Abstract
Objective
We studied associations of MRI-measured SFA occlusions with functional performance, leg symptoms, and collateral vessel number in PAD. We studied associations of collateral vessel number with functional performance in PAD.
Background
Associations of magnetic resonance imaging (MRI)-detected superficial femoral artery (SFA) occlusion and collateral vessel number with functional performance among individuals with peripheral artery disease (PAD) have not been reported.
Methods
457 participants with an ankle brachial index (ABI) < 1.00 had MRI measurement of the proximal SFA with twelve consecutive 2.5 millimeter cross-sectional images. An occluded SFA was defined as an SFA in which at least one segment was occluded. A non-occluded SFA was defined as absence of any occluded slices. Collateral vessels were visualized with magnetic resonance angiography (MRA). Lower extremity functional performance was measured with the six-minute walk, four-meter walking velocity at usual and fastest pace, and the short physical performance battery (SPPB) (0-12 scale, 12=best).
Results
Adjusting for age, sex, race, comorbidities, and other confounders, the presence of an SFA occlusion was associated with poorer six-minute walk performance (1,031 vs. 1,169 feet, P=0.006), slower fast-paced walking velocity (1.15 vs. 1.22 meters/second, P =0.042), and lower SPPB score (9.07 vs. 9.75, P=0.038) compared to the absence of an SFA occlusion. More numerous collateral vessels were associated with better six-minute walk performance (0-3 collaterals-1,064 feet, 4-7 collaterals-1,165 feet, ≥ 8 collaterals-1,246 feet, P trend=0.007), faster usual-paced walking speed (0-3 collaterals-0.84 meters/second, 4-7 collaterals-0.88 meters/second, ≥ 8 collaterals-0.91 meters/second, P trend=0.029), and faster rapid-paced walking speed (0-3 collaterals-1.17 meters/second, 4-7 collaterals-1.22 meters/second, ≥ 8 collaterals-1.29 meters/second, P trend=0.002), adjusting for age, sex, race, comorbidities, ABI, and other confounders.
Conclusions
Among PAD participants, MRI-visualized occlusions in the proximal SFA are associated with poorer functional performance, while more numerous collaterals are associated with better functional performance.
Clinical Trial ID
NCT00520412
Keywords: atherosclerotic plaque, intermittent claudication, peripheral arterial disease, physical functioning
CONDENSED ABSTRACT
Among 457 men and women with peripheral artery disease (PAD), we studied associations of magnetic resonance imaging (MRI)-measured occlusions in the superficial femoral artery (SFA) and lower extremity collateral vessel number with functional performance in PAD. The presence of an occluded SFA was associated with poorer six-minute walk performance, slower walking velocity at rapid pace, and a lower short physical performance battery (SPPB) score. More numerous collateral vessels were associated with better six-minute walk performance and faster walking velocity at usual and fastest pace.
Data from humans and animals demonstrate that pre-existing collateral vessels enlarge in response to shear stress following lower extremity arterial occlusions (1-3). Collateral vessels serve as a natural bypass system, are thought to protect against critical limb ischemia, and may reduce leg symptoms in people with PAD (1-3). Occlusion of a major lower extremity artery is a primary stimulus to the enlargement of pre-existing collateral vessels, and the superficial femoral artery (SFA) is the most common site of lower extremity arterial occlusions (4). However, these collateral vessels typically do not restore lower extremity perfusion to normal (2). Whether larger or more numerous lower extremity collateral vessels protect against functional impairment and functional decline in people with PAD is unknown.
Among individuals with PAD, we studied associations of occlusions in the SFA with leg symptoms, functional performance, and collateral vessel characteristics. We hypothesized that occlusions in the SFA would be associated with more leg symptoms and greater functional impairment. However, we also hypothesized that larger and more numerous lower extremity collateral vessels would mitigate against PAD-related functional impairment. Specifically, we hypothesized that for a given level of PAD severity, more numerous and larger collateral vessels would be associated with better functional performance in PAD.
METHODS
Subjects
Participants were those in the Walking and Leg Circulation Study (WALCS) III cohort (5). Participants were identified from among consecutive PAD patients in four Chicago-area medical centers. Participants were also identified from among lists of consecutive patients with a diagnosis of PAD in the vascular surgery, cardiology, endocrinology, general medicine, and geriatric practices at Northwestern Medical Faculty Foundation and in the vascular surgery practice at the Jesse Brown Veterans Administration. A small number of participants were identified from among men and women age 70 and older in Northwestern’s largest general internal medicine practice who were screened with the ABI and found to have an ABI <1.00. The protocol was Institutional Review Board-approved by Northwestern University and all participating sites. Participants gave written informed consent. Rates of participation and reasons for exclusion among those contacted for WALCS III participation have been reported (5).
Inclusion and Exclusion Criteria
The inclusion criterion was an ABI < 1.00. This criterion was selected because normal ABI values are 1.10-1.40 (6).
Exclusion Criteria
Potential participants with dementia or a mini-mental status examination score < 23 (7) were excluded. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because of severely impaired functioning. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Individuals who required oxygen therapy, had contraindications to MRI testing, stopped the six-minute walk test due to shortness of breath, had recent major surgery, or with severe knee osteoarthritis were excluded (8). Potential participants with bilateral SFA stents were excluded, because stents interfere with plaque imaging.
Ankle Brachial Index Measurement
After participants rested supine for five minutes, a hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) was used to measure systolic pressures in the right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Pressures were repeated in reverse order. The ABI was calculated in each leg by dividing average pressures in each leg by the average of the four brachial pressures (9). Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets, and the two brachial pressures differed by 10 or more mm Hg in at least one measurement set.
Magnetic Resonance Imaging
We imaged the SFA because it is the most common site of lower extremity atherosclerosis (10) and because it supplies calf muscle, which is typically symptomatic in patients with PAD. The leg with lowest ABI was imaged. If the leg with lowest ABI had an SFA stent, the opposite leg was imaged. MRI data were obtained with a 1.5 Tesla (Siemens) platform using four-element phased-array surface coils. The bifurcation of the common femoral artery served as the reference point. Twelve consecutive 2.5 millimeter cross-sectional images were obtained, moving distally from the most proximal point of the SFA, using 2-dimensional bright blood time-of-flight and proton-density weighted images. Fat suppression was applied in black-blood sequences to improve image quality. This method has excellent test re-test reliability (5).
CASCADE software (Seattle, WA) was used by two physician reviewers to trace the outer boundary and the lumen of each cross-sectional image of the SFA. A non-occluded SFA was defined as absence of any occluded segments in the imaged proximal SFA. An occluded SFA was defined as presence of at least one occluded segment.
Images for each participant were assigned to one primary reviewer, and arterial tracings were reviewed by the second reviewer to ensure accuracy. A six percent subsample of participants returned on a second day for test re-test reliability assessment of MRI measurements. The coefficient of variation percent values for these test re-test reliability assessments were 5.7 for mean plaque area, 8.9 for maximum plaque area, 8.0 for mean percent lumen area, and 12.9 for minimum percent lumen area (5).
Magnetic Resonance Angiography
A 1.5T Siemens Espree (Siemens Medical Solutions, Erlangen, Germany) MRI scanner was employed for MRA image acquisition. A 12 channel surface array coil (Siemens Medical Solutions, Erlangen, Germany) was used for signal reception. Dynamic MRA images from one station, the groin to the knee, were acquired from both legs, including the common femoral artery, the profunda femoris, the SFA, and the popliteal artery. Dynamic Images were acquired with the TWIST (Time resolved angiography With Interleaved Stochastic Trajectories) pulse sequence (11). Parallel imaging with Generalized Autocalibrating Partially Parallel Acquisitions (12) image reconstruction provided a 2-fold increase in frame rate. Following a three-plane localizer image, the TWIST sequence was applied in the coronal orientation. A 10 ml bolus of nondiluted gadopentetate dimeglumine (0.5 mmol/mL, Magnevist; Berlex, Montville, NJ, USA) was administered intravenously at 2 mls/sec. Imaging parameters were: TR/TE/Flip angle = 3.3 ms/TE, 1.3 ms/25°; rectangular field of view (rFOV), 246 × 375 mm; matrix, 210 × 320; 88 partitions; voxel size after zero interpolation, 1.2 × 1.2 × 1.2 mm3 (true voxel size, 1.2 × 1.2 × 2.0 mm3); acceleration factor: 2.
MRA Image Analysis
A single radiologist (AK), blinded to all other participant characteristics, read all MRA images on a workstation (GE Healthcare, Milwaukee, Wis, USA). A validated scoring system was used to grade the number and size of collateral vessels, based on previous study (13,14). This method has excellent intra-rater reliability (mean 85.7% agreement) (14). Based on previously reported methods (13,14), small collaterals were defined as those occupying less than 25% of the length of the imaged thigh and less than 50% of the diameter of the SFA. Large collaterals were defined as occupying more than 25% of the length of the imaged thigh and greater than 50% of the diameter of the SFA. Categories were used to classify the number and size of collateral vessels, based on previous study (13,14). For collateral vessel size, Grade 1 was defined as ≤ 5 small collateral vessels, Grade 2 was defined as > 5 small collateral vessels, Grade 3 was defined as ≤ 5 large ± small collateral vessels, and Grade 4 was defined as > 5 large ± small collateral vessels (18). For collateral vessel number, Category 1 was defined as 0-3 collateral vessels, Category 2 was defined as 4-7 collateral vessels, and Category 3 was defined as ≥8 collateral vessels (14). Our MRA analyses did not include identifying proximal SFA occlusions.
FUNCTIONAL MEASURES
Six-minute walk
Participants walk up and down a 100-foot hallway for six minutes after instructions to cover as much distance as possible (5,15).
Four-meter walking velocity
Walking velocity was measured with a four-meter walk performed at “usual” and “fastest” pace, based on previous study (5,15). Each walk was performed twice. The faster walk in each pair was used in analyses.
Short Physical Performance Battery
The SPPB combines data from usual paced four-meter walking velocity, time to rise from a seated position five times, and standing balance (16). Individuals receive a zero score for each task they are unable to complete. Scores of one to four are assigned for remaining tasks, according to established methods. Scores are summed to obtain the SPPB, ranging from 0 to 12 (16).
Repeated chair rises
Participants sit in a straight-backed chair with arms folded across their chest and stand five times consecutively as quickly as possible. Time to complete five chair rises is measured (16).
Standing balance
Participants are asked to hold three increasingly difficult standing positions for ten seconds each: the side-by-side stand, semi-tandem stand (standing with feet parallel and the heel of one foot touching the base of the 1st toe of the opposite foot), and the full tandem stand (standing with one foot directly in front of the other) (16). Scores range from zero (unable to hold the side-by-side stand for ten seconds) to four (able to hold the full tandem stand for ten seconds) (19).
Comorbidities
Algorithms developed for the Women’s Health and Aging Study and the Cardiovascular Health Study were used to document comorbidities (17). These algorithms combine data from patient report, physical examination, medical record review, medications, laboratory values, and a primary care physician questionnaire (17). Comorbidities assessed were angina pectoris, diabetes mellitus, myocardial infarction, stroke, heart failure, pulmonary disease, cancer, spinal stenosis, and disk disease. Criteria from the American College of Rheumatology were used to diagnose knee and hip osteoarthritis (8,18).
Leg Symptoms
Leg symptoms were classified using the San Diego claudication questionnaire (19). Intermittent claudication was defined as exertional calf pain that does not begin at rest, causes the participant to stop walking, and resolves within ten minutes of rest (15,19). Participants without any exertional leg pain were classified as asymptomatic (15,19). Participants with exertional leg pain who walked through their pain were classified as “leg pain carry on”, those with exertional leg pain that sometimes begins at rest were classified as “pain on exertion and rest.” Remaining participants with exertional leg symptoms who did not meet criteria for the aforementioned leg pain categories were classified as “atypical exertional leg pain” (15,19).
Other Measures
Height and weight were measured at the study visit. Body mass index (BMI) was calculated as weight (kg)/(height (meters))2. Cigarette smoking history was measured with self-report.
Statistical Analyses
Clinical characteristics were compared between PAD participants with vs. without an occluded SFA using chi square tests for categorical variables and analyses of variance for continuous variables. Clinical characteristics and functional performance measures were compared between participants with vs. without an occluded SFA using analyses of covariance, adjusting for age, sex, and race (Model 1). Analyses involving nominal variables were performed using logistic regression. Functional performance analyses were repeated with additional adjustment for comorbidities, BMI, and smoking (Model 2). Finally, Model 2 was additionally adjusted for the ABI (Model 3).
Functional performance measures were compared across collateral vessel size (grade) and collateral vessel number (category), adjusting for age, sex, race, smoking, diabetes, BMI, comorbidities, and ABI. These analyses were repeated separately among participants with vs. without SFA occlusions. Because of small sample size in the 0-3 collateral vessel group among participants with an SFA occlusion, participants with an SFA occlusion and 0-3 collateral vessels were combined with those with 4-7 collateral vessels.
RESULTS
Of 473 PAD participants in the WALCS III cohort with SFA imaging, 457 had good quality images and are included in this report. Of these, 59 (12.9%) had an occluded SFA. Mean ages were 68.3 ± 9.1 and 69.4 ± 10.3 (P=0.409), the prevalence of males was 72.9% vs. 64.3% (P=0.197), and the prevalence of African-Americans was 35.6% vs. 32.9% (P =0.684) among participants with vs. without one or more occlusions in the proximal SFA, respectively. Figure 1 shows an occluded SFA with corresponding collateral vessels.
Figure 1. Representative images of an occluded superficial femoral artery and corresponding collateral vessels.
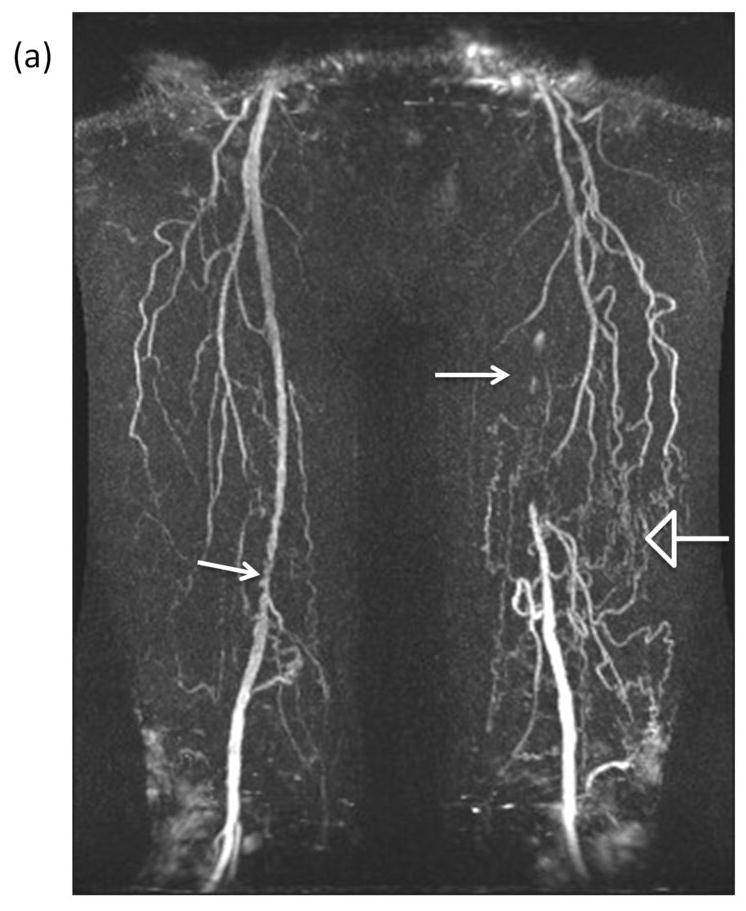
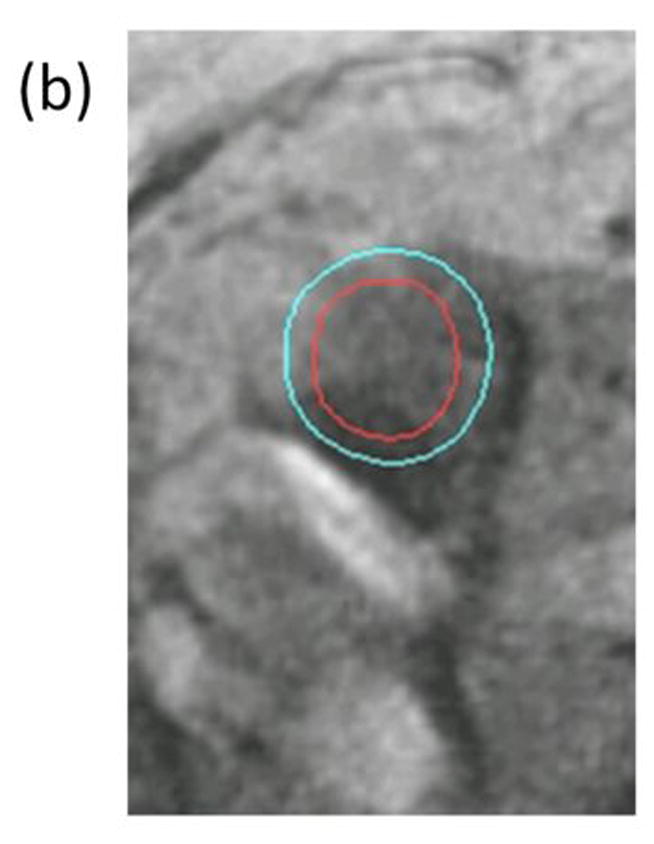
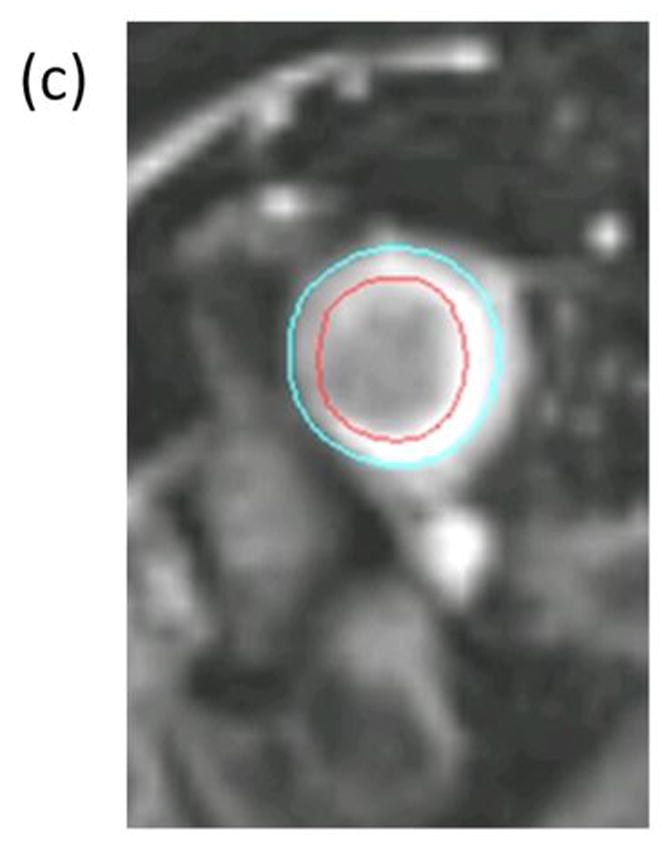
(a) Contrast enhanced magnetic resonance angiogram (MRA) shows segment of irregular severe narrowing in distal right superficial femoral artery (solid arrow) and complete occlusion of left superficial femoral artery (solid arrow). Multiple large and small collateral vessels (open arrow) reconstitute left superficial femoral artery at level of adductor canal. Cross sectional images acquired at the SFA bifurcation in the same patient with (b) Time-of-Flight (TR/TE=38 ms/8.7 ms) and (c) proton-density weighting (TR/TE= 2160 ms/5.7 ms) are shown with the corresponding inner (red- outlining lumen) and outer (blue)-outlining outer vessel wall) contours. Note that the lack of intravascular signal in the TOF image resulting from the lack of blood flow in the occluded SFA does not compromise the ability to quantify wall lumen and wall areas.
Adjusting for age, race, and sex, participants with one or more occlusions in the proximal SFA had a lower ABI, a lower prevalence of asymptomatic PAD, a higher prevalence of leg pain/carry-on, higher mean plaque area and lower mean lumen area, compared to participants without any occlusions (Table 1).
Table 1.
Adjusted Associations of Participant Characteristics and Superficial Femoral Artery Occlusion among Individuals with Peripheral Artery Disease (N=457)*
| No occlusions in the superficial femoral artery (N= 398) |
One or more occlusions in the superficial femoral artery (N=59) |
P value | |
|---|---|---|---|
| Ankle brachial index | 0.68 (0.01) | 0.58 (0.02) | <0.001 |
| Body Mass Index | 29.3 (0.3) | 28.9 (0.8) | 0.573 |
| Diabetes Mellitus | 39.4% (2.4%) | 34.3% (6.3%) | 0.453 |
| Hypertension | 90.7% (1.5%) | 88.4% (3.8%) | 0.566 |
| Current Smoking | 24.2% (2.0%) | 26.9% (5.2%) | 0.577 |
| Angina | 21.6% (2.1%) | 28.5% (5.4%) | 0.238 |
| Myocardial infarction | 19.8% (2.0%) | 30.8% (5.3%) | 0.053 |
| Heart failure | 12.6% (1.7%) | 15.0% (4.4%) | 0.597 |
| Stroke | 16.6% (1.9%) | 16.5% (4.9%) | 0.993 |
| Mean plaque volume | 0.65 (0.01) | 1.04 (0.01) | <0.001 |
| Mean lumen area | 0.36 (0.01) | 0.08 (0.01) | <0.001 |
| Leg Symptoms | |||
| Intermittent claudication | 22.1% (2.1%) | 30.5% (5.5%) | 0.159 |
| Asymptomatic | 22.2% (1.9%) | 5.93% (5.1%) | 0.007 |
| Pain/Carry on | 8.8% (1.5%) | 17.1% (3.9%) | 0.049 |
| Atypical exertional leg pain | 19.9% (2.0%) | 18.3% (5.2%) | 0.782 |
| Pain on exertion and rest | 27.0% (2.2%) | 28.2% (5.7%) | 0.833 |
| Collateral Grade† | |||
| No occlusions in the superficial femoral artery (N= 251) |
Occlusions in superficial femoral artery (N=40) |
||
| Grade 1 | 50.5% (2.9%) | 11.7% (7.4%) | <0.001 |
| Grade 2 | 18.6% (2.4%) | 9.01% (6.0%) | 0.158 |
| Grade 3 | 6.00% (1.7%) | 20.0% (4.2%) | 0.004 |
| Grade 4 | 24.9% (2.8%) | 59.3% (6.9%) | <0.001 |
| Collateral Vessel Number† | |||
| 0-3 collateral vessels | 24.5% (2.5%) | 6.56% (6.3%) | 0.02 |
| 4-7 collateral vessels | 51.0% (3.2%) | 47.2% (8.0%) | 0.649 |
| 8 or more collateral vessels | 24.5% (2.7%) | 46.3% (6.8%) | 0.003 |
Mean values (standard error) are shown after adjustment for age, sex, and race.
291 participants underwent a magnetic resonance angiogram for measuring collateral vessels.
Among the 457 participants with PAD, 291 were eligible for and consented to an MRA. Participants who underwent MRA were younger (68.5±10.4 vs. 70.7±9.6, P=0.025), included a higher proportion of men (70.5% vs. 56.6%, P=0.003), and included a higher proportion of current smokers (28.6% vs. 17.5%, P=0.008) compared to those who did not undergo MRA. Adjusting for age, sex, and race, participants with an occlusion in the proximal SFA had a higher prevalence of large collateral vessels (i.e. Grade 3 and Grade 4) and a lower prevalence of smaller collateral vessels (i.e. Grade 1), compared to participants without occlusions (Table 1). Adjusting for age, sex, and race, participants with an occlusion in the proximal SFA had a lower prevalence of 0-3 collateral vessels and a higher prevalence of ≥ 8 collateral vessels, compared to participants without proximal SFA occlusions (Table 1).
Presence of an occlusion in the proximal SFA was associated with shorter six-minute walk distance, compared to individuals without any occlusions, adjusting for age, sex, and race (Model 1, Table 2). After additional statistical adjustment for comorbidities, BMI, and cigarette smoking (Model 2), participants with an occlusion in the proximal SFA had poorer six-minute walk performance, slower fast-paced four-meter walking velocity, and lower SPPB scores compared to participants without any occlusions (Table 2, Model 2). When Model 2 results in Table 2 were additionally adjusted for the ABI, there were no significant associations of an occluded SFA with functional performance (data not shown). Thus, after taking into account differences in ABI values between participants with vs. without SFA occlusions, there were no significant associations of SFA occlusions with functional performance.
Table 2.
Adjusted Associations of Superficial Femoral Artery Occlusion and Functional Performance among Individuals with Peripheral Artery Disease (N=457)*
| No SFA Occlusions (N=398) | SFA Occlusions (N=59) | P value | |
|---|---|---|---|
| Six Minute Walk | |||
| Model I | 1168 (20) | 1029 (51) | 0.012 |
| Model II | 1169 (18) | 1031 (47) | 0.006 |
| Usual-Paced Four-Meter Walking Velocity | |||
| Model I | 0.88 (0.01) | 0.85 (0.02) | 0.177 |
| Model II | 0.88 (0.01) | 0.85 (0.02) | 0.157 |
| Fast-Paced Four-Meter Walking Velocity | |||
| Model I | 1.22 (0.01) | 1.15 (0.04) | 0.054 |
| Model II | 1.22 (0.01) | 1.15 (0.03) | 0.042 |
| Short Physical Performance Battery | |||
| Model I | 9.74 (0.12) | 9.12 (0.32) | 0.073 |
| Model II | 9.75 (0.12) | 9.07 (0.30) | 0.038 |
Mean values (standard error) are shown after statistical adjustment. Model I adjusts for age, sex, and race. Model II adjusts for variables in Model I and also comorbidities, BMI, and smoking.
Within the entire cohort, more numerous collateral vessels were associated with better six-minute walk performance and faster walking velocity at usual and fastest pace, adjusting for age, sex, race, smoking, comorbidities, ABI, and BMI (Table 3). Similar findings were observed among participants without SFA occlusions (Table 3). Among PAD participants with an SFA occlusion, more numerous collateral vessels were associated with better six-minute walk performance (953 feet for participants with < 8 collateral vessels vs. 1,335 feet for participants with ≥ 8 collateral vessels, p=0.027) and faster rapid-paced four-meter walking velocity (1.07 meters/second for participants with < 8 collateral vessels vs. 1.43 meters/second for participants with ≥ 8 collateral vessels, p<0.001), adjusting for age, sex, race, comorbidites, smoking, ABI, and BMI. There were no associations of collateral vessel number with usual-paced four-meter walking velocity (data not shown) or the SPPB score (9.17 vs. 10.82, p=0.082) among participants with an SFA occlusion.
Table 3.
Adjusted Associations of Collateral Vessel Number and Functional Performance in Participants with Peripheral Artery Disease
| 0-3 Collateral Vessels (N=64) | 4-7 Collateral Vessels (N=146) | ≥8 Collateral Vessels (N=80) | Trend P value | |
|---|---|---|---|---|
| All Study Participants | ||||
| Six-minute walk (feet) | 1,064 (47) | 1,165 (28) | 1,246 (41) | 0.007 |
| Four-meter walking velocity (usual pace) (meters/second) | 0.84 (0.02) | 0.88 (0.01) | 0.91 (0.02) | 0.029 |
| Four-meter walking velocity (fastest pace) (meters/second) | 1.17 (0.03) | 1.22 (0.02) | 1.29 (0.03) | 0.002 |
| Short Physical Performance Battery | 9.21 (0.33) | 9.80 (0.19) | 10.11 (0.27) | 0.059 |
| Participants without Superficial Femoral Artery Occlusion | ||||
| 0-3 Collateral Vessels (N=62) | 4-7 Collateral Vessels (N=127) | ≥8 Collateral Vessels (N=61) | ||
| Six-minute walk (feet) | 1,046 (46) | 1,193 (30) | 1,249 (45) | 0.003 |
| Four-meter walking velocity (usual pace) (meters/second) | 0.83 (0.02) | 0.88 (0.01) | 0.90 (0.02) | 0.033 |
| Four-meter walking velocity (rapid pace) (meters/second) | 1.15 (0.04) | 1.24 (0.02) | 1.27 (0.03) | 0.010 |
| Short Physical Performance Battery (0-12 scale, 12=best) | 9.17 (0.33) | 9.84 (0.21) | 10.03 (0.32) | 0.064 |
*Mean values (standard error) are shown after statistical adjustment. Model adjusts for age, sex, race, comorbidities, smoking, ankle brachial index, and BMI.
There were no significant associations of collateral size (grade) with functional impairment (data not shown).
DISCUSSION
Among 457 participants in the WALCS III cohort with ABI < 1.00, 13% had one or more occlusions in the proximal SFA of the leg with lowest ABI. There were no differences in age, race, sex, or prevalence of smoking or diabetes mellitus between PAD participants with an occlusion and those without any occlusions. PAD participants with an occlusion in the proximal SFA had poorer six-minute walk performance, slower fast-paced walking velocity, and a lower SPPB score, compared to PAD participants without any proximal SFA occlusions after adjusting for age, sex, race, BMI, smoking, diabetes, comorbidities, and other confounders. Our results also show that presence of more numerous collateral vessels is associated with better functional performance compared to fewer collateral vessels among participants with PAD. In contrast, we found no significant associations of collateral size (grade) with functional performance.
Occlusions are relatively common in lower extremity arteries and are an important stimulus to the development of collateral vessels. Yet to our knowledge, no prior studies have evaluated associations of MRI-visualized occlusions or collateral vessels with functional performance measures in individuals with PAD. Collaterals develop from pre-existing arterioles that enlarge in response to sheer stress forces precipitated by arterial occlusion (20). Sheer stress activates mechanosensors, resulting in molecular signaling, modulation of gene expression, and cytokine release including monocyte chemoattractant protein-1, granulocyte-macrophage colony-stimulating factor and cellular adhesion molecules (21-23). Influx of inflammatory cells is followed by release of vascular growth factors, remodeling of the vessel wall, enlargement of vessel diameter, and migration of smooth muscle cells into the artery wall (23,24). Animal evidence suggests that following arterial occlusion, vessels first increase rapidly in number and later enlarge in diameter (23). In animal models, exercise training is associated with collateral vessel enlargement and increased collateral vessel compliance (2). In animals, maximal collateral vessel enlargement occurs during exercise (3). This phenomenon may explain our finding that greater collateral vessel number, but not collateral vessel size (i.e. diameter), was associated with better functional performance in PAD, since our images were obtained during rest and thus may not have captured maximal vessel diameter.
Our study has limitations. First, data are cross-sectional. Associations reported cannot be construed as causal. Second, we imaged a short segment of the SFA. We did not image atherosclerotic plaque in the aorto-iliac or femoro-popliteal arterial segments. Nonetheless, we found that presence of any occluded segments in the proximal SFA was associated with greater functional impairment than absence of occluded segments in the proximal SFA. Third, our collateral vessel data are limited to those who were eligible for and consented to MRA. Our findings regarding collateral vessels may not be generalizable to individuals who did not undergo MRA testing. Fourth, a relatively small proportion of participants had occlusions of the proximal SFA, limiting statistical power to detect meaningful differences between participants with vs. without occlusions in the proximal SFA. Fifth, MRA has lower spatial resolution than computed tomographic angiography or digital subtraction angiography. Some collateral vessels may be too small for identification on MRA (25). Sixth, lack of collateral vessel measurement during exercise may have limited our ability to identify associations of vessel size with functional performance.
Our results demonstrate that PAD participants with one or more occluded segments in the proximal SFA have greater functional impairment than PAD participants without proximal SFA occlusions. More numerous collateral vessels are associated with better functional performance in PAD. Our findings are important in part because of current interest in identifying therapies to increase lower extremity perfusion in individuals with PAD.
Abbreviation List
- MRI
magnetic resonance imaging
- SFA
superficial femoral artery
- PAD
peripheral artery disease
- ABI
ankle brachial index
- WALCS
walking and leg circulation study
- SPPB
short physical performance battery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Source of Funding Supported by the National Heart Lung and Blood Institute (R01-HL083064), the Intramural Research Program of the National Institute on Aging, and the Jesse Brown VA Medical Center.
Conflict of Interest Disclosures Chun Yuan receives research support from VP Diagnostics and from Philips Healthcare. Christopher M. Kramer receives research support from Siemens Healthcare. There are no disclosures from other authors.
References
- 1.Schoop W. Limb collaterals. In: Schaper W, Schaper J, editors. Collateral Circulation: Heart, Brain, Kidneys, Limbs. Kluwer Academic Publishers; Boston, MA: 1993. pp. 317–327. [Google Scholar]
- 2.Ziegler MA, Distasi MR, Bills RG, et al. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation. 2010;17:3–20. doi: 10.1111/j.1549-8719.2010.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prior BM, Lloyd PG, Ren J, et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol. 287:H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 4.Walden R, Adar R, Rubinstein ZJ, Bass A. Distribution and symmetry of arteriosclerotic lesions of the lower extremities: An arteriographic study of 200 limbs. Cardiovasc Intervent Radiol. 1985;8:108–182. doi: 10.1007/BF02552893. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Liu K, Carroll TJ, et al. Superficial femoral artery plaque and functional performance in peripheral arterial disease: Walking and Leg Circulation Study (WALCS III) JACC Cardiovascular Imaging. 2011;4:730–739. doi: 10.1016/j.jcmg.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 7.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368–380. doi: 10.1002/(sici)1099-1166(199806)13:6<368::aid-gps775>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Altman R, Ashe E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 10.Lindbom A. Arteriosclerosis and arterial thrombosis in the lower limb: A roentgenological study. Acta Radiol Suppl. 1950;80:1–80. [PubMed] [Google Scholar]
- 11.Lim RP, Shapiro M, Wang EY, et al. 3D time-resolved MR angiography (MRA) of the carotid arteries with time-resolved imaging with stochastic trajectories: comparison with 3D contrast-enhanced Bolus-Chase MRA and 3D time-of-flight MRA. AJNR Am J Neuroradiol. 2008;29:1847–54. doi: 10.3174/ajnr.A1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–10. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 13.Keeling AN, Carroll TJ, McDermott MM, et al. Clinical correlates of size and number of collateral vessels in peripheral arterial disease. Vasc Med. doi: 10.1177/1358863X12446213. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgartner I, Thoeny HC, Kummer O, et al. Leg ischemia: assessment with MR angiography and spectroscopy. Radiology. 2005;234:833–41. doi: 10.1148/radiol.2343031440. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick E, Salive ME, Wallace RB. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication No. 95-4009, Appendix E. [Google Scholar]
- 18.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and noninvasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 20.Palmer-Kazen U, Wahlberg E. Arteriogenesis in peripheral arterial disease. Endothelium. 2003;10:225–232. doi: 10.1080/10623320390246360. [DOI] [PubMed] [Google Scholar]
- 21.Arras M, Ito WD, Scholz D, et al. Monocyte activation in angiogenesis and collateral growth in the rabbit hind limb. Journal of Clinical Investigation. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosaki K, Ando J, Korenaga R, et al. Fluid shear stress increases the production of granulocyte-macrophage colony stimulating factor by endothelial cells via mRNA stabilization. Circulation Research. 1998;92:794–802. doi: 10.1161/01.res.82.7.794. [DOI] [PubMed] [Google Scholar]
- 23.Shyy JY, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proceedings National Academic Scienc USA. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldfarb M. Signaling by fibroblast growth factors: The inside story. Sciences STKE. 106:PE37. doi: 10.1126/stke.2001.106.pe37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeshita S, Isshiki T, Mori H, et al. Use of synchrotron radiation microangiography to assess development of small collateral arteries in a rat model of hindlimb ischemia. Circulation. 1997;95:805–8. doi: 10.1161/01.cir.95.4.805. [DOI] [PubMed] [Google Scholar]


