Abstract
CONFLICT OF INTEREST: NONE DECLARED
Breast implants are medical devices that are used to augment breast size or to reconstruct the breast following mastectomy or to correct a congenital abnormality. Breast implants consist of a silicone outer shell and a filler (most commonly silicone gel or saline). Approximately 5 to 10 million women worldwide have breast implants. Histomorphometric study to evaluate the biological tissue compatibility of silicone implants suitable for plastic surgery and the adverse effects and risks of this material. Thirty Wistar white rats received subcutaneous implants and the revestiment of silicone gel Silimed ®®, and randomized into six groups of five animals each, according to the type of implanted material and the time of sacrifice. Eight areas of 60.11mm2 corresponding to the obtained surgical pieces were analyzed, counting mesenchymal cells, eosinophils, and foreign body giant cells, observing an acceptable biocompatibility in all implants, for subsequent statistical analysis by Tukey test. Silicone gel showed inflammation slightly greater than for other groups, with tissue reactions varying from light to moderate, whose result was the formation of a fibrous capsule around the material, recognized by the organism as a foreign body. Despite frequent local complications and adverse outcomes, this research showed that the silicone and top layer presented an acceptable chronic inflammatory reaction, which did not significantly differ from the control group. In general, it is possible to affirm that silicone gel had acceptable levels of biocompatibility, confirmed the rare presence of foreign body giant cells, and when of the rupture, formed a fibrous capsule around the material, separating the material of the organism.
Key Words: Silicone Gels, Breast Implantation, Materials Testing, Rats, Wistar
1. INTRODUCTION
Silicone (polysiloxane) has a repeated unity of monomer consisting of a dimethylsiloxane group which is known as polydimethylsiloxane (PMDS). This material has been extensively used in medical areas, in several products such as breast implants, contact lenses, lubricants, sealers, artificial cardiac tubes and valves, urethral and venous catheters, membranes for blood oxygenation, dialysis tubes, orthopedic applications and facial reconstructions (1, 2).
The perfect material to be utilized to recover the mass of soft tissues must have characteristics such as ideal texture, the ability to expand and a color similar to the surrounding tissues, it must be inert, remain in the implantation area, must not spread or cause diseases and it must be integrated in the host. The connective tissue response to the implantation of a biomaterial is manifested as a foreign body inflammatory reaction. This determines the need for the implant not to be toxic or antigenic, porous, and to have the suitable size and shape, demanding a correct implantation to resist fragmentation. All these factors collaborate to decrease the natural response of the host to a foreign body and, consequently, improve biocompatibility (3, 4).
It is known that the formation of a fibrous capsule surrounding the implant is generally a natural occurrence, an unpreventable result of the organism’s defense mechanism called as a foreign body reaction. Researchers have been accomplished about this reaction in animals, evidencing that it consists in a series of interrelated processes whose final result can vary, depending on the susceptibility of the foreign body to phagocytosis, incorporation by giant cells, inflammation or isolation through fibrosis, whose process of capsule formation is similar in animals and human models, and the etiology of the abnormal hardening surrounding human implants is still unknown (5, 6, 7).
According to Siggelkow et al. (8), Mendes et al. (9) (2008) and Palhares et al. (10) (2009), through clinical and histopathological studies respectively, the capsular contracture depends on the implantation time, expansion type, implant surface characteristics, patient’s age as well as other factors such as hematoma, foreign body reaction and subclinical infections. Tavazzani et al. (11) (2005) analyzing histologically the tissue surrounding silicone gel implants observed a chronic inflammation with drops of silicone gel enclosed by giant cells or embodied by macrophages, fibrosis areas and necrosis. Poveda et al. (12) (2006) after implantation, in humans, of cosmetic substances including silicone gel, histologically observed the reaction of foreign body with multinucleated giant cells.
According Food and Drug Administration (FDA) (13), the local complications observed in the silicone gelfilled breast implant post-approval studies are consistent with complications noted at the time of approval. The longer a woman has silicone gelfilled breast implants, the more likely she is to experience local complications or adverse outcomes. As many as 1 in 5 primary augmentation patients and 1 in 2 primary reconstruction patients require implant removal within 10 years of implantation. Limitations in the post-approval studies to date preclude the detection of very rare rates of complications. However, post-approval studies to date do not show evidence that silicone gel-filled breast implants cause connective tissue disease or reproductive problems. Differences in study design, clinical endpoints and definitions, and patient populations preclude direct comparisons of the post-approval study results for the silicone gel-filled breast implants. Patient follow-up rates are lower than anticipated, limiting the ability to draw definitive conclusions and to detect rare complications.
The purpose of this study is to histomorphometrically observe the subcutaneous cellular tissue of rats, after a medical silicone gel implantation, Silimed®, separating the top layer from the internal content, visualizing the biocompatibility of the tissues, the adverse effects and risks in the presence of the material.
2. MATERIALS AND METHOD
The experiments relating to this research were approved by the Ethics Committee of Animal Experiments from Araçatuba School of Dentistry, UNESP, protocol number 13/04, and were developed with the utilization of thirty Wistar white rats, with mean age of 90 days and initial weight around 250 grams, clinically free of any disease, randomly divided into six groups of five animals each.
For the experimental procedures, the animals were anesthetized with intraperitoneal injection of a 5% ketamine hydrochloride solution associated to a muscular relaxant, analgesic and sedative, Xilazine dosed in 0,050 ml of anesthetic and 0,050 ml of the relaxant, for each 100 grams of the animal weight.
After trichotomy at the bilateral upper medium dorsal region, in an area of 10 cm2, antisepsis with 10% PVP-I solution was done (aqueous solution of polyvinylpyrrolidone). Two longitudinal incisions of 2 cm were accomplished with a #15 interchangeable scalpel blade fitted in a #3 Bard-Parker scalpel, one of each side of the animal and parallel to each other, 2 cm distant one from another in order to avoid the manipulation of the middle region, aiming, this way, to decrease the inflammatory response. The skin divulsion was bilaterally done in the subcutaneous space with a straight scissors with rounded tip, in such way that a tunnel was obtained to receive the studied implants, being distant approximately 1,5 cm from the incision. The utilized implants consisted of fragments with approximately 1,0 cm x 1.0 cm x 0,5 cm previously sterilized in a autoclave at 136°C for 20 minutes. In Groups 1, 2 and 3, the silicone gel (Silimed®) was introduced at the left side and the right side served as a control. The same procedure was accomplished in Groups 4, 5 and 6, excepting for the material implanted that, in this case, was the top layer of the silicone gel at the left side and the right side served as a control for the analysis.
After the implantation, both surgical wounds were sutured and, after 7 post-operative days (Groups 1 and 4), 15 post-operative days (Groups 2 and 5) and 30 post-operative days (Groups 3 and 6), the animals were euthanized and the subjacent tissues were removed for the histopathological processing and qualitativequantitative analysis. The histomorphometrical study was accomplished in a computer through the software LEICA QWin, from Leica Imaging Systems Limited, Cambridge, England. For recording and standardization of the histological cuts analysis, a criterion of selecting eight areas of each animal was used, with size of 60,11 mm2. The analysis was done through a100X immersion objective, beginning at the surface adjacent to the cavity represented by the implant. Counting was done by a single examiner, and the same procedure was repeated twice with a three days interval. Results were statistically treated by Tukey test.
3. RESULTS
In the qualitative analysis of the control group, at seven postoperative days, through the absence of the implant of exogenous material, it was possible to observe a complete epithelial repair in the incision area, showing a discrete area of granulation tissue with the predominance of mononuclear inflammatory cells. Meanwhile, when the newly formed tissue surrounding the silicone gel implants and their top layers was observed, we verified a granulation tissue where the nearest band of the implant area exhibited intense cellularity, characterized by mesenchymal, mononuclear inflammatory cells, some multinucleated cells and rare eosinophils. (Figure 1 A, B and C).
Figures 1.
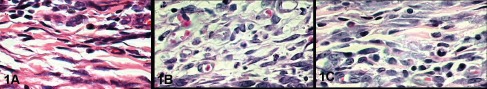
A) Control Group, B) Silicone gel and C) Top Layer Silicone at 7 days. H.E. 1000X
In the period of fifteen days, the epithelial regeneration was complete in the control group and also in the treated groups, where collagen still presented a discrete mononuclear inflammatory infiltrate and the subjacent connective tissue presented some fibroblasts and few mononuclear inflammatory cells (Figure 2 A, B and C).
Figures 2.
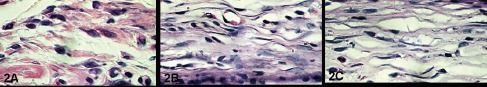
A) Control Group, B) Silicone gel and C) Top Layer Silicone at 15 days. H.E. 1000X
At thirty days, all materials were involved by newly formed tissue that surrounded the implanted material, constituted by several fusiform fibroblastic cells dispersed in a collagenized matrix, containing some mononuclear cells (Figure 3 A, B and C).
Figures 3.
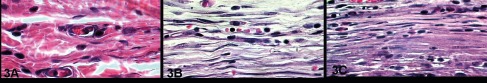
A) Control Group, B) Silicone gel and C) Top Layer Silicone at 30 days. H.E. 1000X
The quantitative histological analysis was done through the means of the countings accomplished in each group and time studied, aiming the analysis of occurrences at short, medium and long term, establishing a coincidence with the follow-up periods of the cutaneous wounds repair in normal rats.
Tukey test, applied to the results observed in the three studied groups and three post-operative periods, showed, concerning the mesenchymal cells, that the control group and the top layer group presented significant differences with each other (Table 1 A), while the silicone gel group was not significantly different from the other two groups. Concerning the mononuclear inflammatory cells, the silicone gel top layer presented significant differences in comparison with control and silicone gel groups (Table 1B). The eosinophils results did not present significant differences among the studied groups (Table 1 C).
Table 1.
Tukey test: Mean of the mesenchymal cells (A); Mononuclear inflammatory cells (B); and eosinophils (C).
| Group | Samples | Mean A | Mean B | Mean C | |
|---|---|---|---|---|---|
| Control | 15 | 18,2480 | 3,8060 | 0,05032 | |
| Silicone gel | 15 | 16,6700 | 3,9847 | 0,04784 | |
| Top Layer | 15 | 15,0840 | 5,6027 | 0,04199 |
The means of the cells identified at the respective operative periods, as well as the standard deviation (SD) and standard error (SE), are represented in Figures 4 to 6.
Figure 4.
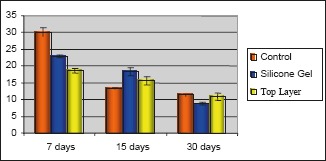
Means and standard error – mesenchymal cells
Figure 6.
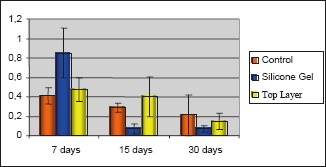
Means and standard error – eosinophils
4. DISCUSSION
Safety and efficiency of materials used in oral maxillofacial prosthesis include the histopathological knowledge of the implant/tissue interface and the possibility of the occurrence of chronic inflammation and granulomatous response through experimental studies, aiming to obtain as low as possible a reaction of the host, as well as the tissues restoration (14).
In the present study, the control group, through the absence of the exogenous material implant, presented at seven days a complete epithelial repair in the incision area, showing a discrete area of granulation tissue with the predominance of mononuclear inflammatory cells. Meanwhile, when observing the newly formed tissue surrounding the silicone gel implants and top layers, we verified a granulation tissue where the nearest band of the implant area exhibited intense cellularity, characterized by mesenchymal cells, mononuclear inflammatory cells, some multinucleated cells and rare eosinophils.
In the period of fifteen days, the epithelial regeneration was complete in the control group and also in the treated groups, where collagen still presented a discrete mononuclear inflammatory infiltrate and the subjacent connective tissue presented some young fibroblasts and few mononuclear inflammatory cells.
It was also possible to observe a chronic infiltrate at the initial time that was disappearing at the other post-operative periods, with the silicone inclusion forming a fibrous capsule composed by fibroblasts that became more mature from fifteen days in substitution of the granulation tissue, with the permanence of few inflammatory cells. This capsule, whose cause is still unclear, could be hypothetically explained by the poor adhesion of the implant surface to the tissues, eventually contracting and avoiding the implant function (7, 15).
In the present study, at fifteen days in the animals implanted with silicone gel and its top layer, it was noticed a more mature tissue, less cellularized, but with moderate quantity of mononuclear inflammatory cells, lymphocytes and plasma cells, in process of repair more delayed than the control group. Rarely, giant cells were observed near the light of the implanted material, what suggests the good acceptance of the material by the implanted tissues.
At thirty days, in the treated groups, the newly formed tissue that surrounded the implanted material was constituted by abundant fusiform fibroblastic cells dispersed in a collagenized matrix, containing some mononuclear cells. Focal areas which presented fragments of silicone showed a more intense mononuclear inflammatory infiltrate, similar to an inflammatory process reactivation.
The clinical observation of the animal allowed verifying that the silicone is a substance that does not induce an acute inflammatory reaction. Moreover, it did not leak nor dislocated from the implantation area, allowing the morphometric study, which showed in different quantities for each material, the maintenance of the repair process for a prolonged time in comparison of the repair was without implant. Concerning the inflammatory cells and eosinophils, there was no significant differences in the studied groups. After analyzing the four parameters, we concluded that the silicone gel caused an inflammatory process slightly higher in comparison with the other groups, but in acceptable levels of biocompatibility, confirmed by the rare presence of foreign body giant cells.
At thirty days, the examination of the specimens revealed a decrease in the number of several cell types, specially lymphocytes and plasma cells, with macrophages saw in few histological cuts as well as foreign body giant cells. All materials were involved by a thin reactive fibrous capsule constituted by fibrocytes, collagen and blood vessels. Experimental studies in rats have defined important aspects of inflammatory response of several types of implants. Histological and functional aspects of the granulation tissue formed surrounding foreign bodies are a results of the interaction between the implants properties and the tissue capacity to react to the stimulus. The shape geometry, physical state, surface type and chemical composition of the foreign body are important factors that define the quantity and cell types involved in the process. The geometric shape serves as a space orientation of the cells positioning and of the collagen deposition. The physical state and the material surface (wrinkled or plane) have an important role in the induction of the cells types involved in the inflammatory reaction. The capsular tissue formed surrounding the wrinkled surfaces reduces the contractile power of this capsule. Several tissues seem to react to the same foreign body with different intensities. The interaction of all these factors will determine the response level of the tissue to the implanted material (3, 6).
Our findings agree with several authors that based on the totality of the evidence, believes that silicone gel-filled reconstruction of body areas have a reasonable assurance of safety and effectiveness when used as labeled. Despite frequent local complications and adverse outcomes, the benefits and risks of implants are sufficiently well understood for patients to make informed decisions about their use (16, 17). The longer a human has silicone gel-filled implants, the more likely he is to experience local complications or adverse outcomes. People with reconstruction of maxillofacial structures or other areas will need to monitor their implants for local complications for the rest of their lives (18, 19).
Thus, considering the significant increase of people who receive silicone gel implants for expansion or reconstruction of body areas, the pertinent literature and the results obtained in this study, we believe that the evaluated material so their use in humans can be done with safety and confiance biological bases but device failure studies are necessary to further characterize the modes and the causes of failure of explanted devices over a 10-year period (20, 21).
Currently the FDA recommends that women with silicone gel-filled implants get their first breast MRI 3 years after they receive the implants and every 2 years thereafter to detect silent ruptures (13). Long-term studies are difficult to undertake for a number of reasons but they are, however, essential from an industry perspective both for the provision of information and supporting audit and professional standing (13, 22).
5. CONCLUSIONS
Despite frequent local complications and adverse outcomes, this research showed that the silicone and top layer presented an acceptable chronic inflammatory reaction, which did not significantly differ from the control group.
These informations are extremely importance to do patients make the decision to use or not to use implants.
A long-term follow-up of participants who receive silicone gel-filled implants is a differential to prevent complications and adverse outcomes.
Figure 5.
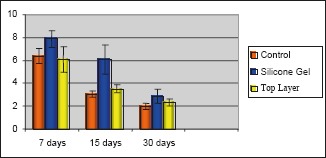
Means and standard error – inflammatory cells
REFERENCES
- 1.Quatela VC, Chow J. Synthetic facial implants. Facial Plast Surg Clin North Am. 2008; 16(1): 1-10. [DOI] [PubMed] [Google Scholar]
- 2.Chin T, Kobe K, Hyakusoku H, Uekusa K, Hirakawa K, Ohno Y. Experimental analysis of silicone leakage. J Nippon Med Sch. 2009; 76(2): 109-112. [DOI] [PubMed] [Google Scholar]
- 3.Kelemen O, Hegedus G, Kollár L, Menyhei G, Seress L. Morphological analysis of the connective tissue reaction in linear hypertrophic scars treated with intralesional steroid or silicone-gel sheeting. A light and electron microscopic study. Acta Biol Hung. 2008; 59(2): 129-145. [DOI] [PubMed] [Google Scholar]
- 4.Flassbeck D, Pfleiderer B, Klemens P, Heumann KG, Eltze E, Hirner AV. Determination of siloxanes, silicon, and platinum in tissues of women with silicone gel-filled implants. Anal Bioanal Chem. 2003; 375(3): 356-362. [DOI] [PubMed] [Google Scholar]
- 5.Bal BT, Yılmaz H, Aydın C, Karakoca S, Tokman B. Histopathologic study of rat connective tissue responses to maxillofacial silicone elastomers. J Mater Sci Mater Med. 2009; 20: 1901-1907. [DOI] [PubMed] [Google Scholar]
- 6.Haddad Filho D, Zveibel DK, Alonso N, Gemperli R. Comparison between textured silicone implants and those bonded with expanded polytetrafluoroethylene in rats. Acta Cir Bras. 2007; 22(3): 187-194. [DOI] [PubMed] [Google Scholar]
- 7.Kałuzny JJ, Jóźwicki W, Wiśniewska H. Histological biocompatibility of new, non-absorbable glaucoma deep sclerectomy implant. J Biomed Mater Res B Appl Biomater. 2007; 81(2): 403-409. [DOI] [PubMed] [Google Scholar]
- 8.Siggelkow W, Faridi A, Spiritus K, Klinge U, Rath W, Klosterhalfen B. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials. 2003. Mar; 24(6): 1101-1109. [DOI] [PubMed] [Google Scholar]
- 9.Mendes FH, Viterbo F, DeLucca L. The influence of external ultrasound on the histologic architecture of the organic capsule around smooth silicone implants: experimental study in rats. Aesthetic Plast Surg. 2008; 32(3): 442-450. [DOI] [PubMed] [Google Scholar]
- 10.Palhares A, Schellini SA, Pellizzon CH, Padovani CR, Dorsa P. Evaluation of low intensity laser’s action on silicone mammary implant pseudocapsules in rats. Acta Cir Bras [serial on the Internet]. 2009; 24(1): 7-12. [DOI] [PubMed] [Google Scholar]
- 11.Tavazzani F, Xing S, Waddell JE, Smith D, Boynton EL. In vitro interaction between silicone gel and human monocyte-macrophages. J Biomed Mater Res A. 2005; 72(2): 161-167. [DOI] [PubMed] [Google Scholar]
- 12.Poveda R, Bagán JV, Murillo J, Jiménez Y. Granulomatous facial reaction to injected cosmetic fillers - a presentation of five cases. Med Oral Patol Oral Cir Bucal. 2006; 11(1): E1-5. [PubMed] [Google Scholar]
- 13.FDA Update on the Safety of Silicone Gel-Filled Breast Implants. U.S. Food and Drug Administration. Center for Devices and Radiological Health. June, 2011. [Google Scholar]
- 14.Wang XY, Baba A, Taniguchi K, Hagio M, Miyazaki K. Study on rat subcutaneous reaction to experimental polyurethane elastomers. Dent Mater J. 2004; 23(4): 512-516. [DOI] [PubMed] [Google Scholar]
- 15.Passos AHR, Costa F, Marchese LT, Guimarães SAC, Oreini WA. Fibrosis in tubulized skin flaps in rats using silicon catheters of two different flexibility: experimental model. Acta Cir Bras. 2008; 23(3): 243-246. [DOI] [PubMed] [Google Scholar]
- 16.Lipworth L, Tarone RE, Friis S, Ye W, Olsen JH, Nyren O, McLaughlin JK. Cancer among Scandinavian women with cosmetic breast implants: a pooled long-term followup study. Int J Cancer. 2009; 124(2): 490-493. [DOI] [PubMed] [Google Scholar]
- 17.Cash TF, Duel LA, Perkins LL. Women’s psychosocial outcomes of breast augmentation with silicone gel-filled implants: a 2-year prospective study. Plast Reconstr Surg. 2002; 109(6): 2112-2121. [DOI] [PubMed] [Google Scholar]
- 18.Hvilsom GB, Holmich LR, Henriksen TF, Lipworth L, McLaughlin JK, Friis S. Localcomplications after cosmetic breast augmentation: results from the Danish Registry for Plastic Surgery of the breast. Plast Reconstr Surg. 2009; 124(3): 919-925. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin JK, Lipworth L, Murphy DK, Walker PS. The safety of silicone gel-filled breast implants: a review of the epidemiologic evidence. Ann Plast Surg. 2007; 59(5): 569-580. [DOI] [PubMed] [Google Scholar]
- 20.Holmich LR, Lipworth L, McLaughlin JK, Friis S. Breast implant rupture and connective tissue disease: a review of the literature. Plast Reconstr Surg. 2007; 120: 62-69. [DOI] [PubMed] [Google Scholar]
- 21.Song JW, Kim HM, Bellfi LT, Chung KC. The effect of study design biases on the diagnostic accuracy of magnetic resonance imaging for detecting silicone breast implant ruptures: a meta-analysis. Plast Reconstr Surg. 2011; 127(3): 1029-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry MG, Stanek JJ. The PIP mammary prosthesis: A product recall study. J Plast Reconstr Aesthet Surg. 2012. Mar 9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


