Abstract
In adults, nucleoside reverse transcriptase inhibitor (NRTI)-only antiretroviral regimens (NOARs) with ≥ three NRTIs are less potent than highly active antiretroviral therapy (HAART). However published pediatric experience with NOARs is limited.
Methods
We analyzed data from NOAR-treated participants in LEGACY, a multicenter observational cohort study of HIV-infected children and adolescents. NOAR-treated case-participantswere matched to participantswithout prior NOAR who initiated HAART during the same year for comparison.
Results
Of 575 participants with data from time of HIV diagnosis through 2006, 67 (12%) received NOARs for at least 24 weeks; most (46%) received the fixed dose combination of zidovudine/lamivudine/abacavir. NOAR use peaked in 2001-2002. NOAR-treated participants were significantly older and more treatment-experienced than HAART-treated participants. Virologic outcomes, including the percentage of participants with a plasma HIV RNA viral load <400 copies/mL at week 24 (47% vs. 34%) and the mean 24-week change in log10 plasma HIV RNA viral load from baseline (−0.63 vs. −1.02) were similar between NOAR- and HAART-treated participants, but virologic rebound was more likely in NOAR-treated participants (77% vs. 54%, P = 0.02). Increase in CD4 percentage points from baseline to 24 weeks was negligible in NOAR-treated participants compared with HAART-treated participants (0.95% vs. 10.1%, P <0.001). Anemia and leukopenia were more commonly reported with NOARs than HAART.
Discussion
Week 24 virologic outcomes were similar between NOAR- and HAART-treated participants, but NOAR durability was poorer and their use was associated with less immunologic reconstitution. NOARs should play a limited role in pediatric and adolescent ART.
Keywords: Pediatric, HIV, reverse-transcriptase inhibitors, therapy
Antiretroviral treatment regimens that consist of at least three nucleoside reverse transcriptase inhibitors (NRTIs) without any other co-administered class of antiretroviral drug are termed NRTI-only regimens (NOARs). In the late 1990s, NOARs gained favor in antiretroviral-naïve HIV-infected adults as well-tolerated alternatives to more complex regimens that included protease inhibitors (PIs) or non-NRTIs (NNRTIs).[1-3] Although several clinical trials demonstrated inferior virologic suppression of NOARs compared to NRTI- or PI-based regimens,[4] NOARs can be effective as maintenance therapy started after a period of complete virologic suppression.[5-7] Nevertheless, NOARs are no longer generally recommended for adult therapy except under specific conditions, such as to avoid drug-drug interactions with rifampin-based therapy for tuberculosis.[8]
Published experience with NOARs in children is very limited, but suggests that NOARs have modest immunologic and virologic benefits among ARV-naïve children[9,10] or when used in children to maintain virologic suppression achieved with NNRTI- or PI-based regimens.[11] Since existing pediatric data are scant, the objective of this analysis was to use the LEGACY cohort of HIV-infected children and adolescents to describe the use of NOARs and relevant clinical outcomes in this population.
Methods
LEGACY study
The Longitudinal Epidemiologic Study to Gain Insight into HIV/AIDS in Children and Youth (LEGACY) study is funded by the Centers for Disease Control and Prevention (CDC) and is an observational, prospective cohort study of HIV-infected children and adolescents enrolled between birth and 24 years of age from HIV-specialty clinics across the U.S. Details of the cohort have been published elsewhere.[12]
NOAR sub-study selection criteria
We examined records from HIV-infected children and adolescents in the LEGACY cohort with abstracted medical record data from birth through December 31, 2006. We defined case-patients as participants who had been treated with a NOAR for at least 24 weeks. We defined control-patients as participants who had never received a NOAR but who had been treated with HAART, which may have included NRTIs. Our goal was to conduct a 1:1 match of control- with case-patients by the year of regimen initiation as a means of reducing bias related to changes in available medications over time. This group of case- and control-patients formed the “overall study cohort”.
Outcome measures and definitions
Our primary outcome was the percentage of participants with a VL >400 copies/mL 24 weeks after beginning therapy. This definition was consistent with the US Department of Health and Human Services criteria for virologic failure.[13] Secondary outcomes included changes in VL and CD4+ counts and CD4+ percentage points from baseline after 12, 24 and 48 weeks of therapy, the percentage of participants with rebound viremia (i.e., one or more VLs ≥400 copies/mL after previous measurements <400 copies/mL), percentage of participants with immunologic failure (i.e., a drop in CD4+ lymphocyte percentage points of at least 5 percentage points),[13] the prevalence of NRTI mutations prior to, during or after therapy, and the prevalence of potential antiretroviral-related toxicities.
The study regimen for analysis purposes for each case-patient was the initial NOAR used to treat the patient for at least a 24-week period. The study regimen for each matching control-patient was the HAART initiated closest in time to the initiation of the matching case-patient's NOAR. Study treatment duration was defined as the difference between stop and start dates for each regimen (i.e., NOAR or HAART), with all dates right censored at December 31, 2006. Values for VLs and CD4+ lymphocyte percentage points obtained within 28 days of weeks 12, 24 and 48 of treatment were used for multiple logistic regression analysis, while all available data were used for survival analyses (see Data Analysis section below). Baseline VL and CD4+ counts and percentage points were those measured closest to, but not after the start of the study regimen.
Toxicity data were not formally graded in LEGACY; clinical and laboratory abnormalities were recorded as present at each visit if indicated in the clinical record. NRTI mutations were defined according to the Stanford HIV Drug Resistance database (http://hivdb.stanford.edu/).[14]
Data analysis
For the primary outcome, we included only those participants in the overall study cohort with evaluable data within a window of 28 days before or after the date on which the participant completed 24 weeks of therapy. This was the “primary outcome cohort.” We used multiple logistic regression to examine the association of NOAR versus HAART with virologic failure. In order to avoid unstable or potentially misleading estimates, our final multivariable model included approximately one covariate for every ten case- or control-patients, whichever group was smaller.[15] We used multiple covariate selection procedures to inform covariate selection, including forward selection and backward elimination and change in the primary effect by more than 20%, regardless of the P-value for the covariate effect.[16]
We analyzed the broader overall study cohort data in several ways. We performed Cox proportional hazards analysis[17] with all available virologic data to test the cumulative percentage of overall study cohort with detectable baseline viral load who never achieved a viral load <400 copies/mL. For this analysis we did not account for rebound after the first VL <400 copies/mL.
We analyzed changes in VLs and CD4+ percentage points from baseline to completion of 12, 24 and 48 weeks of therapy as continuous variables, comparing these changes and other continuous variables by treatment group using the Student's T-test. When necessary for numerical calculations, viral loads <400 copies/mL were considered to be 400 copies/mL. Categorical variables were compared using chi square or Fisher's exact tests.
Finally, we used a trapezoidal approximation to calculate the areas under the change-from-baseline curves (AUCΔ) for both log10 VL and CD4+ percentage points from participants with at least two measurements of each variable as a more global measure of virologic and immunologic response to therapy. We compared AUCΔs by treatment group using Student's T-test.
Results
Subjects
The LEGACY cohort had complete medical record abstraction from birth through December 31, 2006 on 575 children and adolescents. Of these, 92 (16%) participants were prescribed a NOAR, and 67 were prescribed a NOAR for at least 24 weeks. This smaller subset of 67 case-participants was used for comparison with HAART-treated control-patients. We were able to identify 71 participants treated with HAART matched by year of regimen initiation to case-participants, of whom 56 were treated for at least 24 weeks and thus included in our analysis as control-patients. Characteristics of the overall study cohort of 67 case-participants and 56 control-patients are in SDC 1 (table). The primary outcome cohort with available data at week 24 comprised 36 of the 67 case-patients and 32 of the 56 control-patients, and their virologic and immunologic outcomes are included in SDC 1 (table).
For the 25 NOAR-treated case-participants who were treated with fewer than 24 weeks (168 days) of therapy, the average (Standard Deviation [SD]) duration was 73 (54) days, with a range of 4 to 167 days. These 25 participants did not differ significantly from the 67 NOAR-treated case-participants in the overall study cohort with regard to age, race/ethnicity, gender, number of treatment-naïve participants, and number of prior regimens in non-naïve participants (data not shown).
As seen in SDC 1 (table), NOAR-treated participants were significantly older and less likely to be treatment-naïve than HAART-treated participants prior to initiating the study regimen used for this analysis. The groups were similar with regard to gender, race/ethnicity, duration of NOAR/HAART, and number of VL and CD4+ samples per participant. The earliest recorded year of treatment with a NOAR was 1995. Initiation of NOARs peaked from 2000 until 2002, which were the starting years for 54% of the participants even though this window only spanned 28% of the total time frame from 1995 to 2006.
Medications
ARV regimens among both NOAR- and HAART-treated groups were highly variable in composition. There were 19 different regimens in the 67 NOAR patients. Of 67 NOAR case-patients, 63 (94.0%) received triple-drug NOAR, 3 (4.5%) received quadruple-drug NOAR, and 1(1.5%) received a quintuple-drug NOAR. The most common triple-NRTI regimens included zidovudine, lamivudine, and abacavir, separately or in combination as Trizivir® [n=42 case-patients (63%)], zidovudine, lamivudine and didanosine [n=4 (6.0%)], lamivudine, stavudine and abacavir [n=3 (4.5%)], lamivudine, didanosine and stavudine [n=3 (4.5%)], zidovudine, lamivudine and tenofovir [n=3 (4.5%)]. Eight case-participants were each treated with other triple-NRTI regimens. Quadruple-NRTI regimens included zidovudine, lamivudine, tenofovir and didanosine [n=2 case-participants (3.0%)], and zidovudine, lamivudine, abacavir, and tenofovir [n=2 (1.5%)]. A single patient received the quintuple-NRTI regimen of zidovudine, lamivudine, abacavir, didanosine and tenofovir. Overall, ten (15%) case-participants received regimens that are specifically not recommended according to the current DHHS guidelines[13]: one participant received stavudine and zidovudine, six participants received stavudine plus didanosine, and three participants received tenofovir and didanosine. These numbers were too small to assess the impact of type of NOAR on clinical outcomes.
HAART in control-patients was similarly variable. All 56 patients were prescribed three-drug regimens including two NRTIs and a either a PI [n=40 control-patients (71.4%) or an NNRTI [n=16 (28.6%]. The PIs prescribed included nefinavir [n=23 control-patients (41%)], ritonavir as a primary PI [n=4 (7.2%)], ritonavir-boosted lopinavir [n=5 (8.9%)]. ritonavir-boosted atazanvir [n=4, (7.2%)], ritonavir-boosted fosamprenavir [n=2, (3.6%)], amprenavir [n=1, (1.8%)], and indinavir [n=1, (1.8%]. The NNRTIs prescribed included efavirenz [n=8, (14.3%)], and nevirapine [n=8, (14.3%)]. Nine patients received didanosine plus stavudine as an NRTI backbone, which is not recommended according to current guidelines, as mentioned above.[13] No patients received zidovudine with stavudine, or tenofovir with didanosine.
Viral load
Virologic outcomes are summarized in SDC 1 (table). The primary cohort consisted of 36 (53%) NOAR-treated and 32 (57%) HAART-treated participants with a VL measurement available at 24 ±4 weeks. Of these, 19 (53%) NOAR-treated participants and 21 (66%) HAART-treated participants experienced virologic failure with a VL ≥400 copies/mL (P = 0.28). In the overall cohort, by Cox proportional hazards analysis, there was no significant association with having been prescribed a NOAR and achieving virologic control using all available VL data up to 96 weeks of therapy (P = 0.45).
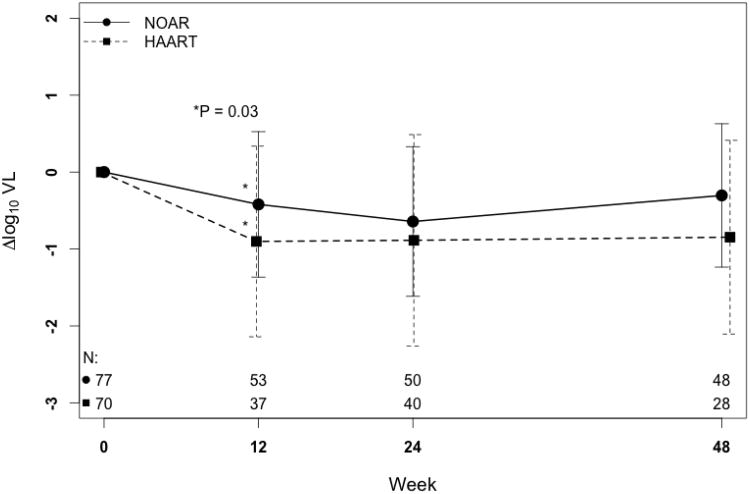
In the primary cohort, the mean change in viral load from baseline to completion of 24 weeks of treatment was -0.63 log10 copies/mL in NOAR-treated participants vs. -1.02 log10 copies/mL in HAART-treated participants (P = 0.13). Figure 1A shows that the mean decrease in viral load in the overall cohort was less in the NOAR-treated group compared with the HAART-treated group at all time points, although this difference was only statistically significant at 12 weeks: -0.47 vs. -1.02 log10 copies/mL, respectively (P = 0.027). However, as shown in SDC 1 (table), significantly more NOAR-treated participants rebounded after achieving <400 copies/mL. For NOAR-treated participants, time to rebound was shorter and their viral AUCΔ was smaller, though neither difference was statistically significant, suggesting less overall change in viral load from baseline compared with HAART-treated participants.
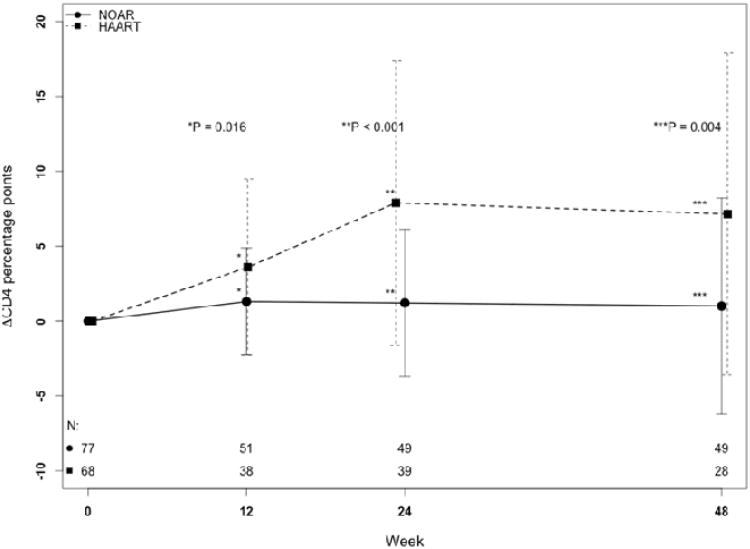
Figure 1.
Mean change in (A) log10 plasma HIV RNA viral load and (B) CD4+ lymphocyte percentage point from baseline to 48 weeks in the total study cohort after starting a nucleoside reverse transcriptase inhibitor only antiretroviral regimen (NOAR) or highly active antiretroviral therapy(HAART). Error bars indicate standard deviations. The numbers of subjects with available observations at each time (N) are indicated at the bottom of both plots, with the NOAR group on top. LEGACY cohort, 2001-2006.
(A) Note: P-values for weeks 24 and 48 are ≥ 0.05.
Factors associated with 24-week virologic failure in the primary cohort are summarized in Table 1. The odds of virologic failure were not significantly different in the NOAR-treated or HAART-treated participants by multivariate analysis controlling for any reported treatment-related toxicity, age, and baseline viral load. Younger age and higher baseline viral load were most strongly associated with the odds of virologic failure (Table 1).
Table 1.
Univariate and multivariate analysis of factors associated with virologic suppression in the primary cohort after 24 weeks treatment with a nucleoside only antiretroviral regimen (NOAR) or highly active antiretroviral therapy (HAART) among all participants with available virologic data at 24 weeks (N = 68). LEGACY cohort, 2001-2006.
| Week 24 | Week 24 | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|
| VL <400 copies/mL | VL ≥ 400 copies/mL | VL ≥ 400 copies/mL* | VL ≥ 400 copies/mL* | |||||
| n = 28 | n = 40 | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Regimen, n (%) | ||||||||
| NOAR | 17 | (47%) | 19 | (53%) | 0.58 (0.21-1.54) | 0.28 | 1.08 (0.20-3.63) | 0.90 |
| HAART | 11 | (34%) | 21 | (66%) | ||||
| Age (years) | ||||||||
| Mean (SD) [range] | 13.5 (6.1) [3-23] | 8.9 (5.8) [0-23] | 0.88 (0.80-0.96) | 0.004 | 0.89 (0.80-0.98) | 0.03 | ||
| Sex, n (%) | ||||||||
| Female | 15 | (37%) | 1822 | (55%) | 0.71 (0.27-1.87) | 0.49 | ||
| Male | 13 | (45%) | (63%) | |||||
| Race/ethnicity, n (%) | ||||||||
| Black, non- Hispanic | 17 | (40%) | 26 | (60%) | 1.02 (0.12-6.78) | 0.84 | ||
| Hispanic | 9 | (45%) | 11 | (55%) | 0.81 (0.09-6.00) | 0.98 | ||
| White, non-Hispanic/Other | 2 | (40%) | 3 | (60%) | ||||
| Baseline VL log10 copies/mL, mean (SD) | 3.52 | (1.14) | 4.41 | (1.18) | 1.92 (1.24-3.11) | 0.005 | 1.62 (0.99-2.75) | 0.06 |
| Toxicity, n (%) | ||||||||
| Any | 27 | (46%) | 32 | (54%) | 0.11 (0.008-0.88) | 0.08 | 0.12 (0.005-0.89) | 0.08 |
| None | 1 | (11%) | 8 | (89%) | ||||
| Treatment naïve at start of ART, n (%) | ||||||||
| Yes | 18 | (40%) | 27 | (60%) | ||||
| No | 10 | (43%) | 13 | (57%) | 1.15 (0.41-3.19) | 0.78 | ||
| Tenofovir-based regimen, n (%) | ||||||||
| Yes | 1 | (50%) | 1 | (50%) | 0.69 (0.03-18.01) | 0.80 | ||
| No | 27 | (41%) | 39 | (59%) | ||||
Abbreviations: SD=standard deviation; CD4+=CD4+ lymphocyte percentage; VL= HIV RNA plasma viral load
An odds ratio greater than 1 indicates increased odds of VL ≥400 copies/mL at 24 weeks, i.e. incomplete suppression of viral replication.
Percentages are row.
CD4+ lymphocyte percentage points
Immunologic outcomes are summarized in SDC 1 (table). CD4+ lymphocyte percentage points increased by a mean of 10.1% in the HAART group compared with only 0.95% in the NOAR group (P <0.001). In Figure 1B, at all time points, the NOAR-treated group experienced little change in CD4+ lymphocyte percentage points, while the HAART-treated group experienced significant gains. The number of participants who experienced immunologic failure (SDC 1, table) did not differ statistically by treatment regimen, although no HAART-treated participant experienced a CD4+ lymphocyte decline >5 percentage points at week 24. Furthermore, the CD4+ lymphocyte AUCΔ was markedly larger for HAART-treated participants.
Toxicity
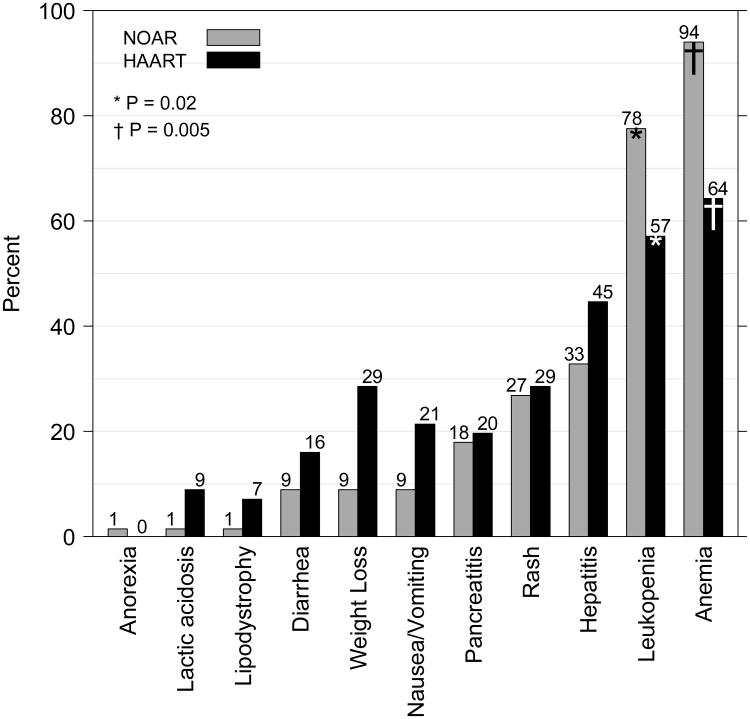
Ungraded toxicities were common among LEGACY participants. Neither causality nor the incidence of treatment-limiting toxicities was estimable from the chart abstraction. Therefore the absolute numbers and proportions of participants experiencing treatment-related, dose-limiting toxicity were presumably smaller; nonetheless, overall, at some point during treatment, 50 (75%) NOAR-treated participants experienced clinical or laboratory abnormalities, compared with 39 (70%) HAART-treated participants (P = 0.68). The most commonly reported toxicities were hematologic, as shown in Figure 2. Among participants who experienced clinical or laboratory abnormalities, anemia was reported in 94% of NOAR-treated participants versus 64% of the HAART-treated participants (P = 0.005) and leukopenia in 78% and 57%, respectively, (P = 0.02). Nausea or vomiting and weight loss were reported more frequently in HAART-treated participants but these differences were not statistically significant (P = 0.74 and P = 0.37, respectively).
Figure 2.
Percentages of nucleoside reverse transcriptase inhibitor only antiretroviral regimen (NOAR)-treated and highly active antiretroviral therapy(HAART)-treated participants with adverse events reported at any time during the treatment period. LEGACY cohort, 2001-2006.
Note: Only P-values < 0.05 are shown. P-values noted above represent comparisons between the NOAR and HAART columns for leukopenia(*) and anemia(†).
P-values for other adverse events are ≥ 0.05.
Mutations
In the LEGACY cohort genotype testing was sporadic, and it was not possible to systematically compare mutational patterns before and after treatment. However, among 83 treatment-naïve participants with no baseline genotype who were assumed to have wild-type virus, 31 (37%) subsequently started a NOAR, and 52 (63%) started HAART. At some point after treatment initiation, 40 genotypes were obtained from 40 of the 83 (48%) participants, including 13 NOAR-treated participants and 27 HAART-treated participants. and all genotypes showed at least one NRTI mutation. Therefore, among 40 participants with NRTI mutations, 13 (33%) were NOAR-treated, and 27 (67%) were HAART-treated. The proportions of NOAR- versus HAART-treated participants among those with NRTI mutations did not differ significantly from the initial distribution of treatment-naïve participants (P = 0.60), suggesting that the risk of NRTI mutation was not mitigated by HAART versus the risk incurred by a NOAR.
Discussion
The present report describes clinical experience using nucleoside-only antiretroviral regimens (NOARs) in HIV-infected children and adolescents enrolled in the LEGACY cohort. This study is a unique window on the prevalence of and outcomes associated with NOAR therapy in the pediatric population.
The use of NOARs was less common than we expected; only 12% of the cohort had been prescribed a NOAR for six months or longer. The use of NOARs declined sharply after 2002, when early reports of sub-optimal virologic efficacy in adults were first published. In this cohort, NOARs were significantly more likely to be used in older participants who were treatment-experienced. This finding may reflect the fact that the most commonly prescribed NOAR was the fixed dose combination of AZT/3TC/ABV, which is not approved for use in patients who weigh less than 40 kg, typically those below 11 years of age. The use of NOARs in treatment-experienced patients may well be a strategy of regimen simplification with partially effective treatment to overcome adherence difficulties associated with more complex HAART that was in use at that time.
A higher proportion of NOAR-treated than HAART-treated participants achieved a plasma HIV RNA viral load of <400 copies/mL after approximately 6 months of therapy, but this difference was not significant. Lower proportions of NOAR-treated compared with HAART-treated participants experienced viral suppression as seen in other measures including the 24-week reduction in viral load from baseline among all treated participants and treatment-naïve participants, and time-dependent virologic suppression measured as the area under the curve for the change in viral load, however these differences were also not significant. The percentage of participants who rebounded to a plasma HIV RNA viral load >400 copies/mL after having achieved <400 copies/mL was significantly greater for NOAR-treated than HAART-treated participants. NOAR-treated compared with HAART–treated participants also experienced shorter rebound times, however the differences were not significant. Thus, any benefits of NOARs, such as reduced pill burden, were outweighed by the inferior potency compared with HAART. This conclusion must be tempered, however, by the observation that most control-patients were treatment-naïve prior to initiating their HAART regimen, compared with approximately half of the NOAR-treated participants. Therefore, one would expect long-term virologic suppression to be biased in favor of the HAART-treated participants. Due to the limitations of retrospective sampling, we were unable to ensure that all participants had baseline screening for resistance mutations.
Immunologic benefits of NOARs were also inferior to HAART in our analysis. HAART-treated participants experienced both a greater mean increase in CD4+ lymphocyte percentage (10-fold higher than for NOAR-treated participants) and larger area under the curve for the change in CD4+ lymphocyte percentage. Moreover, the only participants who experienced a decline in CD4+ lymphocyte percentage >5% were three NOAR-treated participants. Again, conclusions about immunologic potency of NOARs may be biased by the higher proportion of treatment-experienced patients receiving NOARs and the diminishing numbers of evaluable patients with time.
Even though NOARs tended to have reduced virologic efficacy and were immunologically inferior to HAART, successful therapeutic outcomes for HAART-treated children were also less than ideal. Treatment of HIV-infected children and adolescents poses challenges distinct from those of treating adults.[18] In a meta-analysis of HAART efficacy among pediatric patients in developed countries, rates of virologic suppression to <400 copies/mL after 24 weeks of treatment ranged from approximately 45% to 85%.[19] The overall rate of 41% observed in the sample of participants we have analyzed is at the low end of this range, but consistent with a previous study of adolescents that found an adequate virologic response in only one-third of 154 patients who initiated HAART.[20] These rates compare poorly with those reported from adult cohorts of more than 10,000 patients in developed and developing countries, in which >70% of participants achieved a plasma HIV RNA viral load <400 copies/mL within 24 weeks of HAART initiation.[21,22] Indeed, in this study, increasing age was associated with improved odds of virologic suppression, highlighting the need to consider sociodemographic variables in addition to clinical outcomes when studying antiretroviral treatment issues surrounding HIV-infected pediatric patients.
The majority of both NOAR-treated and HAART-treated participants in our study experienced at least one potential treatment-related toxicity. Grading of severity or a rigorous assessment of causality would have provided a better estimate of clinically meaningful adverse events. Nonetheless, significantly more NOAR-treated participants experienced hematologic toxicities in the forms of leukopenia and anemia as expected with the greater exposure to NRTIs.
The similarity in distribution of NOAR and HAART strategies among treatment-naïve participants who subsequently developed at least one NRTI resistance mutation suggests that the addition of a PI or NNRTI did not protect the NRTI backbone. In other words, when virologic resistance developed, even participants on HAART developed NRTI mutations.
Both virologic and immunologic efficacies of NOAR are inferior to HAART and hematologic toxicity is greater. Using NOARs as partially suppressive treatment of children or adolescents who are failing therapy due to adherence difficulties remains an untested challenge. Alternatives such as lamivudine monotherapy have only been studied in adults, and their value compared to maintenance of the failing regimen is unclear. [23-26] In our small sample, the prevalence of treatment-associated NRTI resistance mutations was no worse with NOARs than HAART; nonetheless, the strategy of NOARs as partial suppression cannot be endorsed without explicit investigation due to the risk of ongoing accumulation of mutations that is likely higher than with lamivudine monotherapy.
The strengths of our study include data from a large multi-site cohort representative of HIV-infected youth receiving care in intermediate and large-sized facilities in the United States. The major limitations of this study are related to its design. Although the LEGACY data were collected prospectively from the enrolled cohort, only information in the clinical record was available for each patient. This meant that not all subjects had complete data, and therefore for this case-control study it was not possible to match on further criteria such as age or treatment regimen number and the numbers in the primary cohort and for analyses of the overall cohort beyond 24 weeks were small. These factors may have introduced biases into our analyses, although we believe that in general NOARs are inferior to HAART both virologically and immunologically in children and adolescents, just as for adults.
In conclusion, the immunologic and virologic efficacy of NOAR compared with HAART in the LEGACY cohort supports current pediatric HIV antiretroviral treatment guidelines that NOARs should not be used as initial therapy for pediatric patients who have alternative treatment options.[13] Although NOAR may result in reasonable short-term virologic response even in ART-experienced patients, the response is not sustained, does not result in comparable immunologic benefit and leads to more hematologic toxicity.
Acknowledgments
We thank investigators and abstractors at the LEGACY study sites: Edward Handelsman, Hermann Mendez, Jeffrey Birnbaum, Betsy Eastwood, Diana Mason, Ava Dennie, Gail Joseph (SUNY Downstate, Brooklyn NY*), Ninad Desai, Liberato Lao (Kings County Hospital Center, Brooklyn NY*) Andrew Wiznia, Joanna Dobroszycki, Tina Alford (Jacobi Medical Center, Bronx NY**), Lisa-Gaye Robinson, Tina Alford (Harlem Hospital, New York City NY**), Arry Dieudonne, Peggy Latortue (University of Medicine and Dentistry of New Jersey, Newark NJ*), Tamara Rakusan, Sarah McLeod, Deborah Rone (Children's National Medical Center, Washington DC**), Richard Rutstein, Ariane Adams, Rebecca Thomas, Olivia Prebus (The Children's Hospital of Philadelphia, Philadelphia PA**), George K. Siberry, Allison Agwu, Rosanna Setse, Jenny Chang (The Johns Hopkins Medical Institutes, Baltimore MD**), Steven Nesheim, Sheryl Henderson, Vickie Grimes, Julianne Gaston (Emory University, Atlanta GA**), Clemente Diaz, Francisca Cartagena (University of Puerto Rico, San Juan PR**), Delia Rivera, Dianne Demeritte (University of Miami, Miami FL**), Jose Carro, William Blouin, Vivian Hernandez-Trujillo, Marcelo Laufer, Dianne Demeritte (Miami Children's Hospital, Miami FL**), Ana Puga, Yanio Martinez (Children's Diagnostic and Treatment Center, Fort Lauderdale FL*), Patricia Emmanuel, Janet Sullivan, AJ Sikes, (University of South Florida, Tampa FL*), Mobeen Rathore, Ana Alvarez, Ayesha Mirza, Kristy Champion, Ellen Trainer (University of Florida, Jacksonville FL**), Mary E. Paul, Samuel B. Foster, Amy Leonard (Baylor College of Medicine/Texas Children's Hospital, Houston TX**), Gloria Heresi, Gabriela del Bianco (University of Texas-Houston, Houston TX**), Theresa Barton, Janeen Graper (University of Texas-Southwestern/Children's Medical Center, Dallas TX**), Toni Frederick, Andrea Kovacs, Suad Kapetanovic, Michael Neely, LaShonda Spencer, Mariam Davtyan, Uhma Ganesan (University of Southern California, Los Angeles CA**), Marvin Belzer, Molly Flaherty (Children's Hospital of Los Angeles, Los Angeles CA*), Jane Bork, Mariam Davtyan (Loma Linda University Medical Center, Loma Linda CA*), Dean A. Blumberg, Lisa Ashley, Molly Flaherty (University of California Davis Children's Hospital, Sacramento CA**).
In addition, we thank Kathy Joyce, Julie Davidson, Sharon Swanigan, Patrick Tschumper, Amanda Fournier and Kathleen Paul at Westat Inc. (Rockville MD) for contractual support, site monitoring and data management support. We also thank Vicki Peters (New York City Department of Health and Mental Hygiene) who has served as a consultant to the LEGACY project.
We are also grateful to the patients and caregivers who consented to participate in LEGACY, as well as the administrative personnel at each study site, Westat and CDC.
Contributed patient data to:
* 2006 cohort only
** All LEGACY cohorts
The LEGACY project was funded by the Centers for Disease Control and Prevention, Atlanta GA, contract number 200-2004-09976
Support: Michael Neely was supported in part by NIH-NIAID 1 K23 AI076106.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kumar PN, Rodriguez-French A, Thompson MA, et al. A prospective, 96-week study of the impact of Trizivir, Combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral-naive patients: effect of sex and ethnicity. HIV Med. 2006;7:85–98. doi: 10.1111/j.1468-1293.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 2.Staszewski S, Keiser P, Montaner J, et al. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: A randomized equivalence trial. JAMA. 2001;285:1155–1163. doi: 10.1001/jama.285.9.1155. [DOI] [PubMed] [Google Scholar]
- 3.Vibhagool A, Cahn P, Schechter M, et al. Triple nucleoside treatment with abacavir plus the lamivudine/zidovudine combination tablet (COM) compared to indinavir/COM in antiretroviral therapy-naïve adults: results of a 48-week open-label, equivalence trial (CNA3014) Curr Med Res Opin. 2004;20:1103–1114. doi: 10.1185/030079904125004006. [DOI] [PubMed] [Google Scholar]
- 4.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. New Engl J Med. 2004;350:1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 5.Clumeck N, Goebel F, Rozenbaum W, et al. Simplification with abacavir-based triple nucleoside therapy versus continued protease inhibitor-based highly active antiretroviral therapy in HIV-1-infected patients with undetectable plasma HIV-1 RNA. AIDS. 2001;15:1517–1526. doi: 10.1097/00002030-200108170-00009. [DOI] [PubMed] [Google Scholar]
- 6.Katlama C, Fenske S, Gazzard B, et al. TRIZAL study: switching from successful HAART to Trizivir (abacavir-lamivudine-zidovudine combination tablet): 48 weeks efficacy, safety and adherence results. HIV Med. 2003;4:79–86. doi: 10.1046/j.1468-1293.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 7.d'Ettorre G, Zaffiri L, Ceccarelli G, et al. Simplified maintenance therapy with abacavir/lamivudine/zidovudine plus tenofovir after sustained HIV load suppression: four years of follow-up. HIV Clin Trials. 2007;8:182–188. doi: 10.1310/hct0803-182. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Treatment of Tuberculosis: guidelines for national programmes. http://www.who.int/tb/publications/tb\_treatmentguidelines/en/index.html.
- 9.Wells C, Sharland M, Smith C, et al. Triple nucleoside analogue therapy with zidovudine (AZT), lamivudine (3TC) and abacavir (ABC) in the paediatric HIV in London South (PHILS-Net) cohort A 72 week update. Bangkok, Thailand: 2004. abstract no. TuPeB4399. [Google Scholar]
- 10.Saez-Llorens X, Nelson RP, Emmanuel P, et al. A randomized, double-blind study of triple nucleoside therapy of abacavir, lamivudine, and zidovudine versus lamivudine and zidovudine in previously treated human immunodeficiency virus type 1-infected children. The CNAA3006 Study Team. Pediatrics. 2001;107:E4. doi: 10.1542/peds.107.1.e4. [DOI] [PubMed] [Google Scholar]
- 11.Palma P, Romiti ML, Cancrini C, et al. Successful simplification of protease inhibitor-based HAART with triple nucleoside regimens in children vertically infected with HIV. AIDS. 2007;21:2465–2472. doi: 10.1097/QAD.0b013e3282f1560b. [DOI] [PubMed] [Google Scholar]
- 12.Siberry GK, Frederick T, Emmanuel P, et al. Methicillin-Resistant Staphylococcus aureus Infections in Human Immunodeficiency Virus-Infected Children and Adolescents. AIDS Res Treat. 2012;2012:627974. doi: 10.1155/2012/627974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. [Accessed 20 May 2011];2010 :1–219. Available at: http://aidsinfo.nih.gov/contentfiles/PediatricGuidelines.pdf.
- 14.Shafer RW. Rationale and uses of a public HIV drug-resistance database. J Infect Dis. 2006;194(1):S51–8. doi: 10.1086/505356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox D. Regression models and life-tables. J Roy Stat Soc B Met. 1972;34:187–220. [Google Scholar]
- 18.Garvie PA, Flynn PM, Belzer M, et al. Psychological factors, beliefs about medication, and adherence of youth with human immunodeficiency virus in a multisite directly observed therapy pilot study. J Adolesc Health. 2011;48:637–640. doi: 10.1016/j.jadohealth.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART Outcomes in Pediatric Populations: Comparison of Resource-Limited and Developed Countries. Pediatrics. 2011;127:e423–e441. doi: 10.1542/peds.2009-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding H, Wilson CM, Modjarrad K, McGwin G, Tang J, Vermund SH. Predictors of suboptimal virologic response to highly active antiretroviral therapy among human immunodeficiency virus-infected adolescents: analyses of the reaching for excellence in adolescent care and health (REACH) project. Arch Pediatr Adolesc Med. 2009;163:1100–1105. doi: 10.1001/archpediatrics.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20:2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 22.Barth RE, van der Loeff MFS, Schuurman R, Hoepelman AIM, Wensing AMJ. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 23.Castagna A, Danise A, Menzo S, Galli L, Gianotti N. Lamivudine monotherapy in HIV-1-infected patients harbouring a lamivudine-resistant virus: a randomized pilot study (E-184V study) AIDS. 2006:795–803. doi: 10.1097/01.aids.0000218542.08845.b2. [DOI] [PubMed] [Google Scholar]
- 24.Castagna A, Cossarini F, Spagnuolo V. Prior therapy influences the efficacy of lamivudine monotherapy in patients with lamivudine-resistant HIV-1 infection. J Acquir Immune Defic Synd. 2011;56:e34–5. doi: 10.1097/QAI.0b013e3181fbcc96. [DOI] [PubMed] [Google Scholar]
- 25.Opravil M, Klimkait T, Louvel S, et al. Prior therapy influences the efficacy of lamivudine monotherapy in patients with lamivudine-resistant HIV-1 infection. J Acquir Immune Defic Syndr. 2010;54:51–58. doi: 10.1097/QAI.0b013e3181bef889. [DOI] [PubMed] [Google Scholar]
- 26.Landman R, Descamps D, Peytavin G, et al. Early virologic failure and rescue therapy of tenofovir, abacavir, and lamivudine for initial treatment of HIV-1 infection: TONUS study. HIV Clin Trials. 2005;6:291–301. doi: 10.1310/9DQP-R7JA-75ED-RBCP. [DOI] [PubMed] [Google Scholar]