Abstract
Objective
Carotid artery stenting (CAS) vs endarterectomy (CEA) remains controversial and has been the topic of recent randomized controlled trials. The purpose of this study was to compare the practice and outcomes of CAS and CEA in a real world setting.
Methods
This is a retrospective analysis of 7649 CEA and 430 CAS performed at 17 centers from 2003 to 2010 within the Vascular Study Group of New England (VSGNE). The primary outcome measures were (1) any in-hospital stroke or death and (2) any stroke, death, or myocardial infarction (MI). Patients undergoing CEA in conjunction with cardiac surgery were excluded. Multivariate logistic regression was performed to identify predictors of stroke or death in patients undergoing CAS.
Results
CEA was performed in 17 centers by 111 surgeons, while CAS was performed in 6 centers by 30 surgeons and 8 interventionalists. Patient characteristics varied by procedure. Patients undergoing CAS had a higher prevalence of coronary artery disease, congestive heart failure, diabetes, and prior ipsilateral CEA. Embolic protection was used in 97% of CAS. Shunts were used in 48% and patches in 86% of CEA. The overall in-hospital stroke or death rate was higher among patients undergoing CAS (2.3% vs 1.1%; P = .03). Overall stroke, death, or MI (2.8% CAS vs 2.1% CEA; P = .32) were not different. Asymptomatic patients had similar rates of stroke or death (CAS 0.73% vs CEA 0.89%; P = .78) and stroke, death, or MI (CAS 1.1% vs CEA 1.8%; P = .40). Symptomatic patients undergoing CAS had higher rates of stroke or death (5.1% vs 1.6%; P = .001), and stroke, death, or MI (5.8% vs 2.7%; P = .02). By multivariate analysis, major stroke (odds ratio, 4.5; 95% confidence interval [CI], 1.9–10.8), minor stroke (2.7; CI, 1.5–4.8), prior ipsilateral CEA (3.2, CI, 1.7–6.1), age >80 (2.1; CI, 1.3–3.4), hypertension (2.6; CI, 1.0–6.3), and a history of chronic obstructive pulmonary disease (1.6; CI, 1.0–2.4) were predictors of stroke or death in patients undergoing carotid revascularization.
Conclusions
In our regional vascular surgical practices, the overall outcomes of CAS and CEA are similar for asymptomatic patients. However, symptomatic patients treated with CAS are at a higher risk for stroke or death. (J Vasc Surg 2012;56:990-6.)
There are conflicting data regarding the outcomes of patients undergoing carotid artery stenting (CAS) compared with carotid endarterectomy (CEA) from recent, large randomized controlled trials. As a consequence, debate surrounds the appropriate use for CAS relative to CEA. In the 2010 International Carotid Stenting Study (ICSS), 1713 symptomatic patients from 50 centers in Europe, Australia, New Zealand, and Canada were randomized to CAS or CEA.1 Thirty-day results showed a combined stroke, death, myocardial infarction (MI) rate of 7.4% for CAS, and 4.0% for CEA, P < .006, an effect primarily driven by an increased stroke rate of 7.0% for CAS vs 3.3% for CEA.1
In the Carotid Revascularization Endarterectomy vs Stent Trial (CREST), 2502 patients from 117 centers in the United States and Canada were randomized to CAS or CEA.2,3 Fifty-three percent of patients were symptomatic and 47% were asymptomatic. The 30-day combined stroke, death, and MI rate was 5.2% for CAS and 4.5% for CEA, P > .05. However, like ICSS, the stroke rate was higher in CAS patients, 4.1% vs 2.3%. One explanation for the differences in outcomes is that the populations studied were different, with ICSS containing only symptomatic patients, whereas nearly half of the CREST cohort was symptomatic.
While the Center for Medicare and Medicaid Services (CMS) has approved reimbursement for CAS in symptomatic “high risk” patients, there currently are no operational definitions for the term “high-risk” in the setting of CEA. Several groups have reported risk factors associated with stroke or death following CEA, with the intent of improving preoperative risk assessment and patient selection.4–8 Across these studies, the most consistent risk factor found to predict stroke or death following CEA has been preoperative neurologic symptoms. Other variables associated with increased operative risk have included emergent operation,4 renal failure,8 and diabetes.6 While CAS is generally performed in patients who may be considered high risk for CEA based on these risk factors, there is no clear evidence suggesting that the risks with CAS are any lower.
In the setting of this controversy, the purpose of this study was to analyze the practice and outcomes of CAS compared with CEA in our region, using the experience captured in the Vascular Study Group of New England (VSGNE) database. Specifically, our aims were to perform a stratified analysis of outcomes across asymptomatic and symptomatic patients and to develop a risk prediction model for stroke or death in patients undergoing CAS.
METHODS
Subjects and database
Data from the VSGNE database were used for this analysis. The VSGNE is a regional cooperative quality improvement initiative developed in 2002 and currently involves over 180 physicians at 28 centers (14 academic, 14 community). The group aims to study and improve regional outcomes in vascular surgery and has prospectively collected over 140 detailed patient demographic, operative, and clinical outcome variables for CEA since 2003 and CAS since 2005.9 Trained nurses or clinical data abstractors enter data and research analysts are blinded to patient, surgeon, and hospital identity. Further details on this registry have been published previously and are available at http://www.vascularweb.org/regionalgroups/vsgne.
VSGNE data have been validated for completeness using biennial audits of discharge claims data from each participating institution. These audits ensure complete inclusion of all procedures performed in participating hospitals. In addition, components for our main outcome measure, postoperative stroke or death, specifically, ICD-9 codes for CEA (38.12), and postoperative iatrogenic stroke (997.02), as well as discharge status (alive, dead), have been validated using hospital administrative claims data.
Validation analyses found initially that 92% of CEAs that had been performed by participating surgeons during the specified time interval at all centers had been entered into the VSGNE database. Data from the remaining 8% of patients were then retrieved from hospital charts. Thus, this dataset represents 100% of CEAs performed by VSGNE members during the specified time period. An audit of cases with administrative codes for postoperative iatrogenic stroke revealed that all of these patients were properly recorded in the VSGNE database. Additionally, three strokes were reported to VSGNE that had not been coded in claims data. No postoperative strokes captured in claims data had been “missed” by the VSGNE data reporting mechanism.9 We have not formally audited CAS procedures across the VGSNE. However, based on audits for other procedures (lower extremity bypass and abdominal aortic aneurysm repair), we have not identified any reporting bias by procedure. Additionally, we have not identified any mortality bias by cases not initially captured.
Our study sample included all patients who underwent CEA (excluding those combined with coronary bypass grafting) and CAS between 2003 and 2010 at VSGNE centers. This included 7649 CEA, from 17 centers performed by 111 surgeons, and 430 CAS performed at 6 centers by 30 vascular surgeons and 8 interventionalists.
Outcome measures
Outcomes were stratified by symptomatic status. Symptomatic patients are defined as having a neurologic event, including any hemispheric or ocular transient ischemic attack, major or minor stroke preceding the intervention ipsilateral to the treated lesion. This definition is similar to ICSS and CREST, although ICSS lesions were considered symptomatic for up to 1 year preceding intervention and CREST limited to 180 days preop.
The primary outcome measures were (1) any stroke (major or minor, ipsilateral or contralateral) or death; and (2) any stroke, death, or MI. All outcomes were in-hospital. Transient ischemic attack was not included in the primary outcome measures but was captured as a secondary measure. Postoperative major strokes were defined as cortical, vertebrobasilar, or ocular disability resulting in nonindependent living status, or blindness; otherwise strokes were defined as minor. Neurologists did not routinely examine patients postop, though this is part of the protocol for CAS at several of the participating institutions. Myocardial infarctions included clinical, electrocardiogram (EKG), and troponin-only MI. Indications for obtaining postoperative troponin are institution dependent and variable. No centers routinely screened all postoperative patients for MI with troponin. Cranial nerve injuries, a secondary outcome measure, were deemed permanent by persistence of a deficit at 1-year follow-up.
Statistical analysis
Demographic and outcomes data were compared using a t-test for continuous variables and χ2 with Fisher exact correction (where needed) for categorical or dichotomous variables. To predict in-hospital postoperative stroke or death after carotid revascularization, we initially performed univariate comparisons between our main outcome measures and patient level variables (eg, symptomatic status, congestive heart failure) as well as intraoperative factors (eg, stent architecture, protamine use), to develop the most robust risk prediction model available. Univariate predictors that were significant at P < .10 were then entered into a multivariate model using backwards stepwise multivariate logistic regression, which was used to generate odds ratios and 95% confidence intervals for in-hospital stroke or death.
All analyses were performed using Microsoft Excel (Redmond, Wash) and Stata (College Station, Tex). The Institutional Review Board at Dartmouth College approved the use of de-identified data for this study. All tests of significance were performed at the .05 level.
RESULTS
Patient demographics are shown in Table I. Symptomatic status did not differ by procedure, with 34% of CEA patients and 36% of CAS patients exhibiting preoperative ipsilateral hemispheric or ocular symptoms. However, there were significant differences in patients selected for CAS compared with CEA. The proportion of male patients was higher for CAS than CEA. Additionally, there was a significantly higher prevalence of tobacco use, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease (COPD), and previous ipsilateral endarterectomy among patients undergoing CAS. Stent patients were also more likely to be on antiplatelet therapy preoperatively and less likely to be on a preoperative β-blocker. Embolic protection was used in 97% of stents, predilation in 67%, and an open cell stent was used in 82%. Shunts were used in 47% of CEA, a patch in 86%, general anesthesia in 88%, and a completion duplex in 31%.
Table I.
Patient demographics
| CEA (n = 7649) | CAS (n = 430) | P value | |
|---|---|---|---|
| Age | 70 | 69 | .13 |
| Male | 60% | 66% | .019 |
| Elective | 89% | 90% | .51 |
| Any symptoms | 34% | 36% | .33 |
| TIA/amaurosis | 24% | 25% | .53 |
| Minor stroke | 8% | 8% | .80 |
| Major stroke | 2% | 3% | .35 |
| Hypertension | 88% | 88% | .73 |
| Any smoking history | 80% | 85% | .014 |
| Coronary artery disease | 33% | 45% | .001 |
| Positive stress test | 11% | 13% | .16 |
| CHF | 8% | 17% | .001 |
| Diabetes | 31% | 34% | .20 |
| COPD | 23% | 30% | .002 |
| Renal insufficiency | 6% | 8% | .070 |
| Prior ipsilateral CEA | 2% | 33% | .001 |
| Antiplatelet therapy | 90% | 97% | .001 |
| Current β-blocker therapy | 81% | 72% | .001 |
| Current statin therapy | 76% | 78% | .22 |
| White race | 99% | 99% | .53 |
CEA, Carotid endarterectomy; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack.
The overall rate of stroke or death was significantly higher in patients undergoing CAS, 2.3%, compared with CEA, 1.1% (P = .028). The overall rate of stroke, death, or MI (2.8% CAS, 2.1% CEA; P = .319) was not significantly different. Among asymptomatic patients (CEA = 5043; CAS = 273), the rates of stroke or death, and stroke, death, or MI did not differ between CAS and CEA (Fig 1). Among symptomatic patients (CEA = 2605; CAS = 156), the stroke or death rate was significantly higher in patients undergoing CAS, 5.1%, compared with CEA, 1.6% (P = .001). The rate of stroke, death, or MI was also higher for CAS, 5.8%, compared with CEA, 2.7% (P = .022; Fig 2). In patients who had undergone prior ipsilateral CEA (CEA = 172, CAS = 144), the rate of stroke or death, and stroke, death, or MI, did not differ significantly (Fig 3).
Fig 1.
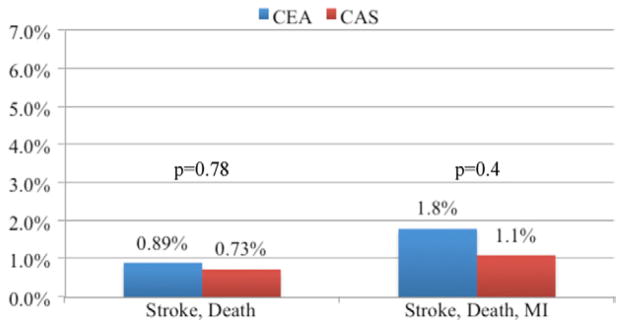
Primary outcomes for asymptomatic patients undergoing carotid artery stent (CAS) and carotid endarterectomy (CEA) in the Vascular Study Group of New England (VSGNE). (CEA = 5043; CAS = 273). MI, Myocardial infarction.
Fig 2.
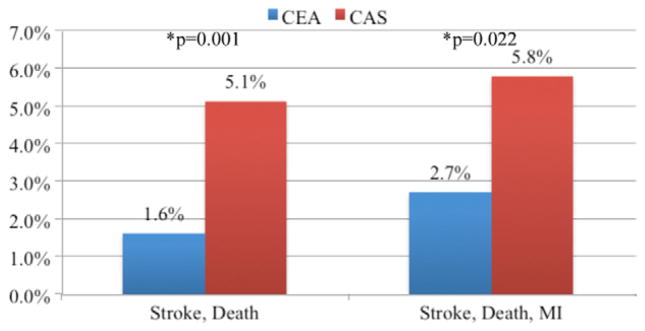
Primary outcomes for symptomatic patients undergoing carotid artery stent (CAS) and carotid endarterectomy (CEA) in the Vascular Study Group of New England (VSGNE). (CEA = 2605; CAS = 156). MI, Myocardial infarction.
Fig 3.
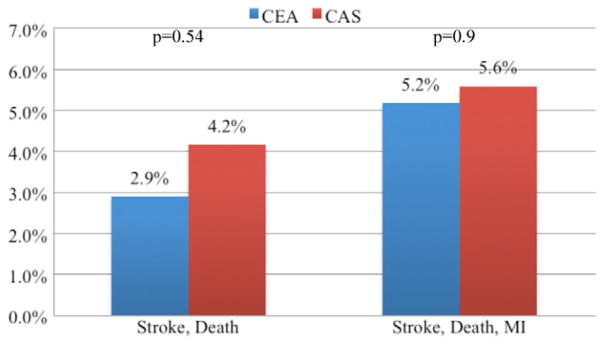
Primary outcomes for patients with prior ipsilateral carotid endarterectomy (CEA) undergoing carotid artery stent (CAS) and CEA in the Vascular Study Group of New England (VSGNE). (CEA = 172; CAS = 144). MI, Myocardial infarction.
Among asymptomatic patients, events were infrequent for both CAS and CEA, and there were no statistically significant differences in any of the secondary outcome measures–ipsilateral stroke, major stroke, minor stroke, transient ischemic attack, MI, death, or permanent cranial nerve injury (Table II, A). Neurologic outcomes among symptomatic patients, however, were worse for CAS compared with CEA. This included higher rates of ipsilateral stroke (3.8% vs 1.2%; P = .004), major stroke (2.6% vs 0.6%; P = .005), and minor stroke (2.6% vs 0.8%) (Table II, B). Among patients with a history of prior ipsilateral CEA, there were no differences in secondary outcomes between CEA and CAS (Table II, C).
Table II.
Secondary outcomes
| A. Asymptomatic (CEA = 5043; CAS = 253)
| |||||||
|---|---|---|---|---|---|---|---|
| Ipsilateral stroke | Major stroke | Minor stroke | TIA | MI | Death | CNI | |
| CAS | 0.4% | 0.4% | 0.5% | 0.5% | 0.7% | 0.4% | 0% |
| CEA | 0.6% | 0.3% | 0.4% | 0.3% | 1.0% | 0.2% | 0.9% |
| P value | .58 | .90 | .74 | .77 | .69 | .42 | .11 |
| B. Symptomatic (CEA = 2605; CAS = 156)
| |||||||
|---|---|---|---|---|---|---|---|
| Any ipsilateral stroke | Any major stroke | Any minor stroke | TIA | MI | Death | CNI | |
| CAS | 3.8% | 2.6% | 2.6% | 0.7% | 1.3% | 1.3% | 0% |
| CEA | 1.2% | 0.6% | 0.8% | 0.6% | 1.3% | 0.2% | 1.1% |
| P value | .004 | .005 | .019 | .99 | .99 | .20 | .19 |
| C. Previous ipsilateral CEA (CEA = 172; CAS = 144)
| |||||||
|---|---|---|---|---|---|---|---|
| Any ipsilateral Stroke | Any major stroke | Any minor stroke | TIA | MI | Death | CNI | |
| CAS | 2.8% | 1.4% | 2.8% | 0.6% | 2.8% | 1.4% | 0% |
| CEA | 1.7% | 1.2% | 1.7% | 0.7% | 2.3% | 1.2% | 0.9% |
| P value | .53 | .86 | .53 | .9 | .77 | .86 | .26 |
CEA, Carotid endarterectomy; CHF, congestive heart failure; CNI, cranial nerve injury; MI, myocardial infarction; TIA, transient ischemic attack. Bold indicates significant difference (P < .05).
A risk prediction model for stroke or death following carotid revascularization was developed using multivariate logistic regression. Factors tested by univariate analysis are shown in Table III. Factors achieving significance in the final multivariate model were age >80, major stroke, minor stroke, COPD, hypertension, and a history of prior CEA (Table IV). Due to colinearity with prior CEA, CAS was not entered into the multivariate model. The model was used to develop predicted rates of stroke or death in the CAS and CEA cohorts given the differences in patient demographics between these groups. Patients undergoing CAS had a significantly higher predicted rate of stroke or death than patients undergoing CEA (2.7% vs 1.8%; P = .001), reflecting the fact that this was a high-risk population. The actual rate of stroke or death among symptomatic patients undergoing CAS, however, was still significantly higher than predicted (5.1% vs 2.7%; P < .001; Fig 4).
Table III.
Univariate associations with any stroke or death after carotid revascularization
| OR | P value | |
|---|---|---|
| TIA/amaurosis fugax | 1.6 | .04 |
| Minor stroke | 2.8 | .001 |
| Major stroke | 4.4 | .001 |
| Age >80 years | 2.2 | .001 |
| Age >75 years | 0.6 | .007 |
| Age >70 years | 0.5 | .001 |
| Age >65 years | 0.6 | .08 |
| CAS | 2.1 | .03 |
| Previous ipsilateral CEA | 3.1 | .001 |
| Hypertension | 2.6 | .04 |
| CHF | 1.9 | .03 |
| COPD | 1.6 | .03 |
| Renal insufficiency | 1.1 | .8 |
| Positive stress test | 0.8 | .4 |
| Statin use | 0.8 | .3 |
| Protamine | 0.9 | .6 |
| Plavix | 1.1 | .6 |
| Aspirin | 0.9 | .7 |
| β-blocker | 1.1 | .8 |
| Current or prior tobacco use | 0.9 | .8 |
| Male sex | 1.1 | .8 |
| Diabetes | 1.0 | .9 |
CEA, Carotid endarterectomy; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; OR, odds ratio; TIA, transient ischemic attack.
Table IV.
Multivariate predictors of any stroke or death after carotid revascularization
| OR | 95% CI | P value | ||
|---|---|---|---|---|
| Major stroke | 4.5 | 1.9 | 10.8 | .001 |
| Minor stroke | 2.7 | 1.5 | 4.8 | .001 |
| TIA/amaurosis fugax | 1.6 | 1.0 | 2.5 | .064 |
| History of ipsilateral CEA | 3.2 | 1.7 | 6.1 | .001 |
| HTN | 2.6 | 1.0 | 6.3 | .041 |
| Age >80 years | 2.1 | 1.3 | 3.4 | .001 |
| COPD | 1.6 | 1.0 | 2.4 | .035 |
CEA, Carotid endarterectomy; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HTN, hypertension; OR, odds ratio; TIA, transient ischemic attack.
Receiver operator curve = 0.6750.
Fig 4.
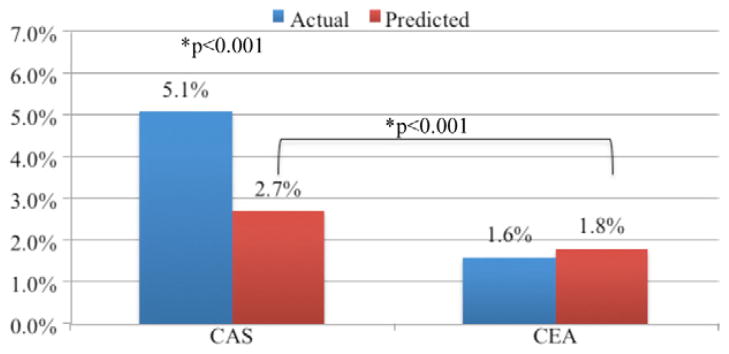
Actual and predicted rates of stroke or death among symptomatic patients. CAS, Carotid artery stent; CEA, carotid endarterectomy.
DISCUSSION
Our study shows that there is an increased risk of stroke or death in symptomatic patients undergoing CAS compared with CEA in the VSGNE even after accounting for differences in comorbidities. In contrast, for asymptomatic patients and patients undergoing prior ipsilateral CEA, there is no difference in the risk of stroke or death between CAS or CEA. According to our risk prediction model, age >80, major stroke, minor stroke, COPD, hypertension, and a history of prior CEA all predicted stroke or death following carotid revascularization (Table IV).
To determine how the results of our regional practice captured in the VSGNE compare with the recent randomized controlled trial (RCTs), we compared them with CREST and ICSS.1,2 In both the VSGNE and CREST cohorts, there was no difference in stroke, death, or MI rates between CEA and CAS. The rate of stroke, death, or MI in the VSGNE for both CEA (2.1%) and CAS (2.8%) was about half of that in CREST (4.5% and 5.2%, respectively).2,3 There was a higher proportion of asymptomatic patients in the VSGNE cohort (65% vs 47%). Stroke, death, or MI in asymptomatic patients from both the VSGNE and CREST were low and not different between CAS and CEA (Fig 5). ICSS randomized only symptomatic patients, and the results are similar to the symptomatic subgroup of VSGNE patients. Again, the stroke, death, or MI rates were slightly lower in the symptomatic VSGNE cohort compared with the ICSS cohort for both CEA and CAS. However, in both the symptomatic VSGNE (CAS 5.8% vs CEA 2.7%; P = .022) and ICSS (CAS 7.4% vs CEA 4.0%; P = .006) cohorts, the stroke, death, or MI rates were significantly higher for patients undergoing CAS than CEA.1 In CREST, however, the rate of stroke, death, or MI did not differ significantly between symptomatic patients undergoing CAS (6.7%) and CEA (5.4%). Of note, this was due to a higher event rate in patients undergoing CEA–event rates in symptomatic patients undergoing CAS were very similar in all three cohorts (Fig 6). Although our results do not represent randomized control level data, they demonstrate that in “real world” practice, similar outcomes in carotid revascularization are observed.
Fig 5.
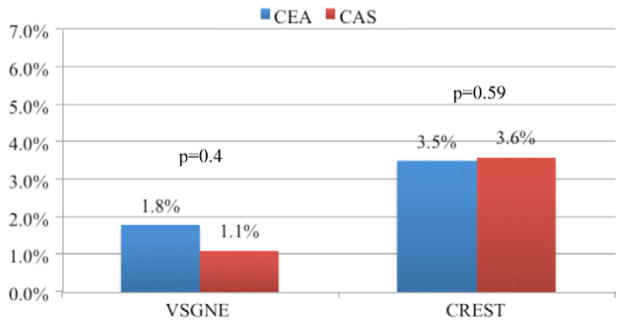
Stroke, death, or myocardial infarction (MI) for asymptomatic patients undergoing carotid endarterectomy (CEA) and carotid artery stent (CAS) in the Vascular Study Group of New England (VSGNE) and Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) cohorts.
Fig 6.
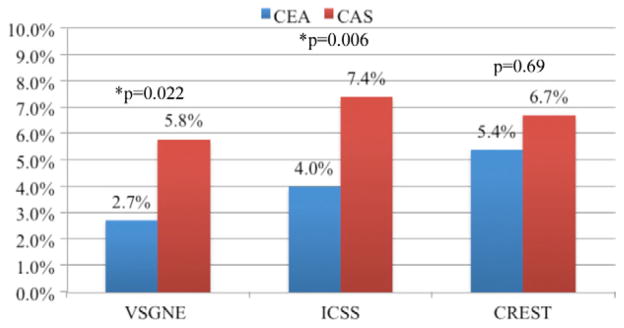
Stroke, death, or myocardial infarction (MI) for carotid endarterectomy (CEA) and carotid artery stent (CAS) in symptomatic patients from the Vascular Study Group of New Eng-land (VSGNE), International Carotid Stenting Study (ICSS), and Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) cohorts.
The populations of patients undergoing CAS and CEA in the VSGNE are significantly different. CAS patients had significantly more medical comorbidities, including congestive heart failure, coronary artery disease, and COPD (with renal insufficiency approaching significance). Additionally, the percentage of patients who had undergone a previous ipsilateral CEA was much higher in patients undergoing CAS, a finding that differentiates our cohort from randomized controlled trials where baseline demographics are well matched through the randomization process. Though CAS is approved for reimbursement by CMS for symptomatic high-risk patients, it is interesting to note that symptomatic status did not seem to influence the decision to perform CAS to the extent that medical comorbidites and prior CEA did; that is, CAS was most often used in asymptomatic patients with higher comorbidities or restenosis after prior CEA in the VSGNE cohort. There was no difference in the percentage of symptomatic patients undergoing CAS compared with CEA, approximately 35%, which also differentiates this cohort from ICSS (100% symptomatic) and CREST (53% symptomatic).
In our sample, symptomatic patients undergoing CAS did considerably worse with regard to neurologic outcomes than patients undergoing CEA. Symptomatic patients treated by CAS had significantly higher rates of any ipsilateral stroke, any major stroke, any minor stroke, and any stroke or death. These findings are consistent with those of ICSS,2 as well as Endarterectomy vs Stenting in Patients with Symptomatic Severe Carotid Stenosis study (EVA-3S)10 and the Stent-Supported Percutaneous Angioplasty of the Carotid Artery vs Endarterectomy study (SPACE),11 though the latter is often criticized for low usage of embolic protection devices. In fact, the stroke rate with CAS was higher in CREST, even with a substantial proportion of asymptomatic patients in the cohort.2 However, when considering MI in the outcome measure, symptomatic patients had similar outcomes with CAS and CEA. The inclusion of MI in composite end points for CEA and CAS has been criticized, and it is questionable to assume that a major or minor stroke is equivalent to an asymptomatic MI. It would seem as though the answer is no, at least from a quality of life standpoint, yet this is the end point traditionally used in CAS analyses. This is likely due to the fact that patients undergoing vascular surgery experiencing an asymptomatic MI based on troponin elevation appear to have lower short-term and long-term survival.12,13 In a study of patients undergoing aortic or infrainguinal revascularization, or amputation, those with a troponin leak had a 27-fold increased risk of MI and a sixfold increased risk of 6-month mortality. Troponin leak size was positively correlated with mortality rates. Additionally, life-table analysis demonstrated that these patients had significantly lower survival up to 2 years after surgery.13 This has been confirmed in vascular patients in follow-up to 5 years as well.12
In contrast to symptomatic patients, the results of CAS in asymptomatic patients were quite good. Cerebral outcomes were equivalent to CEA. The finding that asymptomatic patients do considerably better with CAS than symptomatic patients may bridge the disparity between the findings reported in CREST and the European trials. Though operator experience and particularly the use of embolic protection may also contribute to these differences in outcomes of patients undergoing CAS,14 it appears clear in our data that symptomatic status is an important predictor. This is consistent with results from an analysis of the Nationwide Inpatient Sample showing that symptomatic patients did disproportionately worse with CAS than CEA.15
The results of our risk prediction model for stroke or death following carotid revascularization show that neurologic symptoms, hypertension, COPD, and age >80 predict poor outcomes. These findings are similar to other prior risk prediction models for stroke or death following CEA.16–18 Advanced age and preoperative symptomatic lesions had similar associations with poor outcomes following CEA as in the current analysis.17 The finding that age is an important independent predictor of outcomes with CAS has also been previously shown.2,19 These data show that risk factors often thought to place patients at high risk for CEA may place them at equally high, or greater risk, for CAS.
Our study has several limitations. First, our relatively low event rate, particularly for CAS where the sample size is also relatively small, may have resulted in a type II error. This may have limited our ability to identify other predictors of outcome after CAS such as a benefit from statin use, stent cell design, types of embolic protection, or operator volume. Also, small sample size limits our ability to detect a meaningful volume effect. However, an analysis of event rates in operators performing greater than 10 vs less than 10 cases showed no differences in event rates. Second, outcomes in the VSGNE are self-reported, though some CAS outcomes were neurologist-adjudicated (per protocol at several institutions). The event rates are consistently lower in the VSGNE cohorts compared with both CREST and ICSS. It is possible that this is due to under-reporting of events; however, there is unlikely to be bias in the reporting of events between procedure (CAS vs CEA) or patient strata (symptomatic vs asymptomatic). Additionally, neurologists did not examine all patients postoperatively as in the randomized controlled trials, and there was no standard protocol for screening for MI, both of which may account for slightly lower rates of events than in the randomized controlled trials. Additionally, all outcomes were in-hospital and not at 30 days. However, our audit identified no missed strokes based on claims data indicating that few, if any, strokes were missed. Finally, the VSGNE database does not contain information about arch and carotid lesion anatomy or calcification, limiting our ability to make inferences about the impact of these factors on outcomes with CAS.
Despite these limitations, our results provide an important perspective about the outcomes of CEA vs CAS as performed in New England, at both academic and community centers. They show that in real-world practice, with patient selection for each procedure, that neurologic outcomes with CAS are worse than CEA in symptomatic and older patients. The outcomes in asymptomatic patients are not significantly different. These data suggest that CAS may be best suited for asymptomatic, younger patients, although even larger population studies are required to validate this.
Footnotes
Author conflict of interest: none.
Presented at the Thirty-eighth Annual Meeting of the New England Society for Vascular Surgery, Providence, RI, September 16-18, 2011.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: BN, PG, AS, CK, JC
Analysis and interpretation: BN, RD, PG, AS, CK, JC
Data collection: BN, PG, AS, DH, DB, CK, JC
Writing the article: BD, RD, JC
Critical revision of the article: BN, RD, DH, DB, CK, JC
Final approval of the article: BN, RD, DH, DB, CK, JC
Statistical analysis: BN, PG
Obtained funding: Not applicable
Overall responsibility: BN
References
- 1.Ederle J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (international carotid stenting study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–97. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantese VA, Timaran CH, Chiu D, Begg RJ, Brott TG CREST Investigators. The carotid revascularization endarterectomy versus stenting trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke. 2010;41(10 Suppl):S31–4. doi: 10.1161/STROKEAHA.110.595330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotidartery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musser DJ, Nicholas GG, Reed JF., III Death and adverse cardiac events after carotid endarterectomy. J Vasc Surg. 1994;19:615–22. doi: 10.1016/s0741-5214(94)70034-6. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1999;353:2105–10. doi: 10.1016/s0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]
- 6.Tu JV, Wang H, Bowyer B, Green L, Fang J, Kucey D, et al. Risk factors for death or stroke after carotid endarterectomy: observations from the Ontario Carotid Endarterectomy Registry. Stroke. 2003;34:2568–73. doi: 10.1161/01.STR.0000092491.45227.0F. [DOI] [PubMed] [Google Scholar]
- 7.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Risk factors. Stroke. 1997;28:1507–17. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 8.Kakkos SK, Nicolaides A, Griffin M, Sabetai M, Dhanjil S, Thomas DJ, et al. Factors associated with mortality in patients with asymptomatic carotid stenosis: results from the ACSRS study. Int Angiol. 2005;24:221–30. [PubMed] [Google Scholar]
- 9.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VS-GNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. Discussion:101–2. [DOI] [PubMed] [Google Scholar]
- 10.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 11.Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. Thirty-day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised noninferiority trial. Lancet. 2006;368:1239–47. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 12.Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–54. doi: 10.1016/j.jacc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Kim LJ, Martinez EA, Faraday N, Dorman T, Fleisher LA, Perler BA, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation. 2002;106:2366–71. doi: 10.1161/01.cir.0000036016.52396.bb. [DOI] [PubMed] [Google Scholar]
- 14.Nallamothu BK, Gurm HS, Ting HH, Goodney PP, Rogers MA, Curtis JP, et al. Operator experience and carotid stenting outcomes in Medicare beneficiaries. JAMA. 2011;306:1338–43. doi: 10.1001/jama.2011.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giles KA, Hamdan AD, Pomposelli FB, Wyers MC, Schermerhorn ML. Stroke and death after carotid endarterectomy and carotid artery stenting with and without high risk criteria. J Vasc Surg. 2010;52:1497–504. doi: 10.1016/j.jvs.2010.06.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvillo-King L, Xuan L, Zhang S, Tuhrim S, Halm EA. Predicting risk of perioperative death and stroke after carotid endarterectomy in asymptomatic patients: derivation and validation of a clinical risk score. Stroke. 2010;41:2786–94. doi: 10.1161/STROKEAHA.110.599019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodney PP, Likosky DS, Cronenwett JL Vascular Study Group of Northern New England. Factors associated with stroke or death after carotid endarterectomy in northern New England. J Vasc Surg. 2008;48:1139–45. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaides AN, Kakkos SK, Griffin M, Sabetai M, Dhanjil S, Tegos T, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg. 2005;30:275–84. doi: 10.1016/j.ejvs.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi S, Matsumura JS, Gray W, Xu C, Verta P CAPTURE 2 Investigators and Executive Committee. Carotid artery stenting in octogenarians: periprocedural stroke risk predictor analysis from the multicenter carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events (CAPTURE 2) clinical trial. Stroke. 2010;41:757–64. doi: 10.1161/STROKEAHA.109.569426. [DOI] [PubMed] [Google Scholar]


