Abstract
Background
There has been increasing use of various synthetic and biologically derived materials in surgery. Biologic surgical materials are used in many plastic surgery procedures, ranging from breast reconstruction to hernia repairs. In particular, acellular dermal matrix (ADM) material has gained popularity in these applications. There is a paucity of data on how ADM compares to other surgical materials as a substrate for bacterial adhesion, the first step in formation biofilm, which occurs in prosthetic wound infections. We have designed a high throughput assay to evaluate Staphylococcus aureus adherence on various synthetic and biologically derived materials.
Methods
Clinical isolates of Staphylococcus aureus (strains SC-1 and UAMS-1) were cultured with different materials and bacterial adherence was measured using a resazurin cell vitality reporter microtiter assay. Four materials that are commonly utilized in reconstructive procedures were evaluated: prolene mesh, vicryl mesh, and two different ADM preparations (AlloDerm®, FlexHD®). We were able to develop a high throughput and reliable assay for quantifying bacterial adhesion on synthetic and biologically derived materials.
Results
The resazurin vitality assay can be reliably used to quantify bacterial adherence to acellular dermal matrix material, as well as synthetic material. S. aureus strains SC-1 and UAMS-1 both adhered better to ADM materials (AlloDerm® vs. FlexHD®) than to the synthetic material prolene. S. aureus also adhered better to vicryl than to prolene. Strain UAMS-1 adhered better to vicryl and ADM materials than did strain SC-1.
Conclusion
Our results suggest that S. aureus adheres more readily to ADM material than to synthetic material. We have developed an assay to rapidly test bacterial formation on surgical materials, using two S. aureus bacterial strains. This provides a standard method to evaluate existing and new materials with regard to bacterial adherence and potential propensity for infection. This assay is particularly important in the clinical context of the severe sequelae of post-operative infection.
Keywords: acellular dermal matrix, bacterial adhesion, prosthetic infection, resazurin cell vitality assay
INTRODUCTION
Synthetic materials have revolutionized many areas of modern surgery. Over the past 15 years, biologically derived materials have gained increasing popularity and will continue to change the practice of reconstructive plastic surgery. Biologically derived materials are used in many plastic surgery procedures, ranging from breast reconstruction to abdominal wall repair. One of the first materials to gain broad application was AlloDerm® (LifeCell Corp., Branchburg, NJ), which is a patented acellular dermal matrix (ADM) originally developed in 1994 as a graft for burn patients.1,2 Acellular dermal matrix is derived from cadaveric skin after exposure to high-ionic strength solutions used to disrupt bonds between the epidermal and dermal junction. The dermis is subsequently decellularized by exposure to sodium deoxycholate resulting in a complex matrix of extracellular matrix and basement membrane containing human collagen.3,4
Since the development of AlloDerm®, numerous companies have developed similar ADM products with various claims regarding susceptibility to infection. Although the general processing of the various materials is broadly understood, there is no standardization in the processing of ADM products currently available for use. It is well accepted that the efficacy of ADM is dependent on its low antigenicity, capacity for rapid vascularization, and stability as a dermal template.5,6 To this extent, the literature demonstrates that differences in the preparation of ADM results in variable retention of dermal architectural components such as vimentin, desmin and cell associated antigens (HLA-ABC, HLA-DR).3 There have been numerous studies comparing different properties of biomaterials materials including holding strength, tissue adhesion formation and histologic tissue responses, but few have investigated how different materials withstand bacterial contamination and infection.
Bacterial colonization occurs in approximately one-third of all surgical materials, synthetic or biologically derived, with or without clinical signs of infection. This may occur even years after implantation.7 Colonization of prosthetic materials is highly dependent on successful adherence of the bacteria. The capacity for bacteria to adhere has been directly linked to the capacity to form a matrix of extracellular polymeric substance, and consequently, leads to the development of biofilm (Fig. 1).8-10 Genetic studies in a number of different bacteria indicate that matrix production is critical for bacterial adhesion to both biotic and abiotic materials.11 The changes that contribute to these functional alterations of attached microorganisms have not been elucidated, but it has been suggested that they are almost entirely induced by the surrounding environment.8,9,12,13 In general, matrix-forming microorganisms survive better on implant surfaces than non-matrix formers and therefore, extracellular matrix formation can be considered a major pathogenic property of bacteria.14,15 Bacteria in an aggregate biofilm colony may additionally have antibiotic resistance up to 1000 times that of non-matrix producing bacteria. This is attributed to a number of mechanisms, including altered metabolism, activation of toxin-antitoxin systems, and decreased diffusion of small molecules through the extracellular matix.13,16
Figure 1.
Biofilm life cycle: 1. Bacteria individual cells populate material. 2. Extracellular polymeric substance is produced and serves as a scaffolding or glue to hold biofilm together. 3. Attachment becomes irreversible. 4. Biofilm architecture develops and matures. 5. Bacteria can convert from sessile biofilm to planktonic form to seed new infections.
Numerous clinical studies have been published demonstrating that the incidence of infection with synthetic mesh depends heavily on mesh type and surgical technique applied.17-20 Although ADM material is increasingly utilized in plastic surgery, there is a paucity of information on the relative susceptibility of these biologically derived materials versus that of commonly used synthetic materials to bacterial infection.21,22 New data continues to emerge that suggests a statistically significant higher rate of seroma and infection in ADM-based breast reconstruction versus techniques without ADM.23,24 However, some contradictory data suggests that ADM material may be used safely in breast reconstruction as well as in contaminated or infected high-risk wounds.25-31
Here, we present what we believe to be the first study to quantify and characterize bacterial adhesion on different synthetic and biologically derived acellular dermal matrix materials. This bacterial adhesion assay is rapid, reproducible and lends itself to broad application for assessment of existing and emerging biomaterials.
METHODS
Bacterial strains, culture media, and growth conditions
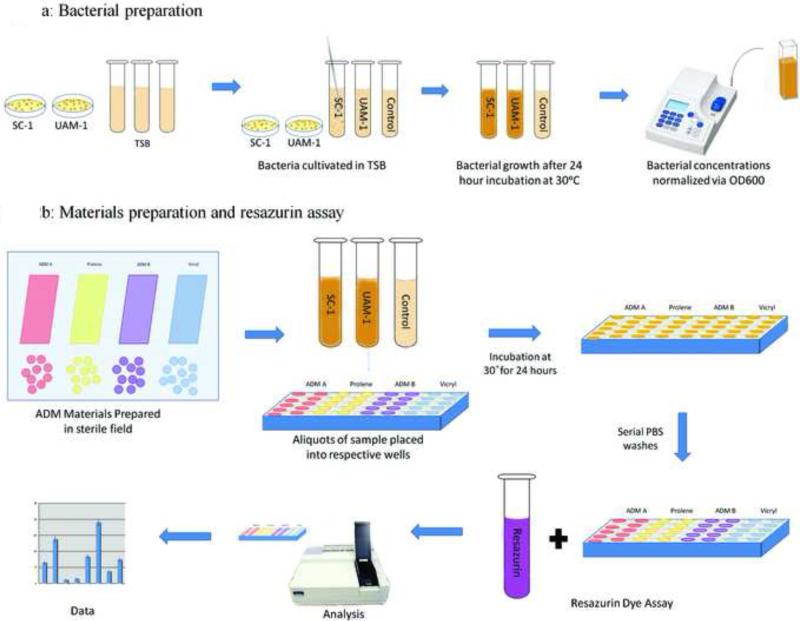
Staphylococcus aureus strains SC-1 and UAMS-1 were used. Both are originally clinical isolates and are characterized for bacterial adhesion.32,33,34,35,36 The UAMS-1 isolate was chosen specifically for its wild-type SigB stress response, unlike many domesticated lab strains of S. aureus that are derived from NCTC 8325.37 In a quantitative analysis, the SigB stress response has been shown to modulate cell wall metabolism and the expression of important adhesion molecules.38 This is likely to enhance the virulence of S. aureus strains in its host niche. Both strains were recovered from frozen stocks by growth on 3% Bacto™ tryptic soy broth (TSB; Becton, Dickinson and Co.) 1.5% Bacto agar medium at 30°C for 48 h. A single colony of each S. aureus strain was then cultivated in 2 ml of TSB at 30°C for 24 hours in rolling culture.39 This rolling culture method provides a distinct advantage over culture plates in allowing potentially adherent bacteria to remain in suspension throughout the growth phase. Following bacterial growth, 500μl each was used to determine the optical density at 600 nm (OD600) of the rolling culture. For each strain, an aliquot of the rolling culture was then diluted into TSB containing 0.5% glucose (TSBG) to an OD600 of approximately 0.025 to initiate the bacterial adhesion assay (Fig. 2).40 An additional sample of TSBG without bacteria was processed in the same manner as a negative control.
Figure 2.
Graphic representation of resazurin cell vitality assay protocol. The different biologically derived ADM (Alloderm®, FlexHD® in this study) and synthetic (prolene, vicryl) materials are placed in quadruplicates for each bacteria strain (SC1, UAMS1) and control (TSBG) cultures. The steps of the culture, washes, resazurin assay are diagrammed.
Surgical biomaterial and synthetic material preparation
For our study, the commercially available synthetic meshes prolene (polypropylene; Ethicon Inc, Somerville, NJ) and vicryl (polygalactin-910; Ethicon Inc, Somerville, NJ) were purchased. The commonly used human derived acellular dermal matrix (ADM) materials AlloDerm® (LifeCell Corp., Branchburg, NJ), and FlexHD® (MTF/Ethicon Inc., Somerville, NJ) were similarly obtained. Both ADM sheets are composed of cadaveric human dermis that has been processed and sterilized under proprietary methods. Utilizing sterile technique, multiple sterile 10-mm circular samples were punched out of each mesh or ADM sheet using a sterilized metal hole puncher. Samples were placed in a 48-well-plate (Clear 48 Multiwell Plate, BD Falcon™, Becton Dickinson and Co., Franklin Lakes, NJ) and sequentially pre-washed with sterile phosphate buffered saline (PBS) solution. Fetal calf serum (FCS) was then added to each respective material and allowed to incubate for 24 hours at 4°C. After the FCS was discarded, 500μl of each bacterial culture (preparation described above) was added to each well containing a mesh sample. On each plate for each material four additional samples without bacteria were prepared in identical manner and used as controls. Bacteria were then grown in a standing culture at 30°C for 24 hours. To assay for bacterial adherence to the mesh, the liquid culture was removed from each well and mesh materials were washed with 500 μl of PBS followed by 250 μl of PBS to remove all non-adherent bacteria.
Resazurin cell vitality assay
The resazurin dye-based cell vitality assay was used to determine the total quantity of bacteria adherent to each mesh specimen.41,42 All specimens were transferred to new 48-well-plates (Black with Clear Bottom 48 Multiwell Plate. BD Falcon™ Becton Dickinson and Co., Franklin Lakes, NJ) and washed three times with 150 μl of PBS to further remove non-adherent bacteria. After adequate removal of all supernatant, 200 μl of resazurin dye (50 ng/μl) was placed in each well and incubated at room temperature for 30 mins (Fig. 2). Using a SpectraMax M2 plate reader equipped with SoftMax Pro software (Molecular Devices), samples were excited at 550 nm and fluorescence intensities were measured at an emission of 590 nm (Fig. 3). The fluorescence values of respective mesh samples prepared without bacterial colonization served as controls. Each material mixed with bacteria was treated as an independent assay and sets of assays were done on three separate days to account for any variation introduced by the rolling culture. The fluorescence values from the four controls wells for each specific material were averaged and this average value was used as the denominator to calculate the fold-increase in resazurin fluorescence signal for each assay well with that specific material using Microsoft Excel 2008 for Mac. Increased relative fluorescence intensities above the control levels were indicative of higher S. aureus adhesion.
Figure 3.
Sample resazurin assay plate. The different biologically derived (Alloderm®, FlexHD®) and synthetic (prolene, vicryl) materials are placed in quadruplicates for each bacteria strain (SC1, UAMS1) and control (TSBG) cultures.
Statistical analysis
Statistical analyses were performed using SigmaPlot 11. The means of fold-increase in resazurin fluorescence when bacteria were present relative to the non-bacterial control wells for each strain on all materials were compared using Kruskal-Wallis ANOVA on ranks followed by Tukey's multiple comparison test set at 0.05. The fold-increase in fluorescence apparent for UAMS-1 versus SC-1 on each material was compared separately in a pair-wise manner using a t-test and differences were considered to be statistically significant if p < 0.05.
RESULTS
Use of crystal violet to stain microbial aggregates has become a standard method for quantification of the relative amount of bacteria on abiotic surfaces.13,42 Unfortunately, crystal violet effectively stains acellular dermal matrix materials (data not shown). Therefore, it was imperative to develop an alternative method for quantification of bacterial attachment to ADMs. For all four materials tested, we observed a measurable increase in the resazurin fluorescence signal when bacteria were added to the materials in medium compared to control assays lacking bacteria (Fig. 3).
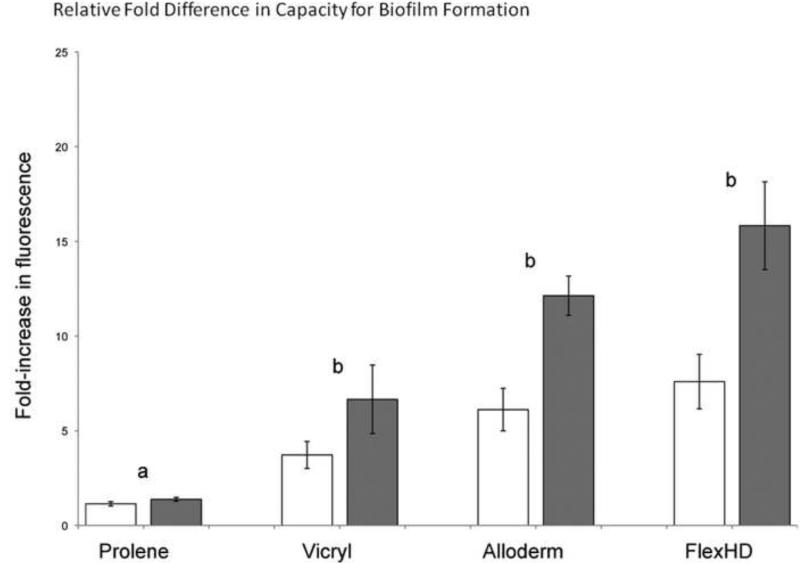
For both strains of S. aureus tested, we observed that the fold-increase in the resazurin fluorescence signal above the non-bacteria control wells was greater with the ADM products, FlexHD® and AlloDerm®, than with the non-biologic synthetic compounds vicryl and prolene (Fig. 4). For each strain, when attachment to all the materials were compared the increase in S. aureus adhesion to the ADM products over prolene© was statistically significant (ANOVA on ranks with Tukey's multiple comparison test, P<0.05). This indicates that S. aureus adhered better to the ADM materials. The trend of increased S. aureus adhesion to the ADM materials relative to vicryl did not reach statistical significance.
Figure 4.
Relative fold difference in capacity for bacterial adherence as measure using a resazurin vitality assay. S. aureus strains SC-1 (white bars) and UAMS-1 (gray bars). Letters indicate significant differences in the means (a is different from b). Error bars represent standard errors of the mean.
Based on the respective physical properties of the two synthetic materials, we hypothesized that bacteria would adhere more readily on, vicryl, a braided material, than prolene, a monofilament. 43,44,45 Of the materials tested, prolene indeed had the lowest capacity for bacterial adhesion with only a 1.14 ± 0.4 (standard deviation; SC-1) and 1.38 ± 0.35 (UAMS-1) fold increase in the fluorescence signal relative to the control (Fig. 4). In contrast, vicryl appeared to have an increased capacity to support S. aureus adhesion with 3.72 ± 2.46 (SC-1) and 6.66 ± 6.26 (UAMS-1) fold higher fluorescence values relative to the control. This difference was statistically significant (p<0.05) and suggests that among synthetic materials, differences in microscopic characteristics might influence the capacity to support a coat of serum components and subsequent bacterial adhesion.
For three of the materials, vicryl and both ADMs, the addition of the S. aureus strain UAMS-1 (gray bars in Figure 4) resulted in higher fluorescence readings than did the SC-1 strain (white bars in Figure 4). When the averaged fold-increase in the fluorescence readings for the two strains were compared only to each other on each specific material, this difference was statistically significant for the ADM materials (t-test, p < 0.05), but not for vicryl. This implies that some strains of S. aureus might bind better to acellular dermal matrix and have a greater propensity for bacterial adhesion and hence infection than others. It is possible that this difference reflects genetic variability in adhesins. This also suggests that the resazurin assay can be reliably used to identify more virulent strains of bacteria with regards to bacterial adhesion on different surgical materials.
DISCUSSION
Since the development of AlloDerm® a number of new ADM materials manufactured under variable proprietary methods have emerged. Although numerous concerns and controversies surround the risk of infection in implant-based reconstruction with ADM products, the use of ADM in reconstruction appears to be increasing.23,26-29 Given this trend, there is a critical need to develop a standard for testing ADM products currently available on the market for inherent capacity to support bacterial adhesion, which is a critical step in establishing prosthetic material-associated infection. Here we describe what we believe to be the first reliable high throughput assay for quantifying bacterial colonization of ADM via bacterial adherence. The reliability of this assay is dependent on the concentration of biofilm formed. This may decrease the sensitivity of the assay in bacteria with poor ability to form biofilm however this method of quantifying bacterial adhesion has been shown to be a good screening assay.46 Bacterial adherence on different substrates has been correlated to bacterial virulence and increased resistance to antimicrobial agents.8,9,12-15 Based on our data we have demonstrated the increased capacity of two commercially available ADM products (AlloDerm® and FlexHD®) to support bacterial attachment relative to prolene. Our data further shows a similar trend observed relative to vicryl. Beyond the individual results of our study, the methods used serves as a reliable standard to test and compare the bacterial adhesion capacity of bio-synthetic and synthetic materials used for surgical reconstruction. It is our hope that this will provide a common language to measure and discuss the risks associated with the various products that are rapidly becoming commercially available.
The association between ADM products and infections remains a highly controversial topic. The arguments for and against the use of biosynthetic materials versus older synthetic products is well supported on both sides by numerous randomized controlled studies.23-31 Although many respected authors have reported clinical findings based on their experience and case volumes there is still no consensus on the topic. Furthermore, there is a paucity of data that is based on basic scientific evidence and independent of retrospective clinical observations. In this paper we demonstrate a clear difference between bacterial adherence to ADM compounds versus a non-biologic synthetic compound. Although various authors report contradictory rates of infection this is the first study to demonstrate an association between ADM products and a known virulence factor such as surface adhesion. Given the concern for infection in ADM based reconstruction, this paper demonstrates a potential mechanism by which these materials might be predisposed to post-operative infections.
SUMMARY
The controversy over infection rates with biomaterials has many implications in surgery. Numerous questions regarding the safety of ADM produced in implant-based reconstruction persist. The current body of literature on infection rates in ADM mediated reconstruction is based heavily on contradictory retrospective clinical observations and case reports. There is a paucity of basic scientific evidence that is independent of observation and practice bias. In this study, we demonstrate the utility of the easily reproducible and reliable resazurin vitality assay to accurately quantify bacterial adherence in an in vitro assay. Based on this, we are preparing animal protocols to assess these findings with in vivo model systems. The findings presented here underscore potential concerns with using biomaterials in implant-based reconstruction without having established a standardized approach for processing and testing ADM materials.
Acknowledgements
This work was generously supported by the Plastic Surgery Educational Foundation. This work was also supported in part by a Mentored Clinical Scientist Development Award to K.P.L. (K08 AI070561) and by grant GM58213 to R.K. Strains of S. aureus graciously donated by Dr. Mark S. Smeltzer and his laboratory. E.C.L. received funding support from the American Surgical Association Research Fellowship, the March of Dimes Basil O'Connor Starter Scholar Award, and the Shriners Hospitals for Children.
Funding for this work was generously provided by the Plastic Surgery Educational Foundation.
Footnotes
DISCLOSURE
None of the authors have any commercial associations or financial interests to disclose.
REFERENCES
- 1.Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17(2):124–136. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–248. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 3.Walter RJ, Matsuda T, Reyes HM, et al. Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burns. 1998;24(2):104–113. doi: 10.1016/s0305-4179(97)00110-1. [DOI] [PubMed] [Google Scholar]
- 4.Nanchahal J, Ward CM. New graft for old? A review of alternative autologous skin. Br. J. Plastic Surg. 1992;45:354–363. doi: 10.1016/0007-1226(92)90004-h. [DOI] [PubMed] [Google Scholar]
- 5.Nolte SV, Xu W, Rennekampff HO, et al. Diversity of fibroblasts--a review on implications for skin tissue engineering. Cells Tissues Organs. 2007;187:165–76. doi: 10.1159/000111805. [DOI] [PubMed] [Google Scholar]
- 6.Wang HJ, Pieper J, Schotel R, et al. Stimulation of skin repair is dependent on fibroblast source and presence of extracellular matrix. Tissue Eng. 2004;10:1054–64. doi: 10.1089/ten.2004.10.1054. [DOI] [PubMed] [Google Scholar]
- 7.Klosterhalfen B, Klinge U, Hermanns B, et al. Pathology of traditional surgical nets for hernia repair after long-term implantation in humans. Chirurg. 2000;71(1):43–51. doi: 10.1007/s001040050007. [DOI] [PubMed] [Google Scholar]
- 8.Stoodley P, Sauer K, Davies DG, et al. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 9.Bhinu VS. Insight into biofilm-associated microbial life. J Mol Microbiol Biotechnol. 2005;10:15–21. doi: 10.1159/000090344. [DOI] [PubMed] [Google Scholar]
- 10.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: Its production and regulation. Int J Artif Organs. 2005;28:1062–8. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 11.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 12.Abele-Horn M, Schupfner B, Emmerling P, et al. “Persistent wound infection after herniotomy associated with small-colony variants of staphylococcus aureus.”. Infection. 2000;28:53–54. doi: 10.1007/s150100050014. [DOI] [PubMed] [Google Scholar]
- 13.López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OToole G, Kaplan HB. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Gristina AG, Naylor P, Myrvik Q. Infections from biomaterials and implants: A race for the surface. Med Prog Technol. 1988;14:205–24. [PubMed] [Google Scholar]
- 16.Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 17.Dunne JR, Malone DL, Tracy JK, et al. Abdominal wall hernias: Risk factors for infection and resource utilization. J Surg Res. 2003;111:78–84. doi: 10.1016/s0022-4804(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 18.Andersson B, Hallén M, Leveau P, et al. Laparoscopic extraperitoneal inguinal hernia repair versus open mesh repair: A prospective randomized controlled trial. Surgery. 2003;133:464–472. doi: 10.1067/msy.2003.98. [DOI] [PubMed] [Google Scholar]
- 19.Sohail MR, Smilack JD. Hernia repair mesh-associated mycobacterium goodii infection. J Clin Microbiol. 2004;42:2858–60. doi: 10.1128/JCM.42.6.2858-2860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumayer L, Giobbie-Hurder A, Jonasson O, et al. Open mesh versus laparoscopic mesh repair of inguinal hernia. N Engl J Med. 2004;350:1819–27. doi: 10.1056/NEJMoa040093. [DOI] [PubMed] [Google Scholar]
- 21.Brown GL, Richardson JD, Malangoni MA, et al. Comparison of prosthetic materials for abdominal wall reconstruction in the presence of contamination and infection. Ann Surg. 1985;201:705–11. doi: 10.1097/00000658-198506000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelsman AF, van der Mei HC, Ploeg RJ, et al. The phenomenon of infection with abdominal wall reconstruction. Biomaterials. 2007;28:2314–27. doi: 10.1016/j.biomaterials.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–36. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 24.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–8. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- 25.Tung CS, Zighelboim I, Scott B, et al. Human acellular dermal matrix for closure of a contaminated gynecologic wound. Gynecol Oncol. 2006;103:354–356. doi: 10.1016/j.ygyno.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: A head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1741–2. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 27.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–53. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 28.Namnoum JD, M D. Expander/Implant reconstruction with AlloDerm: Recent experience. Plastic & Reconstructive Surgery. 2009;124:387–394. doi: 10.1097/PRS.0b013e3181aee95b. [DOI] [PubMed] [Google Scholar]
- 29.Topol BM, Dalton EF, Ponn T, et al. Immediate single-stage breast reconstruction using implants and human acellular dermal tissue matrix with adjustment of the lower pole of the breast to reduce unwanted lift. Ann. Plast. Surg. 2008;61:494–499. doi: 10.1097/SAP.0b013e31816d82d9. [DOI] [PubMed] [Google Scholar]
- 30.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2007;60:1214–1218. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–25. doi: 10.1007/s00266-008-9128-8. [DOI] [PubMed] [Google Scholar]
- 32.Carbonell AM, Matthews BD, Dréau D, et al. The susceptibility of prosthetic biomaterials to infection. Surg Endosc. 2005;19:430–5. doi: 10.1007/s00464-004-8810-4. [DOI] [PubMed] [Google Scholar]
- 33.Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–78. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 34.Gillaspy AF, Hickmon SG, Skinner RA, et al. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–80. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in staphylococcus aureus limits biofilm formation. Infect Immun. 2003. 2003;71:4206–11. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goetz MB, Mulligan ME, Kwok R, et al. Management and epidemiologic analyses of an outbreak due to methicillin-resistant staphylococcus aureus. Am J Med. 1992;92:607–14. doi: 10.1016/0002-9343(92)90778-a. [DOI] [PubMed] [Google Scholar]
- 37.Cassat J, Dunman PM, Murphy E, et al. Transcriptional profiling of a staphylococcus aureus clinical isolate and its isogenic agr and sarA mutants reveals global differences in comparison to the laboratory strain RN6390. Microbiology. 2006;152:3075–90. doi: 10.1099/mic.0.29033-0. [DOI] [PubMed] [Google Scholar]
- 38.Hempel K, Pané-Farré J, Otto A, et al. Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen staphylococcus aureus via biotinylation approach. J Proteome Res. 2010;9:1579–90. doi: 10.1021/pr901143a. [DOI] [PubMed] [Google Scholar]
- 39.Johansson S, Bohman S, Radesäter AC, et al. Salmonella lipopolysaccharide (LPS) mediated neurodegeneration in hippocampal slice cultures. Neurotox Res. 2005;8:207–20. doi: 10.1007/BF03033974. [DOI] [PubMed] [Google Scholar]
- 40.Melchior MB, van Osch MH, Graat RM, et al. Biofilm formation and genotyping of staphylococcus aureus bovine mastitis isolates: Evidence for lack of penicillin-resistance in agr-type II strains. Vet Microbiol. 2009;237:83–9. doi: 10.1016/j.vetmic.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Perrot S, Dutertre-Catella H, Martin C, et al. A new nondestructive cytometric assay based on resazurin metabolism and an organ culture model for the assessment of corneal viability. Cytometry A. 2003;55:7–14. doi: 10.1002/cyto.a.10067. [DOI] [PubMed] [Google Scholar]
- 42.Xiao J, Zhang Y, Wang J, et al. Monitoring of cell viability and proliferation in hydrogel-encapsulated system by resazurin assay. Appl Biochem Biotechnol. 2010 doi: 10.1007/s12010-010-8975-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Jones DJ. Inguinal hernia repair: Which suture?. Ann R Coll Surg Engl. 1986;68:323–5. [PMC free article] [PubMed] [Google Scholar]
- 44.Leknes KN, Selvig KA, Bøe OE, et al. Tissue reactions to sutures in the presence and absence of anti-infective therapy. J Clin Periodontol. 2005;32:130–8. doi: 10.1111/j.1600-051X.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 45.Kathju S, Nistico L, Lasko LA, et al. Bacterial biofilm on monofilament suture and porcine xenograft after inguinal herniorrhaphy. FEMS Immunol Med Microbiol. 2010;59:405–9. doi: 10.1111/j.1574-695X.2010.00691.x. [DOI] [PubMed] [Google Scholar]
- 46.Sandberg ME, Schellmann D, Brunhofer G, et al. Pros and cons of using resazurin staining for quantification of viable staphylococcus aureus biofilms in a screening assay. J Microbiol Methods. 2009;78:104–6. doi: 10.1016/j.mimet.2009.04.014. [DOI] [PubMed] [Google Scholar]