Abstract
BACKGROUND
Limited information is available regarding genetic contributions to valvular calcification, which is an important precursor of clinical valve disease.
METHODS
We determined genomewide associations with the presence of aorticvalve calcification (among 6942 participants) and mitral annular calcification (among 3795 participants), as detected by computed tomographic (CT) scanning; the study population for this analysis included persons of white European ancestry from three cohorts participating in the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium (discovery population). Findings were replicated in independent cohorts of persons with either CT-detected valvular calcification or clinical aortic stenosis.
RESULTS
One SNP in the lipoprotein(a) (LPA) locus (rs10455872) reached genomewide significance for the presence of aorticvalve calcification (odds ratio per allele, 2.05; P = 9.0×10−10), a finding that was replicated in additional white European, African-American, and Hispanic-American cohorts (P<0.05 for all comparisons). Genetically determined Lp(a) levels, as predicted by LPA genotype, were also associated with aorticvalve calcification, supporting a causal role for Lp(a). In prospective analyses, LPA genotype was associated with incident aortic stenosis (hazard ratio per allele, 1.68; 95% confidence interval [CI], 1.32 to 2.15) and aortic-valve replacement (hazard ratio, 1.54; 95% CI, 1.05 to 2.27) in a large Swedish cohort; the association with incident aortic stenosis was also replicated in an independent Danish cohort. Two SNPs (rs17659543 and rs13415097) near the proinflammatory gene IL1F9 achieved genomewide significance for mitral annular calcification (P = 1.5×10−8 and P = 1.8×10−8, respectively), but the findings were not replicated consistently.
CONCLUSIONS
Genetic variation in the LPA locus, mediated by Lp(a) levels, is associated with aorticvalve calcification across multiple ethnic groups and with incident clinical aortic stenosis. (Funded by the National Heart, Lung, and Blood Institute and others.)
Valvular calcification precedes the development of valvular stenosis and may represent an important early phenotype for valvular heart disease. Although aortic sclerosis is frequently considered to be a benign condition, it is associated with progression to clinical aortic stenosis1,2 and with increased cardiovascular morbidity and mortality.3 In addition, mitral annular calcification is associated with a risk of cardiovascular disease that is increased by nearly 50%.4 Currently, there are no treatments that prevent or slow the progression of valve disease.
Although genetic factors may influence the development of valvular calcification, which tends to run in families,5 the role of common genetic variation in valvular calcification remains unknown. Knowledge of the genetic determinants of valvular calcification may help elucidate the mechanisms underlying valvular heart disease and could foster the development of new therapies. We performed a genomewide association study of aorticvalve calcification and mitral annular calcification in three population-based cohorts. The results were confirmed in additional multiethnic cohorts by means of computed tomographic (CT) assessment of valvular calcification or identification of clinically apparent valvular heart disease.
METHODS
STUDY DESIGN AND POPULATION
This investigation was initiated within the Cohorts for Heart and Aging Research in Genome Epidemiology (CHARGE) consortium. The CHARGE consortium is an ongoing investigator-driven collaboration among several large, population-based cohort studies in which genomewide genotype data have been obtained and comprehensive individual phenotyping for a variety of clinical characteristics has been performed. Details of this collaboration have been described previously.6
We performed a two-stage analysis to discover the associations of genetic loci with the presence of mitral annular calcification and aorticvalve calcification and to replicate the findings. In the discovery stage, we obtained genomewide association data from the Framingham Heart Study (FHS) cohort, the Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-RS) cohort, and white European participants in the Multi-Ethnic Study of Atherosclerosis (MESA) for the initial meta-analysis.7–10 All the cohort participants in the discovery stage were of white European ancestry and had undergone genotyping and CT scanning for the presence of aorticvalve calcification and mitral annular calcification (in the FHS and MESA) or aorticvalve calcification alone (in the AGES-RS).
In the replication stage, significant findings from the initial meta-analysis were tested in additional European and multiethnic cohorts, including white European participants in the Heinz Nixdorf Recall Study (HNR); African-American, Chinese-American, and Hispanic-American participants in the MESA and MESA Family; and Swedish and Danish participants with registrydefined clinical aortic stenosis in the Malmö Diet and Cancer Study (MDCS) and the Copenhagen City Heart Study (CCHS), respectively.11–13
Before participating in their respective cohorts, all participants provided written informed consent, including consent for genotyping and analysis. The relevant study protocol was approved by the local review board at each participating site. The last three authors vouch for the accuracy of the data. Detailed information about all the study cohorts is provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
DEMOGRAPHIC AND COVARIATE DATA
In the FHS, AGES-RS, MESA, and HNR, clinical and demographic data on individual participants were collected at the time of CT scanning; a comprehensive medical history and anthropometric measurements were obtained, vital signs were assessed, and fasting blood samples were analyzed. In the FHS and AGES-RS, plasma lipoprotein(a) [Lp(a)] concentrations were measured (see the Supplementary Appendix) with the use of an enzymelinked immunosorbent assay and a turbidimetric immunoassay (Denka Seiken), respectively, neither of which is affected by apolipoprotein(a) isoform size.
ASSESSMENT OF VALVULAR AND CORONARY CALCIFICATION AND CLINICAL AORTIC STENOSIS
All the participants underwent standard CT scanning, and images were analyzed for the presence of coronary-artery calcification, aortic-valve calcification, and mitral annular calcification (see the Supplementary Appendix) with the use of off-line digital software. Using standard Agatston methods,14 we considered three or more contigu-ous pixels with a brightness of at least 130 Hounsfield units to indicate the presence of calcium. Standard methods for assessing coronary-artery calcification were used.15,16 Lesions were classified as aortic-valve calcification if they resided within the aortic-valve leaflets or commissures, excluding the aortic annulus, proximal aorta, and coronary arteries. Mitral annular calcification was detected in a similar way and included calcification along the mitral annulus circumferentially, exclusive of the mitral leaflets (except in the FHS, which made no such exclusion). Clinical aortic stenosis and aortic-valve replacement surgery in the MDCS and CCHS were identified from clinical diagnosis and procedure codes (see the Supplementary Appendix).
GENOTYPING AND IMPUTATION
Investigators in the discovery cohorts independently performed participant-level genotyping using genetic platforms (Table 1) that varied among the sites but that included standard quality-control measures for genotyping and data acquisition (see the Supplementary Appendix). High-density genotyping results were also available in the FHS and MESA for approximately 50,000 single nucleotide polymorphisms (SNPs) in approximately 2000 cardiovascular candidate genes from the Candidate Gene Association Resource (CARe).17
Table 1.
Baseline Characteristics of Participants in the Discovery and Replication Cohorts.*
| Characteristic | Discovery Cohorts | Replication Cohorts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FHS | AGES-RS | MESA | MESA | HNR | MDCS | CCHS | |||
|
White European |
White European |
White European |
African American |
Hispanic American |
Chinese American |
White European |
White European |
White European |
|
| Genotyping platform | Affymetrix, version 5.0 |
Illumina Hu370CNV |
Affymetrix, version 6.0 |
Affymetrix, version 6.0 |
Affymetrix, version 6.0 |
Affymetrix, version 6.0 |
Illumina HumanOmnil- Quad |
LifeSciences ABI 7900HT |
LifeSciences ABI 7900HT |
| Imputation software | MACH | MACH, version 1.0.16 |
IMPUTE, version 2.1.1 |
IMPUTE, version 2.1.1 |
IMPUTE, version 2.1.1 |
IMPUTE, version 2.1.1 |
IMPUTE, version 2.1.2 |
NA | NA |
| Country of origin | United States | Iceland | United States | United States | United States | United States | Germany | Sweden | Denmark |
| No. of participants | 1298 | 3120 | 2527 | 2497 | 2027 | 774 | 745 | 28,193 | 10,400 |
| Age — yr | 60±9 | 76±5 | 63±10 | 61±10 | 61±10 | 62±10 | 60±8 | 58±8 | 56±16 |
| Female sex — no. (%) | 616 (47) | 1811 (58) | 1321 (52) | 1395 (56) | 1094 (54) | 394 (51) | 379 (51) | 17,008 (60) | 5796 (56) |
| Presence of aortic-valve calcium — no. (%) |
510 (39) | 1338 (43) | 397 (16) | 263 (11) | 243 (12) | 67 (9) | 91 (12) | 308 (1)† | 192 (2)† |
| Presence of mitral annular calcium — no. (%) |
259 (20) | NA | 309 (12) | 175 (7) | 197 (10) | 37 (5) | 18 (2) | NA | NA |
Plus-minus values are means +SD. AGES-RS denotes Age, Gene/Environment Susceptibility-Reykjavik Study, CCHS Copenhagen City Heart Study, FHS Framingham Heart Study, HNR Heinz Nixdorf Recall Study, MDCS Malmo Diet and Cancer Study, MESA Multi-Ethnic Study of Atherosclerosis, and NA not applicable.
Included are data from participants with incident aortic stenosis over a median follow-up period of 14 years in the MDCS and 17 years in the CCHS.
To standardize genotyping across cohorts and allow for meta-analysis, participant-level genotype data were imputed to more than 2.5 million SNPs described in the Centre d’Etude du Polymorphisme Humain (CEU) HapMap samples.18,19 Imputed and directly genotyped SNPs were used to examine the association between genetic loci and the presence of valvular calcium.
STATISTICAL ANALYSIS
In the discovery stage, the associations between each SNP and the presence of valvular calcium were analyzed independently in each cohort, with the use of logistic-regression equations and generalized estimating equations adjusted for participants’ age and sex. Results from the individual cohorts were combined with the use of fixed-effect meta-analysis with inverse-variance weighting. Genomewide significance was prespecified as P<5.0×10−8; SNP associations at P<1.0×10−5were deemed to be hypothesis-generating but were not tested further.
In the replication stage, SNPs with genome- wide significance were retested in independent cohorts, with the use of logistic-regression equations and generalized estimating equations adjusted for the participants’ age, sex, and ancestry (as necessary). In the MDCS and CCHS, Cox proportional-hazards regression was used to test whether the SNPs associated with aortic-valve calcification were also associated with incident clinical aortic stenosis, after adjustment for the participants’ age, sex, current smoking status, and body-mass index (BMI). The diagnostic validity for aortic stenosis in the MDCS was high, with a positive predictive value of greater than 90% (as described in the Supplementary Appendix), and most cases of stenosis were moderate to severe. In the replication analyses, P values of less than 0.05 were considered to indicate statistical significance.
To test the hypothesis of a causal association between the SNP located within the apolipoprotein(a) gene (LPA) and aortic-valve calcification, an instrumental variable (i.e., mendelian randomization)20 analysis was performed. In our analyses, genetically determined Lp(a) concentrations (as predicted by the number of LPA SNP copies) were regressed against the presence of aortic-valve calcification. A two-stage Murphy–Topel variance estimator was used to compute 95% confidence intervals. Results from the individual studies were then combined with the use of a randomeffects meta-analysis with inverse variance weighting, owing to heterogeneity across cohorts. Complete details of the statistical analysis are provided in the Supplementary Appendix.
RESULTS
DISCOVERY AND REPLICATION POPULATIONS
The baseline characteristics of the participants in the discovery stage, all of whom were white Europeans, are provided in Table 1; data were included from 1298 participants in the FHS, 3120 in the AGES-RS, and 2527 in the MESA. Data on aortic-valve calcification were available for 6942 participants in the three cohorts, and data on mitral annular calcification were available for 3795 participants in the FHS and MESA cohorts. The replication stage included data from 745 participants in the HNR and from the non-European ethnic groups in the MESA and MESA Family (2497 African Americans, 774 Chinese Americans, and 2027 Hispanic Americans). In addition, with the use of data from 28,193 participants in the MDCS and 10,400 participants in the CCHS, we evaluated whether the SNP most strongly associated with aortic-valve calcification was also associated with incident aortic stenosis.
In the discovery sample, the observed distribution of P values for the correlations of SNPs with the two valvular phenotypes matched the expected distribution (see the quantile–quantile plots in Fig. S1A and S1B in the Supplementary Appendix), with a modest excess of significant associations. This finding suggests that neither genotyping artifact nor systematic differences in allele frequencies owing to ethnic group (i.e., population stratification) created substantial bias in the analysis.
SNPs ASSOCIATED WITH AORTIC-VALVE CALCIFICATION
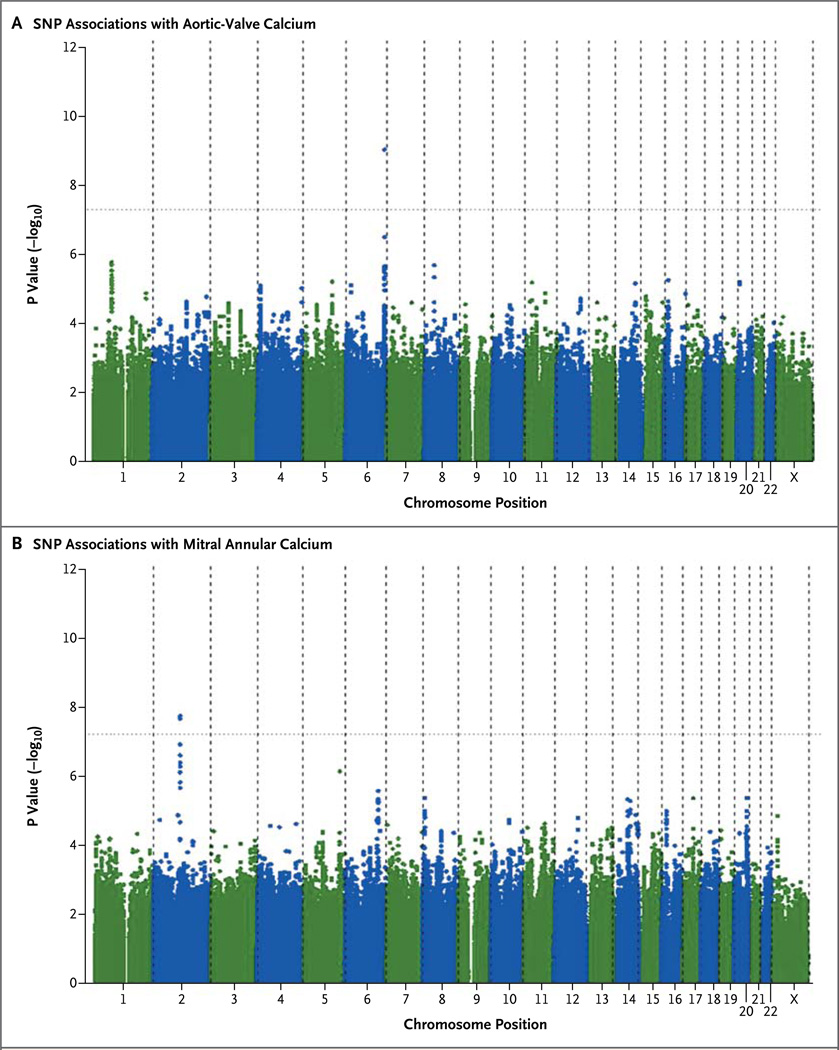
We identified one SNP associated with aortic-valve calcification at a P value of less than 5.0×10−8, surpassing our prespecified threshold for genomewide significance. This SNP, rs10455872, is located within intron 25 of LPA on chromosome 6 (Fig. 1A, and Fig. S2A in the Supplementary Appendix). After adjustments for participants’ age and sex, the risk allele (G) was associated with odds for aortic-valve calcification that were increased by a factor of 2 (odds ratio, 2.05; P = 9.0×10−10) (Table 2). The direction of effect for the risk allele was consistent — and of similar magnitude — in all the discovery-stage cohorts (Table 3). These results with the use of imputed SNPs were confirmed when the direct, densely genotyped SNP data that were available in the FHS and MESA were analyzed; the associations between the SNP and aortic-valve calcification were stronger when the data from directly genotyped SNPs were used than when imputed SNPs were used in the same population sample (FHS: odds ratio, 2.41; P = 1.8×10−5; MESA: odds ratio, 1.93; P = 2.6×10−4). A total of 44 additional SNPs with evidence suggestive of an association (P<1×10−5) are listed in Table S1 in the Supplementary Appendix. A full results data set is available on the Framingham Heart Study website (www.framinghamheartstudy.org/research/resresults.html).
Figure 1. Associations between Each Single-Nucleotide Polymorphism (SNP) and Aortic-Valve Calcium or Mitral Annular Calcium, According to Chromosomal Position.
Manhattan plots show that a single SNP on chromosome 6 (rs10455872) has genomewide significance for aortic-valve calcium (P = 9.0×10−10) (Panel A) and that two SNPs on chromosome 2 (rs17659543 and rs13415097) have genomewide significance for mitral annular calcium (P = 1.5×10−8and P = 1.8×10−8, respectively) (Panel B). The X on the x axis in each panel indicates the X sex chromosome.
Table 2.
Genomewide Significant Associations of Single Nucleotide Polymorphisms (SNPs) with Aortic-Valve and Mitral Annular Calcium.*
| SNP | Phenotype | Chromosome | Minor Allele |
Minor Allele Frequency |
Odds Ratio (95% CI) |
P Value | No. of Studies |
Nearest Gene |
|---|---|---|---|---|---|---|---|---|
| rs10455872 | Aortic-valve calcium | 6 | G | 0.07 | 2.05 (1.63–2.57) | 9.0×10−10 | 3 | LPA |
| rs17659543 | Mitral annular calcium | 2 | T | 0.16 | 1.66 (1.39–1.98) | 1.5×10−8 | 2 | IL1F9 |
| rs13415097 | Mitral annular calcium | 2 | C | 0.16 | 1.66 (1.39–1.98) | 1.8×10−8 | 2 | IL1F9 |
The SNPs were identified in a meta-analysis of data from the FHS, AGES-RS, and MESA during the discovery stage of the study. CI denotes confidence interval.
Table 3.
SNP rs10455872 in LPA and Its Association with Aortic-Valve Calcium in White Europeans in the Discovery and Replication Cohorts.
| Cohort | Minor Allele Frequency |
No. of Participants |
Odds Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| Discovery | ||||
| FHS | 0.07 | 1298 | 2.33 (1.42–3.81) | 7.9×10−4 |
| AGES-RS | 0.06 | 3120 | 2.04 (1.52–2.74) | 1.9×10−6 |
| MESA | 0.06 | 2527 | 1.80 (1.09–2.97) | 0.022 |
| Pooled FHS, AGES-RS, and MESA cohorts* |
0.07 | 6942 | 2.05 (1.63–2.57) | 9.0×10−10 |
| Replication | 0.06 | 745 | 2.04 (1.13–3.67) | 0.018 |
| HNR | ||||
| Pooled FHS, AGES-RS, MESA, and HNR cohorts† |
0.07 | 7687 | 2.05 (1.66–2.53) | 2.8×10−11 |
The method of genomic control was used to correct for heterogeneity among populations in these cohorts (i.e., population stratification).
No genomic-control correction was used for the meta-analysis of these pooled cohorts.
SNPs ASSOCIATED WITH MITRAL ANNULAR CALCIFICATION
We identified two SNPs associated with mitral annular calcification at a P value of less than 5.0×10−8. These two SNPs, which are in high linkage disequilibrium (i.e., highly correlated), were identified on chromosome 2 near the IL1F9 gene cluster between IL1F7 and IL1F9; they are designated as rs17659543 (P = 1.5×10−8 for the association with mitral annular calcification) and rs13415097 (P = 1.8×10−8) (Fig. 1B and Table 2, and Fig. S2B in the Supplementary Appendix). A total of 26 additional SNPs with evidence sugges- tive of an association (P<1×10−5) are listed in Table S2 in the Supplementary Appendix.
INDEPENDENT REPLICATION OF SNP ASSOCIATIONS
Owing to the relatively small sample available for replication, only SNPs with genomewide significance in the discovery stage were evaluated in the replication stage. In the independent replication sample from the HNR, rs10455872 was significantly associated with aortic-valve calcification (P = 0.02), with a similar effect size and the same direction of effect as in the discovery sample, providing independent evidence of an association (Table 3). The combined effect estimate for the discovery and replication samples remained highly significant (P = 2.79×10−11).
The association between rs10455872 and aortic-valve calcification was also evaluated across additional races and ethnic groups in the MESA (Table S3 in the Supplementary Appendix). There was evidence for an association in African Americans (odds ratio, 3.57; P = 0.007) and in Hispanic Americans (odds ratio, 2.75; P = 0.004). The association was not significant in Chinese Americans (P = 0.96), but the power to detect an association was limited by the small sample and low minor allele frequency (0.5%).
In contrast, in 745 white European participants from the HNR among whom the prevalence of mitral annular calcification was 2.4%, there was no significant association between rs17659543 and mitral annular calcification (P = 0.42). In replication analyses stratified according to ethnic group, the association between rs17659543 reached significance only among Hispanic Americans (P = 0.04) (Table S3 in the Supplementary Appendix).
LPA ASSOCIATIONS WITH AORTIC-VALVE AND CORONARY-ARTERY CALCIFICATION
Several additional analyses performed on data from the discovery cohort showed that the association of rs10455872 with aortic-valve calcification was independent of coronary-artery calcification and clinical coronary artery disease. Details of the results of these analyses are provided in the Supplementary Appendix.
CORRELATION OF GENETICALLY DETERMINED Lp(a) LEVELS WITH AORTIC-VALVE CALCIFICATION
In the FHS and AGES-RS, two studies in which data on Lp(a) concentrations were available, rs10455872 was strongly associated with Lp(a) levels, and Lp(a) levels were associated with the presence of aortic-valve calcification (Table 4, and Table S4 in the Supplementary Appendix). After adjustment for Lp(a) levels, the association between the rs10455872 SNP and aortic-valve calcification was attenuated in both cohorts and became nonsignificant when the cohorts were pooled for a meta-analysis (P = 0.09). On the basis of pooled data from the two cohorts, the causal effect of genetically determined Lp(a) levels was estimated as a 62% increase in the odds of aortic-valve calcification per log-unit increase in plasma Lp(a) levels (odds ratio 1.62; P = 1×10−4).
Table 4.
Mendelian Randomization Analysis of Lp(a) and the Presence of Aortic-Valve Calcium.*
| Variable | FHS (N = 885) |
AGES-RS (N = 2785) |
Meta-Analysis |
|---|---|---|---|
| Association of rs10455872 with plasma Lp(a) | |||
| β coefficient for linear regression (95% CI) | 1.38 (1.12–1.63) | 1.75 (1.59–1.91) | 1.58 (1.21–1.94) |
| P value | <0.001 | <0.001 | <0.001 |
| Association with aortic-valve calcium | |||
| rs10455872 | |||
| Odds ratio (95% CI) | 2.63 (1.53–4.52) | 2.04 (1.5–2.77) | 2.17 (1.66–2.83) |
| P value | <0.001 | <0.001 | <0.001 |
| rs10455872, adjusted for Lp(a) levels | |||
| Odds ratio (95% CI) | 2.27 (1.26–4.09) | 1.29 (0.92–1.79) | 1.62 (0.94–2.81) |
| P value | 0.006 | 0.14 | 0.09 |
| Plasma Lp(a) levels† | |||
| Odds ratio (95% CI) | 1.21 (1.05–1.39) | 1.34 (1.25–1.43) | 1.29 (1.18–1.42) |
| P value | 0.008 | <0.001 | <0.001 |
| Genetically determined Lp(a) levels | |||
| Odds ratio (95% CI) | 1.97 (1.33–2.93) | 1.5 (1.26–1.79) | 1.62 (1.27–2.06) |
| P value | 0.001 | <0.001 | <0.001 |
This analysis shows that LPA SNP rs10455872 is associated with both lipoprotein(a) [Lp(a)] levels and aortic-valve calcium and that the association between LPA genotype and aortic-valve calcium is attenuated with adjustment for Lp(a). Furthermore, Lp(a) levels as predicted by LPA genotype — that is, genetically determined Lp(a) — are associated with aortic-valve calcium.
Plasma Lp(a) levels were non-normally distributed and log-transformed.
VARIATION IN LPA AND INCIDENT AORTIC STENOSIS
In the MDCS, over a median follow-up period of 14 years, there were 308 participants (1%) with incident aortic stenosis, with 133 of these participants (43%) undergoing aortic-valve replacement. Clinical factors independently associated with incident aortic stenosis included increasing age, male sex, increasing BMI, and current smoking (P<0.001 for all comparisons). After adjustment for these risk factors, rs10455872 was strongly and independently associated with incident aortic stenosis (hazard ratio, 1.68 per risk allele; 95% confidence interval [CI], 1.32 to 2.15; P = 3×10−5) (Table S5A in the Supplementary Appendix). In a sensitivity analysis in which the outcome was limited to aortic stenosis leading to aortic-valve replacement, similar results were observed (hazard ratio, 1.54 per risk allele; 95% CI, 1.05 to 2.27; P = 0.03). In additional analyses that excluded the 67 participants with antecedent myocardial infarction, there was minimal change in the results with respect to incident aortic stenosis (hazard ratio, 1.69; 95% CI, 1.28 to 2.22; P = 0.0002) or valve replacement (hazard ratio, 1.82; 95% CI, 1.22 to 2.69; P = 0.003). Similar results with respect to the association of rs10455872 with incident aortic stenosis were obtained among participants in the CCHS during a median follow-up period of 17 years (hazard ratio, 1.60; 95% CI, 1.12 to 2.28; P = 0.01) (Table S5B in the Supplementary Appendix).
DISCUSSION
In this genomewide association study, we found that the SNP rs10455872 in the gene LPA was strongly associated with the presence of aortic-valve calcification in participants of European descent. This finding was replicated in independent cohorts from multiple ethnic groups. In two independent prospective cohorts, the G allele was associated with an increase of 60 to 68% per allele in the risk of clinical aortic stenosis, clearly linking genetic variation at the LPA locus with aortic-valve calcification as detected on CT scans and clinical calcific aortic-valve disease. We confirmed the association between rs10455872 and Lp(a) levels and showed that Lp(a) levels mediate the effect of this SNP on aortic-valve calcification. Using a mendelian randomization approach, we found that genetically determined Lp(a) levels were causally associated with an increase of approximately 62% in the odds of aortic-valve calcification per log-unit increment in Lp(a) levels. Our results suggest that lifelong elevations in Lp(a) levels lead to a markedly increased prevalence of aortic-valve calcification in adulthood and implicate Lp(a) in the development of aortic-valve disease.
We also identified an association between a SNP and mitral annular calcification that had genomewide significance. This finding was replicated in a Hispanic-American cohort but could not be replicated in white Europeans in the HNR or in African Americans in the MESA. Whether this represents a true positive finding remains unclear, and efforts to replicate this SNP in larger samples are warranted.
We used a subclinical phenotype, valvular calcium detected by means of CT scanning, rather than clinically defined valvular disease, for our discovery genomewide association study. Aortic-valve calcification represents an early protophenotype for subsequent aortic-valve disease.2,21 By reducing phenotypic (and thus, genetic) heterogeneity, the use of “deep phenotypes” on the basis of a biologic process (e.g., calcification) rather than clinical diagnosis may improve the detection of genetic signals.22 This method has been used to identify nongenetic correlates of aortic-valve calcification in both the FHS and the MESA.23,24 We replicated the rs10455872 association in a cohort with clinical aortic stenosis, thus supporting this strategy of identifying genetic signals for clinical disease.
Although calcific valvular disease is known to cluster within families,5,25,26 the genetic factors explaining this heritability remain elusive. Prior linkage studies have identified NOTCH1 on chromosome 9q34–35 as a susceptibility locus for bicuspid aortic-valve and accelerated valvular calcification.27 Polymorphisms within other candidate genes have also been associated with valvular disease in single reports.28–31 However, candidate gene studies have been criticized for high false positive rates due to small samples and for the lack of independent replication.32,33 In contrast, our study of genomewide association with aortic-valve calcification features a large sample of well-phenotyped persons, uses contemporary criteria for statistical significance, and includes replication in independent cohorts.
Lp(a) is a cholesterol-rich particle consisting of a covalently linked molecule of apolipoprotein B100 with a molecule of apolipoprotein(a).34 Lp(a) has long been considered to be a risk factor for coronary artery disease, and evidence has convincingly shown a causal role for this particle35,36 through intimal deposition of Lp(a)37 and oxidized lipids38 in the vessel wall and induction of a prothrombotic state.39 Similar mechanisms could play a role in aortic-valve disease, especially at areas of mechanical injury where Lp(a) may be preferentially retained.40 Plasma Lp(a) levels are largely genetically determined by variation in the number of kringle IV type 2 (KIV-2) repeats at the LPA locus, which encodes apolipoprotein(a).34 In a previous study, rs10455872 was shown to be in linkage disequilibrium with the KIV-2 polymorphism and to be strongly associated with both plasma Lp(a) levels and the risk of coronary artery disease.35
An association between increased Lp(a) levels and aortic-valve disease has been observed in previous studies.41–44 Lp(a) has been shown to accumulate in both early-stage and end-stage aortic-valve lesions and to colocalize with calci- um deposition.41–45 However, prior studies could not determine whether Lp(a) was a cause or simply a marker of disease. Our results provide evidence for a causal relationship between Lp(a) and calcific aortic-valve disease and strongly implicate genetic variation at the LPA locus in the pathogenesis of the disease. Further studies are needed to evaluate whether lowering Lp(a) levels during early-stage aortic-valve disease, with the use of either niacin46 or novel specific Lp(a) in-hibitors,47 can slow the progression to aortic stenosis.
In conclusion, in a genomewide association study, we have identified a SNP in the LPA locus that is significantly correlated with aortic-valve calcification. Our findings implicate genetic variation at the LPA locus, through elevated plasma Lp(a) levels, in the development of aortic-valve disease. Further studies are needed to evaluate whether lowering Lp(a) levels will reduce the incidence or progression of aortic-valve disease.
Supplementary Material
Acknowledgments
The Framingham Heart Study was supported by a grant (N01-HC-25195) from the National Heart, Lung, and Blood Institute (NHLBI) and an NHLBI contract (N02-HL-6-4278) with Affymetrix for genotyping services; the Age, Gene/Environment Susceptibility–Reykjavik Study, by a grant from the National Institute on Aging (N01-AG-12100) and by the National Eye Institute, the National Institute on Deafness and Other Communication Disorders, the NHLBI, the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (Icelandic parliament); the MESA, MESA Family, MESA CARe, and the MESA SHARe project, by grants (N01-HC-95159 through 95169, RR-024156, R01-HL-071739, R01-HL-071051, R01-HL-071205, R01-HL-071250, R01-HL-071251, R01-HL-071252, R01-HL-071258, R01-HL-071259, N02-HL-6-4278, and N01-HC-65226) from the NHLBI; the Heinz Nixdorf Recall Study, by the Heinz Nixdorf Foundation and the German Foundation of Research (DFG); the Malmö Diet and Cancer study, by the Swedish Cancer Society, the Swedish Medical Research Council, the Swedish Dairy Association, the Albert Påhlsson and Gunnar Nilsson Foundations, and the Malmö city council; and the Copenhagen City Heart Study, by the Danish Heart Foundation. Detailed information on support for individual authors and analyses is available in the Supplementary Appendix.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the Cohorts for Heart and Aging Research in Genome Epidemiology (CHARGE) Consortium for its essential role in the development and support of this study.
APPENDIX
The affiliations of the authors are as follows: Framingham Heart Study: Department of Medicine and the Research Institute, McGill University Health Centre, Montreal (G.T.), National Heart Lung and Blood Institute (NHLBI) Division of Intramural Research (S.-J.H., C.J.O.) and the NHLBI Framingham Heart Study, Framingham, MA (G.T., G.M.P., S.-J.H., L.A.C., S.K., C.J.O.), Department of Biosta-tistics, Boston University School of Public Health, Boston (L.A.C.), and Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston (G.M.P., R.M., S.K., C.J.O.); Multi-Ethnic Study of Atherosclerosis: Department of Medicine, John Hopkins University, Baltimore (C.Y.C., W.S.P.), Cedars–Sinai Medical Center, Los Angeles (J.I.R.), Los Angeles Biomedical Research Institute at Harbor–UCLA, Los Angeles (M.J.B.), Wake Forest School of Medicine, Winston-Salem, NC (Y.L.), Departments of Biostatis-tics (K.F.K., Q.W.) and Epidemiology (S.R.H.), School of Public Health, University of Washington, Seattle, Department of Family and Preventive Medicine University of California, San Diego, La Jolla (M.A.A., M.H.C.), and Department of Medicine, University of Washington, Seattle (K.D.O.); Age Gene/Environment Susceptibility Study: Department of Medicine, University of Washington, Seattle (D.S.O., K.D.O.), Iceland Heart Association, Kopavogur, Iceland (A.V.S., T.A., S.S., V.G.), University of Iceland, Reykjavik (A.V.S., T.A., V.G.), National Institute on Aging, Bethesda, MD (T.B.H.), Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, United Kingdom (M.C.), and Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom (E.D.A., J.D.); Malmö Diet and Cancer Study: Program in Medical and Population Genetics, Broad Institute, Cambridge, MA (J.G.S.), Department of Cardiology, Lund University, Lund, Sweden (J.G.S., J.P.), and Department of Clinical Sciences, Lund University, Malmö, Sweden (M.S., O.M.); Heinz Nixdorf Recall Study: Institute for Medical Informatics, Biometry and Epidemiology, University of Essen, Essen (S.P.), Department of Cardiology, West German Heart Center, Essen (H.K., R.E.), and Department of Genomics, Life and Brain Center and Institute of Human Genetics (T.W.M., M.M.N.) and German Center for Neurodegenerative Diseases (M.M.N.), University of Bonn, Bonn — all in Germany; Copenhagen City Heart Study: Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital (P.R.K.), Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, and Faculty of Health Sciences, University of Copenhagen (B.G.N.), and Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, and Faculty of Health Sciences, University of Copenhagen (A.T.-H.) — all in Copenhagen.
REFERENCES
- 1.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 2.Cosmi JE, Kort S, Tunick PA, et al. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med. 2002;162:2345–2347. doi: 10.1001/archinte.162.20.2345. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Vasan RS, Parise H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- 5.Bella JN, Tang WH, Kraja A, et al. Genome-wide linkage mapping for valve calcification susceptibility loci in hyper- tensive sibships: the Hypertension Genetic Epidemiology Network Study. Hypertension. 2007;49:453–460. doi: 10.1161/01.HYP.0000256957.10242.75. [DOI] [PubMed] [Google Scholar]
- 6.Psaty BM, O’Donnell CJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circulation. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmermund A, Möhlenkamp S, Stang A, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Am Heart J. 2002;144:212–218. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 12.Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study: design and feasibility. J Intern Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 13.Schnohr P, Jensen G, Lange P, Scharling H, Appleyard M. The Copenhagen City Heart Study — Østerbroundersø-gelsen: tables with data from the third examination, 1991–1994. Eur Heart J. 2001;3(Suppl):H1–H83. [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of MultiEthnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Fram-ingham Heart Study) Am J Cardiol. 2008;102:1136–1141. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musunuru K, Lettre G, Young T, et al. Candidate Gene Association Resource (CARe): design, methods, and proof of concept. Circ Cardiovasc Genet. 2010;3:267–275. doi: 10.1161/CIRCGENETICS.109.882696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 19.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton D, McKeigue PM. Epidemio-logical methods for studying genes and environmental factors in complex diseases. Lancet. 2001;358:1356–1360. doi: 10.1016/S0140-6736(01)06418-2. [DOI] [PubMed] [Google Scholar]
- 21.Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 22.Tracy RP. “Deep phenotyping”: characterizing populations in the era of ge-nomics and systems biology. Curr Opin Lipidol. 2008;19:151–157. doi: 10.1097/MOL.0b013e3282f73893. [DOI] [PubMed] [Google Scholar]
- 23.Thanassoulis G, Massaro JM, Cury R, et al. Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol. 2010;55:2491–2498. doi: 10.1016/j.jacc.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens DS, Katz R, Johnson E, et al. Interaction of age with lipoproteins as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:1200–1207. doi: 10.1001/archinte.168.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horne BD, Camp NJ, Muhlestein JB, Cannon-Albright LA. Evidence for a heritable component in death resulting from aortic and mitral valve diseases. Circulation. 2004;110:3143–3148. doi: 10.1161/01.CIR.0000147189.85636.C3. [DOI] [PubMed] [Google Scholar]
- 26.Probst V, Le Scouarnec S, Legendre A, et al. Familial aggregation of calcific aortic valve stenosis in the western part of France. Circulation. 2006;113:856–860. doi: 10.1161/CIRCULATIONAHA.105.569467. [DOI] [PubMed] [Google Scholar]
- 27.Garg V, Muth AN, Ransom JF, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 28.Ortlepp JR, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P. The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. Heart. 2001;85:635–638. doi: 10.1136/heart.85.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novaro GM, Sachar R, Pearce GL, Sprecher DL, Griffin BP. Association between apolipoprotein E alleles and calcific valvular heart disease. Circulation. 2003;108:1804–1808. doi: 10.1161/01.CIR.0000097560.96431.3E. [DOI] [PubMed] [Google Scholar]
- 30.Nordström P, Glader CA, Dahlén G, et al. Oestrogen receptor α gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J Intern Med. 2003;254:140–146. doi: 10.1046/j.1365-2796.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 31.Ortlepp JR, Schmitz F, Mevissen V, et al. The amount of calcium-deficient hexagonal hydroxyapatite in aortic valves is influenced by gender and associated with genetic polymorphisms in patients with severe calcific aortic stenosis. Eur Heart J. 2004;25:514–522. doi: 10.1016/j.ehj.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Gaudreault N, Ducharme V, Lamon-tagne M, et al. Replication of genetic association studies in aortic stenosis in adults. Am J Cardiol. 2011;108:1305–1310. doi: 10.1016/j.amjcard.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 33.Morgan TM, Krumholz HM, Lifton RP, Spertus JA. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. JAMA. 2007;297:1551–1561. doi: 10.1001/jama.297.14.1551. [Erratum, JAMA 2007;298:973.] [DOI] [PubMed] [Google Scholar]
- 34.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 36.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 37.Kreuzer J, Lloyd MB, Bok D, et al. Li-poprotein (a) displays increased accumulation compared with low-density lipo-protein in the murine arterial wall. Chem Phys Lipids. 1994;67–68:175–190. doi: 10.1016/0009-3084(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 38.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipopro-tein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 39.Miles LA, Fless GM, Levin EG, Scanu AM, Plow EF. A potential basis for the thrombotic risks associated with lipo-protein(a) Nature. 1989;339:301–303. doi: 10.1038/339301a0. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen LB, Stender S, Kjeldsen K, Nordestgaard BG. Specific accumulation of lipoprotein(a) in balloon-injured rabbit aorta in vivo. Circ Res. 1996;78:615–626. doi: 10.1161/01.res.78.4.615. [DOI] [PubMed] [Google Scholar]
- 41.Bozbas H, Yildirir A, Atar I, et al. Effects of serum levels of novel atherosclerotic risk factors on aortic valve calcification. J Heart Valve Dis. 2007;16:387–393. [PubMed] [Google Scholar]
- 42.Glader CA, Birgander LS, Soderberg S, et al. Lipoprotein(a), Chlamydia pneu-moniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur Heart J. 2003;24:198–208. doi: 10.1016/s0195-668x(02)00385-8. [DOI] [PubMed] [Google Scholar]
- 43.Gotoh T, Kuroda T, Yamasawa M, et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocar-diography (the JMS Cardiac Echo and Cohort Study) Am J Cardiol. 1995;76:928–932. doi: 10.1016/s0002-9149(99)80263-x. [DOI] [PubMed] [Google Scholar]
- 44.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcif-ic aortic valve disease: Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien KD, Reichenbach DD, Marco-vina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 46.Capuzzi DM, Guyton JR, Morgan JM, et al. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998;82:74U–81U. doi: 10.1016/s0002-9149(98)00731-0. [DOI] [PubMed] [Google Scholar]
- 47.Merki E, Graham M, Taleb A, et al. Antisense oligonucleotide lowers plasma levels of apolipoprotein (a) and lipopro-tein (a) in transgenic mice. J Am Coll Car-diol. 2011;57:1611–1621. doi: 10.1016/j.jacc.2010.10.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.