Abstract
As broadly demonstrated for the formation of a functional skeleton, proper mineralization of periodontal alveolar bone and teeth – where calcium phosphate crystals are deposited and grow within an extracellular matrix – is essential to dental function. Mineralization defects in tooth dentin and cementum of the periodontium invariably lead to a weak (soft or brittle) dentition such that teeth become loose and prone to infection and are lost prematurely. Mineralization of the extremities of periodontal ligament fibres (Sharpey's fibres) where they insert into tooth cementum and alveolar bone is also essential for the function of the tooth suspensory apparatus in occlusion and mastication. Molecular determinants of mineralization in these tissues include mineral ion concentrations (phosphate and calcium), pyrophosphate, small integrin-binding ligand N-linked glycoproteins (SIBLINGs), and matrix vesicles. Amongst the enzymes important in regulating these mineralization determinants, two are discussed at length here with clinical examples given, namely tissue-nonspecific alkaline phosphatase (TNAP) and phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX). Inactivating mutations in these enzymes in humans and in mouse models lead to the soft bones and teeth characteristic of hypophosphatasia (HPP) and X-linked hypophosphatemia (XLH), respectively, where levels of local and systemic circulating mineralization determinants are perturbed. In XLH, in addition to renal phosphate wasting causing low circulating phosphate levels, phosphorylated mineralization-regulating SIBLING proteins such as matrix extracellular phosphoglycoprotein (MEPE) and osteopontin (OPN), and the phosphorylated peptides proteolytically released from them such as the acidic serine- and aspartate-rich motif (ASARM) peptide, may accumulate locally to impair mineralization in this disease.
Introduction
In the craniofacial region, the tooth and its periodontium represent a remarkable tooth-suspension and masticatory apparatus, functionalized by four key mineralized tissues – enamel, dentin, cementum and bone –; and a periodontal ligament (Fig. 1), the loss of any one of which soon renders the entire apparatus nonfunctional. Loss of functionality within teeth and periodontal tissues can be inherited or caused by degenerative or infectious disease, by trauma, by dietary deficiencies, or as a consequence of surgical, radiological or chemical/drug treatments. Maintaining the integrity of the periodontium as a whole is central to retaining teeth in the oral cavity and to keeping them appropriately positioned for the proper occlusion. Proper positioning of teeth requires not only an intact and structurally and functionally sound suspensory periodontal ligament, but also bone modeling and remodeling events in the surrounding alveolar bone that initially facilitate tooth eruption in children, and then act to maintain and stabilize the tooth via the periodontal ligament during adulthood.
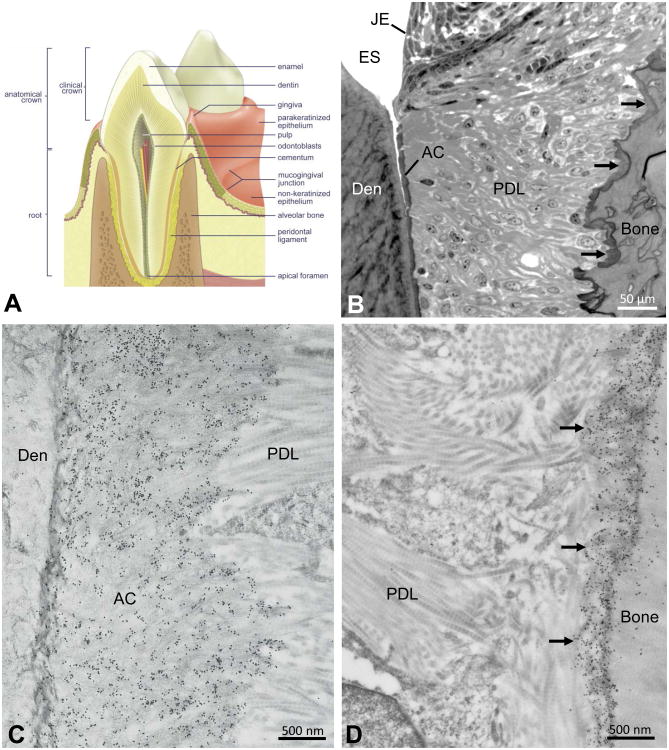
Figure 1.
(A) Anatomical relationships of mineralized tissues of the tooth and surrounding hard and soft tissues of the periodontium. (B) Light microscopic relationships of periodontal tissues near the cemento-enamel junction. Junctional epithelium (JE) abuts against the enamel (here enamel space [ES] after sample decalcification), and a thin layer of acellular cementum (AC) interfaces with the dentin (Den) at the dentino-enamel junction. Periodontal ligament (PDL) collagen fibres surround the tooth, inserting at one end into the acellular cementum and inserting at the other end into a fringe of darkly stained bone (arrows) lining the alveolus. (C,D) Electron microscopy after colloidal-gold immunolabeling (small black particles) for osteopontin of periodontal ligament (PDL) collagen fibrils inserting into the acellular cementum (AC) apposed to the dentin (Den) at the dentino-enamel junction (left panel), and into the fringe of alveolar bone (arrows) lining the alveolus (right panel). Images obtained from the first molar of a 1-month-old mouse.
Essential to the proper development and functioning of these key tooth and periodontal structures (including bone) is mineralization – the deposition of inorganic, calcium- and phosphate-containing crystals (calcification) into the extracellular matrices of teeth and bone (120, 121). Mineralization of teeth and periodontal tissues has as their key supportive structural/functional element nanosized apatitic crystals acting collectively in large numbers to harden and stabilize the collagenous matrices within which they reside (with the exception of enamel, which has only noncollagenous matrix proteins). The inorganic phase that mineralizes tissues by permeating their extracellular matrices serves to counteract compressive forces (47, 172). Any deviation from normal in crystal number, size, shape, orientation and/or ultrastructural matrix location leads to tissue fragility that compromises tooth and periodontal tissue function.
Overview of extracellular matrices of the teeth and periodontium
In mature erupted tooth enamel, whose pre-existing organic matrix component essential to its formation and mineralization are almost completely removed by enzymatic degradation, the final mineralized state of the (very hard) enamel is critical for resisting mechanical abrasion and chemical (mostly dietary and bacterial) attack. This unique and incredible hardness of enamel (∼96% mineral) (159) is quite unlike the other “softer” mineralized tissues of the tooth (54, 136), and bone, whose extracellular matrices have a permanently resident, intermingled and fibrillar ductile collagenous protein phase which along with other noncollagenous proteins provides for the substantial deformability required for these tissues (15, 16). Tooth crown dentin must flex readily when considerable masticatory forces are placed upon the enamel, and the amalgamation of root dentin and cementum across the cemento-dentinal junction likewise must accommodate the strains placed upon it by masticatory forces transduced along the suspensory periodontal ligament (82, 86, 116, 132). Interfaces between these distinct tissues also play an important role in maintaining tooth integrity, just as they do in remodeling bone (118). The embedded and mineralized portions of the extremities of the periodontal ligament fibres (Sharpey's fibres) that insert into both cementum and alveolar bone complete the union of the suspensory apparatus and allow for the dissipation of potentially disruptive masticatory forces across the whole periodontium. None of this would be achieved without Nature's hardening strategy – mineralization – for vertebrate extracellular matrices.
Described briefly below in a categorical way are three of the four extracellular matrices related to the periodontium that mineralize – dentin, cementum and bone [enamel extracellular matrix will not be covered here, but has been recently reviewed by (67, 88)]. Periodontal ligament structure, organization and function have recently been reviewed (20). However, some discussion is given here concerning the terminal ends of periodontal ligament collagen fibres known as Sharpey's fibres as they mineralize at their extremities where they insert into alveolar bone at one end, and into tooth root cementum at the other end.
Jaw bone in the craniofacial complex is generally not unlike bone elsewhere (121), but its origins are different, with much of it deriving from a neural crest cell contribution (130). Another unique characteristic is its high turnover (remodeling) rate (24, 168), presumably resulting from substantially large and frequent mastication forces exerted primarily by the masseter muscle. Such forces transduced in part via the tooth and the suspensory periodontal ligament to the bone result in the requirement for particularly ductile bone lining the tooth alveolus. The bulk of the alveolar bone likely has both an organic and inorganic composition similar to bone found elsewhere (121), but the bone immediately lining the osseous alveolus has a unique structure appearing variably as either a thin “fringe” of bone accommodating the many insertion sites of the periodontal ligament (Fig. 1), or as so-called bundle bone (14) where it is thicker and more developed. Whereas the extensive attachment of the periodontal ligament insertions as Sharpey's fibres deep into the alveolar bone to form bundle bone is evident in terms of its providing a robust suspensory attachment function for bony anchorage of the tooth, the functional attachment mechanism for the “fringe” bone is not so readily obvious. Given what is likely a common adhesion mechanism for the two extremities of Sharpey's fibres which mineralize, the alveolar bone fringe resembles in several ways the acellular cementum found at the tooth root surface – both have a similar thickness, ultrastructural organization and noncollagenous protein composition (116). In particular, both acellular cementum and this surface-residing alveolar bone “fringe” layer are rich in osteopontin (Fig. 1) (32, 36, 84, 101, 116, 119, 186), where it is thought that this protein guides mineralization locally in such a way as to be optimal for this type of attachment. Alternatively, or in addition, osteopontin at these insertion sites might be part of creating a robust, tough and flexible organic extracellular matrix by virtue of it being a substrate for the homo- and heterotypic covalent crosslinking activity of transglutaminase enzymes (93, 94).
Bone and dentin are similar in several respects, especially regarding the composition of their respective extracellular matrices. However, unlike bone, dentin is not resorbed as part of a remodelling process, and thus it is not involved in the regulation of systemic mineral ion homeostasis as occurs through release of mineral ions during osteoclastic resorption of bone (134). Bone, dentin and cementum are all rich in type I collagen as the scaffolding base of the extracellular matrix, and all are 50-70% calcified with a carbonate-substituted apatitic mineral phase (136). During osteogenesis, dentinogenesis and cementogenesis, extracellular matrix assembly and subsequent mineralization occur through successive, highly ordered steps with a lag time existing between matrix deposition and mineralization. As part of this process, extracellular matrix proteins are secreted, sometimes modified or cleaved by enzymes, and organized in some cases into macromolecular assemblies, which altogether is then structured into a mature fibrillar matrix receptive to mineral deposition (120). These remarkable tissue construction events are orchestrated by the tissue-forming “blast” cells – the osteoblasts, the odontoblasts (a misnomer, in fact should be dentinoblasts) and the cementoblasts – with each being associated with a thin layer of unmineralized matrix which subsequently mineralizes at the “mineralization front” to form the completed tissue. Additional mineralization and mineral changes (such as further carbonate substitution into the hydroxyapatite lattice) (40) slowly occur over time (30), along with changes in the organic phase of the extracellular matrix (93, 94, 171) and proteolytic degradation, in a maturation process that ultimately provides an adequate final functional state to provide for the biomechanical demands placed upon each tissue.
General concepts for mineralization of collagenous matrices
The hardness and toughness of the collagenous mineralized tissues derive from their being composite materials, with each consisting of an interconnected (and often crosslinked) organic matrix network of proteins whose macromolecular assemblies interweave as a scaffold for mineral deposition (120). Calcium and phosphate complex together as crystalline salts to mineralize (calcify) extracellular matrix in bones and teeth. This biomineralization process is not haphazard, but in fact occurs in a way that is incredibly controlled at the molecular level – indeed, not surprisingly – like most cellular processes. The inorganic phase (mostly a crystalline, substituted form of hydroxyapatite) (144) that forms in the skeleton provides exactly the required hardness, while at the same time ensuring requisite toughness by intimate intercalation of nanocrystals of mineral with the organic scaffolding extracellular matrix (120). In fact, it is precisely this nanoscale deposition of (at least) billions of inorganic, tiny plate-like crystallites within a softer organic matrix that provides for the inherent flexibility of tough bones that allows deformation during mechanical challenge, and then a return to their original shape characteristic for any given skeletal element.
Heterogeneous mineral deposition events occur at discrete locations within, at the surface of, and between, collagen fibrils (100, 120) during mineralization of the teeth and of the alveolar bone of the periodontium (and bone elsewhere). The composite nature of these mineralized tissues is thus determined in a way that spans most dimensional scales starting from the protein/organic-nanocrystal interface and extending into the anatomical macroscale structure of skeletal and tooth structures. The collagen fibrils with interwoven noncollagenous protein assemblies, together with interconnected pores extending throughout the structured extracellular matrix that are ultimately filled with apatitic crystals, all have nanoscale dimensions. Mineral is nucleated not only in nanoscale volumes within fibrillar collagens, but also interfibrillarly where crystal dimensions are slightly larger and where noncollagenous proteins are abundant (117). These two structurally and biochemically distinct extracellular compartments provide different constraints on biomineralization, yet despite this, at each site, mineral is nucleated and crystal growth is subsequently regulated such that bone crystals do not grow beyond the nanometre range. Initially, to reach an apatitic mineral phase, critical nuclei of mineral ions reach a size and stability that subsequently mature to form crystals having some degree of long-range order that ultimately possess welldefined crystallographic faces characteristic of apatite. Initial nucleation events are controlled locally by concentrations of mineral ions and by the presence, or absence, of mineral nucleators and inhibitors.
In bones and teeth, mineral crystals typically are platelet-shaped crystallites with dimensions that vary depending on their physical location within the structure of the preestablished extracellular matrix (100) where there are different local chemical environments defined by matrix composition that will in part define crystal properties at those sites. Charge groups and matrix interaction domains in fibrillar type I collagen have been characterized (158), and mineral identified within the holes zones in the interior of the collagen fibrils, but much less is known about the chemical environment contributing to mineral crystal nucleation and propagation between the fibrils. Of interest, the mineral-binding proteins comprising the SIBLING family of proteins (see below), that are generally thought to guide the mineralization process in bones and teeth, are located primarily between the collagen fibrils. A recent high-resolution transmission electron microscopy study of dense, human cortical femur bone sections prepared by cryogenic ion-milling both parallel and perpendicular to the long axis of the bone revealed that most of the mineral (approximately 80%) is located external to the collagen fibrils in the form of plate-like mineral structures with dimensions of 5 nm thick, 65 nm wide and over 200 nm long (123). While crystal deposition within the collagen fibril is generally thought to occur independently from that occurring between the fibrils, a recent proposal suggests that crystals formed at the surface of collagen fibrils might migrate to locations within the fibril (166), but further work needs to be done to confirm this unusual possibility.
Protein/genes related to skeletal and dental mineralization are listed in Table 1, and are discussed in greater detail below.
Table 1. Human proteins/genes associated with mineralization.
| Protein abbreviation | Gene name(s) | Protein function |
|---|---|---|
| TNAP | Tissue-nonspecific alkaline phosphatase (ALPL) | TNAP is a membrane-bound phosphatase prominent in osteoblasts, chondrocytes and odontoblasts, as well as in their matrix vesicles (126). In the skeleton, it hydrolyzes the mineralization inhibitor pyrophosphate, also producing phosphate locally in the matrix. TNAP is also found in other organs (liver, kidney, skin), however little is known about its function in these tissues. |
| PHEX, PEX | Phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) | PHEX is a membrane-bound endopeptidase found predominantly in osteoblasts, osteocytes and odontoblasts (21, 73, 152, 153). Mutations in PHEX lead to X-linked hypophosphatemia, which is accompanied by an increase in circulating levels of FGF23 resulting in renal phosphate wasting (141, 147). PHEX protects MEPE from cleavage (74), as well as cleaving the physiologically relevant mineralization inhibitors osteopontin (19) and the MEPE ASARM and OPN ASARM peptides (4, 6). |
| ANK | Progressive ankylosis protein human (ANKH) | ANK is a transmembrane protein that acts as pyrophosphate transporter, exporting intracellular pyrophosphate into the extracellular space to inhibit mineralization (3). ANK loss-of-function mutations lead to soft-tissue calcification (85) while ANK gain-of-function mutations in humans leads to calcium pyrophosphate disease characterized by the formation of calcium pyrophosphate crystals in cartilage extracellular matrix (3). |
| NPP1 | Ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1) | NPP1 is a type II transmembrane ecto-enzyme that hydrolyzes nucleotide triphosphates to generate extracellular pyrophosphate to inhibit mineralization (164). A loss-of-function mutation in the gene encoding this enzyme leads to idiopathic infantile arterial calcification (154). |
| PHOSPHO1 | Phosphatase orphan 1 (PHOSPHO1) | PHOSPHO1 is an enzyme prominent in mineralized tissue cells that exhibits phosphatase activity towards phosphoethanolamine and phosphocholine (146). PHOSPHO1 is also present in matrix vesicles and is therefore thought to play a role in the initiation of matrix mineralization (145). |
| OPN (Osteopontin) | Secreted phosphoprotein 1 (SPP1) | OPN is produced by many cell types, and in bone by osteoblasts, osteocytes and osteoclasts, and in tooth by odontoblasts and cementoblasts (118). Many in vitro and in vivo studies have shown that OPN has a strong inhibitory role in mineralization that is largely phosphorylation dependent (5, 31, 64). OPN is also thought to play a role in osteoclast development and function (57), cell adhesion and migration (103), as well as several immune-related functions (173). |
| BSP (Bone sialoprotein) | Integrin-binding sialoprotein (IBSP) | BSP is prominent in mineralized tissue extracellular matrices such as bone, dentin and mineralized cartilage, where it is produced mainly by osteoblasts, osteocytes and chondrocytes (60, 137). Reported functions include hydroxyapatite nucleation (78) as well as promotion of osteoclastogenesis (113). |
| MEPE | Matrix extracellular phosphoglycoprotein (MEPE) | MEPE is mainly produced by osteocytes (129) and osteoblasts (12), and is upregulated during osteoblast matrix mineralization. MEPE plays a role in matrix mineralization by enhancing osteoblastic activity (71) and indirectly suppressing osteoclastogenesis (48). Its ASARM peptide, derived from a C-terminal cleavage, has been shown to be a potent mineralization inhibitor (6) and an indirect promoter of renal phosphate excretion (151). |
| DMP1 | Dentin matrix protein 1 (DMP1) | DMP1 is a prominent matrix protein in teeth (predentin, dentin and cementum - odontoblasts and cementoblasts), bones (osteocytes) and to some extent in certain soft tissues (108, 138). Generally, it is processed to 37-kDa N-terminal and 57-kDa C-terminal fragments (139). The full-length and fragment forms of DMP1 have been shown to have many biological functions including as a hydroxyapatite nucleator (full-length and C-terminal fragment) (59) and as a promoter of cell differentiation via its RGD interaction with cell surface integrin/CD44 receptors (96). DMP1 is also thought to control phosphate homeostasis through the FGF23 pathway (51), as well as osteogenesis and dentinogenesis (138). |
| DSPP | Dentin sialophosphoprotein (DSPP) | DSPP is found predominantly in dentin (and to a lesser extent in bone), but is immediately cleaved into its active fragments dentin phosphoprotein and dentin sialoprotein (110, 140). Dentin phosphoprotein contains a large number of aspartic acid and phosphoserine repeats which allows it to bind to hydroxyapatite and is thought to initiate and modulate dentin mineralization (135). The function of dentin sialoprotein is not yet known. |
| FGF23 | Fibroblast growth factor 23 (FGF23) | FGF23 is a secreted protein mainly expressed by osteoblasts and osteocytes that acts as a circulating factor to control serum phosphate levels (142). FGF23 binds to FGF receptor 1, 3 or 4 and its co-receptor Klotho in the proximal tubule of the kidney, which decreases the expression of sodium-dependent phosphate cotransporter 2, thus reducing phosphate reabsorption (142, 157). It also suppresses 1,25-dihyroxyvitamin D levels by downregulating renal 25-hydroxyvitamin D-1α-hydroxylase. |
| KLOTHO | KLOTHO (KL) | KLOTHO is produced in a limited number of tissues, with the highest being in the distal convoluted tubules of the kidney (98). Here, KLOTHO acts as a membrane-bound co-receptor for FGF23, allowing it to bind to fibroblast growth factor receptors 1, 3, and 4, and contribute to phosphate homeostasis (99). Although less common, KLOTHO can be found in a secreted form where it is thought to activate cell surface ion channels, ion transporters, and growth factor receptors (97). |
| SLC34A3, Na/Pi-IIc (NPT2C) | Solute carrier family 34 (sodium phosphate), member 3 protein; Renal sodium-phosphate cotransporter, Na/Pi-IIc (SLC34A3) | SLC34A3 is prominent in the apical membrane of proximal tubular cells in the kidney (155). Like other sodium-dependent phosphate cotransporters, it plays a major role in extracellular phosphate homeostasis. |
Pyrophosphate and the phosphate/pyrophosphate ratio in mineralization
Homeostasis of inorganic phosphate is essential for the normal development and maintenance of the mineralized tissues of the skeleton and dentition (55). Pyrophosphate (PPi) – composed of two molecules of phosphate (Pi) – pivotally regulates physiologic mineralization and pathologic calcification by acting as a potent inhibitor of crystal precipitation and growth (5, 53, 124, 128). Local tissue concentrations of pyrophosphate are controlled by a number of regulatory enzymes and transporters. The ectoenzyme tissue-nonspecific alkaline phosphatase (TNAP) hydrolyzes pyrophosphate (126), thus providing a mechanism to control the concentration of this potent mineralization inhibitor. TNAP is highly expressed by cells resident in bones and teeth, and it is critical for proper skeletal mineralization (128, 175). Loss-of-function mutations in the human TNAP gene ALPL cause hypophosphatasia (HPP), a disease marked by poor bone mineralization, rickets, and osteomalacia, as well as tooth phenotypes (143, 177). Ablation of the homologous mouse gene Alpl leads to increased inhibitory pyrophosphate and osteopontin leading to (76, 77) mineralization defects that closely phenocopy human infantile HPP (125, 131). Furthermore, two proteins can increase pyrophosphate locally in tissues – the progressive ankylosis protein (ANK) and the enzyme ectonucleotide pyrophosphatase phosphodiesterase 1 (NPP1). ANK encodes a multipass transmembrane protein that regulates transport of intracellular pyrophosphate to the extracellular space (75, 85, 92). NPP1 increases extracellular PPi by hydrolysis of nucleotide triphosphates (91). Pyrophosphate removal by TNAP activity thus antagonizes provision of PPi by ANK and NPP1, thereby creating a concerted regulation of phosphate and pyrophosphate levels to ultimately regulate mineralization (76). TNAP also can remove phosphate from osteopontin, reducing its mineralization-inhibiting function (5). Thus, in the extracellular matrix of bone, TNAP's enzymatic degradation of pyrophosphate (together with dephosphorylation of osteopontin and potentially other matrix proteins) controls the phosphate/pyrophosphate (Pi/PPi) ratio to favor proliferation of hydroxyapatite crystals outside the matrix vesicles (for matrix vesicles, see next section) and along and between the collagen fibrils. Indeed, mineralization in bone is determined partly by the ability of osteoblasts (and possibly early osteocytes as well) to remove inhibitory pyrophosphate from their adjacent extracellular matrix via expression of TNAP, and by the presence of a fibrillar collagen-rich matrix network (128). Thus, the co-expression of TNAP and a fibrillar collagenous scaffold appear to be necessary and sufficient to cause mineralization of an extracellular matrix. Enamel, which will not be discussed at length here, appears to be an interesting exception to this general rule, where TNAP function is likewise important for mineralization (185) but where a collagenous matrix is lacking.
Matrix vesicles and mineralization
Mineralization of bones and teeth occurs by a series of physicochemical and biochemical processes that together facilitate the deposition of apatitic crystals at specific sites within the extracellular matrix. Crystals reside within and between collagen fibrils of the extracellular matrix (68), and also within the lumen of chondrocyte-, osteoblast- and odontoblast-derived matrix vesicles shed from the cells (8, 9, 70, 182). Early studies on matrix vesicles used transmission electron microscopy and serial sections of growth plate cartilage to show that matrix vesicles form through exfoliation of vesicles from the plasma membrane of growth plate chondrocytes (26), findings which were subsequently confirmed by freeze-fracture analyses (27, 41, 183). Additional evidence that matrix vesicles arise from the plasma membrane derives from comparative lipid and protein studies of the vesicles and the plasma membrane of the epiphyseal chondrocyte and osteoblasts (181). Overall, cell membranes contain similar components to matrix vesicles, but in different proportions. While investigators in the bone mineralization field are divided between accepting collagen-mediated and matrix vesicle-mediated mechanisms of mineralization, there is no obligatory incompatibility between these two mechanisms. Biochemical and genetic studies (10, 11, 45, 76, 77, 83, 128, 184), using single- and double-knockout mice to probe the function of key molecules involved in the control of phosphate/pyrophosphate ratio including TNAP, NPP1, ANK, and phosphatase orphan 1 (PHOSPHO1), are compatible with a sequence of events involving both mechanisms described briefly as follows. Initially, formation of hydroxyapatite crystals inside matrix vesicles is favored by phosphate accumulation through two mechanisms, PHOSPHO1-mediated intravesicular production and transporter-mediated influx of extravesicular phosphate produced primarily by the adenosine triphosphatase activity of either TNAP or NPP1 (184). Organophosphate compounds such as adenosine triphosphatase, polyphosphates and perhaps also pyrophosphate might be the source of phosphate for this initial step of mineralization (133, 184). TNAP and NPP1 also support the next phase of collagen-mediated extravesicular calcification, although it is the pyrophosphatase, rather than the adenosine triphosphatase or polyphosphatase, activity of these enzymes that is predominant in this step (184). Extravesicular mineralization is then driven by the extracellular phosphate/pyrophosphate ratio and by the presence of a collagenous fibrillar scaffold, with further regulation of crystal growth by noncollagenous proteins of the SIBLING family (see below), most notably osteopontin. This model, which is compatible with most available experimental data, takes into account the roles of both organic and inorganic phosphates in skeletal and tooth dentin mineralization, and unifies the matrix vesicle-mediated and collagen-mediated models of mineralization as two separate, but linked, steps during osteogenesis and dentinogenesis. These interactions regulating mineralization are summarized in Figure 2.
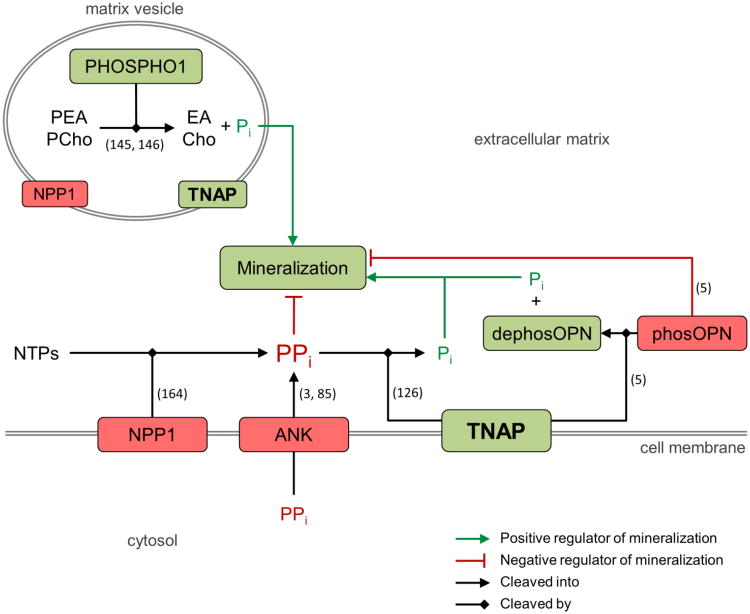
Figure 2.
Determinants of normal mineralization relevant to hypophosphatasia. The extracellular phosphate/pyrophosphate ratio is regulated by phosphatase orphan 1 (PHOSPHO1), ectonucleotide pyrophosphatase phosphodiesterase 1 (NPP1), progressive ankylosis protein (ANK), and tissue-nonspecific alkaline phosphatase (TNAP). Inactivating mutations in the enzyme TNAP result in an increase in extracellular pyrophosphate that inhibits mineralization and is a key factor in causing hypophosphatasia. Similar molecular determinants are thought to occur related to matrix vesicles. In the extracellular matrix, phosphorylated osteopontin (phosOPN) inhibits mineralization, and dephosphorylation of osteopontin (dephosOPN; and possibly other matrix proteins) by TNAP may contribute to extracellular phosphate levels. Green boxes indicate positive regulators of mineralization, while red boxes indicate negative regulators of mineralization. Citations supporting the indicated functions are shown by the numbers in parentheses. PEA, phosphoethanolamine; EA, ethanolamine; PCho, phosphocholine; Cho, choline; Pi, phosphate; PPi, pyrophosphate; NTPs, nucleotide triphosphates.
Noncollagenous extracellular matrix proteins and mineralization
While the local removal from the extracellular matrix of mineralization inhibitors such as pyrophosphate is central to the induction of mineralization, the exquisite biological control regulating crystal growth in mineralized tissues appears to reside at the molecular/atomic level of mineral-nucleating and mineral-inhibiting noncollagenous proteins (and their released peptides) (63) to transform unmineralized “oid” matrix into mineralized matrix (137). Many of the noncollagenous proteins in the skeleton and in teeth (and also in many other biomineralized invertebrate tissues) are highly acidic phosphoproteins that bind strongly to mineral – often through negatively charged phosphate groups – to regulate crystal growth. Substantial evidence now exists for the role of acidic, negatively charged mineral-binding proteins (and their peptides) as being important in regulating at least the interfibrillar mineralization process, and this family of proteins (also having cell-signaling properties) is referred to as the SIBLING family (small integrin-binding ligand N-linked glycoproteins) (52), being a subcategory of the secreted calcium-binding phosphoprotein (SCPP) family (95).
The SIBLING family, encoded by a series of genes located on chromosome 4 in humans (loci 20-21), includes dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), osteopontin (OPN), statherin, and matrix extracellular phosphoglycoprotein (MEPE). In various mineralized tissues (including pathologically mineralizing soft tissues), once initiated, crystal growth is regulated (inhibited) by direct binding of certain inhibitory SIBLING proteins/peptides (for example, osteopontin and its ASARM-containing peptides; see below) to crystal surfaces (4, 66). Some SIBLING proteins are associated with specific sites on collagen molecules, possibly to promote the nucleation and growth of apatite crystals related to collagen (81, 167). SIBLING genes are well-conserved during the evolution of vertebrates, especially genomic regions coding for the arginine-glycine-aspartate (RGD) motif and for long stretches of acidic amino acids, with these motifs having proven roles in cell adhesion and mineral binding, respectively (17, 18, 112, 148). In MEPE, the arginine-glycine-aspartate tripeptide is associated with a glycosaminoglycan attachment motif serine-glycine-aspartate-glycine (SGDG) to form a region called dentonin, and a synthetic dentonin peptide has been shown to stimulate new bone formation (80) and to enhance dental pulp progenitor proliferation (104, 160). Finally, transgenic mouse studies on the SIBLINGs, together with the identification of human SIBLING gene mutations linked to diseases showing mineralization defects, have confirmed the importance of these proteins in regulating bone and tooth mineralization (7, 48, 71, 161-163).
Hypophosphatasia, tissue-nonspecific alkaline phosphatase and the periodontium
Deficiency of TNAP activity characterizes hypophosphatasia (HPP), which is a heritable disorder featuring hypomineralization of the skeleton and teeth (58, 176, 178). Clinical manifestations of hypophosphatasia vary from stillbirth with complete absence of skeletal mineralization, to early tooth loss as the only symptom. The typical and striking oral manifestation of hypophosphatasia is premature loss of primary teeth (176, 178). Expression of TNAP in bone, dentin, and cementum has been well-characterized by immunohistochemistry and in situ hybridization (22, 72, 79, 87, 169, 180). Dysplasia or aplasia of cementum has been well-documented histologically in hypophosphatasia, and this abnormality is considered the cause for the early exfoliation of teeth (38, 50, 89). Irregular dentin mineralization and enlarged pulp chambers have also been described (25, 50, 90).
Cementum was first linked to pyrophosphate metabolism in the condition hypophosphatasia, where premature tooth exfoliation was discovered to result from developmental cementum aplasia or hypoplasia, and thus poor periodontal ligament attachment (38, 170, 175). Intriguingly, studies to date suggest the acellular cementum (acellular extrinsic fiber cementum) of the cervical portion of the root is the most severely affected by the pyrophosphate dysregulation, while the apically located cellular cementum (cellular intrinsic fiber cementum) is generally unaffected, or at least much less so. Proper cementum formation is critical for dento-alveolar function, though cementogenesis remains relatively poorly understood (compared to other mineralized tissues) in terms of associated cells and their origin, their activities and the regulatory factors involved in cementum formation and mineralization. This is especially true with regard to differences between the acellular and cellular varieties of cementum, and how cementum differs developmentally from the other hard tissues. Pyrophosphate serves as an essential regulator of tooth root acellular cementum development and mineralization, and is a key determinant defining the hard-soft interface between the cementum and the periodontal ligament (56). Loss of TNAP caused severe underdevelopment, or even an absence of acellular cementum, and this was prevented by enzyme replacement therapy with mineral-targeting TNAP (122). In contrast, loss of either ANK or NPP1 results in loss of control of cementum apposition and mineralization, causing an extensive hypercementosis (56). With TNAP, ANK and NPP1 adjusting extracellular pyrophosphate levels, the observations described above strongly support pyrophosphate as being a key mechanistic factor uniting the cementum phenotypes in all three mouse models, together suggesting that pyrophosphate regulates acellular cementum in a molecular “rheostat” fashion, i.e. that cementum thickness relates inversely to pyrophosphate production.
While one might suspect that enamel mineralization could also depend on the local regulation of phosphate and pyrophosphate metabolism, there have been no conclusive reports of enamel defects in hypophosphatasia patients, although some papers have alluded to enamel hypoplasia in this disease (89, 111, 143, 174). Recently, Yadav et al. mapped the expression of TNAP in amelogenesis in healthy mice to maturation stage ameloblasts and to the stratum intermedium cell layer (185). Furthermore, these authors showed that deficiency of TNAP in Alpl-/- mice leads to enamel defects. Of note here is that enzyme replacement therapy with mineral-targeting TNAP can prevent the dentin (125), cementum (122) and enamel defects (185) in the Alpl-/- model of infantile hypophosphatasia. Clinical trials of mineral-targeting TNAP (Asfotase Alfa) in patients with life-threatening hypophosphatasia indicate improved survival and correction of the rickets in these patients (179). A study of changes in the teeth, and tooth retention, as a result of enzyme replacement therapy, is yet to be conducted in these subjects.
The ASARM peptide and mineralization
Proteins of the SIBLING family have an acidic serine- and aspartate-rich motif (ASARM) which is highly conserved across species (114, 148). The ASARM region contains serine residues which can be phosphorylated, and which are interspersed with abundant acidic aspartate and glutamic acid residues (149). The conserved ASARM motif is located in the C-terminal region of all SIBLINGs except osteopontin where it is located in the mid-region of the protein. In most vertebrates, ASARM appears to have evolved to regulate mineralization, extending from eggshell to mammalian bone (17). In mammals, its function appears to have been extended to both transduce and suppress fibroblast growth factor 23 (FGF23) signalling (105, 148). In normal osteogenesis and dentinogenesis, MEPE can be enzymatically cleaved resulting in the release of free ASARM peptide into the extracellular matrix (114), and related recent work for osteopontin also shows enzymatic cleavage to produce ASARM-containing peptides (19). These acidic peptides are generally highly resistant to proteolysis, and are potent inhibitors of bone and dentin mineralization (4, 6, 151). However, ASARM peptides can be selectively cleaved and thus cleared from the local matrix environment where mineralization is destined to occur by the enzyme PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome), a transmembrane zinc-dependent endopeptidase highly expressed by osteoblasts, osteocytes and odontoblasts (4, 6, 19). PHEX can bind to MEPE (and possibly also to other SIBLING proteins(49)) at the ASARM site, protecting these proteins from proteolytic cleavage by other enzymes (74, 150). Once released, the ASARM peptide itself becomes a substrate for PHEX with multiple internal cleavage sites (4, 6), thus promoting mineralization. In this way, together with our recent report on the essentially complete degradation of inhibitory full-length osteopontin by PHEX (19), this enzyme tightly controls mineralization at the local level in the extracellular matrix (4, 150). Furthermore, PHEX can negatively or positively influence FGF23 expression through binding DMP1 or through cleaving ASARM, respectively, which in turn influences systemic circulating phosphate levels (49, 114, 115). In addition, it has also been suggested that PHEX indirectly influences cleavage of FGF23, probably via KLOTHO, thus controlling the negative effects of this hormone on phosphatemia (13, 115, 148). However, the complex links between PHEX, FGF23, DMP1, ASARM, KLOTHO and MEPE in the control of phosphate homeostasis still need further study.
In chronic inflammatory diseases that affect bones and teeth, and more specifically in the case of periodontal disease, excessive pathologic bone resorption occurs in the alveolar bone of the periodontium (46). In this disease process, bone mineral is dissolved, extracellular matrix is degraded, and total bone volume is lost, all of which threatens the stability and longevity of adjacent teeth. As part of this extensive matrix degradation and mineral dissolution, mineral-bound proteins and peptides such as ASARM might be released, redistribute in tissue fluids and ultimately circulate systemically (35). While tissue-resident PHEX may be able to degrade some of this increased inhibitory ASARM (4, 6) to promote bone healing and mineralization, and positively influence phosphatemia, on other occasions the enzymatic capacity of PHEX might be overwhelmed such that bone healing is impaired and circulating phosphate levels are negatively affected. In this context, ASARM peptides might be considered as target molecules for the treatment of chronic inflammatory diseases of bone.
X-linked hypophosphatemia, PHEX and the periodontium
Familial hypophosphatemic rickets (e.g. X-linked hypophosphatemic rickets, or X-linked hypophosphatemia, XLH) are genetic disorders whose major symptom is a soft (hypomineralized) skeleton and dentition. In 1995, the PEX gene located on chromosome Xp22.1-22.2 in humans and later renamed PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) was identified as the cause of XLH (MIM-307800) (1). Recently, PHEX mutations have been diagnosed in 87% of familial cases, and also in 72% of sporadic cases, in a large cohort of 118 pedigrees representing 56 familial cases and 62 sporadic cases of X-linked hypophosphatemia (61). Regarding other genes associated with familial hypophosphatemic rickets, FGF23 mutations were identified as the cause of autosomal dominant hypophosphatemic rickets (2), and DMP1 mutations (108) and SLC34A mutations (23, 107) as causes of recessive autosomal hypophosphatemic rickets. In addition, loss-of-function mutations in the NPP1 gene and a translocation involving the KLOTHO gene were assumed to be also associated with hypophosphatemic rickets (37, 102, 106).
In X-linked hypophosphatemia, impaired bone mineralization manifests in children as rickets with severe skeletal deformities, and in adults as osteomalacia (39). A major feature in these same patients is the occurrence of spontaneous tooth abscesses both in the deciduous and permanent dentition, and in teeth without any signs of trauma or decay (43, 156) (Fig. 3). Despite the fact that teeth of patients with X-linked hypophosphatemia look clinically normal, X-rays show a thin enamel layer and a radiolucent dentin layer, the latter associated with enlarged pulp chambers resembling classic taurodontism and prominent pulp horns extending up to the dentino-enamel junction (43) (Fig. 3). Histologic examination show extensive enamel cracking and fissuring, and unmerged dentin calcospherites separated by large nonmineralized interglobular spaces (42, 127) where the dentin matrix contains degraded fragments of MEPE, DMP1 and osteopontin, and also the MEPE-ASARM peptide (33). Abnormal dentin mineralization and enamel cracks may lead to rapid pulp necrosis with periapical complications, and with bacterial ingress into the pulp being facilitated to cause infections of the teeth and surrounding periodontium (44).
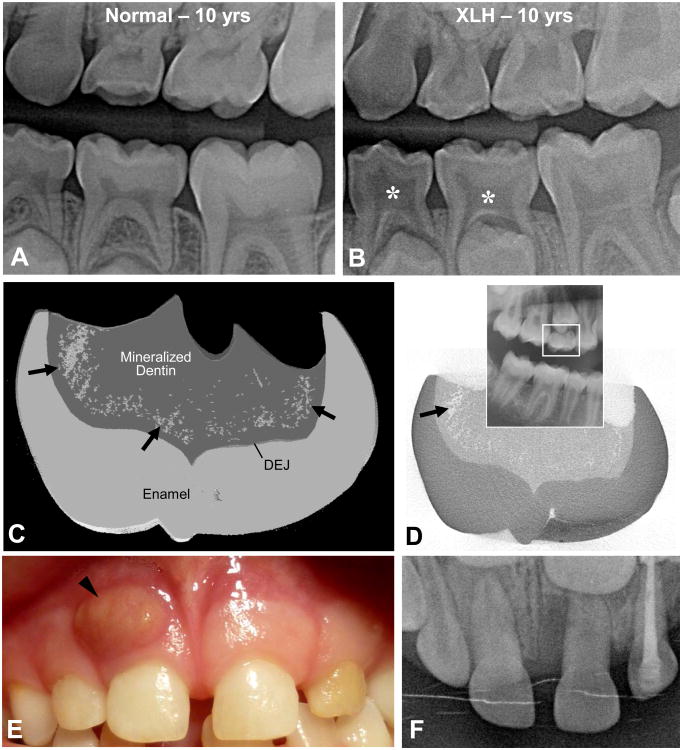
Figure 3.
(A,B) Tooth radiographs from a normal, 10-year-old male, and from a 10-year-old male patient with X-linked hypophosphatemia (XLH) caused by an inactivating mutation in the PHEX gene. In deciduous molars, note the enlarged pulp chambers (asterisks), the prominent pulp horns, and the radiolucency of the hypomineralized dentin that mineralized prior to the onset of systemic treatment. (C,D) Micro-computed tomography of a crown from the upper-right, second deciduous molar (seen on the panoramic X-ray inset) of a 13-year-old female X-linked hypophosphatemia female patient with a mutation in the PHEX gene. Mineralization voids (the spotty areas indicated by the arrows in panel C accumulating in the dentin near the dentino-enamel junction (DEJ) were rendered in grey using 3D reconstruction software to compile volumetric data from the 2D X-ray “slices,” one of which is depicted in panel D where the mineralization voids appear white. (E,F) Photograph and occlusal X-ray of the upper central right deciduous incisor from a 6-year-old X-linked hypophosphatemia male patient with a mutation of the PHEX gene. A large abscess (arrowhead) is observed related to the incisor root; the tooth was extracted after systemic treatment with antibiotics.
The major dental focus in X-linked hypophosphatemia patients has been on the tooth abnormalities alone, and only a few studies have extended this by exploring their overall periodontal status. Recently, a study has reported the periodontal condition of ten adults with familial hypophosphatemic rickets (188). They showed that 60% of the patients presented periodontal bone loss – a figure much higher than reported values for periodontitis prevalence of 3.6% to 7.3% (28, 29) – suggesting that patients with hypophosphatemic rickets are prone to increased periodontal bone loss and thus require more comprehensive examination by dental care providers. Our unpublished clinical data support the results of this study, and we generally observe symptoms of periodontal disease in roughly 70% of our adult X-linked hypophosphatemia patients (data from the “Centre de Référence des maladies rares du métabolisme du phosphore et du calcium,” Assistance Publique-Hôpitaux de Paris, France). Consistent with this, mice lacking DMP1 develop severe periodontal defects related to defective alveolar bone and cementum (187). Of note in this regard, proteolytic processing of DMP1 results in two major fragments (139) (a C-terminal 57 kDa and a 37 kDa N-terminal fragment) having different functions, the former inhibiting FGF23 expression (109), and both promoting hydroxyapatite formation (65).
Since the 1970s, a treatment has been introduced for X-linked hypophosphatemia which combines the administration of oral phosphate salts and 1α,25-dihydroxyvitamin D3, the hormonal form of vitamin D (in X-linked hypophosphatemia, vitamin D metabolism is perturbed). This treatment for X-linked hypophosphatemia patients has considerably improved their growth and bone mineralization, and it has decreased or prevented bone malformations thus reducing the need for surgical intervention (39, 69). This combined treatment has also had a substantial beneficial impact on oral health, especially for the permanent teeth, which mineralize after birth and for which the treatment has some effect. In 2003, we reported that the decayed, missing and filled teeth index of patients treated since early childhood was similar to the index of healthy, age-matched controls (43). Moreover, we showed that tooth examination formed an important part of the evaluation of the benefits of systemic treatment on mineralization (34, 44, 62). Indeed, permanent tooth analysis from patients treated since early childhood showed a rescue by this treatment resulting in normal dentin and a generally healthy overall tooth phenotype. In terms of periodontal disease, more work is required to determine the treatment effects on the periodontal status of these patients.
Specifically referring to X-linked hypophosphatemia, and as discussed in detail in the preceding section, PHEX is involved in mineral ion homeostasis and in the binding and proteolytic processing of the proteins and peptides regulating mineralization. Important to this are the high levels of expression of these proteins (relative to other cells types) by osteoblasts, osteocytes and odontoblasts (153, 165). In normal conditions, it has been described that PHEX protectively binds to MEPE to prevent ASARM peptide release by other proteases (74, 105), but when free inhibitory ASARM has been released, PHEX degrades it to promote mineralization (4, 6). PHEX also completely degrades full-length inhibitory osteopontin to promote mineralization, and in the absence of PHEX activity, an osteopontin protein fragment accumulates in Hyp mouse bone (19) – the murine mouse homolog of X-linked hypophosphatemia. With PHEX mutations in X-linked hypophosphatemia, excessive ASARM and inhibitory osteopontin fragments accumulate to inhibit mineralization. High levels of ASARM peptide have been detected in the serum of patients with X-linked hypophosphatemia, and in Hyp mice (35), and the peptide accumulates in the kidneys (35) where it impairs phosphate uptake from the urine and consequently induces hypophosphatemia (49). More recently, MEPE-derived ASARM peptides have been shown to inhibit in a dose-dependent manner the mineralization of human primary osteoblast cultures grown under differentiating conditions (13). Consistent with this, experiments both in vivo and in vitro have confirmed the capacity of MEPE-derived ASARM peptides to inhibit both dentin mineralization and odontoblast differentiation (unpublished data). In schematic format, key determinants of normal mineralization are summarized as they relate to X-linked hypophosphatemia (Fig. 4).
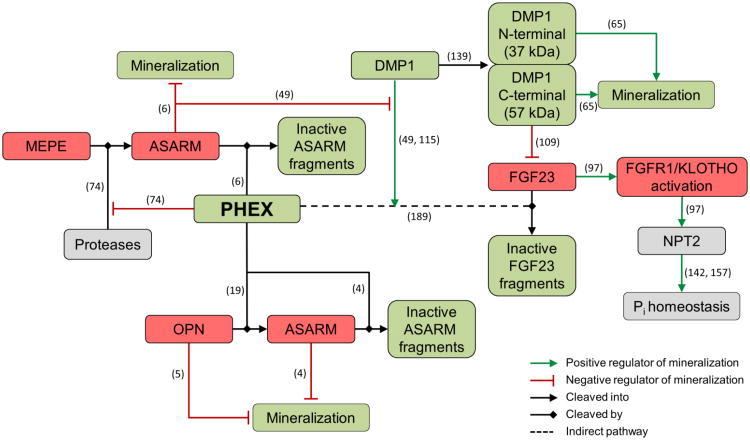
Figure 4.
Determinants of normal mineralization relevant to X-linked hypophosphatemia. Local and systemic regulation of mineralization is controlled by phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX). PHEX regulates mineralization locally at the level of the extracellular matrix by directly degrading mineralization inhibitors such as osteopontin and ASARM peptides. Systemic regulation involves influencing phosphate homeostasis indirectly through fibroblast growth factor 23 (FGF23), a key circulating factor that directs sodium-dependent phosphate transporters (NPT2) in the kidney and intestine, thus controling phosphate reabsorption. Green boxes indicate positive regulators of mineralization, red boxes indicate negative regulators of mineralization, and grey boxes indicate indirect regulators of mineralization. Citations supporting the indicated functions are shown by the numbers in parentheses.
Conclusions
The structure and function of the periodontium – including the teeth, bone and the soft surrounding tissues – act collectively to provide for occlusion and mastication. Essential to these activities is mineralization of bone and tooth extracellular matrices, and mineralization of the extremities of the periodontal ligament fibres where they insert either into alveolar bone at one end, and into cementum at the other end. The proper function of this suspensory apparatus relies on this essential mineralization, and diseases where bone and tooth mineralization is altered – such as X-linked hypophosphatemia and hypophosphatasia – invariably lead to malformed and weak bones and teeth, and premature tooth loss and tooth infections. Substantial progress has been made in recent years in understanding the underlying mechanisms contributing to mineralization diseases where bones are soft (hypomineralized), and some new clinical treatments are showing great promise in promoting mineralization, particularly of bone. Additional work is now required to extend this progress to the treatment of diseased dental tissues.
Acknowledgments
The authors thank their many laboratory members, and their basic science and clinical colleagues, as well as the patients and their families, for their contributions which have all in some way contributed over the years to the data, interpretations and new ideas presented in this review. Much of this work was funded by the Canadian Institutes of Health Research, the University Paris Descartes and Assistance Publique-Hôpitaux de Paris (France), the Fundacao de Amparo a Pesquisa do Estado de Sao Paulo and the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, and the National Institutes of Health Research (USA). The authors thank the Molson Medical Informatics project team at McGill University for creating schematic Figure 1A. Marc D. McKee has received research grants and consulting honoraria from Enobia Pharma (now Alexion Pharmaceuticals).
Footnotes
Guest Editors: Mark Bartold and Christopher McCulloch (Topical Issue for Perio 2000: Information generation and processing to regulate periodontal structure and function)
References
- 1.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 2.Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 3.Abhishek A, Doherty M. Pathophysiology of articular chondrocalcinosis--role of ANKH. Nat Rev Rheumatol. 2011;7:96–104. doi: 10.1038/nrrheum.2010.182. [DOI] [PubMed] [Google Scholar]
- 4.Addison WN, Masica DL, Gray JJ, McKee MD. Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res. 2010;25:695–705. doi: 10.1359/jbmr.090832. [DOI] [PubMed] [Google Scholar]
- 5.Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282:15872–15883. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 6.Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- 7.Alford AI, Hankenson KD. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Ali SY, Sajdera SW, Anderson HC. Isolation and Characterization of Calcifying Matrix Vesicles from Epiphyseal Cartilage. Proceedings of the National Academy of Sciences. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci. 2005;10:822–837. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 11.Anderson HC, Sipe JB, Hessle L, Dhamyamraju R, Atti E, Camacho NP, Millán JL. Impaired Calcification Around Matrix Vesicles of Growth Plate and Bone in Alkaline Phosphatase-Deficient Mice. The American Journal of Pathology. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argiro L, Desbarats M, Glorieux FH, Ecarot B. Mepe, the gene encoding a tumorsecreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001;74:342–351. doi: 10.1006/geno.2001.6553. [DOI] [PubMed] [Google Scholar]
- 13.Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM. Sclerostin is a locally acting regulator of lateosteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res. 2011;26:1425–1436. doi: 10.1002/jbmr.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery JK. Histology of the periodontium: aveolar bone, cementum and periodontal ligament. In: Avery JK, editor. Histology of the periodontium: aveolar bone, cementum and periodontal ligament. New York, NY, USA: Thieme; 2002. pp. 226–242. [Google Scholar]
- 15.Bar-On B, Daniel Wagner H. Enamel and dentin as multi-scale bio-composites. J Mech Behav Biomed Mater. 2012;12:174–183. doi: 10.1016/j.jmbbm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Bar-On B, Wagner HD. Elastic modulus of hard tissues. J Biomech. 2012;45:672–678. doi: 10.1016/j.jbiomech.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Bardet C, Delgado S, Sire JY. MEPE evolution in mammals reveals regions and residues of prime functional importance. Cell Mol Life Sci. 2010;67:305–320. doi: 10.1007/s00018-009-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardet C, Vincent C, Lajarille MC, Jaffredo T, Sire JY. OC-116, the chicken ortholog of mammalian MEPE found in eggshell, is also expressed in bone cells. J Exp Zool B Mol Dev Evol. 2010;314:653–662. doi: 10.1002/jez.b.21366. [DOI] [PubMed] [Google Scholar]
- 19.Barros NMT, Hoac B, Neves RL, Addison WN, Assis DM, Murshed M, Carmona AK, McKee MD. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1766. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Bartold PM. Bone and tooth interface: periodontal ligament. In: McCauley LK, Somerman MJ, editors. Bone and tooth interface: periodontal ligament. Ames, IA, USA: Wiley-Blackwell; 2012. pp. 219–230. [Google Scholar]
- 21.Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99:1200–1209. doi: 10.1172/JCI119276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beertsen W, VandenBos T, Everts V. Root Development in Mice Lacking Functional Tissue Non-specific Alkaline Phosphatase Gene: Inhibition of Acellular Cementum Formation. Journal of Dental Research. 1999;78:1221–1229. doi: 10.1177/00220345990780060501. [DOI] [PubMed] [Google Scholar]
- 23.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertoldo F, Santini D, Lo Cascio V. Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nat Clin Pract Oncol. 2007;4:711–721. doi: 10.1038/ncponc1000. [DOI] [PubMed] [Google Scholar]
- 25.Beumer J, Silverma S, Trowbrid Ho, Eisenber E. Childhood hypophosphatasia and premature loss of teeth - clinical and laboratory study of 7 cases. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics. 1973;35:631–640. doi: 10.1016/0030-4220(73)90028-5. [DOI] [PubMed] [Google Scholar]
- 26.Bonucci E. Fine structure and histochemistry of “calcifying globules” in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103:192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- 27.Borg TK, Runyan RB, Wuthier RE. Correlation of freeze-fracture and scanning electron microscopy of epiphyseal chondrocytes. Calcified Tissue International. 1978;26:237–241. doi: 10.1007/BF02013264. [DOI] [PubMed] [Google Scholar]
- 28.Borrell LN, Crawford ND. Social disparities in periodontitis among United States adults 1999-2004. Community Dent Oral Epidemiol. 2008;36:383–391. doi: 10.1111/j.1600-0528.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 29.Borrell LN, Burt BA, Taylor GW. Prevalence and trends in periodontitis in the USA: the [corrected] NHANES, 1988 to 2000. J Dent Res. 2005;84:924–930. doi: 10.1177/154405910508401010. [DOI] [PubMed] [Google Scholar]
- 30.Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89:1333–1348. doi: 10.1177/0022034510377791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 32.Bosshardt DD, Zalzal S, McKee MD, Nanci A. Developmental appearance and distribution of bone sialoprotein and osteopontin in human and rat cementum. Anat Rec. 1998;250:13–33. doi: 10.1002/(SICI)1097-0185(199801)250:1<13::AID-AR3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Boukpessi T, Septier D, Bagga S, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int. 2006;79:294–300. doi: 10.1007/s00223-006-0182-4. [DOI] [PubMed] [Google Scholar]
- 34.Boukpessi T, Gaucher C, Leger T, Salmon B, Le Faouder J, Willig C, Rowe PS, Garabedian M, Meilhac O, Chaussain C. Abnormal presence of the matrix extracellular phosphoglycoprotein-derived acidic serine- and aspartate-rich motif peptide in human hypophosphatemic dentin. Am J Pathol. 2010;177:803–812. doi: 10.2353/ajpath.2010.091231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bresler D, Bruder J, Mohnike K, Fraser WD, Rowe PS. Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): implications for phosphaturia and rickets. J Endocrinol. 2004;183:R1–9. doi: 10.1677/joe.1.05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bronckers AL, Farach-Carson MC, Van Waveren E, Butler WT. Immunolocalization of osteopontin, osteocalcin, and dentin sialoprotein during dental root formation and early cementogenesis in the rat. J Bone Miner Res. 1994;9:833–841. doi: 10.1002/jbmr.5650090609. [DOI] [PubMed] [Google Scholar]
- 37.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruckner RJ, Rickles NH, Porter DR. Hypophosphatasia with premature shedding of teeth and aplasia of cementum. Oral Surg Oral Med Oral Pathol. 1962;15:1351–1369. doi: 10.1016/0030-4220(62)90356-0. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26:1381–1388. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cazalbou S, Combes C, Eichert D, Rey C, Glimcher MJ. Poorly crystalline apatites: evolution and maturation in vitro and in vivo. J Bone Miner Metab. 2004;22:310–317. doi: 10.1007/s00774-004-0488-0. [DOI] [PubMed] [Google Scholar]
- 41.Cecil RNA, Clarke Anderson H. Freeze-fracture studies of matrix vesicle calcification in epiphyseal growth plate. Metabolic Bone Disease and Related Research. 1978;1:89–95. doi: 10.1016/0221-8747(83)90014-0. [DOI] [PubMed] [Google Scholar]
- 42.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 43.Chaussain-Miller C, Sinding C, Wolikow M, Lasfargues JJ, Godeau G, Garabedian M. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets: prevention by early treatment with 1-hydroxyvitamin D. J Pediatr. 2003;142:324–331. doi: 10.1067/mpd.2003.119. [DOI] [PubMed] [Google Scholar]
- 44.Chaussain-Miller C, Sinding C, Septier D, Wolikow M, Goldberg M, Garabedian M. Dentin structure in familial hypophosphatemic rickets: benefits of vitamin D and phosphate treatment. Oral Dis. 2007;13:482–489. doi: 10.1111/j.1601-0825.2006.01326.x. [DOI] [PubMed] [Google Scholar]
- 45.Ciancaglini P, Yadav MC, Simão AMS, Narisawa S, Pizauro JM, Farquharson C, Hoylaerts MF, Millán JL. Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. Journal of Bone and Mineral Research. 2010;25:716–723. doi: 10.1359/jbmr.091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79:1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 47.Cole JH, van der Meulen MC. Whole bone mechanics and bone quality. Clin Orthop Relat Res. 2011;469:2139–2149. doi: 10.1007/s11999-011-1784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David V, Martin A, Hedge AM, Rowe PS. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology. 2009;150:4012–4023. doi: 10.1210/en.2009-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.David V, Martin A, Hedge AM, Drezner MK, Rowe PS. ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am J Physiol Renal Physiol. 2011;300:F783–791. doi: 10.1152/ajprenal.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Labban NG, Lee KW, Rule D. Permanent teeth in hypophosphatasia: light and electron microscopic study. Journal of Oral Pathology & Medicine. 1991;20:352–360. doi: 10.1111/j.1600-0714.1991.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 51.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(1):33–40. [PubMed] [Google Scholar]
- 53.Fleisch H, Bisaz S. Mechanism of calcification: inhibitory role of pyrophosphate. Nature. 1962;195:911. doi: 10.1038/195911a0. [DOI] [PubMed] [Google Scholar]
- 54.Foster BL, Somerman MJ. Cementum. In: McCauley LK, Somerman MJ, editors. Cementum. Ames, IA, USA: Wiley-Blackwell; 2012. pp. 169–181. [Google Scholar]
- 55.Foster BL, Tompkins KA, Rutherford RB, Zhang H, Chu EY, Fong H, Somerman MJ. Phosphate: known and potential roles during development and regeneration of teeth and supporting structures. Birth Defects Res C Embryo Today. 2008;84:281–314. doi: 10.1002/bdrc.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster BL, Nagatomo KJ, Nociti FH, Jr, Fong H, Dunn D, Tran AB, Wang W, Narisawa S, Millán JL, Somerman MJ. Central Role of Pyrophosphate in Acellular Cementum Formation. PLoS ONE. 2012;7:e38393. doi: 10.1371/journal.pone.0038393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franzen A, Hultenby K, Reinholt FP, Onnerfjord P, Heinegard D. Altered osteoclast development and function in osteopontin deficient mice. J Orthop Res. 2008;26:721–728. doi: 10.1002/jor.20544. [DOI] [PubMed] [Google Scholar]
- 58.Fraser D. Hypophosphatasia. The American Journal of Medicine. 1957;22:730–746. doi: 10.1016/0002-9343(57)90124-9. [DOI] [PubMed] [Google Scholar]
- 59.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 60.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 61.Gaucher C, Walrant-Debray O, Nguyen TM, Esterle L, Garabedian M, Jehan F. PHEX analysis in 118 pedigrees reveals new genetic clues in hypophosphatemic rickets. Hum Genet. 2009;125:401–411. doi: 10.1007/s00439-009-0631-z. [DOI] [PubMed] [Google Scholar]
- 62.Gaucher C, Boukpessi T, Septier D, Jehan F, Rowe PS, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin noncollagenous matrix proteins in familial hypophosphatemic rickets. Cells Tissues Organs. 2009;189:219–223. doi: 10.1159/000151382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 2008;108:4670–4693. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sorensen ES, Boskey AL. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey AL. Different forms of DMP1 play distinct roles in mineralization. J Dent Res. 2010;89:355–359. doi: 10.1177/0022034510363250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 67.Gibson CW, Snead ML. Enamel fabrication: the story of amelogenesis. In: McCauley LK, Somerman MJ, editors. Enamel fabrication: the story of amelogenesis. Ames, IA, USA: Wiley-Blackwell; 2012. pp. 153–161. [Google Scholar]
- 68.Glimcher MJ. Bone: Nature of the Calcium Phosphate Crystals and Cellular, Structural, and Physical Chemical Mechanisms in Their Formation. Reviews in Mineralogy and Geochemistry. 2006;64:223–282. [Google Scholar]
- 69.Glorieux FH, Marie PJ, Pettifor JM, Delvin EE. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980;303:1023–1031. doi: 10.1056/NEJM198010303031802. [DOI] [PubMed] [Google Scholar]
- 70.Golub EE. Role of matrix vesicles in biomineralization. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790:1592–1598. doi: 10.1016/j.bbagen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 72.Groeneveld MC, Everts V, Beertsen W. Alkaline Phosphatase Activity in the Periodontal Ligament and Gingiva of the Rat Molar: Its Relation to Cementum Formation. Journal of Dental Research. 1995;74:1374–1381. doi: 10.1177/00220345950740070901. [DOI] [PubMed] [Google Scholar]
- 73.Guo R, Quarles LD. Cloning and sequencing of human PEX from a bone cDNA library: evidence for its developmental stage-specific regulation in osteoblasts. J Bone Miner Res. 1997;12:1009–1017. doi: 10.1359/jbmr.1997.12.7.1009. [DOI] [PubMed] [Google Scholar]
- 74.Guo R, Rowe PS, Liu S, Simpson LG, Xiao ZS, Quarles LD. Inhibition of MEPE cleavage by Phex. Biochem Biophys Res Commun. 2002;297:38–45. doi: 10.1016/s0006-291x(02)02125-3. [DOI] [PubMed] [Google Scholar]
- 75.Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79:1017–1029. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harmey D, Johnson KA, Zelken J, Camacho NP, Hoylaerts MF, Noda M, Terkeltaub R, Millán JL. Elevated Skeletal Osteopontin Levels Contribute to the Hypophosphatasia Phenotype in Akp2−/− Mice. Journal of Bone and Mineral Research. 2006;21:1377–1387. doi: 10.1359/jbmr.060619. [DOI] [PubMed] [Google Scholar]
- 78.Harris NL, Rattray KR, Tye CE, Underhill TM, Somerman MJ, D'Errico JA, Chambers AF, Hunter GK, Goldberg HA. Functional analysis of bone sialoprotein: identification of the hydroxyapatite-nucleating and cell-binding domains by recombinant peptide expression and site-directed mutagenesis. Bone. 2000;27:795–802. doi: 10.1016/s8756-3282(00)00392-6. [DOI] [PubMed] [Google Scholar]
- 79.Hasselgren G, Franzén A, Hammarström LE. Histochemical characterization of alkaline phosphatase in developing rat teeth and bone. European Journal of Oral Sciences. 1978;86:325–336. doi: 10.1111/j.1600-0722.1978.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 80.Hayashibara T, Hiraga T, Yi B, Nomizu M, Kumagai Y, Nishimura R, Yoneda T. A synthetic peptide fragment of human MEPE stimulates new bone formation in vitro and in vivo. J Bone Miner Res. 2004;19:455–462. doi: 10.1359/JBMR.0301263. [DOI] [PubMed] [Google Scholar]
- 81.He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, Hao J, George A. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herring SW. Biomechanics of teeth in bone: function, movement, and prosthetic rehabilitation. In: McCauley LK, Somerman MJ, editors. Biomechanics of teeth in bone: function, movement, and prosthetic rehabilitation. Ames, IA, USA: Wiley-Blackwell; 2012. pp. 255–267. [Google Scholar]
- 83.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. PNAS. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirata A, Sugahara T, Nakamura H. Localization of runx2, osterix, and osteopontin in tooth root formation in rat molars. J Histochem Cytochem. 2009;57:397–403. doi: 10.1369/jhc.2008.952192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 86.Ho SP, Kurylo MP, Fong TK, Lee SS, Wagner HD, Ryder MI, Marshall GW. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials. 2010;31:6635–6646. doi: 10.1016/j.biomaterials.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoshi K, Amizuka N, Oda K, Ikehara Y, Ozawa H. Immunolocalization of tissue nonspecific alkaline phosphatase in mice. Histochemistry and Cell Biology. 1997;107:183–191. doi: 10.1007/s004180050103. [DOI] [PubMed] [Google Scholar]
- 88.Hu JC, Chun YH, Al Hazzazzi T, Simmer JP. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs. 2007;186:78–85. doi: 10.1159/000102683. [DOI] [PubMed] [Google Scholar]
- 89.Hu JCC, Plaetke R, Mornet E, Zhang C, Sun X, Thomas HF, Simmer JP. Characterization of a family with dominant hypophosphatasia. European Journal of Oral Sciences. 2000;108:189–194. doi: 10.1034/j.1600-0722.2000.108003189.x. [DOI] [PubMed] [Google Scholar]
- 90.Jedrychowski JR, Duperon D. Childhood hypophosphatasia with oral manifestations. J Oral Med. 1979;34:18–22. [PubMed] [Google Scholar]
- 91.Johnson K, Moffa A, Chen Y, Pritzker K, Goding J, Terkeltaub R. Matrix vesicle plasma cell membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res. 1999;14:883–892. doi: 10.1359/jbmr.1999.14.6.883. [DOI] [PubMed] [Google Scholar]
- 92.Johnson K, Goding J, Van Etten D, Sali A, Hu SI, Farley D, Krug H, Hessle L, Millan JL, Terkeltaub R. Linked deficiencies in extracellular PP(i) and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. J Bone Miner Res. 2003;18:994–1004. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- 93.Kaartinen MT, El-Maadawy S, Rasanen NH, McKee MD. Tissue transglutaminase and its substrates in bone. J Bone Miner Res. 2002;17:2161–2173. doi: 10.1359/jbmr.2002.17.12.2161. [DOI] [PubMed] [Google Scholar]
- 94.Kaartinen MT, Sun W, Kaipatur N, McKee MD. Transglutaminase crosslinking of SIBLING proteins in teeth. J Dent Res. 2005;84:607–612. doi: 10.1177/154405910508400705. [DOI] [PubMed] [Google Scholar]
- 95.Kawasaki K, Weiss KM. Evolutionary genetics of vertebrate tissue mineralization: the origin and evolution of the secretory calcium-binding phosphoprotein family. J Exp Zool B Mol Dev Evol. 2006;306:295–316. doi: 10.1002/jez.b.21088. [DOI] [PubMed] [Google Scholar]
- 96.Kulkarni GV, Chen B, Malone JP, Narayanan AS, George A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch Oral Biol. 2000;45:475–484. doi: 10.1016/s0003-9969(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 97.Kuro-o M. Klotho and βKlotho. Adv Exp Med Biol. 2012;728:25–40. doi: 10.1007/978-1-4614-0887-1_2. [DOI] [PubMed] [Google Scholar]
- 98.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 99.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc Res Tech. 1996;33:192–202. doi: 10.1002/(SICI)1097-0029(19960201)33:2<192::AID-JEMT9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 101.Lao M, Marino V, Bartold PM. Immunohistochemical study of bone sialoprotein and osteopontin in healthy and diseased root surfaces. J Periodontol. 2006;77:1665–1673. doi: 10.1902/jop.2006.060087. [DOI] [PubMed] [Google Scholar]
- 102.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM, Giachelli CM. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu H, Li W, Shi S, Habelitz S, Gao C, Denbesten P. MEPE is downregulated as dental pulp stem cells differentiate. Arch Oral Biol. 2005;50:923–928. doi: 10.1016/j.archoralbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 105.Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD. Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol. 2007;192:261–267. doi: 10.1677/joe.1.07059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL, McKee MD, Drezner MK, Bonewald LF, Feng JQ. The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J Bone Miner Res. 2011;26:331–340. doi: 10.1002/jbmr.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 111.Macfarlane JD, Swart JG. Dental aspects of hypophosphatasia: a case report, family study, and literature review. Oral Surg Oral Med Oral Pathol. 1989;67:521–526. doi: 10.1016/0030-4220(89)90266-1. [DOI] [PubMed] [Google Scholar]
- 112.Machado JP, Johnson WE, O'Brien SJ, Vasconcelos V, Antunes A. Adaptive evolution of the matrix extracellular phosphoglycoprotein in mammals. BMC Evol Biol. 2011;11:342. doi: 10.1186/1471-2148-11-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Malaval L, Wade-Gueye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, Laroche N, Roux JP, Burt-Pichat B, Duboeuf F, Boivin G, Jurdic P, Lafage-Proust MH, Amedee J, Vico L, Rossant J, Aubin JE. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med. 2008;205:1145–1153. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McCulloch CA, Lekic P, McKee MD. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol 2000. 2000;24:56–72. doi: 10.1034/j.1600-0757.2000.2240104.x. [DOI] [PubMed] [Google Scholar]
- 117.McKee MD, Nanci A. Postembedding colloidal-gold immunocytochemistry of noncollagenous extracellular matrix proteins in mineralized tissues. Microsc Res Tech. 1995;31:44–62. doi: 10.1002/jemt.1070310105. [DOI] [PubMed] [Google Scholar]
- 118.McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33:141–164. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 119.McKee MD, Zalzal S, Nanci A. Extracellular matrix in tooth cementum and mantle dentin: localization of osteopontin and other noncollagenous proteins, plasma proteins, and glycoconjugates by electron microscopy. Anat Rec. 1996;245:293–312. doi: 10.1002/(SICI)1097-0185(199606)245:2<293::AID-AR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 120.McKee MD, Addison WN, Kaartinen MT. Hierarchies of extracellular matrix and mineral organization in bone of the craniofacial complex and skeleton. Cells Tissues Organs. 2005;181:176–188. doi: 10.1159/000091379. [DOI] [PubMed] [Google Scholar]
- 121.McKee MD, Murshed M, Kaartinen MT. Extracellular matrix and mineralization of craniofacial bone. In: McCauley LK, Somerman MJ, editors. Extracellular matrix and mineralization of craniofacial bone. Ames, IA, USA: Wiley-Blackwell; 2012. pp. 99–109. [Google Scholar]
- 122.McKee MD, Nakano Y, Masica DL, Gray JJ, Lemire I, Heft R, Whyte MP, Crine P, Millán JL. Enzyme Replacement Therapy Prevents Dental Defects in a Model of Hypophosphatasia. Journal of Dental Research. 2011;90:470–476. doi: 10.1177/0022034510393517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McNally EA, Schwarcz HP, Botton GA, Arsenault AL. A Model for the Ultrastructur of Bone Based on Electron Microscopy of Ion-Milled Sections. PLoS ONE. 2012;7:e29258. doi: 10.1371/journal.pone.0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 125.Millán J, Narisawa S, Lemire I, Loisel T, Boileau G, Leonard P, Gramatikova S, Terkeltaub R, Camacho N, McKee M, Crine P, Whyte M. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Millan JL. Mammalian Alkaline Phosphatases. Weinheim: Wiley-VCH Verlag GmbH & Co., KGaA; 2006. [Google Scholar]
- 127.Murayama T, Iwatsubo R, Akiyama S, Amano A, Morisaki I. Familial hypophosphatemic vitamin D-resistant rickets: dental findings and histologic study of teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:310–316. doi: 10.1067/moe.2000.107522. [DOI] [PubMed] [Google Scholar]
- 128.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nampei A, Hashimoto J, Hayashida K, Tsuboi H, Shi K, Tsuji I, Miyashita H, Yamada T, Matsukawa N, Matsumoto M, Morimoto S, Ogihara T, Ochi T, Yoshikaw H. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J Bone Miner Metab. 2004;22:176–184. doi: 10.1007/s00774-003-0468-9. [DOI] [PubMed] [Google Scholar]
- 130.Nanci A, Moffatt P. Embryology of craniofacial bones. In: McCauley LK, Somerman MJ, editors. Embryology of craniofacial bones. Ames, IA, USA: Wiley-Blackwell; 2012. pp. 3–11. [Google Scholar]
- 131.Narisawa S, Frohlander N, Millan JL. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 132.Naveh GR, Lev-Tov Chattah N, Zaslansky P, Shahar R, Weiner S. Tooth-PDL-bone complex: Response to compressive loads encountered during mastication - A review. Arch Oral Biol. 2012 doi: 10.1016/j.archoralbio.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 133.Omelon S, Georgiou J, Henneman ZJ, Wise LM, Sukhu B, Hunt T, Wynnyckyj C, Holmyard D, Bielecki R, Grynpas MD. Control of Vertebrate Skeletal Mineralization by Polyphosphates. PLoS ONE. 2009;4:e5634. doi: 10.1371/journal.pone.0005634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Opsahl Vital S, Gaucher C, Bardet C, Rowe PS, George A, Linglart A, Chaussain C. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone. 2012;50:989–997. doi: 10.1016/j.bone.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prasad M, Butler WT, Qin C. Dentin sialophosphoprotein in biomineralization. Connect Tissue Res. 2010;51:404–417. doi: 10.3109/03008200903329789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qin C, Feng JQ. Dentin. In: McCauley LK, Somerman MJ, editors. Dentin. Ames, IA, USA: Wiley-Blackwell; 2012. pp. 135–141. [Google Scholar]
- 137.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 138.Qin C, D'Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86:1134–1141. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- 139.Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 140.Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 141.Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285:E1–9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- 142.Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reibel A, Manière M, Clauss F, Droz D, Alembik Y, Mornet E, Bloch-Zupan A. Orodental phenotype and genotype findings in all subtypes of hypophosphatasia. Orphanet J Rare Dis. 2009;4:6. doi: 10.1186/1750-1172-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rey C, Combes C, Drouet C, Glimcher M. Bone mineral: update on chemical composition and structure. Osteoporosis International. 2009;20:1013–1021. doi: 10.1007/s00198-009-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roberts S, Narisawa S, Harmey D, Millan JL, Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J Bone Miner Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 146.Roberts SJ, Stewart AJ, Sadler PJ, Farquharson C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem J. 2004;382:59–65. doi: 10.1042/BJ20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rowe PS. The wrickkened pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med. 2004;15:264–281. doi: 10.1177/154411130401500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rowe PS. The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled. Cell Biochem Funct. 2012;30:355–375. doi: 10.1002/cbf.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 150.Rowe PS, Garrett IR, Schwarz PM, Carnes DL, Lafer EM, Mundy GR, Gutierrez GE. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP) Bone. 2005;36:33–46. doi: 10.1016/j.bone.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruchon AF, Marcinkiewicz M, Siegfried G, Tenenhouse HS, DesGroseillers L, Crine P, Boileau G. Pex mRNA is localized in developing mouse osteoblasts and odontoblasts. J Histochem Cytochem. 1998;46:459–468. doi: 10.1177/002215549804600405. [DOI] [PubMed] [Google Scholar]
- 153.Ruchon AF, Tenenhouse HS, Marcinkiewicz M, Siegfried G, Aubin JE, DesGroseillers L, Crine P, Boileau G. Developmental expression and tissue distribution of Phex protein: effect of the Hyp mutation and relationship to bone markers. J Bone Miner Res. 2000;15:1440–1450. doi: 10.1359/jbmr.2000.15.8.1440. [DOI] [PubMed] [Google Scholar]
- 154.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nurnberg P. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 155.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K. Growth-related renal type II Na/Pi cotransporter. J Biol Chem. 2002;277:19665–19672. doi: 10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- 156.Seow WK, Brown JP, Tudehope DA, O'Callaghan M. Dental defects in the deciduous dentition of premature infants with low birth weight and neonatal rickets. Pediatr Dent. 1984;6:88–92. [PubMed] [Google Scholar]
- 157.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Silver FH, Landis WJ. Deposition of apatite in mineralizing vertebrate extracellular matrices: A model of possible nucleation sites on type I collagen. Connect Tissue Res. 2011;52:242–254. doi: 10.3109/03008207.2010.551567. [DOI] [PubMed] [Google Scholar]
- 159.Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, Hu JC. Regulation of dental enamel shape and hardness. J Dent Res. 2010;89:1024–1038. doi: 10.1177/0022034510375829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Six N, Septier D, Chaussain-Miller C, Blacher R, DenBesten P, Goldberg M. Dentonin, a MEPE fragment, initiates pulp-healing response to injury. J Dent Res. 2007;86:780–785. doi: 10.1177/154405910708600818. [DOI] [PubMed] [Google Scholar]
- 161.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 162.Sun Y, Lu Y, Chen L, Gao T, D'Souza R, Feng JQ, Qin C. DMP1 Processing is Essential to Dentin and Jaw Formation. J Dent Res. 2011 doi: 10.1177/0022034510397839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun Y, Prasad M, Gao T, Wang X, Zhu Q, D'Souza R, Feng JQ, Qin C. Failure to process dentin matrix protein 1 (DMP1) into fragments leads to its loss of function in osteogenesis. J Biol Chem. 2010;285:31713–31722. doi: 10.1074/jbc.M110.137059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Terkeltaub R. Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification. Purinergic Signal. 2006;2:371–377. doi: 10.1007/s11302-005-5304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thompson DL, Sabbagh Y, Tenenhouse HS, Roche PC, Drezner MK, Salisbury JL, Grande JP, Poeschla EM, Kumar R. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res. 2002;17:311–320. doi: 10.1359/jbmr.2002.17.2.311. [DOI] [PubMed] [Google Scholar]
- 166.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282:22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 167.Traub W, Arad T, Weiner S. Origin of mineral crystal growth in collagen fibrils. Matrix. 1992;12:251–255. doi: 10.1016/s0934-8832(11)80076-4. [DOI] [PubMed] [Google Scholar]
- 168.Tricker ND, Dixon RB, Garetto LP. Cortical bone turnover and mineral apposition in dentate bone mandible. In: Garetto LP, Turner CH, Duncan RL, Burr DB, editors. Cortical bone turnover and mineral apposition in dentate bone mandible. Indiana University School of Dentistry; 2002. pp. 226–227. [Google Scholar]
- 169.van den Bos T, Beertsen W. Alkaline phosphatase activity in human periodontal ligament: age effect and relation to cementum growth rate*. Journal of Periodontal Research. 1999;34:1–6. doi: 10.1111/j.1600-0765.1999.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 170.van den Bos T, Handoko G, Niehof A, Ryan LM, Coburn SP, Whyte MP, Beertsen W. Cementum and dentin in hypophosphatasia. J Dent Res. 2005;84:1021–1025. doi: 10.1177/154405910508401110. [DOI] [PubMed] [Google Scholar]
- 171.Vetter U, Weis MA, Morike M, Eanes ED, Eyre DR. Collagen crosslinks and mineral crystallinity in bone of patients with osteogenesis imperfecta. J Bone Miner Res. 1993;8:133–137. doi: 10.1002/jbmr.5650080203. [DOI] [PubMed] [Google Scholar]
- 172.Wagner HD, Weiner S. On the relationship between the microstructure of bone and its mechanical stiffness. J Biomech. 1992;25:1311–1320. doi: 10.1016/0021-9290(92)90286-a. [DOI] [PubMed] [Google Scholar]
- 173.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 174.Watanabe H, Umeda M, Seki T, Ishikawa I. Clinical and laboratory studies of severe periodontal disease in an adolescent associated with hypophosphatasia. A case report. J Periodontol. 1993;64:174–180. doi: 10.1902/jop.1993.64.3.174. [DOI] [PubMed] [Google Scholar]
- 175.Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15:439–461. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- 176.Whyte MP. Hypophosphatasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, editors. Hypophosphatasia. New York, NY, USA: McGraw-Hill; 2001. pp. 5313–5329. [Google Scholar]
- 177.Whyte MP. Hypophosphatasia Nature's window to alkaline phosphatase in man. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Hypophosphatasia Nature's window to alkaline phosphatase in man. San Diego, CA: Academic Press; 2002. pp. 1229–1248. [Google Scholar]
- 178.Whyte MP. Hypophosphatasia: nature's window on alkaline phosphatase function in humans. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Hypophosphatasia: nature's window on alkaline phosphatase function in humans. San Diego, CA: Academic Press; 2008. pp. 1573–1598. [Google Scholar]
- 179.Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, Van Sickle BJ, Simmons JH, Edgar TS, Bauer ML, Hamdan MA, Bishop N, Lutz RE, McGinn M, Craig S, Moore JN, Taylor JW, Cleveland RH, Cranley WR, Lim R, Thacher TD, Mayhew JE, Downs M, Millán JL, Skrinar AM, Crine P, Landy H. Enzyme-Replacement Therapy in Life-Threatening Hypophosphatasia. New England Journal of Medicine. 2012;366:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 180.Woltgens JH, Lyaruu DM, Bronckers AL, Bervoets TJ, Van Duin M. Biomineralization during early stages of the developing tooth in vitro with special reference to secretory stage of amelogenesis. Int J Dev Biol. 1995;39:203–212. [PubMed] [Google Scholar]
- 181.Wuthier RE. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1975;409:128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]
- 182.Wuthier RE, Lipscomb GF. Matrix vesicles: structure, composition, formation and function in calcification. Front Biosci. 2011;16:2812–2902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- 183.Wuthier RE, Linder RE, Warner GP, Gore ST, Borg TK. Non-enzymatic isolation of matrix vesicles: characterization and initial studies on 45Ca and 32P-orthophosphate metabolism. Metabolic Bone Disease and Related Research. 1978;1:125–136. [Google Scholar]
- 184.Yadav MC, Simão AMS, Narisawa S, Huesa C, McKee MD, Farquharson C, Millán JL. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: A unified model of the mechanisms of initiation of skeletal calcification. Journal of Bone and Mineral Research. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yadav MC, de Oliveira RC, Foster BL, Fong H, Cory E, Narisawa S, Sah RL, Somerman M, Whyte MP, Millan JL. Enzyme replacement prevents enamel defects in hypophosphatasia mice. J Bone Miner Res. 2012;27:1722–1734. doi: 10.1002/jbmr.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yamamoto T, Domon T, Takahashi S, Arambawatta AK, Anjuman KA, Fukushima C, Wakita M. Determination of two different types of cellular cementogenesis in rat molars: a histological and immunohistochemical study. Matrix Biol. 2005;24:295–305. doi: 10.1016/j.matbio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 187.Ye L, Zhang S, Ke H, Bonewald LF, Feng JQ. Periodontal breakdown in the Dmp1 null mouse model of hypophosphatemic rickets. J Dent Res. 2008;87:624–629. doi: 10.1177/154405910808700708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ye L, Liu R, White N, Alon US, Cobb CM. Periodontal status of patients with hypophosphatemic rickets: a case series. J Periodontol. 2011;82:1530–1535. doi: 10.1902/jop.2011.100736. [DOI] [PubMed] [Google Scholar]
- 189.Yuan B, Feng JQ, Bowman S, Liu Y, Blank RD, Lindberg I, Drezner MK. Hexa-D-Arginine treatment increases 7B2*PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]