Abstract
Although oral delivery of insulin offers a number of unmatched advantages, it nevertheless is beset by the poor permeability of insulin molecules through the epithelial cell membranes of the intestinal mucosal layer. We previously reported the development of low molecular weight protamine (LMWP) as a nontoxic yet potent cell penetrating peptide, of which via covalent linkage was capable of translocating protein cargos through the membranes of almost all cell types. It is therefore hypothesized that LMWP could be practically employed as a safe and effective tool to deliver insulin across the intestinal mucosal membrane, thereby augmenting its absorption through the GI tract. However, formulating 1:1 monomeric insulin/LMWP conjugate presents a tall order of challenge, as the acidic insulin and basic LMWP would automatically form tight aggregates through electrostatic interactions. In this paper, we developed an innovative conjugation strategy to solve this problem, by using succinimidyl-[(N-maleimidopropionamido)-polyethyleneglycol] ester (NHS-PEG-MAL) as an intermediate cross-linker during the coupling process. Both SDS-PAGE and MALDI-TOF mass spectroscopy confirmed the formation of a homogeneous, monomeric (1:1 ratio) insulin/LMWP conjugate without encountering the conventional problem of substrate aggregation. Cell culture studies demonstrated that transport of the Insulin-PEG-LMWP conjugate across the intestinal mucosal monolayer was augmented by almost five folds compared to native insulin. Furthermore, results from the in situ loop absorption tests in rats showed that systemic pharmacological bioavailability of insulin was significantly enhanced after its conjugation with LMWP. Overall, the presented chemical conjugation with LMWP could offer a reliable and safe means to improve the intestinal permeability of therapeutic peptides/proteins, shedding light of the possibility for their effective oral delivery.
Keywords: Cell-penetrating peptide, intestinal absorption, low molecular weight protamine, insulin, monomeric conjugation, permeation enhancement
1. Introduction
Insulin is a universal clinical drug used in the treatment of diabetes. Current methods for insulin administration is primarily based on subcutaneous injection, which may produce peripheral hyperinsulinemia leading to hypertension and atherosclerosis [1]. Oral delivery of insulin has been the most desirable and popular choice, due to its convenience and high patient compliance. Most importantly, oral dosing mimics insulin’s physiological pathway, thus potentially reducing the side-effects [2]. However, oral delivery of peptide/protein drugs such as insulin is beset by the bottleneck of poor bioavailability, which is caused primarily by two barriers: 1) susceptibility to digestion by proteolytic enzymes in the gastrointestinal (GI) tract as well as chemical degradation by the acidic gastric environment; 2) poor permeability through the epithelial cell membranes of the intestinal mucosal layer. To achieve effective GI delivery of these macromolecular drugs [3, 4], these obstacles must be resolved.
At present, a number of approaches have been developed to improve the oral bioavailability of insulin, including encapsulation of insulin in nanoparticles [5, 6] or hydrogel possessing interpenetraing-network [7], enteric coating of insulin-loaded nanoparticles [8], coating of insulin-loaded nanoparticles with protease inhibitors [9], etc. In principle, polymers used in these strategies could provide an inhibition on proteases in the intestinal lumen as well as protection to the insulin molecules against enzymatic degradation. In practice, the paracellular permeability of insulin was, to certain extent, being improved due to opening by the polymer of the tight junctions between intestinal epithelial cells. In reality, however, the permeability of insulin itself remained poor and unaltered. Overall, while the first barrier (i.e. susceptibility to protease digestion) has been largely circumvented by far, the second barrier (poor permeability through mucosal layer) remains as the primary challenge that must be overcome in order to significantly augment the oral bioavailability of insulin.
Recently, the discovery of a class of cell-penetrating peptides (CPP), aka protein transduction domain (PTD) peptides, has shed light of ultimately resolving the membrane barrier problems [10–14]. Both cell culture and animal studies demonstrated that by covalently linking CPP to almost any type of cargos including hydrophilic proteins (MW >150 kDa) or large drug carriers (e.g. liposomes), CPP was able to translocate the linked cargos into almost every cell or tissue types, without causing any detectable membrane perturbation or damage [13, 14].
We previously reported the development of low molecular weight protamine (LMWP) with sequence of VSRRRRRRGGRRRR, a potent yet nontoxic CPP, via enzymatic digestion of natural protamine by thermolysin [10–12]. In cell culture and animal studies, we also demonstrated that LMWP possessed equivalent cellinternalization potency to that of TAT (trans-activator of transcript), the most extensively investigated CPP to-date derived from the human immunodefiency virus (HIV-1) [15]. As reported in the literature, LMWP was successfully used to condense siRNA in improving its uptake by tumor cells and suppressing tumor growth in vivo [16]. More importantly, unlike most other cationic peptides or transfection agents, LMWP was shown to be neither immunogenic [17] nor antigenic [18], and elicited only minor complement activation and non-detectable hypotensive responses in dogs [12]. Based on these findings, we hypothesized that LMWP could be employed as an effective yet safe tool to improve insulin delivery across the intestinal mucosal layer thereby enhancing systemic bioavailability via the oral route.
Herein, we reported the synthesis of homogeneous and monomeric (1:1 ratio) insulin:LMWP conjugates without encountering the conventional problem of charge-induced aggregation, by utilizing succinimidyl-[(N-maleimidopropionamido)-polyethyleneglycol] ester (NHS-PEG-MAL) as a intermediate cross-linker during the coupling process. In vitro characterization and in vivo animal studies were carried out to confirm the monomeric composition of the insulin/LMWP conjugate, as well as to demonstrate the transport property across the intestinal mucosal monolayer and systemic bioavailability of this conjugate when comparing with native insulin without the coupling of LMWP.
2. Materials and methods
2.1. Materials and animals
Unless otherwise stated, salmon protamine, thermolysin, bovine insulin and bovine FITC-labeled insulin (insulin/FITC ≈1:1), dimethylmaleic anhydride (DMMA), and other chemicals were purchased from Sigma (St. Louis, MO, USA). N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP), dithiothreitol (DTT) and Slide-A-Lyzer dialysis cassettes (MWKO 2K) were purchased from Pierce Biotechnology, Inc (Rockford, IL, USA). Hi-Trap heparin columns and desalting columns were obtained from GE Healthcare Bio-Sciences Corp (Piscataway, NJ, USA). Fetal bovine serum (FBS), phosphate-buffered saline (PBS), 0.25% (w/v) trypsin-EDTA, Dulbecco's modified essential medium (DMEM) and 4’, 6-diamidino-2-phenylindole (DAPI) were purchased from Gibco-BRL (Invitrogen, Carlsbad, CA, USA). Heterobifunctional succinimidyl-[(N-maleimidopropionamido)-polyethyleneglycol] ester (NHS-PEG-MAL, MW: ~3600Da) was purchased from JenKem Technology, Inc. (Allen, TX, USA). Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA, USA). Twenty-four well transwell culture plates were from Corning (Corning, NY, USA). The 10–20% Tris-Tricine SDS-PAGE gel and Macro-Prep High Q Column were purchased from Bio-Rad Inc (Hercules, CA, USA). The 24-well BD BioCoat™ HTS Caco-2 assay system was obtained from BD Company (Sparks, MD, USA).
Wistar rats weighing 200±20 g were obtained from Sino-British SIPPR/BK Lab. Animal Co., Ltd. (Shanghai, China). All animal experiments were performed according to the Guiding Principles for the Care and Use of Experiment Animals in Fudan University (Shanghai, China).
2.2. Conjugation of Insulin with LMWP
2.2.1. Preparation of LMWP
LMWP was derived by enzymatic digestion of protamine with thermolysin, according to a previously established protocol [10–12]. In brief, thermolysin and protamine were mixed and incubated for 1 hour at room temperature, followed by quenching the enzyme with e addition of 50 mM EDTA. The product was further purified using a heparin column. The molecular weight and amino acid sequence of the peptide were determined by MALDI-TOF MS analysis, which was performed by the Protein and Carbohydrate Research Center at the University of Michigan. The purity of the peptides was above 96 %.
2.2.2. Synthesis of LMWP-SH
LMWP (5 mg/mL; prepared in 100 mM phosphate buffer at pH 7.4) was modified by reaction with a five-fold molar excess of N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP; dissolved in dimethyl sulfoxide (DMSO)) for 2 h at room temperature. After removal of the excess SPDP using affinity chromatography (HiTrap™ Heparin HP Column), the SPDP-modified LMWP was reduced by 50 mM dithiothreitol (DTT) for 0.5 h to generate the activated LMWP with a free sulfhydryl (-SH) group at the N-terminal. The excess DTT was removed by using affinity chromatography (HiTrap™ Heparin HP Column). The sulfhydryl content of the activated LMWP was measured by using the Ellman’s reagent, according to a well-established procedure [19].
2.2.3. DMMA Protection of Insulin
Protection of insulin was performed by using a slightly modified protocol of previously established methods[20, 21]. Briefly, insulin (25 mg) was dissolved in 5 mL of 50 mM phosphate buffer containing 25 mM EDTA.
Dissolved insulin was then reacted with 3.5 mg of DMMA under gentle rotating at 4 °C for 0.5 h. The pH of the solution was maintained at 6.8 by addition of 1M sodium carbonate solution. Reaction with DMMA was repeated two more times so that a total of 10.5 mg of DMMA was added to insulin at the controlled pH of 6.8. The protected insulin thus prepared was then dialyzed at 4°C, against 4 L of 50 mM phosphate buffer containing 25 mM EDTA over night to remove unreacted DMMA.
2.2.4. Synthesis and Analysis of Insulin-PEG-MAL
Insulin was reacted with NHS-PEG-MAL (MW: ~3600Da) to produce Insulin-PEG-MAL. DMMA-protected insulin was reacted, at five-fold molar excess, with NHS-PEG-MAL for 0.5 h at room temperature, resulting in the formation of an amide bond between the amino group on Insulin and the NHS-group on the crosslinker. Excess NHS-PEG-MAL and unreacted insulin were removed by anion exchange chromatography using a Bio-Rad Macro-Prep High Q Column. The insulin-PEG-MAL product was then eluted with 20 mM phosphate buffer (pH 7.4), and proteins were containing a linear gradient (0–1 M) of NaCl. The elution profile was monitored at a wavelength of 280 nm.
To confirm the modification site, Insulin-PEG-MAL was treated with dithiothreitol (DTT) and trypsin. The obtained fractions were then subject to mass spectroscopic analysis using a Waters Tofspec-2E MALDI-TOF spectrometer. Approximately 10 pmol of samples were mixed with the matrix consisting of 10 mg/mL alpha-cyano-4-hydroxy-cinnamic acid (CHCA) dissolved in 50/50 acetonitrile/ethanol, spotted on the target plate, evaporated to dryness, and then detected in the linear mode using MALDI-TOF.
2.2.5. Synthesis of Insulin-PEG-LMWP Conjugate
The LMWP-SH prepared earlier was added to the above-purified Insulin-PEG-MAL and incubated at room temperature for 2 hours to produce the Insulin-PEG-LMWP conjugate through the maleimide group. Excess LMWP-SH was removed by ultrafiltration (MWCO: 3,000 Da), and the conjugate was then purified by affinity chromatography using a HiTrap™ Heparin HP Column. The final insulin-PEG-LMWP product was eluted with 50 mM phosphate buffer (pH 7.4) containing a step gradient of 0M, 0.5M and 1 M NaCl. The elution profile was monitored at a wavelength of 280 nm. Insulin concentration in the final purified product was determined using an appropriate insulin ELISA kit.
2.3. Characterization of Insulin-PEG-LMWP Conjugate
Both SDS-PAGE and MALDI-TOF methods were employed to assess the purity of the insulin-PEG-LMWP as well as the number of PEG and LMWP chains per insulin molecule. SDS-PAGE was performed under non-reducing conditions using gels containing 10–20% gradient of Tris-Tricine, and protein bands were detected with the Coomassie blue staining method. Mass spectroscopic analysis of the conjugate was carried out using a Waters Tofspec-2E MALDI-TOF Mass Spectrometer, according to the exact protocol described in Section 2.2.4.
2.4. In Vitro Intestinal Absorption Assay on Caco-2 Cell Monolayer
2.4.1. Fluorescence Labeled Conjugate
For cell uptake study, FITC-labeled Insulin was used to replace DMMA-protected insulin in synthesizing the labeled insulin-PEG-LMWP conjugate. The same procedure described in Section 2.2.4 was followed and thus not reiterated here.
2.4.2. Caco-2 Cell Culture
Caco-2 cells at a seeding density for cultivation of 2.5 ×105 cells/flask were cultured in 75 cm2 culturing flasks containing 10 mL Dulbecco’s Modified Medium, (DMEM, Biosource International, Camarillo, CA,). Cells were maintained in an incubator at a controlled atmosphere of 37°C, 95% relative humidity and 5% CO2. The culture medium was replaced with fresh medium every other day for 6 days, until the cells reached 60–80% confluence. Cells were then detached from the culturing flask by trypsin treatment, resuspended in fresh culture medium, and then transferred under the desired seeding density into a new culturing flask or experimental wells. Unless otherwise stated, cells with passage numbers between 60 and 80 were used.
2.4.3. Intestinal Absorption Assay on Caco-2 Cell Monolayer
Caco-2 cell monolayers were grown in 24-well BD BioCoat™ HTS Caco-2 assay system. The integrity of the monolayers was monitored by measuring the transepithelial electrical resistance (TEER) values in Hank's balanced salt solution (HBSS; pH 7.4) before and after the experiment. TEER was determined using a voltmeter with a chopstick electrode (World Precision Instrument, Sarasota, F,). Only cells with TEER values in the range of 250–350 Ω ·cm2 were selected for subsequent transport studies. Briefly, filter inserts were rinsed with transport buffer (the same as the uptake medium; pH 7.4) and allowed to equilibrate at 37°C for 30 min. Experiments were carried out in apical-to-basolateral group and basolateral-to-apical group with samples containing 0.5 µM of FITC-labeled insulin or FITC-labeled Insulin-PEG-LMWP. In the apical-to-basolateral group, the apical buffer was replaced with the sample solutions (0.3 mL) whereas the basolateral buffer with fresh transport buffer (1 mL). At given time intervals, 200 µL aliquot were withdrawn from the basolateral chamber of the transwell and replaced with fresh buffer. In the basolateral-to-apical group, the apical buffer (0.3 mL) was replaced with fresh transport buffer whereas the basolateral buffer with sample solutions (1 mL). At given time intervals, a sample (20 µL) was withdrawn from the apical chamber and replaced with fresh buffer. Each experiment was performed in triplicate. The insulin concentrations of the samples were analyzed using a fluorescence spectrometer.
The apparent permeability coefficient (Peff, cm/s) was calculated according to the following equation:
where dc/dt (µg/s) represents the time derivative of concentration in the receiver chamber, A (cm2) the surface area of the monolayer, VR (cm3) the volume of the receiver chamber, and C0 (µg) the the initial insulin concentration in the donor chamber initial concentration in the apical compartment. Experiments were conducted in triplicate, and results were expressed as mean ± SD.
2.5. In situ absorption experiments
Studies were conducted using a modified protocol of the method developed previously [22]. Briefly, Male Wistar rats weighing 180–220 g were fasted, but with free access to water, for 24 h before the experiments. After intraperitoneal (i.p.) injection of sodium pentobarbital (50 mg/kg), rats were anesthetized and kept in a supine position on a thermostatically controlled board at 37 °C. To maintain anesthesia, additional dosages of sodium pentobarbital (12.5 mg/kg) were given every 1 h by i.p. injections.. The ileum was exposed following a small midline incision in the abdomen, and the 10 cm ileum segment proximal to ileocecal junction was cannulated at both ends. PBS warmed at 37 °C was infused through the cannula at 5.0 mL/min for 1 min using a pump to wash out the intestinal content in the segment. The cannulation tubing was removed, and the segment was then closed tightly and placed back into the peritoneal cavity. Rats were left on the board at 37 °C for another 30 min to recover from the elevated blood glucose concentration resulting from the surgery. After 30 min of rest, 0.5 mL of insulin, insulin and LMWP mixture (molecular ratio=1:1), Insulin-PEG-Mal and LMWP mixture (molecular ratio=1:1), Insulin-PEG-LMWP conjugate, or PBS (negative control) were administered directly into the 6 cm ileal loop from the 10 cm pretreated segment. The insulin dose was controlled at 50 IU/kg body weight for both the insulin and Insulin-PEG-MAL samples, and the mixture was gently shaken and incubated in room temperature for 2 h.
The relative pharmacological bioavailability of enterally administered insulin was calculated relative to that via the subcutaneous (s.c.) route. Briefly, an insulin PBS solution was prepared and s.c. administered at a dose of 1.0 IU/kg body weight. Insulin-PEG-LMWP conjugate was also s.c. administered at a dose of 1.0 IU/kg body weight to test its bioactivity. To maintain the same physical conditions for the rats, identical ileal loop surgical procedures employed in the intestinal absorption study were performed on rats receiving sc. Insulin and Insulin-PEG-LMWP conjugate.
During the experiment, blood samples were withdrawn from the caudal vein before and at predetermined time intervals after dosing. Blood glucose concentration was measured using an Accu-Chek (Roche Diagnostics GmbH, Mannheim, Germany) glucose meter. The change in blood glucose concentration, represented by percentage to the initial value before injection, was plotted against time. The biological activity of insulin was calculated from the corresponding blood glucose concentration and expressed as percentage of the predose glucose concentration in the control group administered with PBS solution. The area above the curve (AAC) was calculated using the trapezoidal method and was used to calculate the pharmacological bioavailability (PA) according to the following equation.
3. Results
Low molecular weight protamine (LMWP) was reported to be a nontoxic yet potent cell penetrating peptide. Via covalent linkage, LMWP was capable of translocating protein cargos through the membranes of almost all cell types. It is therefore hypothesized that LMWP could be practically employed as a safe and effective tool to deliver insulin across the intestinal mucosal membrane, thereby augmenting its absorption through the GI tract. However, formulating 1:1 monomeric insulin/LMWP conjugate presents a daunting challenge, as the acidic insulin and basic LMWP would automatically form tight aggregates through electrostatic interactions. In addition, the innate instability of insulin complicates its chemical modification.
3.1. Synthesis and Purification of Monomeric Insulin-LMWP Conjugate
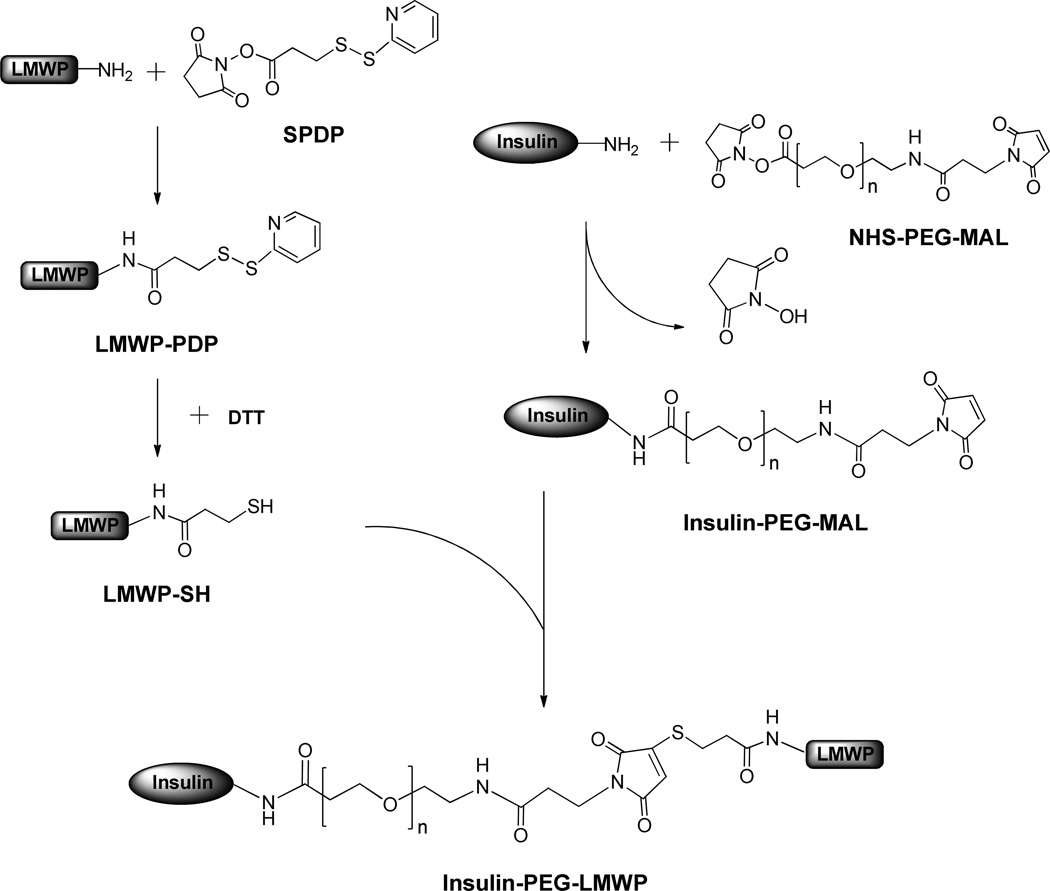
In this paper, we designed an innovative strategy based on the unique and unparallel dynamic movement of the PEG polymer and its subsequently yielded shielding effect to override the aforementioned obstacles. As shown in Scheme 1, LMWP (MW: 1880 Da, Sequence: VSRRRRRRGGRRRR) was first modified with N-succinimidyl-3-(2-pyridyldithio)-propionate (SPDP) and the LMWP-PDP was then reduced by dithiothreitol (DTT) to produce LMWP-SH with a free and reactive sulfhydryl group. In the second step, DMMA was used to selectively protect the amino group at the activity center of the insulin molecule, according to a well-established method[21]. Protected insulin was then reacted with PEG (MW: ~3,600 Da; Note: the MW of the PEG molecule should exceed that of LMWP) containing heterobifunctional reactive groups of NHS (N-hydroxysuccinimide) and maleimide at the two terminuses to yield Insulin-PEG-MAL. In the final step, Insulin-PEG-MAL was reacted with the above-prepared LMWP-SH via its reactive MAL group with the free sulfhydryl group on LMWP-SH to produce the Insulin-PEG-LMWP conjugate. Unlike direct mixing of native insulin or SPDP-activated insulin with LMWP in which significant precipitation of the insoluble complexes were observed, the PEG chain on the Insulin-PEG conjugate could shield insulin from the charge-induced complexation with LMWP during the coupling process, thereby producing a transparent reaction mixture presumably containing monomeric Insulin-PEG-LMWP conjugates.
Scheme 1.
Synthesis of Insulin-PEG-LMWP chemical conjugate
Assay of the sulfhydryl groups using Ellman’s reagent indicated that the yield of LMWP-SH was about 40% of the initial dose of LMWP.
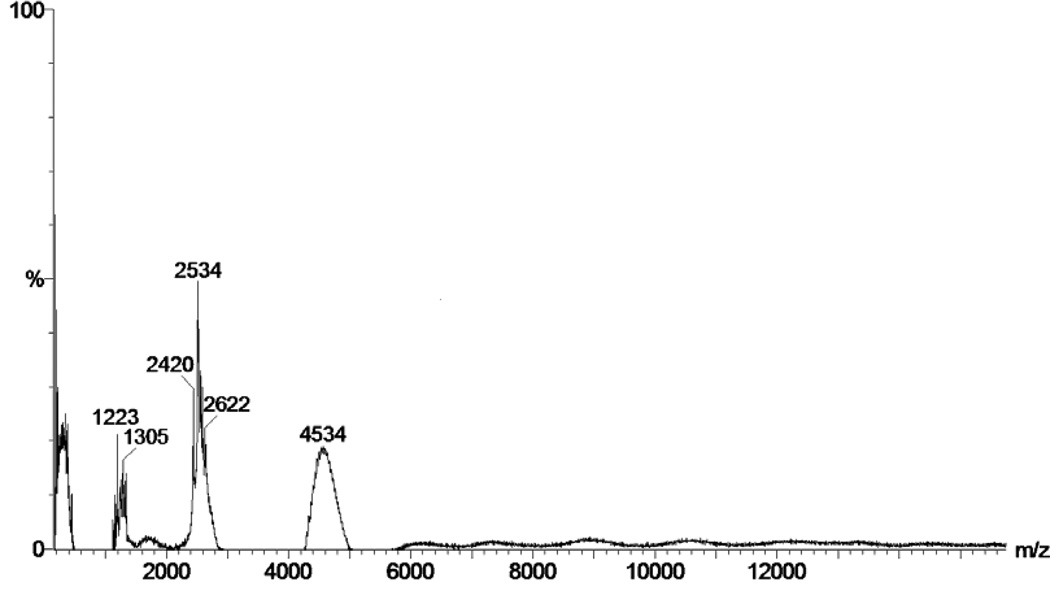
MALDI-TOF mass spectrum of the Insulin-PEG-MAL conjugates after treatment by DTT and trypsin together in Fig. 1 showed the presence of three major peaks with molecular weights of 2450, 2600 and 4530Da, representing the A-chain and N-terminal of the B-chain (B1-B22) of insulin, as well as the PEGylated fragment of B-chain (B23-B30) of the Insulin-PEG-MAL conjugate, respectively. This finding indicated that the terminal primary amino group of insulin were protected by DMMA, consistent with report by other investigators [20, 21] and, as a consequence, did not react with NHS-PEG-MAL. The PEG modification site of insulin appeared to be on LysB29.
Fig 1.
MALDI-TOF mass spectrum of the Insulin-PEG-MAL conjugate being treated with DTT and trypsin. For experimental details, please refer to the “Materials and Methods” section.
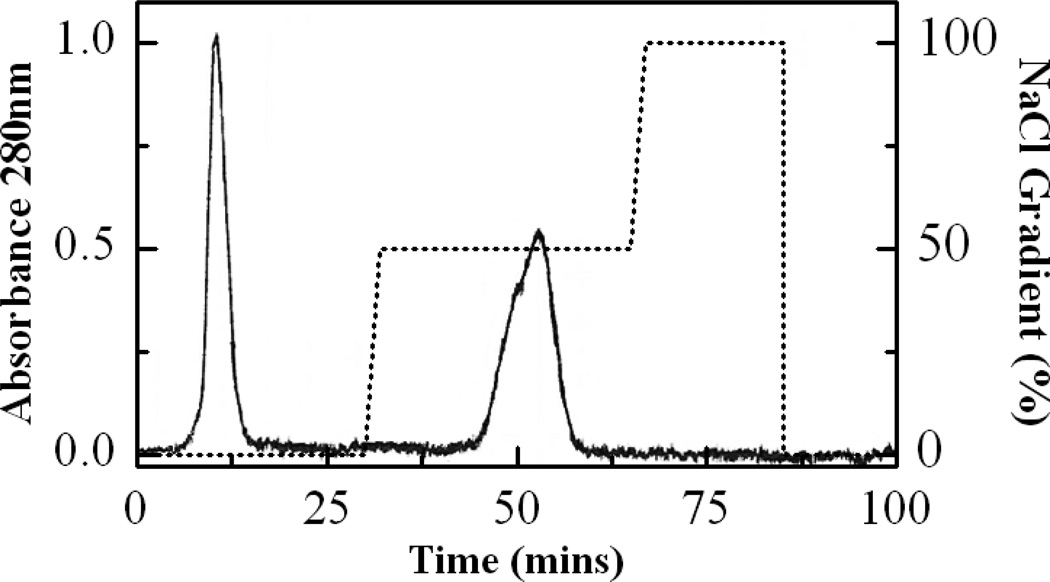
Results from purification of the Insulin-PEG-LMWP conjugates using a heparin affinity column were illustrated in Fig. 2. As seen, excess Insulin-PEG-MAL was eluted at the beginning of the chromatography (Peak1), because it did not have the affinity for heparin. In contrast, the Insulin-PEG-LMWP conjugate was eluted as a homogeneous peak from the column at 0.5 M NaCl (Peak2), presumably attributed to the stronger binding affinity to the heparin column due to presence of the cationic LMWP moiety on the conjugates. Apparently, the heparin column resulted in an effective separation of the desired Insulin-PEG-LMWP product from the other substrates in the conjugation reaction mixture.
Fig 2.
Elution profile of Insulin-PEG-MAL and LMWP-SH reaction mixture through a heparin affinity column (HiTrap™ Heparin HP Column) after removal of excess LMWP-SH with ultrafiltration. Elution solution was 50 mM phosphate buffer containing a stepwise salt gradient (0, 0.5, and 1.0 M NaCl at pH 7.4. Excess Insulin-PEG-MAL was eluted at the beginning of the chromatography (Peak1), while the Insulin-PEG-LMWP conjugate was eluted as a homogeneous peak from the column at 0.5 M NaCl (Peak2). For experimental details, please refer to the “Materials and Methods” section.
3.2. Characterization of the Insulin-PEG-LMWP Conjugates
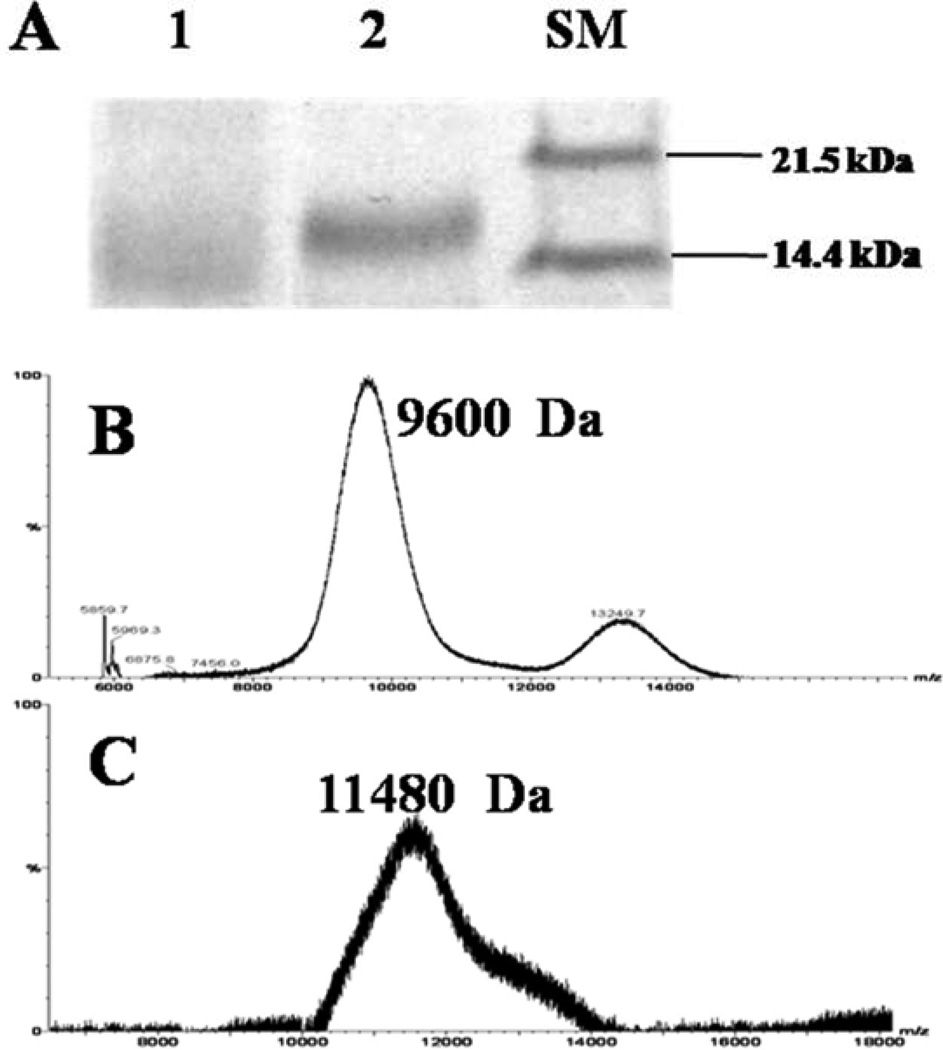
SDS-PAGE analysis of the conjugates after purification by the heparin column revealed a single protein band with slightly reduced gel mobility relative to the Insulin-PEG-MAL conjugate (Fig. 3A), indicating the presence of a homogeneous preparation Insulin-PEG-LMWP product.
Fig 3.
(A) SDS-PAGE analysis of: Insulin-PEG-MAL (Lane 1), Insulin-PEG-LMWP (Lane 2) and size marker (SM); (B) MALDI-TOF mass spectrum of Insulin-PEG-MAL; (C) MALDI-TOF mass spectrum of Insulin-PEG-LMWP. The peaks around 9,600 Da and 13,249 Da in Fig. 3B were thought to belong to PEGylated Insulin containing one and two PEG chains on each Insulin molecule respectively. The peaks around 11,480 Da in Fig. 3C was thought to belong to Insulin-PEG-LMWP. For experimental details, please refer to the “Materials and Methods” section.
The MALDI-TOF MS results demonstrated that the molecular weights of Insulin-PEG-MAL and Insulin-PEG-LMWP were estimated to be 9,600 Da (Fig. 3B) and 11,480 Da (Fig. 3C), respectively. The difference in molecular weight between these two conjugates was almost identical to the molecular weight of a single LMWP molecule (i.e. 1,880 Da), confirming the presence of a 1:1, insulin:LMWP monomeric structure in the conjugate. The peak that appeared around 13,249 Da in Fig. 3B was thought to belong to PEGylated Insulin containing two PEG chains on each insulin molecule. Based on the intensities of these peaks, the major product in the reaction mixture appeared to be the Insulin-PEG-MAL conjugate with a molecular weight showing around 9,600 Da.
3.3. In Vitro Intestinal Absorption Assay using Caco-2 Cell Monolayer Model
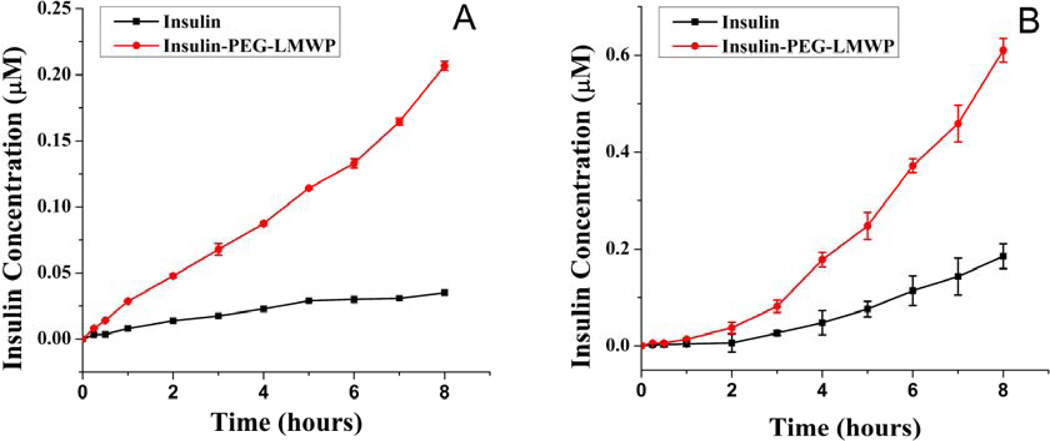
The transporting efficiency of insulin and the Insulin-PEG-LMWP conjugates from both apical-to-basolateral and basolateral-to-apical flux directions were examined. As seen in Fig. 4, the transporting efficiency of Insulin-PEG-LMWP was 4.85 and 4.09 times higher than that of native insulin for apical-to-basolateral and basolateral-to-apical flux respectively, as reflected by the calculated effective permeability (Peff) values obtained for both insulin products (Table 1). Compared to the extremely poor transportation efficiency of free insulin, it was increased dramatically once insulin was conjugated to the LMWP peptide.
Fig 4.
In vitro intestinal absorption of the Insulin-PEG-LMWP conjugates. (A) apical-to-basolateral; and (B) basolateral-to-apical permeability of 0.4 µM of insulin ( ) and 0.4 µM insulin-equivalent of the Insulin-PEG-LMWP conjugate (
) and 0.4 µM insulin-equivalent of the Insulin-PEG-LMWP conjugate ( ) across transwell-grown Caco-2 cell monolayer. Samples were collected at the indicated time points and insulin concentrations were determined by fluorescence spectrometer. Each data point represents average results from three measurements. For experimental details, please refer to the “Materials and Methods” section.
) across transwell-grown Caco-2 cell monolayer. Samples were collected at the indicated time points and insulin concentrations were determined by fluorescence spectrometer. Each data point represents average results from three measurements. For experimental details, please refer to the “Materials and Methods” section.
Table 1.
Calculated permeability coefficients of insulin and the Insulin-PEG-LMWP conjugate across the Caco2 cell monolayers.
| Apical-to-basolateral |
Basolateral-to-apical |
|||
|---|---|---|---|---|
| Insulin | Insulin-PEG-LMWP | Insulin | Insulin-PEG-LMWP | |
| Peff (×106cm/s) | 1.10±0.02 | 5.33±0.03 | 1.21±0.25 | 4.95±0.15 |
3.4. In Situ Loop Absorption Experiments
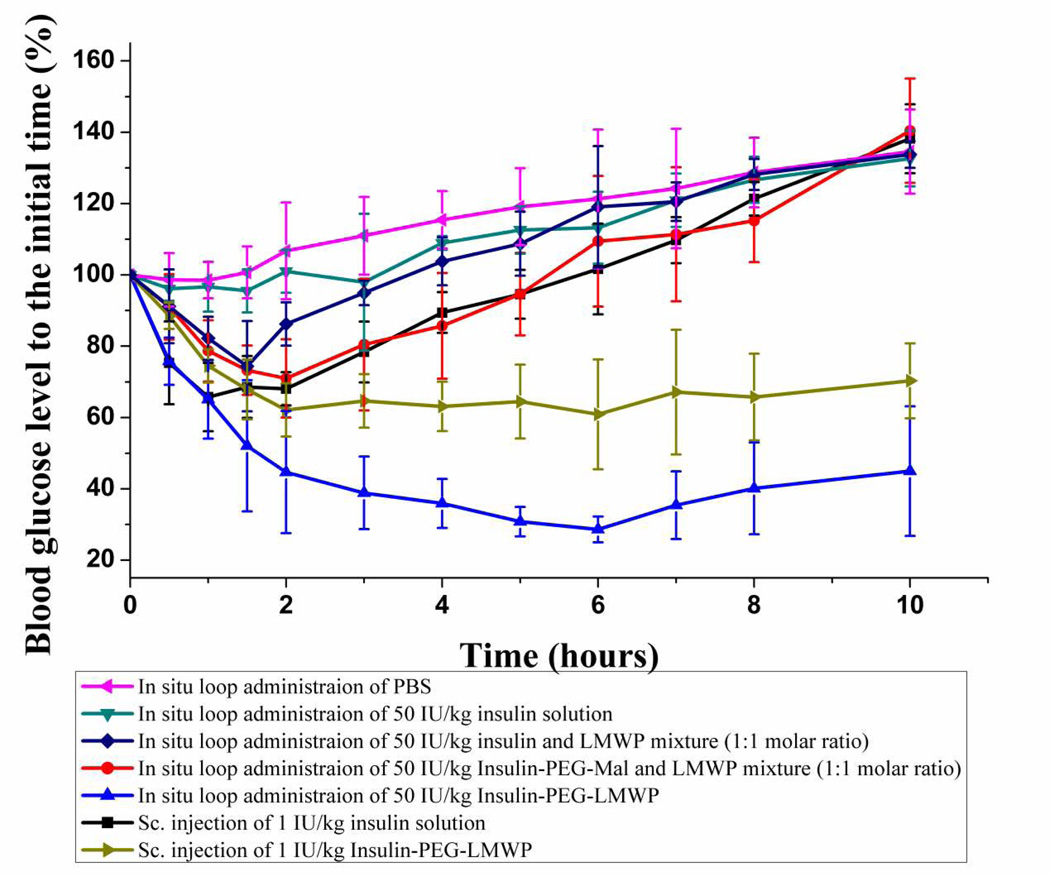
The hypoglycemic effect of insulin solution, PBS (negative control), mixture of insulin and LMWP, mixture of Insulin-PEG-Mal and LMWP, and Insulin-PEG-LMWP conjugate was examined and compared following in situ loop administration of samples in rats. The hypoglycemic effect by subcutaneous injection of 1 IU/kg insulin was also determined as a positive control in order to calculate the relative pharmacological bioavailability of the test compounds. As illustrated in Fig. 5, no hypoglycemic effect was observed following in situ loop administration of the PBS solution (negative control). As expected, the blood glucose level (BGL) decreased significantly soon after subcutaneous injection of 1 IU/kg insulin (positive control). The BGL rapidly reached a minimum (~66%) at 1 h, followed by a gradual increase to the basal level (~100%) at 6 h, and then remained unchanged till the end of the study. Two hours following subcutaneous injection of 1 IU/kg Insulin-PEG-LMWP, the BGL in rats was reduced by about 30% to almost the same extent shown by subcutaneous injection of 1 IU/kg insulin. The BGL then maintained at the level of around 70% till the end of this study (10 h). Although the hyperglycemia effect after s.c. injection of Insulin-PEG-LMWP seemed to start slightly later, yet it lasted much longer than that by s.c. injection of insulin alone, apparently due to the attachment of PEG and LMWP. Based on these results, the bioactivity of insulin appeared not to be impaired by the conjugation with LMWP.
Fig 5.
Plots of the blood glucose level (BGL) against time after in situ loop administration of: PBS ( , negative control); 50 IU/kg insulin solution (
, negative control); 50 IU/kg insulin solution ( ); 50 IU/kg of 1:1 molar ratio of insulin and LMWP mixture (
); 50 IU/kg of 1:1 molar ratio of insulin and LMWP mixture ( ); 50 IU/kg 1:1 molar ratio of Insulin-PEG-Mal and LMWP mixture (
); 50 IU/kg 1:1 molar ratio of Insulin-PEG-Mal and LMWP mixture ( ); 50 IU/kg of the Insulin-PEG-LMWP conjugate (
); 50 IU/kg of the Insulin-PEG-LMWP conjugate ( ); and subcutaneous injection of 1 IU/kg insulin solution (
); and subcutaneous injection of 1 IU/kg insulin solution ( , positive control); and 1 IU/kg Insulin-PEG-LMWP conjugate (
, positive control); and 1 IU/kg Insulin-PEG-LMWP conjugate ( ) to male Wistar rats. Each data point was represented as mean ± s.d. (N=6). For experimental details, please refer to the “Materials and Methods” section.
) to male Wistar rats. Each data point was represented as mean ± s.d. (N=6). For experimental details, please refer to the “Materials and Methods” section.
However, after in situ loop administration of 50 IU/kg insulin, no statistically significant decrease in BGL was observed when comparing to that of PBS administration (i.e. negative control), suggesting that intestinal uptake of insulin itself was completely ineffective. Yet, in situ loop administration of a mixture containing 50 IU/kg insulin and an equal molar dose of LMWP yielded a glucose-lowing effect similar to that of the positive control (s.c. administration of 1 IU/kg insulin) of 1 IU/kg. The BGL was quickly reduced to ~74% of the initial value within 1.5 h; a level significantly lower than the negative control (p < 0.05). This relatively poor intestinal uptake of the physically mixed, presumably ionic insulin/LMWP aggregates was probably due to the weak cell-penetrating ability mediated by the ionically adsorbed LMWP on the aggregates. It has been widely reported that ionic complexation of a gene compound with a CPP, such as LMWP [23, 24], would enhance its effectiveness for cell transfection [25–29]. Nevertheless, this hypoglycemic effect did not last long, as the BGL returned to the baseline in about 4 h. Importantly, Table 2 showed that the pharmacological availability (PA) of the in situ loop administered ionic insulin/LMWP aggregates (0.91%) was only minimally improved over that of insulin alone (0.52%); which was virtually negligible. These findings results demonstrated limitations of the physical mixture of insulin and LMWP in enhancing the intestinal absorption of insulin, due to ionic aggregation between these two compounds thereby markedly reducing the LMWP-mediated mucosa translocation. On the same token, the BGL reduced to a minimum (~71%) in 2 h following in situ loop administration of the physical mixture of 50 IU/kg Insulin-PEG-Mal and LMWP, similar to the event by in situ loop administration of the insulin and LMWP physical mixture; as previously reported. Nevertheless, the hypoglycemic effect of this mixture seemed to last slightly longer than that of the insulin and LMWP mixture, which, despite requiring further proof, might be attributed to the involvement of PEG. In addition, Table 2 showed that PA of this drug mixture (1.81%) also appeared to be slightly improved over that (0.91%) of the insulin and LMWP mixture.
Table 2.
Calculated pharmacodynamic parameters after in situ loop administration of: insulin solution; insulin and LMWP mixture; Insulin-PEG-Mal and LMWP mixture; the Insulin-PEG-LMWP conjugate; and subcutaneous injection of insulin (N=6).
| Parameters | insulin | insulin + LMWP mixture |
Insulin-PEG-Mal + LMWP mixture |
Insulin-PEG- LMWP conjugate |
insulin |
|---|---|---|---|---|---|
| Dose (IU/kg) | 50 | 50 | 50 | 50 | 1 |
| Ways of Administration | in situ loop | in situ loop | in situ loop | in situ loop | sc. |
| BGLmin (%) | - | 74.37 | 70.96 | 28.61 | 65.75 |
| Tmin (h) | - | 1.5 | 2 | 6 | 1 |
| AAC0→10h(%min) | 2751 | 4793 | 9541 | 37338 | 10547 |
| PA0→10h (%) | 0.52 | 0.91 | 1.81 | 7.08 | 100.00 |
BGLmin represents the minimum blood glucose level. Tmin represents the time to reach BGLmin. AAC0→10h represents the area above the curve during 0–100 h. PA0→10h represents the relative pharmacological availability.
In a sharp contrast, the BGL dropped considerably soon after in situ loop administration of 50 IU/kg Insulin-PEG-LMWP conjugate, and after 6 hours reached a minimum (~29%) that was significantly lower than any of the previous groups. More importantly, after reaching the minimum, the BGL levels remained low and relatively unchanged between 30–45% over an extended period of 10 hours; indicating a longlasting hypoglycemic effect that could not be matched by any other experimental groups. In reality, this hypoglycemic effect could have lasted longer than 10 hours had the experiments not been terminated, due to the fact that the rats could not survive longer after the surgery. Furthermore, the PA (7.08%) of this in situ loop administered Insulin-PEG-LMWP conjugate was substantially higher than any other experimental groups.
4. Discussion
Although oral insulin delivery has been the method that most clinicians are yarned for concerning the treatment of diabetes due to the convenience and high patient compliance, its reality for clinical practice has been hindered by two major obstacles of: 1) high susceptibility of insulin to digestion by proteolytic enzymes in the GI track, and 2) poor permeability of insulin through the mucosal barrier. While the former barrier can largely be circumvented via recent advancement in technologies on insulin encapsulation into protective polymer-based nano-carriers, the latter one still remains as the bottleneck problem that must be addressed to achieve sufficient oral insulin bioavailability. Recent discovery of the class of so-called cell-penetrating peptides (CPP) sheds light of the possibility to finally resolve this mucosa barrier. Via covalent linkages, these CPPs were proven to ferry attached large cargos such as proteins, genes, and nano-carriers across the membrane of every cell or tissue types including the blood brain barrier (BBB) [14]. Based on this unprecedented property, we hypothesized that by covalent conjugation of the insulin molecule with a CPP we would be able to prevail over the mucosa barrier in oral insulin delivery.
Despite remarkable accomplishment of cell-penetrating peptides in cell culture investigation, in vivo animal studies have been far less successful, primarily due to ionic aggregation between the cationic CPP and the anionic proteins (e.g. insulin) and gene or siRNA products, rendering both CPP and its carried drug cargos physiologically inactive. Indeed, in a widely cited, authoritative review article, Meade and Dowdy actually phrased the current situation concerning covalent conjugation of the cationic CPP to an anionic drug (siRNA) as the “Holy Grail” task [30, 31], obviously referring to the formidable difficulty of designing this chemical process without forming the charge-associated aggregates. Same here in our study, covalent conjugation of insulin with CPP was not a trivial issue, since the cationic CPP and anionic insulin would self-assemble into insoluble ionic aggregates; a phenomenon observed by us and many other investigators [32]. In agreement with the results reported by many investigators concerning cellular uptake of the ionic aggregates of CPP with anionic species such as siRNAs [26, 29], our animal studies in Fig. 5 and Table 2 clearly demonstrated that the capability of CPP-mediated cell-internalization of the carried anionic cargos was significantly impaired. Hence, the primary objective of this research was to design a strategy that could warrant the synthesis of a covalently-linked, monomeric CPP:insulin (1:1) conjugate in improving insulin’s pharmacological bioavailability (PA), thereby paving the road towards realizing the possibility of oral insulin delivery.
LMWP was first selected as the CPP in our investigation based on several major advantages. Unlike other CPPs that must be produced by organic synthesis and thus their poor yield would be prohibitive for animal testing, LMWP could be produced rapidly and also in mass quantities, according to single step isolation/purification production process developed in our laboratory via enzymatic digestion of natural protamine [10]. On a laboratory scale, a production rate of 10 g per week could be readily achieved. In addition, since LMWP possesses only a single -NH2 group at the N-terminus, its conjugation to a protein drug can be easily carried out using our established SPDP activation method [15]. Most importantly, unlike currently existing CPPs, the toxicological profile of LMWP has been fully documented. Animal studies confirmed that LMWP possessed significantly attenuated antigenicity, mutagenicity, and ability in activating the host complement system, as well as the hemodynamic and hematologic toxicities in dogs normally observed with other cationic peptides [12].
To overcome the aggregation problem caused by ionic complexation, we devised an innovative strategy by using NHS-PEG-MAL as an intermediate cross-linker during the coupling process. This strategy was primarily by taking the advantage of the unique dynamic movement and shielding effect of PEG. As noted, PEG is a nontoxic, nonimmunogenic/antigenic, highly water-soluble polymer that has been approved by FDA for various clinical applications. Conjugation of a protein drug with PEG, a process termed PEGylation, has been wide applied to protect the linked drug from detection by the host immune system as well as from degradation by the circulating proteases, thereby significantly prolonging the plasma half-life of the protein drug[33]. Indeed, Kim and co-workers showed that while site-specific PEGylation of insulin did not alter much of its therapeutic potency in vivo, yet this modification markedly enhanced insulin’s resistance to aggregation and significantly reduced its immunogenicity and antigenicity[34, 35].
Hence, the strategy called for first linking insulin (its active center was pre-protected with DMMA, as discussed below) with a PEG chain (with MW greater than that of LMWP) containing heterobifunctional reactive groups of NHS (N-hydroxysuccinimide) and maleimide (MAL) at the two terminuses to yield the Insulin-PEG-MAL intermediate conjugate. It should be noted that since the maleimide group was less reactive, the predominant reaction between insulin and PEG would be through the more active NHS-end on PEG. This conjugate was then reacted with LMWP, which was pre-thiolated at the N-terminal to contain a reactive -SH group, to yield the final product of Insulin-PEG-LMWP. By mixing native insulinor SPDP-activated insulin with LMWP, significant precipitation of the insoluble ionic aggregate was observed, consistent with the findings by many other investigators [32]. In a sharp contrast, however, a virtually transparent reaction mixture was obtained by using the above strategy; presumably due to the fact that the rapid 3-D movement by the highly flexible PEG chains had shielded insulin from the charge-induced complexation with LMWP during the coupling process.
Prior to insulin conjugation, the lysine residues on the B-chain of insulin where the active center resided must be protected to ensure a full retention of insulin’s biological functions. As reported, three primary amino groups were present on each insulin molecule, the A1-glycine (GlyA1), B1-phenylalanine (PheB1), and B29-lysine (LysB29) [21]. These amino groups could potentially interact with NHS-ester at a pH where most of the amine groups were de-protonated. As noted, insulin modifications involving the primary amine groups were usually directed towards either the PheB1 or LysB29 residues, since modification at the GlyA1 residue was known to significantly impair the biological activity of insulin [36]. In our study, LysB29 was chosen as the position for the attachment of PEG. Therefore, the primary amines of the GlyA1 and PheB1 residues needed to be blocked in order to achieve an optimized site-specific conjugation of PEG. This selective labeling was successfully accomplished by controlling the pH in the reaction solution within the range of 6.8–6.9. The pKa of these amino groups of GlyA1, PheB1, and LysB29 were reported to be 8.4, 7.1, and 9.8, respectively. Hence, at the controlled pH, the terminal amine groups on GlyA1 and PheB1 were de-protonated and thus able to react with DMMA through the nucleophilic attack, while most of the other lysine residues remained intact, since the pH was about two units below the pKa of the lysine residues. It should be noted that the DMMA modification was labile to the acidic environment, and thus this modification could be reversed by making the solution slightly acidic.
Following protection, the DMMA-modified insulin was reacted with the NHS-PEG-MAL cross-linker. Successful synthesis of the Insulin-PEG-MAL conjugate was confirmed by MALDI-TOF analysis after the conjugate was treated with DTT and trypsin. While DTT dissociated the A- and B-chain of insulin, trypsin cleaved insulin at the amide bond between the ArgB22 and GlyB23 residues, yielding a 43-residue peptide containing both the A- and B-chain N-terminals and a smaller eight amino acid peptide consisting of the B chain C-terminal of insulin. On the other hand, access to the site between LysB29 and Ala residues, which was subjected to tryptic cleavage, was blocked by the PEG modification. As depicted in Fig. 1, the Insulin-PEG-MAL conjugate was cleaved into three major fragments consisting of: A-chain (MW: 2,450Da), N-terminal of B-chain (B1-B22) (MW: 2,600Da), and PEGylated B-chain (B23-B30) (MW: 4,530Da). Based on these results, it was confirmed that the PEG modification site was LysB29, instead of the N-terminal primary amines of the A- or B-chain.
Prior to the purification of the desired Insulin-PEG-LMWP conjugate via heparin affinity chromatography, excess LMWP-SH was removed by centrifugation through a filter membrane (MWCO: 3,000Da) into the filtrate, whereas both the Insulin-PEG-MAL and Insulin-PEG-LMWP conjugates were maintained due to their much higher than the membrane cutoff range. Without this filtration step to remove excess LMWP-SH, the fraction of Insulin-PEG-LMWP would co-elute with the fraction of LMWP-SH from the heparin column, due to their relatively similar binding affinity to the heparin column. Fig. 2 displayed that a symmetric protein peak representing the final Insulin-PEG-LMWP conjugate was eluted from the heparin column at a retention time of ~50 min and a salt gradient of 0.5 M NaCl.
Success of obtaining the desired Insulin-PEG-LMWP conjugates was confirmed by results from Fig. 3. Fig. 3A illustrated the SDS-PAGE analysis results of the conjugates. It has been reported that the migration property of PEGylated protein could be very different from that of protein of same molecular weight probably resulting from the complex interaction between PEG chains and SDS micelles, and a simple comparison with the protein standard bands could not give the correct molecular weight information of the products [37]. Therefore, the bands in Fig. 3A did not precisely correlate to the MWs of the conjugates (9,600 and 11,500 Da, respectively) due to the attachment of PEG. However, what’s important here is that Insulin-PEG-LMWP conjugate possess slightly reduced gel mobility relative to the Insulin-PEG-MAL conjugate, indicating the successful attachment of LMWP onto Insulin-PEG-MAL and the presence of a homogeneous Insulin-PEG-LMWP product.
MALDI-TOF MS data also yielded consistent findings. The Insulin-PEG-MAL (Fig. 3B) product contained two components, a major 9,600-Da peak trailed by a minor 13,250Da peak. Based on molecular weight analysis, the primary product appeared to be insulin that was PEGylated with a single PEG molecule whereas the minor by-product was insulin containing two PEG chains. On the other hand, Fig. 3C showed that the Insulin-PEG-LMWP product consisted of a single fraction, although a little bit non-symmetric in shape, with the peak MW being 11,480 Da. The difference in MW between Insulin-PEG-MAL and Insulin-PEG-LMWP was almost identical to the molecular weight of LMWP, corroborating the presence of a monomeric structure (i.e. insulin: LMWP ratio of 1:1) in the final product.
The capability of Insulin-PEG-LMWP to transport across the mucosal barrier was examined against cultured Caco-2 cells. Caco-2 cells, derived from a human colorectal carcinoma, form a highly polarized membrane when grown to confluence on microporous filters and show close morphological and functional similarities to intestinal epithelium. Caco-2 cell monolayers have gained enormous popularity as a reliable and high-throughput in vitro model system for the evaluation of a large number of drug candidates for their intestinal absorption potential [38, 39]. Fig. 4 demonstrated that comparing to native insulin, both the apical-to-basolateral and basolateral-to-apical flux of the Insulin-PEG-LMWP conjugate were notably increased, apparently due to incorporation of the cell-penetrating LMWP into this conjugate. In agreement with these findings, the permeability coefficient of the conjugate across the Caco-2 cell monolayer was estimated to be 4–5 folds higher than the value of native insulin.
It had been reported by us and many other investigators that non-covalent packaging of the cationic cell-penetrating peptide (e.g. TAT) with an anionic compound such as insulin [24, 32, 40] or nucleic acids [24, 41, 42] would still be able to yield a certain degree of enhancement on CPP-mediated cell internalization. However, it was also well recognized that the most effective means for enhancing the cellular uptake would be through covalent linking of the CPP to the anionic drug prior to cellular treatment [30]. This was simply due to the fact that the dense cationic charged motifs present within CPP were crucial for enhancing cellular uptake by engaging the necessary components on the cell surface, and charge neutralization by ionic complexation would inhibit CPP-mediated cell internalization [31]. Furthermore, non-covalent packaging of CPP on the surface of the anionic protein would also likely to shield the access to the active center thereby inhibiting the protein’s biological functions. Our results of the in situ loop insulin absorption provided a strong verification to this phenomenon. Fig. 5 demonstrated that while in situ loop administration of 50 IU/kg insulin alone produced virtually no hypoglycemic effects, in situ loop administration of the physically mixed LMWP and 50 IU/kg insulin or Insulin-PEG-Mal conjugate only slightly reduced the blood glucose level (BGL) by about 30% after 2 hours. More critically, the BGL returned to normal 5 hours following insulin administration. In a sharp contrast, the BGL dropped considerably by 70% soon after in situ loop administration of 50 IU/kg Insulin-PEG-LMWP and, most importantly, the BGL remained stable at this low level (30–45% of the initial, normal value) over a period of longer than 10 hours; reflecting the achievement of the desirable long-lasting hypoglycemic effect.
Conclusions
In this paper, we developed a chemical conjugation strategy (Scheme 1) that, for the first time, yields a homogeneous, monomeric chemical chimera between the highly negatively charged insulin and positively charged LMWP (a potent yet non-toxic CPP). Results presented here provided a full spectrum of proofs including: [1] physical (i.e. formation of a transparent solution during coupling of insulin with LMWP), [2] chemical (i.e. MALDI-TOF (Fig. 1), heparin affinity chromatography (Fig. 2), and SDS-PAGE (Fig. 3) data), [3] biological (apical-to-basolateral and basolateral-to-apical in vitro intestinal absorption profiles (Fig. 4) and permeability coefficient (Table 1) data), and [4] pharmacological (BGL changes following in situ loop administration of insulin samples (Fig. 5) and pharmacological bioavailability and pharmacokinetic data (Table 2)) evidences that all validated the success in synthesizing a 1:1 insulin:LMWP conjugate that possessed unparalleled therapeutic efficacy in lowering BGL. Overall, the current investigation paves the road towards clinical realization of an effective oral insulin delivery system for treatment of diabetes. With the Insulin-LMWP chemical conjugate available, future work gearing towards encapsulation of this conjugate into a chitosan-modified, silica-based mucoadhesive nanocomposites and assessment the in vivo efficacy in a clinically relevant diabetic rat model is currently under development in our laboratories.
Acknowledgments
This work was supported in part by NIH R01 Grant CA114612. We also thank the support from NSFC, China (91029743, 81172996), Shanghai Pu-jiang Scholar Program (11PJ1411800), National Science and Technology Major Project (2012ZX09304004) and the Open Project Program of Key Lab of Smart Drug Delivery (Fudan University), MOE & PLA (SDD2011-02). This research was also partially sponsored by Grant R31-2008-000-10103-01 from the World Class University (WCU) project of the MEST and NRF of South Korea. Victor C. Yang is currently a Participating Faculty in the Department of Molecular Medicine and Biopharmaceutical Sciences, College of Medicine & College of Pharmacy, Seoul National University, South Korea.
Abbreviations
- BGL
Blood Glucose Level
- CPP
Cell Penetrating Peptides
- DMEM
Dulbecco's Modified Essential Medium
- DMMA
Dimethylmaleic Anhydride
- DMSO
Dimethyl Sulfoxide
- DTT
Dithiothreitol
- EDTA
Ethylenediaminetetraacetic Acid
- ELISA
Enzyme-Linked Immunosorbent Assay
- FBS
Fetal Bovine Serum
- FITC
Fluoresceinisothiocyanate
- Insulin-PEG-LMWP
Insulin-Polyethylene Glycol-Low Molecular Weight Protamine Conjugate
- Insulin-PEG-MAL
Insulin-Polyethylene Glycol-Maleimide
- LMWP
Low Molecular Weight Protamine
- MALDI-TOF MS
Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry
- NHS-PEG-MAL
Succinimidyl-[(N-maleimidopropionamido)-polyethyleneglycol] ester
- PA
Pharmacological bioavailability
- PBS
Phosphate Buffered Saline
- PEG
Polyethylene Glycol
- SDS-PAGE
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
- SPDP
N-Succinimidyl 3-(2-Pyridyldithio) Propionate
- STZ
Streptozotocin
- TEER
Transepithelial Electrical Resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. New Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iyer H, Khedkar A, Verma M. Oral insulin - a review of current status. Diabetes Obes Metab. 2010;12:179–185. doi: 10.1111/j.1463-1326.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen MC, Sonaje K, Chen KJ, Sung HW. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011;32:9826–9838. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 4.Khafagy el S, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Deliver Rev. 2007;59:1521–1546. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Lin YH, Sonaje K, Lin KM, Juang JH, Mi FL, Yang HW, et al. Multi-ion-crosslinked nanoparticles with pH-responsive characteristics for oral delivery of protein drugs. J Control Release. 2008;132:141–149. doi: 10.1016/j.jconrel.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Woitiski CB, Veiga F, Ribeiro A, Neufeld R. Design for optimization of nanoparticles integrating biomaterials for orally dosed insulin. Eur J Pharm Biopharm. 2009;73:25–33. doi: 10.1016/j.ejpb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Ding J, Zhang J, He C, Tang C, Yin C. Polymer integrity related absorption mechanism ofsuperporous hydrogel containing interpenetrating polymer networks for oral delivery of insulin. Biomaterials. 2010;31:3347–3356. doi: 10.1016/j.biomaterials.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 8.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, et al. Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31:3384–3394. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 9.Su FY, Lin KJ, Sonaje K, Wey SP, Yen TC, Ho YC, et al. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials. 2012;33:2801–2811. doi: 10.1016/j.biomaterials.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 10.Chang LC, Lee HF, Yang Z, Yang VC. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (I): preparation and characterization. AAPS PHARMSCI. 2001;3:7–14. doi: 10.1208/ps030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang LC, Liang JF, Lee HF, Lee LM, Yang VC. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (II): in vitro evaluation of efficacy and toxicity. AAPS PHARMSCI. 2001;3:15–23. doi: 10.1208/ps030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Wrobleski S, Wakefield T, Yang V. Low molecular weight protamine as nontoxic heparin/low molecular weight heparin antidote (III): Preliminary in vivo evaluation of efficacy and toxicity using a canine model. AAPS PHARMSCI. 2001;3:24–31. doi: 10.1208/ps030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steven R, Schwarze Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 14.Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci. 2000;21:45–48. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 15.Park YJ, Chang L-C, Liang JF, Moon C, Chung C-P, Yang VC. Nontoxic membrane translocation peptide from protamine, LMWP, for enhanced intracellular protein delivery – in vitro and in vivo study. FASEB J. 2005;19:1555. doi: 10.1096/fj.04-2322fje. [DOI] [PubMed] [Google Scholar]
- 16.Xia H, Gao X, Gu G, Liu Z, Zeng N, Hu Q, et al. Low molecular weight protamine-functionalized nanoparticles for drug delivery to the brain after intranasal administration. Biomaterials. 2011;32:9888–9898. doi: 10.1016/j.biomaterials.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Liang JF, Zhen L, Chang L-C, Yang VC. A less toxic heparin antagonist- low molecular weight protamine. Biochemistry-Moscow+ 2003;68:116–120. doi: 10.1023/a:1022109905487. [DOI] [PubMed] [Google Scholar]
- 18.Tsui B, Singh VK, Liang JF, Yang VC. Reduced reactivity towards anti-protamine antibodies of a LMWP analogue. Thromb Res. 2003;101:417–420. doi: 10.1016/s0049-3848(00)00427-8. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson J, Drevin H, Axen R. Protein thiolation reversible protein-protein conjugation N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978;173:723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia CQ, Wang J, Shen W-C. Hypoglycemic effect of insulin-transferrin conjugate in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2000;295:594–600. [PubMed] [Google Scholar]
- 21.Kavimandan NJ, Losi E, Wilson JJ, Brodbelt JS, Peppas NA. Synthesis and Characterization of Insulin-Transferrin Conjugation. Bioconjugate Chem. 2006;17:1376–1384. doi: 10.1021/bc050344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morishita M, Kamei N, Ehara J, Isowa K, Takayama K. A novel approach using functional peptides for efficient intestinal absorption of insulin. J Control Release. 2007;118:177–184. doi: 10.1016/j.jconrel.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Choi YS, Lee JY, Suh JS, Kwon YM, Lee SJ, Chung JK, et al. The systemic delivery of siRNAs by a cell penetrating peptide, low molecular weight protamine. Biomaterials. 2010;31:1429–1443. doi: 10.1016/j.biomaterials.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Park YJ, Liang JF, Ko KS, Kim SW, Yang VC. Low molecular weight protamine as an efficient and nontoxic gene carrier: in vitro study. J Gene Med. 2003;5:700–711. doi: 10.1002/jgm.402. [DOI] [PubMed] [Google Scholar]
- 25.Ignatovich IA, Dizhe EB, Pavlotskaya AV, Akifiev BN, Burov SV, Orlov SV, et al. Complexes of plasmid DNA with basic domain 47–57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem. 2003;278:42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- 26.Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 2003;31:2717–2724. doi: 10.1093/nar/gkg385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandgren S, Cheng F, Belting M. Nuclear targeting of macromolecular polyanions by an HIV-Tat derived peptide Role for cell-surface proteoglycans. J Biol Chem. 2002;277:38877–38883. doi: 10.1074/jbc.M205395200. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph C, Plank C, Lausier J, Schillinger U, Muller RH, Rosenecker J. Oligomers of the arginine- rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278:11411–11418. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 29.Unnamalai N, Kang BG, Lee WS. Cationic oligopeptide-mediated delivery of dsRNA for post- transcriptional gene silencing in plant cells. FEBS Lett. 2004;566:307–310. doi: 10.1016/j.febslet.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv Drug Deliver Rev. 2007;59:134–140. doi: 10.1016/j.addr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Deliver Rev. 2008;60:530–536. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamei N, Morishita M, Eda Y, Ida N, Nishio R, Takayama K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J Control Release. 2008;132:21–25. doi: 10.1016/j.jconrel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 34.Uchio T, Baudyš M, Liu F, Song SC, Kim SW. Site-specific insulin conjugates with enhanced stability and extended action profile. Adv Drug Deliver Rev. 1999;35:289–306. doi: 10.1016/s0169-409x(98)00078-7. [DOI] [PubMed] [Google Scholar]
- 35.Hinds KD, Kim SW. Effects of PEG conjugation on insulin properties. Adv Drug Deliver Rev. 2002;54:505–530. doi: 10.1016/s0169-409x(02)00025-x. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay DG, Shall S. The acetylation of insulin. Biochem J. 1971;121:737–745. doi: 10.1042/bj1210737a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng C, Ma G, Su Z. Native PAGE eliminates the problem of PEG-SDS interaction in SDS-PAGE and provides an alternative to HPLC in characterization of protein PEGylation. Electrophoresis. 2007;28:2801–2807. doi: 10.1002/elps.200600807. [DOI] [PubMed] [Google Scholar]
- 38.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Bioph Res Co. 1991;175:880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 39.Chong S, Dando SA, Soucek KM, Morrison RA. In vitro permeability through Caco-2 cells is not quantitatively predictive of in vivo absorption for peptide-like drugs absorbed via the dipeptide transporter system. Pharm Res. 1996;13:120–123. doi: 10.1023/a:1016045820933. [DOI] [PubMed] [Google Scholar]
- 40.Khafagy el S, Morishita M, Kamei N, Eda Y, Ikeno Y, Takayama K. Efficiency of cell-penetrating peptides on the nasal and intestinal absorption of therapeutic peptides and proteins. Int J Pharmaceut. 2009;381:49–55. doi: 10.1016/j.ijpharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004;11:1165–1175. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Turner JJ, Jones S, Fabani MM, Ivanova G, Arzumanov AA, Gait MJ. RNA targeting with peptide conjugates of oligonucleotides, siRNA and PNA. Blood Cell Mol Dis. 2007;38:1–7. doi: 10.1016/j.bcmd.2006.10.003. [DOI] [PubMed] [Google Scholar]