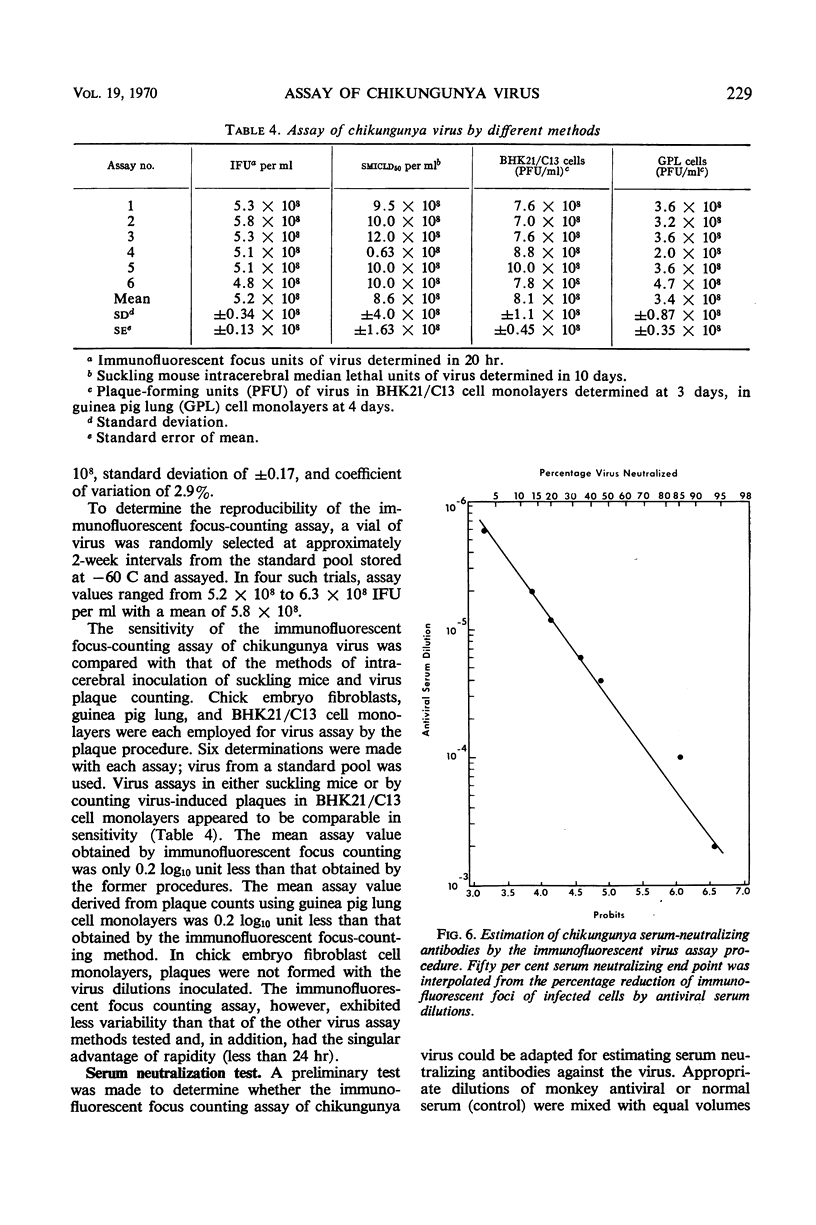
Abstract
Chikungunya virus was quantitatively assayed by counting immunofluorescent foci after infection of BHK21/C13 cell monolayers. The speed and efficiency of virus attachment to cells were markedly enhanced when augmented by centrifugal force. By this procedure, a proportionality was obtained between the number of immunofluorescent foci and the volume of inoculum. Virus penetration into cells was linear and complete within 15 min at 35 C. From observations on the sequential development of viral antigen within cells and immunofluorescent focus counts, foci of infected cells may be enumerated as early as 16 hr after inoculation of cell monolayers. A linear function was demonstrated between immunofluorescent focus counts and relative virus concentration. The immunofluorescent assay was comparable in sensitivity but more precise and rapid than virus assays based on the intracerebral inoculation of suckling mice or on plaque counting. By the immunofluorescent procedure, the 50% neutralizing end point of antiviral serum was rapidly and quantitatively determined.
Full text
PDF

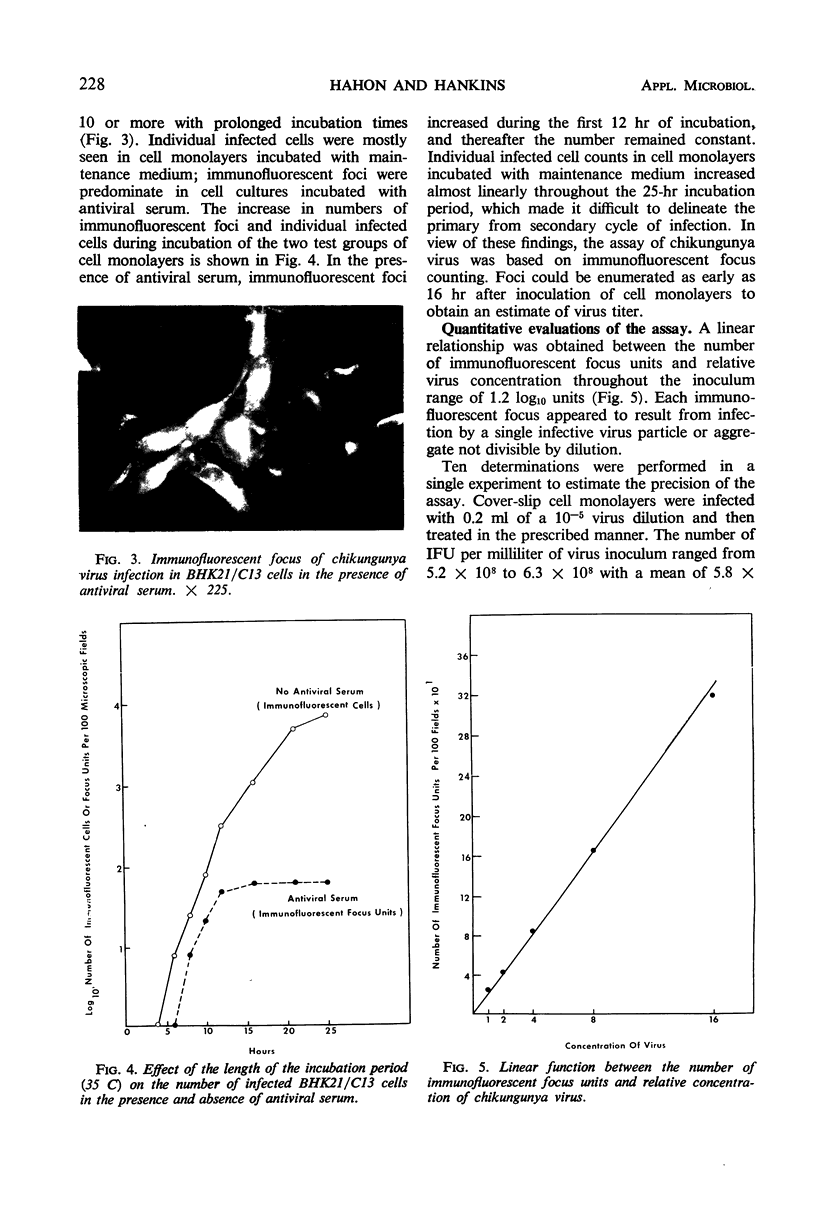


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G. B. The rapid titration of Semliki forest virus in cell monolayers by immunofluorescence. J Gen Virol. 1969 Jan;4(1):139–143. doi: 10.1099/0022-1317-4-1-139. [DOI] [PubMed] [Google Scholar]
- Chiu R. J., Black L. M. Assay of wound tumor virus by the fluorescent cell counting technique. Virology. 1969 Apr;37(4):667–677. doi: 10.1016/0042-6822(69)90285-2. [DOI] [PubMed] [Google Scholar]
- Hahon N., Cooke K. O. Primary virus-cell interactions in the immunofluorescence assay of Venezuelan equine encephalomyelitis virus. J Virol. 1967 Apr;1(2):317–326. doi: 10.1128/jvi.1.2.317-326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N. Fluorescent cell-counting assay of yellow fever virus. J Infect Dis. 1966 Feb;116(1):33–40. doi: 10.1093/infdis/116.1.33. [DOI] [PubMed] [Google Scholar]
- Hahon N. Immunofluorescent cell-counting assay of Rift Valley fever virus. Am J Vet Res. 1969 Jun;30(6):1007–1014. [PubMed] [Google Scholar]
- Mantani M., Igarashi A., Tuchinda P., Kato S. Cytoplasmic RNA synthesis and viral antigen in FL cells infected with Chikungunya virus. Biken J. 1967 Dec;10(4):203–218. [PubMed] [Google Scholar]
- RIGGS J. L., SEIWALD R. J., BURCKHALTER J. H., DOWNS C. M., METCALF T. G. Isothiocyanate compounds as fluorescent labeling agents for immune serum. Am J Pathol. 1958 Nov-Dec;34(6):1081–1097. [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- WEINBREN M. P., HADDOW A. J., WILLIAMS M. C. The occurrence of Chikungunya virus in Uganda. I. Isolation from mosquitoes. Trans R Soc Trop Med Hyg. 1958 May;52(3):253–257. doi: 10.1016/0035-9203(58)90084-1. [DOI] [PubMed] [Google Scholar]