner on site, so it is often difficult for patients who seek treatment at these clinics to receive care for both OSA and insomnia. It might be possible for these practices to align with psychology-based practices or with academic sleep centers that contain BSM practitioners.
Increased attention to the comorbidity between OSA and insomnia could also stimulate a change in the standard of practice. Although there are several practice parameter papers from the American Academy of Sleep Medicine on individual sleep disorders, none have addressed the situation with comorbid sleep disorders. As the literature on comorbid OSA and insomnia matures, it might soon become possible to develop guidelines to help clinicians and patients make informed decisions on which treatments to use and when to initiate them. Such action would bring us closer to the desired goal of personalized medicine.
Acknowledgements
We would like to thank Michael Lederman and Allison Kong for their help in data collection. Portions of the data for the pilot project at the Rush Sleep Center were presented at the Sleep 2012 meeting. Support for preparation of this paper was provided in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health (Award Number R01HL114529). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Al-Jawder SE, Bahammam AS. Comorbid insomnia in sleep-related breathing disorders: an under-recognized association. Sleep Breath. 2011 doi: 10.1007/s11325-011-0513-1. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine . The International Classification of Sleep Disorders-2. Rochester, MN: 2005. [Google Scholar]
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. American Journal of Respiratory and Critical Care Medicine. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association DSM-5 Development: Insomnia Disorder. 2012 http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=65.
- Balk E, Moorthy D, Obadan N, Patel K, Ip S, Chung M, et al. Comparative Effectiveness Review no. 32. Vol. 32. Agency for Healthcare Research and Quality; 2011. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. [PubMed] [Google Scholar]
- Ballester E, Badia JR, Hernandez L, Carrasco E, de Pablo J, Fornas C, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. American Journal of Respiratory and Critical Care Medicine. 1999;159(2):495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- Barbe F, Mayoralas LR, Duran J, Masa JF, Maimo A, Montserrat JM, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Annals of Internal Medicine. 2001;134(11):1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med. 2001;63(4):579–584. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Borak J, Cieslicki J, Szelenberger W, Wilczak-Szadkowska H, Koziej M, Zielinski J. Psychopathological characteristics of the consequences of obstructive sleep apnea prior to and three months after CPAP. Psychiatr Pol. 1994;28(3 Suppl):33–44. [PubMed] [Google Scholar]
- Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130(5):1369–1376. doi: 10.1378/chest.130.5.1369. [DOI] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22(9):1335–1350. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack NS. Sleep apnea and insomnia: sleep apnea plus or sleep apnea minus. Respiration. 2005;72(5):458–459. doi: 10.1159/000087667. [DOI] [PubMed] [Google Scholar]
- Chung KF. Insomnia subtypes and their relationships to daytime sleepiness in patients with obstructive sleep apnea. Respiration. 2005;72(5):460–465. doi: 10.1159/000087668. [DOI] [PubMed] [Google Scholar]
- Drake CL, Day R, Hudgel D, Stefadu Y, Parks M, Syron ML, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26(3):308–311. doi: 10.1093/sleep/26.3.308. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Carwile S, Miller P, Hope V, Mayti C. Psychological status, syndromatic measures, and compliance with nasal CPAP therapy for sleep apnea. Percept Mot Skills. 1994;78(3 Pt 2):1116–1118. doi: 10.2466/pms.1994.78.3c.1116. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Stepanski EJ, Olsen MK, Stechuchak KM, Carney CE, et al. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses. Results of a multitrait-multimethod analysis. Arch Gen Psychiatry. 2011;68(10):992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”. Sleep. 1996;19(5):378–381. doi: 10.1093/sleep/19.5.378. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. American Journal of Respiratory and Critical Care Medicine. 1999;159(2):461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49(3):263–266. doi: 10.1136/thx.49.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. American Journal of Respiratory and Critical Care Medicine. 2002;166(6):855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- Epstein LJ, Kristo D, Strollo PJ, Jr., Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2012;33(4):459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemons WW, Tsai W. Quality of life consequences of sleep-disordered breathing. J Allergy Clin Immunol. 1997;99(2):S750–756. doi: 10.1016/s0091-6749(97)70123-4. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Gehrman PR, Nkwuo JE, Bellamy SL, Schutte-Rodin S, Dinges DF, et al. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166(16):1732–1738. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Davis K, Huynh NT. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31(11):1527–1533. doi: 10.1093/sleep/31.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Palombini L, Poyares D, Chowdhuri S. Chronic insomnia, premenopausal women and sleep disordered breathing: part 2. Comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of chronic insomnia. J Psychosom Res. 2002;53(1):617–623. doi: 10.1016/s0022-3999(02)00463-4. [DOI] [PubMed] [Google Scholar]
- Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary rehabilitation for chronic low back pain: systematic review. BMJ. 2001;322(7301):1511–1516. doi: 10.1136/bmj.322.7301.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson-Frojmark M, Linton SJ. The course of insomnia over one year: a longitudinal study in the general population in Sweden. Sleep. 2008;31(6):881–886. doi: 10.1093/sleep/31.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales A, Caldwell AB, Cadieux RJ, Vela-Bueno A, Ruch LG, Mayes SD. Severe obstructive sleep apnea--II: Associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38(5):427–434. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Coulouvrat C, Hajak G, Roth T, Shahly V, et al. Insomnia and the Performance of US Workers: Results from the America Insomnia Survey. Sleep. 2011;34(9):1161–1171. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow B, Melendrez D, Ferreira E, Clark J, Warner TD, Sisley B, et al. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120(6):1923–1929. doi: 10.1378/chest.120.6.1923. [DOI] [PubMed] [Google Scholar]
- Krakow B, Melendrez D, Lee SA, Warner TD, Clark JO, Sklar D. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8(1):15–29. doi: 10.1007/s11325-004-0015-5. [DOI] [PubMed] [Google Scholar]
- Krell SB, Kapur VK. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005;9(3):104–110. doi: 10.1007/s11325-005-0026-x. [DOI] [PubMed] [Google Scholar]
- Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. American Review of Respiratory Disease. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- Krieger J. Long-term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and nonapneic snorers. Sleep. 1992;15(6 Suppl):S42–46. doi: 10.1093/sleep/15.suppl_6.s42. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J, Jr., et al. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: an update for 2005. Sleep. 2006;29(2):240–243. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–1037. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136(5):1263–1268. doi: 10.1378/chest.09-0811. [DOI] [PubMed] [Google Scholar]
- Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31(9):1310–1316. [PMC free article] [PubMed] [Google Scholar]
- Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Annals of Internal Medicine. 2009;151(10):696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67(3):405–410. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- Loube DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115(3):863–866. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- Luyster FS, Buysse DJ, Strollo PJ., Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2011;6(2):196–204. [PMC free article] [PubMed] [Google Scholar]
- McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. American Journal of Respiratory and Critical Care Medicine. 1999;159(4 Pt 1):1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- Meurice JC, Dore P, Paquereau J, Neau JP, Ingrand P, Chavagnat JJ, et al. Predictive factors of long-term compliance with nasal continuous positive airway pressure treatment in sleep apnea syndrome. Chest. 1994;105(2):429–433. doi: 10.1378/chest.105.2.429. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- Morin CM, Gaulier B, Barry T, Kowatch RA. Patients’ acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15(4):302–305. doi: 10.1093/sleep/15.4.302. [DOI] [PubMed] [Google Scholar]
- Murphy SA, Lynch KG, Oslin D, McKay JR, TenHave T. Developing adaptive treatment strategies in substance abuse research. Drug and Alcohol Dependence. 2007;88(Suppl 2):S24–30. doi: 10.1016/j.drugalcdep.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health State of the Science Conference Statement Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28(9):1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. Jama. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Ong JC, Gress JL, San Pedro-Salcedo MG, Manber R. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J Psychosom Res. 2009;67(2):135–141. doi: 10.1016/j.jpsychores.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JC, Kong A, Lederman M, Park M, Crisostomo MI, Cvengros JA, Wyatt JK. Developing Clinical Profiles and a Multidisciplinary Approach for Patients with OSA and Comorbid Insomnia.. Poster presented at the 26th annual meeting of the Associated Professional Sleep Societies; Boston, MA. 2012. [Google Scholar]
- Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24(5):703–715. doi: 10.1016/j.beem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63–70. doi: 10.1007/s11325-009-0281-3. [DOI] [PubMed] [Google Scholar]
- Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. American Journal of Respiratory and Critical Care Medicine. 1994;149(1):149–154. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- Sanders MH, Gruendl CA, Rogers RM. Patient compliance with nasal CPAP therapy for sleep apnea. Chest. 1986;90(3):330–333. doi: 10.1378/chest.90.3.330. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kramer J, Danko G, Volpe FR. The prevalence and clinical course of sedative-hypnotic abuse and dependence in a large cohort. American Journal of Drug and Alcohol Abuse. 2002;28(1):73–90. doi: 10.1081/ada-120001282. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. American Journal of Respiratory and Critical Care Medicine. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Smith S, Sullivan K, Hopkins W, Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med. 2004;5(5):449–456. doi: 10.1016/j.sleep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Stepanski E. Hypnotics should not be considered for the initial treatment of chronic insomnia. Journal of Clinical Sleep Medicine. 2005;1(2):125–128. [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24(4):411–417. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- Wickwire EM, Collop NA. Insomnia and sleep-related breathing disorders. Chest. 2011;137(6):1449–1463. doi: 10.1378/chest.09-1485. [DOI] [PubMed] [Google Scholar]
- Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: A clinical case series. Sleep Med. 2010;11(8):772–776. doi: 10.1016/j.sleep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Canadian Respiratory Journal. 2008;15(7):365–369. doi: 10.1155/2008/534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CM, Liao YS, Lin CM, Chou SL, Wang EN. Psychological and behavioral factors in patients with comorbid obstructive sleep apnea and insomnia. J Psychosom Res. 2011;70(4):355–361. doi: 10.1016/j.jpsychores.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New England Journal of Medicine. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. American Journal of Respiratory and Critical Care Medicine. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]