Abstract
Despite development of new therapies, metastatic colorectal cancer (mCRC) largely remains an incurable disease. Approximately 2–6% of colorectal cancers harbor NRAS mutations. The anti-VEGF antibody bevacizumab is a backbone of most therapeutic regimens; however, biomarkers predicting its activity are not known. We report two cases of mCRC with a Q61K NRAS mutation that had a favorable response to bevacizumab and the histone deacetylase inhibitor valproic acid. In contrast, none of 10 patients with wild-type NRAS or unknown NRAS status and mutated KRAS (NRAS and KRAS mutations are mutually exclusive) responded to the same regimen. These results suggest that NRAS mutation merits further investigation as a potential biomarker predicting the efficacy of bevacizumab-based treatment.
Keywords: NRAS mutation, Colorectal cancer, Bevacizumab, Q61K
Introduction
Colorectal cancer is the third most frequent cause of death from cancer in men and women in the United States, and it is estimated that more than 49,000 Americans died of this disease in 2011.[1] Metastatic colorectal cancer is not curable, except for a small proportion of patients with isolated liver metastasis, and the median overall survival usually does not exceed 2 years.[2; 3] Standard treatment options include oxaliplatin or irinotecan in combination with 5-fluorouracil/leucovorin or capecitabine. These cytotoxic drugs may be combined with targeted agents such as monoclonal antibodies cetuximab or panitumumab, which target epidermal growth factor receptor (EGFR), or the monoclonal antibody bevacizumab, which targets vascular endothelial growth factor (VEGF).[2; 3; 4] While patients with a mutated KRAS oncogene are known not to derive benefit from anti-EGFR antibodies, there is no marker predicting response to bevacizumab. As a result, bevacizumab is widely used even though only a subset of patients derive benefit from it.
Case reports
Patient 1 was a 55-year-old man who was diagnosed with rectal adenocarcinoma in April 2004. In May 2004, he underwent a low anterior resection. The final pathology reading demonstrated moderate to poorly differentiated adenocarcinoma invading through the muscularis propria with one out of two lymph nodes infiltrated by carcinoma. On imaging (computed tomography [CT] of chest, abdomen, and pelvis), there was no evidence of distant metastasis (pT2pN1M0). The patient received adjuvant chemotherapy with oxaliplatin, 5-fluorouracil, leucovorin (FOLFOX) and adjuvant external chemoradiation with concurrent continuous infusion of 5-fluorouracil. He was disease-free until May 2006, when he was diagnosed with a left hepatic lobe and small pulmonary metastases. He received palliative chemotherapy with FOLFOX in combination with bevacizumab from July to September 2006, with a partial response. In October 2006, he underwent left hepatic metastasectomy, which removed a solitary liver metastasis (moderately differentiated adenocarcinoma with clear margins) and then continued on chemotherapy with FOLFOX in combination with bevacizumab from December 2006 to March 2007. He remained progression-free until September 2007, when he was found to have enlarging pulmonary metastases. Then in October 2007, chemotherapy was initiated with irinotecan, 5-fluorouracil, leucovorin (FOLFIRI) and bevacizumab, resulting in stable disease. Because of poor tolerance his treatment was changed to capecitabine and bevacizumab in March 2008, which continued until progression of pulmonary metastases in March 2009.
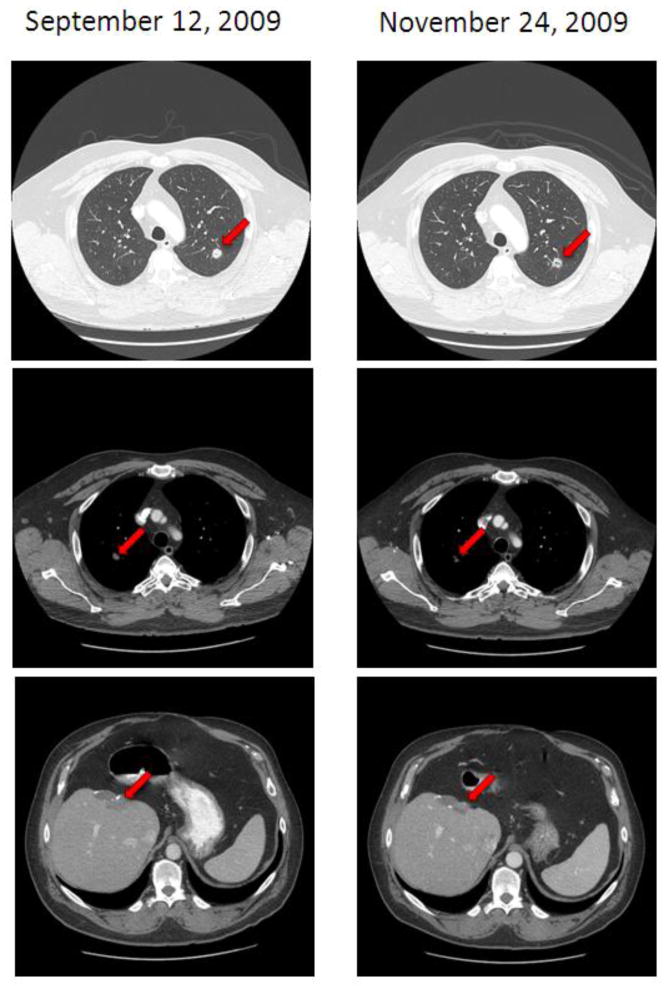
He was then referred to the Clinical Center for Targeted Therapy. The molecular profile of the tumor sample from the left hepatic metastasectomy showed wt KRAS, wt BRAF, wt EGFR, wt KIT, wt PIK3CA, intact PTEN expression and a Q61K NRAS mutation (181C>A). In April 2009, he initiated an investigational therapy with carboplatin and a nucleoside antimetabolite. His tumors were slowly growing until he was found to have unequivocal disease progression in the lungs and liver in September 2009. Then he initiated a clinical trial with the anti-VEGF monoclonal antibody bevacizumab (11 mg/kg IV on days 1 and 15 of 28 days) in combination with the histone deacetylase (HDAC) inhibitor valproic acid (5.3 mg/kg PO daily). The first restaging with CT of chest, abdomen, pelvis showed about 11% improvement per Response Evaluation Criteria in Solid Tumors (RECIST) and this was maintained for 13.5 months until November 2010 (Figure 1).[5] Western blotting of peripheral mononuclear blood cells obtained before the first study drug administration and on day 15 of cycle 1 confirmed increased histone acetylation (data not shown).
Figure 1. Imaging showing response to bevacizumab and vaproic acid in Patient 1.
The baseline CT of chest, abdomen and pelvis shows lung and liver metastases. After 8 weeks of therapy there is improvement shown by reduced density of left lung metastasis in lung windows (top scan) and a reduction in the size of right lung and liver metastases in soft tissue windows (middle and bottom picture).
Patient 2 was a 35-year-old man who was diagnosed with sigmoid adenocarcinoma in August 2005; he then underwent sigmoid resection, which revealed moderately differentiated adenocarcinoma infiltrating through the bowel, and six out of thirteen lymph nodes were infiltrated. A CT of chest, abdomen, and pelvis showed no evidence of distant metastasis (pT3pN2M0). He received 6 months of adjuvant chemotherapy with FOLFOX. He was disease-free until April 2008, when he was diagnosed with left hepatic lobe metastasis. He received palliative chemotherapy with FOLFOX in combination with bevacizumab from April to October 2008, with response shown by attenuated FDG uptake on positron emission tomography (PET). The patient then underwent regional treatment for liver metastasis with a Cyberknife. Eventually, he presented with recurrent liver metastases and resumed chemotherapy with FOLFOX in combination with bevacizumab from July to October 2009, with a response noted in a follow up FDG-PET. In November 2009, he underwent an explorative laparotomy with the intent to remove liver metastasis, but this was aborted due to a perioperative finding of peritoneal carcinomatosis. In December 2009, he initiated chemotherapy with FOLFIRI and bevacizumab, but because of poor tolerance this treatment was changed after 7 cycles to irinotecan, capecitabine (XELIRI) and cetuximab, which continued until disease progression in November 2010. Starting in January 2011, he initiated an experimental Phase I therapy with a recombinant IgG1 antibody product consisting of two antibodies against EGFR. At the time of disease progression in March 2011, he initiated treatment with a p38 mitogen-activated-protein kinase (MAPK) inhibitor and progressed on that in May 2011. Then he was referred to the Clinical Center for Targeted Therapy at MD Anderson Cancer Center. The molecular profile of the tumor sample from the primary tumor showed wt KRAS, and additional analysis from the omental metastasis revealed a Q61K NRAS mutation and V272M + R273C TP53 mutations. The tumor showed wt BRAF, wt EGFR, wt KIT, wt PIK3CA, and no aberrations in the additional 35 oncogenes tested in the panel.
In July 2011, he initiated a clinical trial with the anti-VEGF monoclonal antibody bevacizumab (11 mg/kg IV on days 1 and 15 of 28 days) in combination with the HDAC inhibitor valproic acid (5.3 mg/kg PO daily). The first restaging CT of chest, abdomen, and pelvis after 8 weeks of therapy showed a 27% improvement, and the second restaging after 16 weeks of therapy showed a 35% improvement per RECIST compared to baseline (Figure 2). He continues on therapy for 8+ months.
Figure 2. Imaging showing response to bevacizumab and vaproic acid in Patient 2.
The baseline CT of chest, abdomen and pelvis of Patient 2 shows ascites, right lung pleural based, and peritoneal metastases. After 8 weeks of therapy there is improvement in size of all lesions with resolution of ascites.
We treated an additional 10 patients, who had metastatic colorectal cancer with wt NRAS (n=3) or unknown NRAS status and mutated KRAS (n=7, KRAS and NRAS mutations are mutually exclusive), and none of the patients experienced any tumor shrinkage per RECIST. Their median progression-free survival was 1.8 months (range, 0.3–3.9 months).
Discussion
We report two cases of heavily pretreated patients with metastatic colorectal cancer and a NRAS Q61K mutation treated with anti-VEGF monoclonal antibody bevacizumab and the HDAC inhibitor valproic acid. Both patients were heavily pretreated with standard therapies including bevacizumab. Both patients demonstrated tumor shrinkage per RECIST at the time of first restaging, which lasted for more than 13 months in patient 1. Patient 2 has achieved a partial response and continued on therapy at the time of this report for 8+ months. None of the 10 patients with metastatic colorectal cancer and wt NRAS or mutated KRAS (KRAS mutations do not coexist with NRAS mutations) treated on the same regimen showed prolonged stable disease or tumor regression.
NRAS mutations occur in subsets of diverse solid tumors, with the highest prevalence (14–30%) in malignant melanoma.[6; 7; 8] NRAS mutations have been reported in 2–6% of patients with colorectal cancers and in a retrospective analysis were associated with low responses to cetuximab and chemotherapy.[6; 7; 9] NRAS belongs to a family of small G proteins with three homologues (HRAS, NRAS, KRAS) coded by different RAS gene loci. RAS activates mitogen-activated-protein kinase (MAPK) and PI3K/AKT/mTOR signaling, which are two major pathways involved in the progression of multiple cancers.[10] In vitro studies link NRAS function to antiapoptotic signaling.[11] Preclinical models in a genetically engineered mouse model demonstrated that KRAS mutations in colonic epithelium promote hyperproliferation via MEK signaling, whereas epithelial cells with NRAS mutations suppressed apoptosis.[12] In addition, preclinical models using cell line-derived xenografts with knocked out MEK expression demonstrated decreased angiogenesis and tumor growth compared to xenografts with intact MEK.[13] Therefore, it is conceivable that aberrations activating the MAPK pathway can promote angiogenesis. However, 8 of 10 patients with metastatic colorectal cancer with wt NRAS (n=3) or unknown NRAS status (n=7) had KRAS mutations in codon 12 or 13 and their outcomes on bevacizumab and valproic acid were dismal (of note, 5 of these patients also tested negative for BRAF mutations).
The role of NRAS mutations in selecting therapeutic regimens for cancer is currently unknown. Preclinical models with cell lines from different human cancers with NRAS mutations showed inhibition when treated with the MEK inhibitor CI-1040, and human colon cancer xenografts with NRAS mutations were sensitive to the MEK inhibitor AZD6244.[14; 15] In addition, preclinical data on melanoma cell lines and early clinical observations suggest that melanomas with NRAS mutations are sensitive to HSP90 inhibitors.[16; 17]
Bevacizumab is an anti-VEGF monoclonal antibody, which in most cancers has only modest activity when given as a single agent but prolongs survival in combination with chemotherapy in several metastatic cancers.[4; 18; 19] Survival prolongation is approximately 2 months and it is plausible that a small subset of patients benefit more substantially while most patients do not. Valproic acid, widely used for treating seizures, is also a HDAC inhibitor. Inhibiting histone deacetylase activity results in histone acetylation, which is associated with up-regulated gene transcription.[20] By promoting gene transcription, valproic acid induces differentiation, growth inhibition, and apoptosis.[21] Combinations of antiangiogenic agents and HDAC inhibitors are expected to have synergistic antiangiogenic activity.[22; 23; 24]
The mechanism of action explaining the salutary effect of bevacizumab in these two patients with colorectal cancer and a Q61K NRAS mutation remains unclear. First, the effect may be due to bevacizumab. Indeed these patients were previously treated on bevacizumab-containing regimens for approximately 22 and 13 months, respectively, before showing progression. However, with their prior therapy, the role of bevacizumab versus combination chemotherapy such as FOLFOX or FOLFIRI is unclear. Alternatively, it is possible that the addition of valproic acid contributed to therapeutic benefit in these cases since bevacizumab has only modest activity as single agent.[18] In theory, epigenetic modulation with valproic acid, which promotes transcription of tumor suppressor genes, may contradict the antiapoptotic effect of NRAS mutations, and in combination with bevacizumab, lead to enhanced antiangiogenesis. Alternatively, bevacizumab and valproic acid may induce autophagy to the point of autophagic cell death. Finally, bevacizumab and valproic acid may inhibit a critical, as yet unknown, pathway activated by NRAS. It is however conceivable that the therapeutic effect seen is due to bevacizumab, and the fact that single agent activity occurs in only a minority of patients with colorectal cancer is consistent with this notion, especially if NRAS mutation is a biomarker of response (since NRAS mutations occur in a minority of patients with colorectal cancer). NRAS mutations may serve as potential biomarkers to predict the anticancer effect of bevacizumab and valproic acid. Nevertheless, our observations should be interpreted with caution. Even though it is possible that NRAS mutations can lead to increased angiogenesis, they are not necessarily predictive for response to bevacizumab.
Bevacizumab is an essential component of nearly all standard-of-care regimens for metastatic colorectal cancer.[2; 4] Despite being a targeted agent, which apparently is effective in only a small subset of patients, the relevant target or biomarker predicting its activity has not been identified. To understand the underlying molecular basis of efficacy, a bedside to bench foray has to be applied and preclinical experiments in NRAS mutant colorectal cancer models need to be designed. Further, preclinical and clinical studies are warranted to investigate the role of NRAS mutation in bevacizumab-based therapies in colorectal cancer.
Acknowledgments
Financial support: Supported by Grant Number RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
All treatment and analysis were performed in accordance with MD Anderson Institutional Review Board guidelines. We thank Ms. Joann Aaron for scientific review and editing of this article.
Footnotes
Conflicts of interest: The authors have declared that no competing interests exist.
Ethical statement: All treatment and analysis were approved by MD Anderson Institutional Review Board and were conducted according to the principles expressed in the Declaration of Helsinki.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 5.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 6.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, Teague JW, Campbell PJ, Stratton MR, Futreal PA. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2010;39:D945–950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janku F, Lee JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS, Fu S, Luthra R, Garrido-Laguna I, Kurzrock R. PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PLoS One. 2011;6:e22769. doi: 10.1371/journal.pone.0022769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellerhorst JA, Greene VR, Ekmekcioglu S, Warneke CL, Johnson MM, Cooke CP, Wang LE, Prieto VG, Gershenwald JE, Wei Q, Grimm EA. Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res. 2011;17:229–235. doi: 10.1158/1078-0432.CCR-10-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S, Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff AO, Ciardiello F, Pfeiffer P, Qvortrup C, Hansen TP, Van Cutsem E, Piessevaux H, Lambrechts D, Delorenzi M, Tejpar S. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 10.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 11.Wolfman JC, Palmby T, Der CJ, Wolfman A. Cellular N-Ras promotes cell survival by downregulation of Jun N-terminal protein kinase and p38. Mol Cell Biol. 2002;22:1589–1606. doi: 10.1128/mcb.22.5.1589-1606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, Settleman J, Giovannini M, Jacks T. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006;9:33–44. doi: 10.1016/j.ccr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, Cockerill M, Cartlidge S, Smith PD. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 16.Grbovic OM, Basso AD, Sawai A, Ye Q, Friedlander P, Solit D, Rosen N. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerji U, Affolter A, Judson I, Marais R, Workman P. BRAF and NRAS mutations in melanoma: potential relationships to clinical response to HSP90 inhibitors. Mol Cancer Ther. 2008;7:737–739. doi: 10.1158/1535-7163.MCT-08-0145. [DOI] [PubMed] [Google Scholar]
- 18.Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 21.Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003;9:199–216. doi: 10.1111/j.1527-3458.2003.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, Pili R. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 23.Qian DZ, Wang X, Kachhap SK, Kato Y, Wei Y, Zhang L, Atadja P, Pili R. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64:6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. [DOI] [PubMed] [Google Scholar]
- 24.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]