Abstract
CONFLICT OF INTEREST: NONE DECLARED
The aim of the study was detection of diastolic dysfunction of myocardium with Tissue Doppler Imaging (TDI) in asymptomatic type 2 diabetic patients, in five years duration of disease, and normal cardiac function on conventional echocardiography (CE), according to the performance showed on exercise stress test.
Material and Methods
We studied 300 patients, of them 150 patients with non-obese, normotensive, uncomplicated type 2 diabetes, in five years duration of disease and 150 healthy control subjects. Of all patients, 100 with type 2 diabetes, and 100 patients from the control group underwent exercise test on a treadmill. All participants underwent both CE and TDI echocardiography. With TDI, lateral E’ peak velocity, atrial velocity (A’), their ratio (E’/A’) and systolic velocity (S’) were measured. Diastolic dysfunction was diagnosed by tissue Doppler imaging, and the following criterion was met: E’/A’ ratio <1. Cardiac function with CE was without significant features in the two groups.
Results and Discussion
Using TDI interrogation, diabetic subjects showed a lower E’ velocity (10,75±1,2 vs. 14±3 cm/s, p<0,001), an increased A’ velocity (10,65±1,8 vs. 11±3 cm/s, p<0,02), and a reduced E/A ratio (0,82±0,04 vs. 1,17±1,4, p<0,001), S (8.92±3,80 vs. 9,30±3.30 cm/sec); E/A (1,17±0.55, p<0,01). In diabetic patients, after the exercise stress test performance, the myocardial velocity increase is registered for wave E’=1,27 cm/sec (12,01%), for wave A’=1,7 cm/sec (15,9%), reduced ratio E’/A’ (0.89±0,1 cm/sec 9,0%) and S’=1,3 cm/sec (14,77%). Whereas, mean myocardial velocity values in examined control group after the exercise stress test were higher as follows: E’=2,7 cm/sec (19%), A’=2,1 cm/sec (14%), E’/A’=0,8 cm/sec (12%), and S’=2,7 cm/sec (18%). Myocardial diastolic dysfunction due to reduced exercise tolerance can be evidenced by TDI in type 2 diabetic subjects, even in the presence of a normal cardiac function with CE and symptom free diabetic patients in rest. Therefore, our findings could justify the use of Tissue Doppler imaging for diastolic function assessment in diabetics with otherwise non significant features on CE.
Key words: echocardiography, diabetes mellitus, diastolic dysfunction, tissue Doppler imaging
1. INTRODUCTION
Abnormalities in diastolic function are considered to be an early sign of diabetic cardiomyopathy, and are identified in type 2 diabetic patients without systolic ventricular dysfunction as assessed by conventional methods, and also by tissue Doppler imaging (TDI) (1, 2, 3, 4, 5, 6, 7, 8). The close association of diabetes with high cardiovascular morbidity and mortality is primarily due to an increased rate of ischemic heart disease. Some authors have reported a direct effect of diabetes on the myocardium (diabetic cardiomyopathy) that can lead to congestive heart failure in the absence of coronary atherosclerosis (9, 10, 11). Prevalence of CVD especially of ischemic heart disease in diabetics is more increased when associated with: arterial hypertension, overweight, increased levels of serum lipoproteins etc. (12, 13). Therefore, tissue Doppler imaging, as a new echocardiography tool, based on measurement of wall motion velocities (1, 14, 15, 16, 17, 18), seems better suited for evaluating diastolic function, and is expected to improve the identification of diabetic patients with diastolic dysfunction and early impairment of cardiac performance (1, 19). Recent studies have reported that the addition of TDI to CE increases the ability to identify diastolic dysfunction among diabetic patients (6). In the early stage, diabetic cardiomyopathy is characterized by left ventricular diastolic dysfunction (LVDD), while left ventricular (LV) systolic function impairs later on in the clinical course of diabetes (20).
The aim of the study was to evaluate whether TDI is able to detect abnormalities of diastolic function by using exercise stress test in type 2 diabetic subjects even in the presence of a normal cardiac function with CE and symptom free in rest.
2. MATERIAL AND METHODS
2.1. Subjects
Three hundred subjects of both genders were studied; the study population was selected from two groups of subjects: 150 non-obese, normotensive, uncomplicated type 2 diabetic patients of average age of 50,5±10, in five years duration of disease and the control group which consisted of 150 non-diabetic subjects, of average age of 47,50±8,5, recruited from healthy volunteers. From the study were excluded patients with HTA, obesity, acute ischemic disease, heart failure, heart defects and pulmonary obstructive disease. The diagnosis of diabetes was established according to current World Health Organization criteria (21). Of all subjects, 100 patients with type 2 diabetes, and 100 subjects from the control group performed the same symptom-limited graded exercise test on a treadmill. Bruce treadmill protocol was applied to all patients. Time in seconds on the treadmill was used to evaluate exercise capacity, and the number of metabolic equivalents (MET-s) was estimated using appropriate equations. Blood pressure was recorded with a manual mercury sphygmoma-nometer and heart rate was recorded every 3 minutes during exercise. Raw data and an average 12- lead electrocardiogram monitored cardiac status during the exercise test. Cardiologists blinded to echocardiography diagnoses supervised all exercise tests.
2.2. Echocardiography
Conventional echocardiography. Standard and pulsed wave Doppler echocardiograms were obtained in all diabetic patients and healthy volunteers. All subjects were examined in the left lateral decubitus position, using a commercially available ultrasound system Phillips I/E 33 (Bothell, WA, USA) S5-1 phased-array transducer with M-mode, two-dimensional, pulsed and continuous wave, color-flow, and tissue Doppler capabilities. Measurements of the different cardiac chambers were made according to recommendations of the American Society of Cardiology (21).
Tissue Doppler imaging. Color TDI images were obtained from an apical window, utilizing the 4-chamber and 2-chamber orientations. For prevention of additional artifacts by total cardiac motion during breathing, image acquisition performance has been done during apnea. The image angle was adjusted to ensure a parallel alignment of the sampling window. Early (E) and late (A) diastolic myocardial velocities were obtained and their ratio was derived. The TDI signals were recorded at a speed of 100 mm/s. The velocity profiles were recorded with a sample volume of < 5 mm placed at the lateral corner of mitral annulus, according to recommendations of the American Society of Echocardiography. An average of 3 to 5 consecutive cardiac cycles was taken for the calculation of all echo-Doppler parameters. Diastolic dysfunction was diagnosed by tissue Doppler imaging according to the guidelines of the European Study Group on Diastolic Heart Failure (21), and the following criterion was met: E’/A’ ratio <1.
A team of two experienced sonographers performed all echocardiography measurements. All echocardiography examinations were recorded for offline analysis by a second team of two investigators blinded to the patient’s diagnosis. The study was approved by the Local Ethics Committee and informed consent was obtained from all participants.
2.3. Statistical analysis
Statistical analysis was performed with SPSS for Windows version 11.5 (SPSS Inc., Chicago, IL, USA). Results are given as mean ±SD or number. Means were compared by unpaired Student’s t-test. A P value < 0,5, two-sided, was considered statistically significant.
3. RESULTS
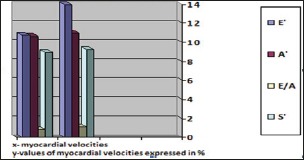
Diabetic subjects, were treated with an association between gliclazide 1-3 mg plus or/and metformin 500-1000 mg, b.d. The median of diabetes duration was 5.0 years. Parameters of both diastolic and systolic function assessed with CE were similar or non significant in diabetic and non diabetic subjects. However, at TDI recordings were registered approximate values between two groups. The echocardiography characteristics of the two groups before the exercise stress test are listed in Table 1.
Table 1.
Tissue Doppler Echocardiography caracteristics of both groups before the stress test
| Parameters | Diabetic patients | Control group | P |
|---|---|---|---|
| E’ (cm/sec) | 10,75±1,2 | 14±3 | P<0,01 |
| A (cm/sec) | 10,65±1,8 | 11±3 | P<0,02 |
| E’/A’ | 0,82+0,04 | 1,17±0,55 | P<0,01 |
| S’ (cm/sec) | 8,93±3,72 | 9,30±3,30 | N.S |
E’ velocity (10,75+1,2 vs. 14±3 cm/s, p<0,01), A’ velocity (10,65±1,8 vs. 11±3 cm/s, p<0,02), E/A ratio (8.93±3.72 vs. 1,17±0,55, 0,01), S’(8,93±3,72 cm/sec vs. 9,30±3,30).
The performance of exercise stress test has showed in diabetics, a lower increase of E’ velocity (12,02±1,6 cm/sec vs. 16,7±1,3 cm/s, p<0,01), slight increase of A’ velocity (12,35±1,8 cm/sec vs. 13,1±1,2 cm/sec and reduced E’/A’ ratio (0,89±0,1 vs. 1,8±1,2, p<0,01) compared with control subjects Table 2.
Table 2.
Tissue Doppler Echocardiography characteristics of both groups after the stress test
| Parameters | Diabetic patients | Control group | P |
|---|---|---|---|
| E’ (cm/sec) | 12,02±1,6 | 16,7±1,3 | P<0,01 |
| A’ (cm/sec) | 12,35±1,8 | 13,1±1,2 | P<0,02 |
| E’/A’ | 0.89±0,1 | 1,8±1,2 | P<0,01 |
| S’ (cm/sec) | 10,22±0,95 | 12.92±1,2 | P<0,01 |
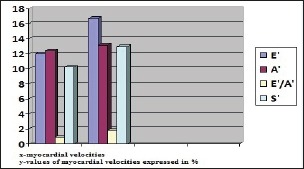
In diabetic patients, after the exercise stress test performance, the mean values of myocardial velocity increase are registered for: wave E’=2,7cm/sec (19%),, for A’=1,7cm/sec (15,9%), for E’/A’=0,8cm/sec (12%) and S’=1,3cm/sec (14,77%), (Figure 2).
Figure 2.
4. DISCUSSION
This article emphasizes high clinical relevance of the detection of left ventricular diastolic dysfunction in type 2 diabetics based on tissue Doppler imaging criteria.
In our study, impairment of diastolic function has been detected by means of TDI. Performing the exercise stress test in type 2 diabetic subjects with five years duration of disease, and normal LV function at CE, with TDI interrogation we managed to unmask the presence of diastolic dysfunction in asymptomatic diabetics, underscoring its relation to reduced exercise tolerance.
In contrast, classical criteria based on CE do not seem to share the same ability. In addition, this abnormality seems to be related to the diabetic cardiomyopathy. It is likely that metabolic abnormalities may play a major role. Experimental data from animal models of diabetes strongly support a causal role of insulin resistance in the development of diastolic dysfunction. Treatment with metformin prevented the development of cardiomyocite dysfunction (22, 23). In an insulin-resistant pre-diabetic rat model, Mizushige et al. observed that the abnormalities of diastolic filling occurred before the development of frank hyperglycaemia.
Histopathology studies evidenced increased myocite fibrosis and collagen deposition, suggesting that these structural alterations play an important role in the development of diastolic dysfunction (24). Another factor that may impair diastolic function is hyperglycaemia. There is experimental evidence that short-term hyperglycemia is able to alter cardiomyocite contraction and relaxation (25). In addition, high glucose concentration causes the formation of advanced glycation endproducts (AGE) that alter collagen structure (26) and interfere with intracellular calcium handling (27). In humans, Fang and colleagues found an increased myocardial fibrosis in diabetic patients with cardiac dysfunction (28). Collectively, these data indicate that the diabetic milieu, i.e. insulin resistance and hyperglycaemia, is able to induce functional and structural changes of cardiomyocites, which lead to progressive deterioration of regional and global diastolic dynamics.
The prevalence of LVDD in type 2 diabetes is variable, ranging from 16% in normoalbuminuric diabetic patients in the Strong Heart Study (29) to 60% in well-controlled type 2 diabetic men (30). To identify the earliest abnormalities, the impact of diabetes per se on the diastolic function, and to rule out the impact of effort on diabetes, we examined a homogeneous group of non-obese, nor-motensive, uncomplicated type 2 diabetic patients with very short duration of disease (median one year). The reduced exercise performance in our studied diabetic subjects with diastolic dysfunction recognized by TDI suggests a potential clinical application for combined TDI and stress test application, in the assessment of diastolic function in diabetics apparently free from heart disease.
Such a finding might as well serve as stimulus for the development of strategies aimed at correcting the causal mechanisms and preventing the progression from tissue Doppler imaging abnormalities to more advanced structural derangements and, ultimately, to symptomatic heart disease.
5. CONCLUSION
Myocardial diastolic dysfunction due to reduced exercise tolerance can be unmasked by TDI in type 2 diabetic patients, even in the presence of a normal cardiac function with CE and symptom free state. Tissue Doppler imaging application might justify routine screening for diastolic dysfunction in diabetic patients presumed to have healthy hearts.
List of Abbreviations
A–atrial velocity, CE–conventional echocardiography, CVD–cardiovascular disease, E–early velocity, LV– left ventricle, LVDD–left ventricular diastolic dysfunction, S–systolic velocity, TDI–tissue Doppler imaging
Figure 1.
REFERENCES
- 1.Carlos A. Roldan. The Ultimate Echo Guide. Lippincot Williams & Wilkins, (Second edition). 2012; 76-81. [Google Scholar]
- 2.Nyström L., Ostman J., Wall S., et al. Mortality of all incident cases of diabetes mellitus in Sweden diagnosed 1983-1987 at age 15-34 years. Diabetes Incidence Study in Sweden (DISS) Group Diabetic Medicine. Diab Med. 1992; 9(5): 422-427. [DOI] [PubMed] [Google Scholar]
- 3.Paillole C, Dahan M, Paycha F., et al. Prevalence and significance of left ventricular filling abnormalities determined by Doppler echocardiography in young type 1 (insulin-dependent) diabetic patients. Am J Cardiol. 1989; 64: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 4.Gorani D, Zahiti B, Dragusha G., et al. Early detection of Diastolic dysfunction in type 2 diabetics with short duration of disease using tissue Doppler imaging. HealthMED. 2010; 4(4, Suppl. 1): 1107-1112. [Google Scholar]
- 5.Shapiro LM, Howat AP, Calter MM. Left ventricular function in diabetes mellitus: Methodology and prevalence and spectrum of abnormalities. Br Heart J. 1981; 45: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarich SW, Arbuckle BE, Cohen LR., et al. Diastolic abnormalities in young asymptomatic diabetic patients assessed by pulsed Doppler echocardiography. J Am Coll. Cardiol. 1988; 12: 114-120. [DOI] [PubMed] [Google Scholar]
- 7.Vinereanu D, Nicolaides E, Tweddel AC., et al. Subclinical left ventricular dysfunction in asymptomatic patients with type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci 2003; 105: 591-599. [DOI] [PubMed] [Google Scholar]
- 8.Boyer JK, Thanigaraj S, Schechtman KB., et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004; 93: 870–875. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham Study. Diabetes Care. 1979; 2: 120-126. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Epidemiology Research International (DERI) Mortality Study Group: International evaluation of cause-specific mortality and IDDM. Diabetes Care. 1991; 14: 55-60. [DOI] [PubMed] [Google Scholar]
- 11.Galderisi M, Anderson KM, Wilson PWF., et al. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol. 1991; 68: 85-89. [DOI] [PubMed] [Google Scholar]
- 12.Mark KH, Faxon DP. Clinical studies on coronary revascularization in patients with type 2 diabetes. Eur Heart J. 2003; 24: 1087-1103. [DOI] [PubMed] [Google Scholar]
- 13.Gibbson RJ, Balady GJ, Beasley JW., et al. ACC/AHA Guidelines for Exercise testing. J. Am Coll Cardiol. 1997; 30: 260-311. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland GR, Stewart MJ, Groundstroem KW., et al. Colour Doppler myocardial imaging: a new technique for the assessment of myocardial function. J Am Soc Echocardiogr. 1994; 23: 1441—1458. [DOI] [PubMed] [Google Scholar]
- 15.Donovan CL, Armstrong WF, Bach DS. Quantitative Doppler tissue imaging of the left ventricular myocardium: validation in normal subjects. Am Heart J. 1995; 130: 100-104. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez L, Garcia M, Ares M., et al. Assessment of mitral annular dynamics during diastole by Doppler tissue imaging: comparison with mitral Doppler inflow in subjects without heart disease and in patients with left ventricular hypertrophy. Am Heart J. 1996; 131: 982-987. [DOI] [PubMed] [Google Scholar]
- 17.Dokainish H. Tissue Doppler imaging in the evaluation of left ventricular diastolic function. Curr Opin Cardiol., 2004; 19: 437-441. [DOI] [PubMed] [Google Scholar]
- 18.Khouri SJ, Maly GT, Suh DD., et al. A practical approach to the echocardiographic evaluation of diastolic function. J. Am.Soc Echocardiogr. 2004; 17: 290-297. [DOI] [PubMed] [Google Scholar]
- 19.Quinines MA, Otto CM, Stoddard M., et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002; 15: 167-184. [DOI] [PubMed] [Google Scholar]
- 20.Di Bonito P, Cuomo S, Moio N., et al. Diastolic dysfunction in patients with Type 2 diabetes of short duration. Diabet Med. 1996; 13: 321-324. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization: Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Organization, 1999: 1112. [Google Scholar]
- 22.Schiller NB, Shah PM, Crawford M, et al. : Recommendations for quantification of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989; 2: 358-367. [DOI] [PubMed] [Google Scholar]
- 23.European Study Group on Diastolic Heart Failure : How to diagnose diastolic heart failure. Eur Heart J. 1998; 19: 990-1003. [DOI] [PubMed] [Google Scholar]
- 24.Schneider SH, Amorosa LF, Khachadurian AK., et al. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non-insulin dependent) diabetes. Diabetologia. 1984; 26: 355-360. [DOI] [PubMed] [Google Scholar]
- 25.Vanoverschelde JJ, Essamri B, Vanbutsele R., et al. Contribution of left ventricular diastolic function to exercise capacity in normal subjects. J Appl Physiol. 1993; 74: 2225-2233. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Davidoff AJ. Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocites. Am J Physiol. 1997; 272: H148-158. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Masurekar MR, Vatner DE., et al. Gycation endproduct cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am J Physiol. 2003; 285: H2587–2591. [DOI] [PubMed] [Google Scholar]
- 28.Bidasee KR, Zhang Y, Shao CH., et al. Diabetes increases formation of advanced glycation end products on Sarco (endo) plasmic reticulum Ca2+-ATPase. Diabetes. 2004; 53: 463-473. [DOI] [PubMed] [Google Scholar]
- 29.Fang ZY, Yuda S, Anderson V., et al. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003; 41: 611-617. [DOI] [PubMed] [Google Scholar]
- 30.Poirier P, Bogaty P, Garneau C., et al. Diastolic dysfunction in normotensive men with well-controlled Type 2 diabetes. Diabetes Care. 2001; 24: 5-10. [DOI] [PubMed] [Google Scholar]


