Abstract
Purpose
Previous mouse studies suggesting that low-fat diets slow prostate cancer (PCa) growth often used corn oil (ω-6), which enhances PCa growth, as their primary fat. Alternatively, using a saturated-fat-based diet, we previously found no significant difference in tumor growth between low-fat- and high-fat-fed SCID mice xenografted with LAPC-4 cells. Whether similar results would hold in a castration model is unclear.
Materials and Methods
A total of 80 male SCID mice were fed a Western diet (40% fat, 44% carbohydrate) and injected with LAPC-4 human PCa cells. When tumors reached 200mm3, mice were castrated and randomized to either an isocaloric Western or low-fat diet (12% fat, 72% carbohydrate). Animals were euthanized when tumors reached 1,000mm3. Serum was collected and assayed for PSA, insulin, IGF-1, and IGFBP-3. Tumors were assayed for total- and phosphorylated-Akt levels.
Results
Mice weights were equivalent across groups. Overall, dietary group was not significantly associated with survival (log-rank, p=0.32). There were no statistically-significant differences in PSA (p=0.53), IGF-axis parameters (all p>0.05), or p-Akt:t-Akt ratios (p=0.22) between groups at sacrifice.
Conclusions
In this xenograft model, we found no difference in tumor growth or survival between low-fat- vs. Western-fed mice, when the fat source was saturated fat. Given these results conflict from those when corn oil is used in which low-fat diets have been shown to delay PCa growth, these findings suggest type of fat may be as important as amount of fat in the setting of PCa.
Keywords: prostatic neoplasms, diet, fat, insulin, IGF-1
INTRODUCTION
Prostate cancer (PCa) is the second leading cause of cancer-related death among men in Western society.1 In contrast, PCa incidence and mortality are much lower in Asian countries.2 These disparities exist despite relatively equal PCa incidence on autopsy studies.3
Some have hypothesized differences in dietary composition, particularly increased dietary fat, may partially account for this disparity.4 This concept that increased dietary fat promotes PCa growth was tested in 1995 by Wang et al, who found reducing dietary fat slowed the growth of androgen-sensitive LNCaP xenografts. Specifically, mice fed diets with 21.2% kcal/fat or less had slower tumor growth and smaller final tumors than mice fed 40.5% kcal/fat.5 In 2003, Ngo et al found severe-combined-immunodeficient (SCID) mice xenografted with LAPC-4 cells fed a low-fat diet had smaller tumors than those fed a high-fat Western-type diet.6 Similarly, in a separate study, Ngo et al found castrated SCID mice xenografted with LAPC-4 fed a low-fat diet survived longer than Western-fed mice.7
When analyzing the broad applicability of these mouse studies to humans, it is important to note that all these prior studies used corn oil (largely comprised of linoleic acid, an ω-6 fatty acid) as their primary dietary fat. Indeed, both human8 and animal9 studies suggest diets high in ω-6 fats may exert a growth-enhancing effect on PCa. As such, it is not surprising that “low-fat” diets which are reduced in growth-promoting ω-6 fat would slow PCa growth. Whether “low-fat” diets derived from alternative fats similarly slow PCa growth was recently tested by the Freedland group, which utilized a saturated-fat-based diet. In an initial study, Freedland et al found no significant difference in tumor growth between low-fat- and high-fat-fed SCID mice xenografted with LAPC-4 cells, when the dietary fat was predominantly saturated fat.10 Whether similar results (i.e. lack of benefit for a low-fat diet when the dietary fat is primarily saturated fat) would hold for a castration model is unknown. Thus, we sought to further explore this question, as well as to elucidate possible mechanisms that may contribute to any differences observed.
MATERIALS & METHODS
Cell Culture
LAPC-4 human PCa cells were a generous gift from William J. Aronson, UCLA School of Medicine. Cells were maintained in Iscove’s modified medium with 10% Fetal Bovine Serum and supplemented with the synthetic androgen R1881 at 1nM. Cells were grown in 5% CO2 at 37°C and harvested by trypsinization at ~80% confluence in log phase growth.
Animal Studies
After approval from the Duke University Institutional Animal Care and Use Committee, 80 male SCID (CB.17 scid/scid) mice, aged 8 weeks, were purchased from Taconic Farms, Inc. (Hudson, NY). Given the importance of energy balance in modulating tumor growth, all mice were housed one mouse per cage to permit precise measurements of caloric intake.11 The diets were prepared by TestDiet (Indianapolis, IN) (table 1). Animals were fed an ad libitum high-fat-diet (40% fat, 44% carb, 16% protein kcals) for a 2 week acclimation period, after which they were injected subcutaneously with 1 × 105 LAPC-4 tumor cells in 0.1mL of Matrigel (Becton Dickinson, Franklin Lakes, NJ). When tumors became palpable, tumor dimensions were measured using calipers. Tumor volumes were calculated using the formula: width × height × length × 0.5236.7 Animals were maintained on the ad lib high-fat diet until tumor size reached 200 mm3. At that time, individual mice were castrated and randomized to either a low-fat (12% fat, 72% carb, 16% protein kcals) or high-fat diet. Low-fat mice were fed ad libitum and high-fat mice fed via a modified paired-feeding protocol to maintain isocaloric intake between the groups.10 Mice were weighed twice weekly to ensure equal body weights across groups.
Table 1.
Ingredients of Experimental Diets**
| Low-fat | High-fat | |||
|---|---|---|---|---|
| Grams | % of energy | Grams | energy | |
| Fat - total | 60.0 | 12.0 | 200.3 | 40.0 |
| Corn oil | 2.9 | 0.6 | 9.5 | 1.9 |
| Milk fat | 28.6 | 5.7 | 95.4 | 19.0 |
| Lard | 28.6 | 5.7 | 95.4 | 19.0 |
| Protein | 197.1 | 15.7 | 197.1 | 15.7 |
| casein | 194.1 | 15.5 | 194.1 | 15.5 |
| DL-methionine | 3.0 | 0.2 | 3.0 | 0.2 |
| Carbohydrate | 815.7 | 72.3 | 500.0 | 44.3 |
| Dextrin | 81.57 | 7.2 | 50.0 | 4.4 |
| Maltodextrin 10 | 163.14 | 14.5 | 100.0 | 8.9 |
| Sucrose | 571 | 50.6 | 350.0 | 31.0 |
| Cholesterol | 1.5 | 0.0 | 1.5 | 0.0 |
| AIN-76 mineral mix | 35.0 | 0.0 | 35.0 | 0.0 |
| AIN-76 vitamin mix | 10.0 | 0.0 | 10.0 | 0.0 |
| Cellulose | 50.0 | 0.0 | 50.0 | 0.0 |
| Calcium carbonate | 4.0 | 0.0 | 4.0 | 0.0 |
| Choline bitartrate | 2.0 | 0.0 | 2.0 | 0.0 |
| Total grams | 1175.4 | 100.0 | 1000.0 | 100.0 |
Based upon amount of food needed to deliver 4509.75 kcal of energy
Though the experimental diets both contain a minor amount of corn oil, the great majority of fat calories come from high-saturated fat animal sources (milk-fat and lard), thus we were reasonably able to treat these diets as being effectively purely animal-fat-based in our analysis
Tumors were also measured twice weekly. At 3 weeks post-tumor-injection, mice were bled via the facial vein to measure blood glucose, using a handheld Accu-Chek Active glucometer (Roche Diagnostics, Indianapolis, IN). At this time, urine was also expressed by gentle suprapubic pressure to measure urinary ketones using Ketostix semi-quantitative urine strips (Bayer Corporation, Elkhart, IN). Animals were euthanized using a lethal dose of pentobarbital when tumors reached 1,000mm3 or when the health of the animal appeared compromised per Duke institutional criteria (ruffled fur, hunched posture, lethargy, weight loss, etc). Serum was obtained via cardiac puncture and tumor samples were snap frozen at -80°C for subsequent analysis. Serum from the median surviving 12 mice from each group (total 24 mice) were assayed for murine levels of insulin, insulin-like growth factor (IGF)-1 and IGFBP-3 using a multiplex mouse-specific enzyme-linked immunoassays (ELISA) (Millipore Corp., Billerica, MA; Diagnostics Systems Laboratory, Webster, TX; and ALPCO Diagnostics, Salem, NH; respectively) and human prostate-specific antigen (PSA), produced by the LAPC-4 xenograft, with ELISA (Abazyme, LLC., Needham, MA). Mouse tumor phospho- (p-Akt) and total-Akt (t-Akt) were determined via Western blot. Western blotting for the detection of Akt was performed using electrophoresis of 50 μg of protein on 8-16% Tris-HCL gel overnight at constant voltage, electroblotting onto nitrocellulose, sequential washings with TBST, followed by treatment with primary and secondary antibodies (Santa Cruz Biotechnology, Inc., and Cell Signaling Technology, Boston, MA) and visualized using the ECL kit (Pierce Protein Research Products, Rockford, IL).
Statistical Analysis
The primary end-point was survival, defined as time from randomization to sacrifice, which we examined using a log-rank test. Graphically, survival was represented using Kaplan-Meier curves. Comparisons in calories consumed and body weights, tumor volumes, PSA levels, and IGF-hormone levels were determined using the Wilcoxon rank-sum test. Comparisons in serum glucose and ketone levels and tumor Akt levels were performed using the t-test. Akt values were not normally distributed and thus were log-transformed prior to analysis. All statistical analyses were performed using STATA 10.0 (Stata Corp., College Station, TX) with p≤0.05 considered statistically significant.
RESULTS
Caloric Intake and Body Weight
At castration, mouse weights were equivalent across groups (p=0.55). A paired-feeding protocol allowed for isocaloric feeding between groups. High-fat-fed mice were slightly smaller on average through much of the study duration; however, this only reached statistically significance on days 52 (p=0.03) and 56 (p=0.01) (figure 1). Over the entire study period, low-fat mice fed ad libitum consumed an average of 11.5 kcal/day, whereas the pair-fed high-fat mice consumed an average of 12.1 kcal/day, a difference of approximately 5% (p=0.08). Overall, all mice consumed their prescribed diets with no observed toxicity in either group at any point.
Figure 1. Mouse Body Weights Do Not Differ Between Groups over Time*.

*each point represents the mean body weight, by group, on the given study day
Three weeks after randomization and castration, there were no significant differences between arms in either serum glucose levels (low-fat average 145 vs. high-fat average 138mg/dL, p=0.57, table 2) or urinary ketone levels (low-fat 7.6 vs. high-fat 8.1mg/dL, p=0.72, table 2).
Table 2.
Comparisons of Serum Glucose, Insulin, IGF-1, IGFBP-3, and PSA and Urinary Ketones, Showing No Significant Difference between Dietary Groups*
| Low-fat | High-fat | p-value | |
|---|---|---|---|
| Mean Serum Glucose (SD) | 145 mg/dL (17) | 138 mg/dL (43) | p=0.57 (t-test) |
| Serum Insulin (SD) | 171 ng/mL (130) | 174 ng/mL (165) | p=0.49 (rank sum) |
| Serum IGF-1 (SD) | 595 ng/mL (69) | 640 ng/mL (79) | p=0.09 (rank sum) |
| Serum IGFBP-3 (SD) | 278 ngm/mL (26) | 264 ng/mL (42) | p=0.33 (rank sum) |
| Median Serum PSA (IQR) | 20.2 ng/mL (16-37) | 37.2 ng/mL (15-61) | p=0.53 (rank sum) |
| Mean Urinary Ketones (SD) | 7.6 mg/dL (5) | 8.1 mg/dL (5) | p=0.72 (t-test) |
IQR: Interquartile Range
Serum glucose and urinary ketones collected on study day 21; Serum insulin, IGF-1, IGFBP-3, and PSA collected at the time of sacrifice.
Tumor Growth and Survival
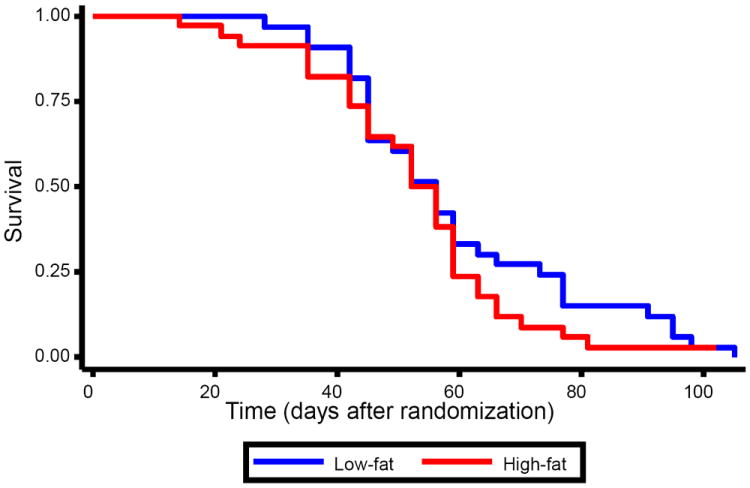
Median tumor volumes at randomization were similar in both groups (233mm3 in the LF group vs. 237mm3 in the high-fat group, p=0.99, figure 2). Throughout the study, median tumor volumes tended to be larger in the high-fat group than the low-fat group, though these differences only reached statistical significance on days 17 (p=0.02), 24 (p=0.03), and 31 (p=0.05). Though the tumor volume curves visually appeared to separate some over the last five days of the study, the p-values remained non-significant at all time points (all p≥0.78). Overall, dietary group was not significantly associated with survival (p=0.32, figure 3).
Figure 2. Mouse Tumor Volumes Do Not Differ between Dietary Groups over Time*.
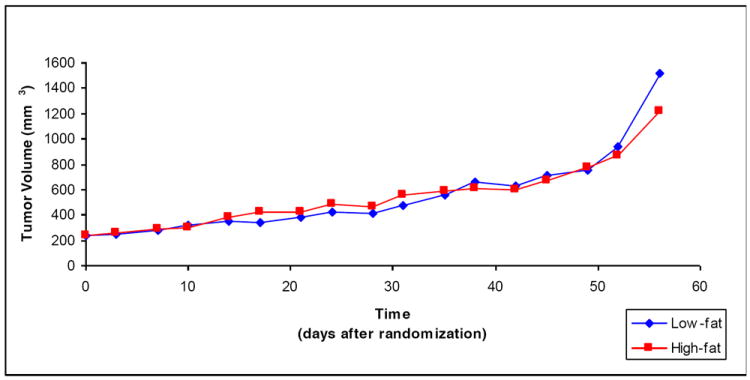
*each point represents the median tumor volume, by group, on the given study day
Figure 3. Kaplan-Meier Survival Plot Showing No Difference in Overall Mouse Survival by Dietary Group.
Serum & Tissue Analyses
The median PSA values at sacrifice were 20.2 and 37.2 ng/mL for low-fat- and high-fat-fed mice, respectively (p=0.53). Likewise, IGF-axis hormones, including serum insulin (p=0.49), IGF-1 (p=0.09), and IGFBP-3 (p=0.33) levels did not differ between groups (table 2), though insulin levels were elevated in both groups (LF=171ng/mL, W=174ng/mL) to levels similar to those of non-castrated high-fat-fed mice in a previous study.10 Furthermore, tumor tissue levels of p-Akt to t-Akt ratio were not statistically different between groups (image not shown, p=0.22).
DISCUSSION
It is often presumed dietary fat plays an important role in modulating PCa growth. Indeed, an isocaloric low-fat diet reduces LAPC-4 tumor growth in both intact and castrated xenografts, when ω-6 fatty acids are the predominant fat.6, 7 However, we previously found lowering fat content of a saturated fat-based diet did not significantly reduce tumor growth or improve survival in an intact PCa xenograft model.10 As such, in this study, we explored the effect of reduced dietary fat on PCa outcomes in a hormone-deprivation model utilizing a predominantly saturated fat diet. Mice fed the low-fat diet failed to demonstrate any difference in tumor growth, serum hormone levels, or survival compared to high-fat-fed mice. The current results combined with our prior results suggest lowering the fat content of a primarily saturated-fat diet offers little survival benefit in either an intact or castrated LAPC-4 xenograft model. Given this is in contrast to the findings when ω-6 fats are used, these results raise the possibility that type of fat may be as important as amount of fat, or perhaps even of greater importance.
In epidemiologic studies, the most consistent dietary component associated with PCa risk is fat intake.12 Indeed, per capita total fat consumption is strongly correlated with national PCa mortality rates.13 In a case-control study of 3300 Caucasian, African-American, and Asian men in the US and Canada, Whittemore et al found a positive, statistically significant association between PCa risk and total fat intake across all ethnic groups.14 Similarly, case-control studies in Spain15 and China16 have also associated higher fat consumption with increased PCa incidence. However, other studies have not identified such a connection.17, 18 Therefore, the role of dietary fat in PCa risk remains unclear.
Previous animal studies have offered mixed results regarding whether low-fat diets improve PCa survival. Wang et al’s seminal study noted decreased growth of established LNCaP tumors in nude mice fed a low-fat diet.5 Similarly, Ngo et al found lowering dietary fat content slowed LAPC-4 tumor xenograft growth in both intact and castrated SCID mice.6, 7 However, the dietary fat in these previous studies came predominantly from corn oil, a primarily ω-6 fatty acid. In contrast, our earlier study, which employed a diet based around saturated animal-derived fats, found intact SCID mice fed a low-fat diet did not demonstrate decreased tumor growth or a survival advantage versus mice fed a high-fat Western diet.10 We hypothesized the type of fat in our earlier study may have been the reason behind the lack of observed benefit. Specifically, we hypothesized that relative to ω-6 fats, saturated fat stimulated tumor growth to a lesser degree if at all. Thus, we sought to validate our experience with a saturated-fat based diet in a hormone-deprivation model.
In the current study, and similar to our results in non-castrated mice, we found feeding with an isocalorically-balanced low-fat diet did not improve survival relative to high-fat fed mice. As noted above, these results contrast with those of prior groups.5-7 Moreover, we found no differences in blood glucose and urinary ketone levels at study day 21 nor serum PSA, insulin, IGF-1, and IGFBP-3 levels and tumor p-Akt expression at sacrifice. The exact explanation for the differences between our study and those of others is not clear, though we surmise the type of fat (i.e. animal fat vs. corn oil) may be involved.
A mechanism has been proposed to explain how linoleic acid, the primary ω-6 fat in corn oil, promotes PCa growth and proliferation. Briefly, linoleic acid is converted to arachadonic acid, which can be converted by cyclooxygenase-1 and -2 to prostaglandin H2. Prostaglandin H2 is then converted to prostaglandin E2, a proinflammatory eicosinoid implicated in the promotion of cell proliferation and angiogenesis, and the inhibition of apoptosis.9 This pathway becomes particularly salient in PCa due to the relative overexpression of cyclooxygenase-2 in PCa tissue.19 Additionally, malignant prostate tissue also overexpresses lipoxygenase-5, which converts arachadonic acid to 5-hydroxyeicosatetraenoic acid, which supports the growth of androgen-sensitive and androgen-insensitive PCa cells.20 Thus, it seems plausible that lowering dietary ω-6 could decrease PCa growth and enhance survival.
However, less is known about the in vivo effect of saturated fat on PCa growth. Some epidemiologic studies suggested high saturated fat diets may increase PCa risk4 and recurrence risk.21 However, two recent studies totaling more than 220,000 subjects found no association between saturated fat intake and PCa risk.17, 18 Several hypotheses have been proposed that may explain a possible mechanism by which saturated fat could worsen PCa outcomes; these center upon effects on IGF-1, hormonal metabolism, and free-radical damage.22 However, potential confounders in the relationship between saturated fat and PCa include the correlation between consumption of heterocyclic amines (found in grilled beef, pork, chicken, lamb, and fish), which may themselves be carcinogenic, and consumption of animal-derived fat.23 Ultimately, any connection between saturated fat consumption and PCa remains unclear and requires further study.
Consumption of a high-fat diet may also contribute to worse cancer outcomes by modulating the insulin/IGF axis. Excessive dietary fat has been demonstrated to promote insulin resistance,24 potentially leading to a hyperinsulinemic state, which increases PCa development.25, 26 Moreover, in prior animal studies, we did find lower insulin and IGF-1 levels in low-fat fed intact mice.10 However, in the current study, we found uniformly elevated levels of serum insulin across the dietary groups. Thus, in the current study where there were no differences in insulin/IGF-1 levels, we saw no difference in tumor growth in contrast to prior studies where dietary changes did alter the IGF axis and did modulate tumor growth, supporting the potentially important role of the IGF axis in PCa growth.6, 26, 27 Interestingly, the levels of insulin in the current castration study were similar to levels observed in intact high-fat fed mice in a previous study.10 Indeed, it is possible the insulin resistance generated by androgen deprivation28 leads to such marked hyperinsulinemia29 that alterations in diet have little effect. If true, it would suggest that while the growth-delaying effects of low-fat diets based upon ω-6 diets in intact mouse models6 may be mediated in part by insulin and the IGF-axis, the effects in castration models may not be,7 though this requires further study.
This study is not without limitations. First, it is a single animal model and further research, both in other models and in human trials, is required to draw any generalizable conclusions. Indeed, in a recent study in LNCaP xenografts using a saturated-fat based diet, we did find a significant survival benefit for low-fat diets suggesting that not only is the amount and type of fat potentially important, but results may vary across models.27 Second, our study modeled a simplified diet in which dietary fat was provided solely from animal sources. Human dietary fat intake is more complex, as humans consume a variety of fats, including both ω-6 and saturated fats, as well as other fats (e.g. ω-3), which may themselves affect PCa outcomes.30 Thus, the applicability of these results to humans is unknown. Third, serum was harvested at the time of sacrifice, from mice with extensive disease. Thus, though we saw no difference between the low-fat and high-fat groups, our study does not exclude the possibility that differences between groups may have existed earlier, when the mice were in better health. Finally, we chose to examine survival, rather than PCa progression, in this study. Given that our data show a trend toward lower tumor volumes in the low-fat group at certain time points, it is possible that a low-fat diet may favorably impact disease progression, which we did not fully elucidate in this study. Indeed, both PCa progression and survival can be meaningful endpoints; however, we believe that survival duration offered more utility in the current study, given that survival served as the endpoint in our previous studies, and we were thus able to make more direct comparisons between the intact and castrated models.10, 27 Moreover, when examining biomarkers obtained from a cross-sectional analysis, should differences in tumor volume exist, it is difficult to determine whether differences observed between experimental groups arise secondary to the treatment intervention or result specifically due to differences in tumor size. Thus, we chose survival as a clear, fixed, dichotomous endpoint for this study.
CONCLUSION
In a PCa xenograft model, when utilizing a saturated-fat diet, reducing dietary fat content did not reduce tumor growth or prolong survival versus high-fat-fed mice. These results were associated with no differences in serum PSA, insulin, IGF-1 and IGFBP-3 levels, or tumor p-Akt levels. Notably, serum insulin levels were elevated in both groups, perhaps suggesting that the metabolic effects of castration (including insulin resistance and hyperinsulinemia) minimize the potential benefits previously observed with reduced dietary fat. These results suggest type of fat may affect PCa growth as much as, or perhaps more than, amount of fat.
Acknowledgments
Supported by the Department of Veterans Affairs; Division of Urology, Department of Surgery, Duke University; Prostate Cancer Foundation; and the National Institutes of Health Training Grant 1 TL1 RR024126.
Abbreviations
- PCa
Prostate Cancer
- SCID
Severe Combined Immunodeficient
- IGF-1
Insulin-like Growth Factor 1
- IGFBP-3
Insulin-like Growth Factor Binding Protein 3
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23:8152. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Corr JG, Thaler HT, et al. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87:1456. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 6.Ngo TH, Barnard RJ, Cohen P, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734. [PubMed] [Google Scholar]
- 7.Ngo TH, Barnard RJ, Anton T, et al. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004;64:1252. doi: 10.1158/0008-5472.can-03-3830. [DOI] [PubMed] [Google Scholar]
- 8.Godley PA, Campbell MK, Gallagher P, et al. Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemiol Biomarkers Prev. 1996;5:889. [PubMed] [Google Scholar]
- 9.Kobayashi N, Barnard RJ, Henning SM, et al. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006;12:4662. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedland SJ, Mavropoulos J, Wang A, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee P, Sotnikov AV, Mangian HJ, et al. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J Natl Cancer Inst. 1999;91:512. doi: 10.1093/jnci/91.6.512. [DOI] [PubMed] [Google Scholar]
- 12.Kolonel LN. Nutrition and prostate cancer. Cancer Causes Control. 1996;7:83. doi: 10.1007/BF00115640. [DOI] [PubMed] [Google Scholar]
- 13.Clinton SK, Giovannucci E. Diet, nutrition, and prostate cancer. Annu Rev Nutr. 1998;18:413. doi: 10.1146/annurev.nutr.18.1.413. [DOI] [PubMed] [Google Scholar]
- 14.Whittemore AS, Kolonel LN, Wu AH, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 15.Ramon JM, Bou R, Romea S, et al. Dietary fat intake and prostate cancer risk: a case-control study in Spain. Cancer Causes Control. 2000;11:679. doi: 10.1023/a:1008924116552. [DOI] [PubMed] [Google Scholar]
- 16.Lee MM, Wang RT, Hsing AW, et al. Case-control study of diet and prostate cancer in China. Cancer Causes Control. 1998;9:545. doi: 10.1023/a:1008840105531. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Murphy SP, Wilkens LR, et al. Fat and meat intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2007;121:1339. doi: 10.1002/ijc.22805. [DOI] [PubMed] [Google Scholar]
- 18.Crowe FL, Key TJ, Appleby PN, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87:1405. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura R, Sano H, Masuda C, et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589. [PubMed] [Google Scholar]
- 20.Gupta S, Srivastava M, Ahmad N, et al. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 21.Strom SS, Yamamura Y, Forman MR, et al. Saturated fat intake predicts biochemical failure after prostatectomy. Int J Cancer. 2008;122:2581. doi: 10.1002/ijc.23414. [DOI] [PubMed] [Google Scholar]
- 22.Stacewicz-Sapuntzakis M, Borthakur G, Burns JL, et al. Correlations of dietary patterns with prostate health. Mol Nutr Food Res. 2008;52:114. doi: 10.1002/mnfr.200600296. [DOI] [PubMed] [Google Scholar]
- 23.Stuart GR, Holcroft J, de Boer JG, et al. Prostate mutations in rats induced by the suspected human carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2000;60:266. [PubMed] [Google Scholar]
- 24.Akiyama T, Tachibana I, Shirohara H, et al. High-fat hypercaloric diet induces obesity, glucose intolerance and hyperlipidemia in normal adult male Wistar rat. Diabetes Res Clin Pract. 1996;31:27. doi: 10.1016/0168-8227(96)01205-3. [DOI] [PubMed] [Google Scholar]
- 25.Hsing AW, Gao YT, Chua S, Jr, et al. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:67. doi: 10.1093/jnci/95.1.67. [DOI] [PubMed] [Google Scholar]
- 26.Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 27.Mavropoulos JC, Buschemeyer WC, 3rd, Tewari AK, et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila Pa) 2009;2:557. doi: 10.1158/1940-6207.CAPR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 29.Dockery F, Bulpitt CJ, Agarwal S, et al. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 30.Berquin IM, Min Y, Wu R, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]


