Abstract
In the present study, we evaluated the effect of largazole (LAR), a marine-derived class I HDAC inhibitor, on tumor necrosis factor-α (TNF-α)-induced expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), and matrix metalloproteinase-2 (MMP-2) activity. LAR (1-5 μM) had no adverse effect on the viability of RA synovial fibroblasts. Among the different class I HDACs screened, LAR (1-5 μM) inhibited the constitutive expression of HDAC1 (0-30%). Surprisingly, LAR increased class II HDAC [HDAC6] by ~220% with a concomitant decrease in HDAC5 [30-58%] expression in RA synovial fibroblasts. SAHA (5 μM), a pan-HDAC inhibitor, also induced HDAC6 expression in RA synovial fibroblasts. Pretreatment of RA synovial fibroblasts with LAR further enhanced TNF-α-induced ICAM-1 and VCAM-1 expression. However, LAR inhibited TNF-α-induced MMP-2 activity in RA synovial fibroblasts by 35% when compared to the TNF-α-treated group. Further, the addition of HDAC6 specific inhibitor Tubastatin A with LAR suppressed TNF-α+LAR-induced ICAM-1 and VCAM-1 expression and completely blocked MMP-2 activity, suggesting a role of HDAC6 in LAR-induced ICAM-1 and VCAM-1 expression. LAR also enhanced TNF-α-induced phospho-p38 and phospho-AKT expression, but inhibited the expression of phospho-JNK and nuclear translocation of NF-κBp65 in RA synovial fibroblasts. These results suggest that LAR activates p38 and Akt pathways and influences class II HDACs, in particular HDAC6, to enhance some of the detrimental effects of TNF-α in RA synovial fibroblasts. Understanding the exact role of different HDAC isoenzymes in RA pathogenesis is extremely important in order to develop highly effective HDAC inhibitors for the treatment of RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease that leads to progressive destruction of bone and joints. Activation of RA synovial fibroblasts with inflammatory cytokines stimulates synthesis and expression of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), which facilitate recruitment and retention of inflammatory cells in the synovium resulting in joint inflammation (Lee and Weinblatt, 2001; Sweeney and Firestein, 2004). Gene transcription of chemotactic and inflammatory mediators is regulated, at least in part, by the tight balance between histone acetylation and deacetylation processes (Grabiec et al., 2011). Numerous genes encoding cytokines, chemokines and the expression of activating or inhibitory factors of immune cells are linked to the persistence of chronic arthritis and result in functional changes in immunoregulatory and cell cycle networks (Criswell, 2010).
Epigenetic regulation of synovial cell proliferation, persistent recruitment, activation, retention and survival of infiltrated immune cells plays an important role in RA pathogenesis (Huber et al., 2007). One of the crucial events in gene expression is histone modification through reversible acetylation which is regulated by histone acetyl transferases (HATs) and histone deacetylases (HDACs) (Grabiec et al., 2011). However, the pathogenic contribution of the different HDACs in RA is still an understudied area of research and warrants further investigation. Chromatin remodeling has been suggested to play a role in the etiology and progression of rheumatoid arthritis (Shuttleworth et al., 2010; Grabiec et al., 2011). Histone acetylation by HATs loosens the chromatin coils, thereby exposing transcription-factor binding sites and promoting the transcription of genes including those for proinflammatory cytokines (Glaser, 2007; Halili et al., 2009). On the contrary, HDACs condense the chromatin and prevent transcription factors from accessing the genome, thus preventing gene transcription.
Based on the structural and functional similarities, HDACs are classified into 4 subfamilies: Class I (HDACs 1, 2, 3, and 8), class II (HDACs 4-7, 9, and 10), class III (Sirtuins 1-7), and class IV (HDAC 11) (McGee-Lawrence and Westendorf, 2011). Among these, classes I and II are well studied. Studies suggest that class I HDACs are broadly expressed and are enzymatically active to deacetylase histones (Lahm et al., 2007). In contrast, class II HDACs demonstrate a more tissue specific expression and respond to the stimulation mediated signaling events.(McGee-Lawrence and Westendorf, 2011). Recent advances in the understanding of the role of HDACs in chronic inflammatory diseases such as cancer and RA has lead to growing interest to develop small molecule chemical inhibitors of HDACs (HDACi) (Dallavalle et al., 2012). Broadly, the HDACi are classified into four chemical groups based on the structural properties: hydroxamates (trichostatin A, TSA; suberoyl anilide bishydroxamic acid, SAHA), cyclic tetrapeptides (romidepsin, FK-228), short-chain fatty acids (phenylbutyrate, valproic acid, VPA), and benzamides (entinostat, MS-275) (Drummond et al., 2005). Recently isolated marine natural product largazole (LAR), a structural congener of FK228, possesses potent and selective anticancer activity (Taori et al., 2008). LAR was first isolated from a marine cyanobacterium of the genus Symploca and has shown remarkable selective antiproliferative activity on transformed cell lines as against non-transformed cell lines (Bowers et al., 2008; Ying et al., 2008). It has also demonstrated selectivity for class I HDAC enzymes (Bowers et al., 2008; Ying et al., 2008).
Synovial fibroblasts are a major player in executing the progressive joint damage in RA by accelerating the infiltration of circulating and resident lymphocytes and monocytes with the help of ICAM-1 and VCAM-1 (Marlor et al., 1992). In addition, activated synovial fibroblasts have been shown to invade the adjacent bone and cartilage through activation of matrix degrading enzymes termed matrix metalloproteinase-2 (MMP-2) (Ahmed et al., 2006). However, there is no established relationship between HDAC activity in RA synovial fibroblasts and inflammation in RA, which underlines the limitation of testing anti-inflammatory HDACi in these cells. Some studies suggest that the expression of class I HDACs (HDAC1 and 2) was decreased in RA synovial tissue as compared to osteoarthritis (OA) or normal synovial tissue (Horiuchi et al., 2009). In contrast several other studies suggest that HDAC1 and 4 mRNA expression was elevated in RA synovial tissue and fibroblasts (Chabane et al., 2009; Kawabata et al., 2010).
Initial screenings of HDACi on RA synovial fibroblasts have shown some promise. Inhibition of HDAC activity by TSA or FK-228 in RA synovial fibroblasts resulted in cell cycle arrest and sensitization of these cells to TRAIL-induced apoptosis (Ito et al., 2005; Nasu et al., 2008). A study aimed at examining the efficacy of HDACi on gene expression of inflammatory proteins using SV40 T-Ag-transformed lines showed that SAHA and MS-275 suppressed lipopolysaccharide (LPS)-induced IL-6, IL-18, MMP-2, MMP-9, and vascular endothelial growth factor (VEGF) expression through inhibition of nuclear factor-κB (NF-κB) nuclear translocation (Choo et al., 2010). However, little or no information is available regarding the role or presence of other classes of HDACs in RA synovial fibroblasts and the effect of HDACi on these HDACs and their possible therapeutic implications. Considering its potency and HDAC class I selectivity, we tested the effect of LAR on tumor necrosis factor-α (TNF-α)-stimulated RA synovial fibroblasts.
MATERIALS AND METHODS
Antibodies and Reagents
Recombinant human TNF-α, goat polyclonal antibodies against human ICAM-1 and VCAM-1 were purchased from R&D Systems (Minneapolis, MN). Rabbit polyclonal antibodies against phosphorylated ERK1/2, JNK/SAPK, and p38, and anti-rabbit and anti-mouse horseradish peroxide-linked secondary antibodies were purchased from Cell Signaling Technologies (Beverly, MA). Rabbit polyclonal antibody against NF-κBp65 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The signaling inhibitors were purchased from EMD Millipore (Billerica, MA). Tubastatin A HCl (HDAC6 inhibitor) was purchased from Selleck Chemicals (Houston, TX). Rabbit anti-β-actin was purchased from Sigma-Aldrich (St. Louis, MO).
Isolation and culture of RA synovial fibroblasts
Fibroblasts were isolated from synovium, obtained according to the University of Toledo Institutional Review Board (IRB) approved protocol in compliance with the Helsinki Declaration, from RA patients who had undergone total joint replacement surgery or synovectomy and fulfilled the criteria set by the American College of Rheumatology. Fresh synovial tissues were minced and digested in a solution of dispase, collagenase, and DNase (Ahmed et al., 2008). RA synovial fibroblasts were grown in RPMI containing 2 mM L-glutamine with 10% FBS, at 37 °C, in a humidified atmosphere with 5% CO2. Cells were used between passages 5-9.
Synthesis of largazole and preparation of LAR test solutions
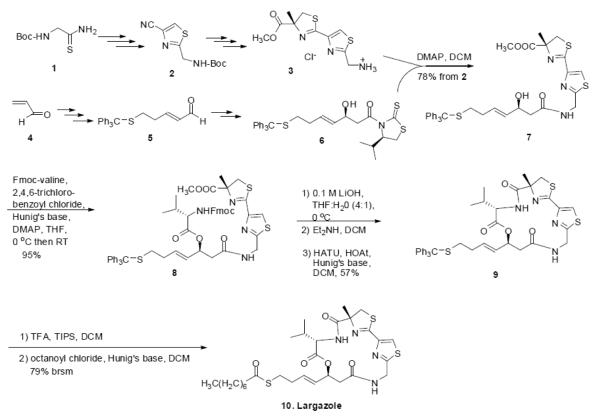
Largazole was synthesized and characterized as described earlier (Bhansali et al., 2011) and shown as a schematic diagram (Fig. 1). Briefly, the thiazole-thiazoline intermediate 3 was synthesized from Bocthioglycinamide 1 via the thiazole nitrile 2. The aldehyde 5, synthesized from acrolein 4, was converted to the alcohol 6 by stereoselective aldol reaction with acetyl Nagao auxillary (Yurek-George et al., 2004; Bhansali et al., 2011). Acyl transfer from 6 to 3 in the presence of 4-N,N-dimethylaminopyridine (DMAP) in dichloromethane (DCM) gave the intermediate 7. It was converted to the ester 8 by Yamaguchi esterification with Fmoc-valine in the presence of Hunig’s base and DMAP. After saponification of the methyl ester group under mild conditions with lithium hydroxide (LiOH) in tetrahydrofuran (THF)-water and the removal of the Fmoc protecting group with diethyl amine, it was cyclized using HATU and HOAt in the presence of Hunig’s base to obtain the depsipeptide 9. The trityl protecting group of 9 was removed with trifluoroacetic acid (TFA) and the resulting thiol was esterified with octanoyl chloride to obtain largazole 10 (described in Fig. 1).
Fig. 1.
Schematic diagram of the synthesis of largazole. Boc = tert-butoxycarbonyl, DMAP = 4-dimethylaminopyridine, DCM = dichloromethane, Fmoc = 9-fluorenylmethyloxycarbonyl, THF = tetrahydrofuran, TFA = trifluoroacetic acid, TIPS = triisopropylsilane, HATU = 2-(7-aza-1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, HOAt = 1-hydroxy-7-azabenzotriazole. Brsm = based on recovered starting material.
A stock solution of 10 mM of LAR was prepared in sterile DMSO and stored at −20° C in aliquots. A fresh LAR solution was used in each experiment and added directly to the culture medium. DMSO at the comparative volume was used as control.
Cell viability assays
To study the effect of LAR on cell viability, RA synovial fibroblasts (2 × 104/well) were plated in 96-well, flat-bottomed tissue culture plates (Corning, Corning, NY) and cultured in RPMI 1640 plus 10% FBS for 6 h. This was then replaced with fresh medium and culture continued for 24 h. LAR (1–5 μM) alone or with TNF-α (20 ng/ml) was added to RA synovial fibroblasts in serum-free medium and the culture was incubated at 37 °C for another 24 h. SAHA (5 μM) alone or in combination with TNF-α was also used as control. Two hours prior to termination of each time point, 20 μl of MTT dye (5 mg/ml in sterile phosphate buffered saline [PBS]; Invitrogen, Carlsbad, CA) was added to each well and further incubated at 37 °C. At the end of incubation, cells were washed twice with PBS, solubilized in 100 μl of DMSO at 37 °C for 5 min, and read at an optical density of 570 nm.
Western immunoblotting
To study the effect of LAR on HDACs, RA synovial fibroblasts were incubated with or without 0.5-5 μM LAR alone or with TNF-α for 24 h, in serum-free RPMI. SAHA (5 μM) was used as the treatment control. Cells were lysed in lysis buffer (100 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM NaP2O4, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mM phenylmethylsulfonyl fluoride), and protease inhibitors (Roche Diagnostics Corporation, IN; 1 tablet/10 ml), and protein was measured using BCA protein assay kits (Pierce Biotechnology Inc., Rockford, IL). Equal amounts of protein (20-25 μg) were separated by SDS/PAGE and transferred onto nitrocellulose membranes (Bio-Rad). Blots were probed with rabbit polyclonal antibodies for the specific protein of interest, followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. Immunoreactive protein bands were visualized by enhanced chemiluminescence, and were densitometrically analyzed using Image-J software (NIH). Blots were stripped and reprobed for β-actin to ensure equal protein loading. Data were statistically analyzed using Image-J software (NCI).
RNA extraction and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from human RA synovial fibroblasts using RNAeasy mini RNA isolation kits in conjunction with QIAshredders (Qiagen Inc., Valencia, CA) following the manufacturer’s protocol. Following isolation, RNA was quantified and checked for purity using a spectrophotometer (Nanodrop Technologies, Wilmington, DE). Complementary DNA (cDNA) was then prepared using a first strand synthesis kit, with anchored oligo-DT RNA primers (Qiagen Inc.) following the manufacturer’s protocol. Quantitative polymerase-chain reaction (PCR) was performed using Platinum SYBR Green qPCR MasterMix (Qiagen Inc.) and specific primer sequences for human ICAM-1 (NM_003183), VCAM-1 (NM_ 001110), and β-actin (NM_001101) (Qiagen Inc.) by the method described in our earlier studies (Ahmed et al., 2008; Marotte et al., 2010). All samples were run in duplicate and analyzed using the Eppendorf software provided with the instrument. For quantification, the relative abundance of each gene was normalized to β-actin.
Preparation of nuclear extracts and NF-κBp65 nuclear translocation study
To study the effect of LAR on the constitutive and TNF-α-induced NF-κBp65 activation, confluent RA synovial fibroblasts, cultured in 60 mm dishes, were untreated or pretreated with LAR (5 μM) for 2 h followed by TNF-α (20 ng/ml) stimulation for 30 min, in RPMI-1% FBS. Upon termination, cells were washed with ice-cold PBS, collected by scraping, and centrifuged at 1,500g for 5 min at 4 °C. Nuclear and cytoplasmic fractions from different treatment groups were prepared as described earlier (Ahmed et al., 2006; Abboud et al., 2008; Ahmed et al., 2009). Equal amount of protein (15 μg) from nuclear fractions was evaluated by Western blotting for studying the level of NFκBp65 expression.
Gelatin zymography
MMP-2 activity in conditioned medium was measured as previously described (Ahmed et al., 2006; Ahmed et al., 2008). Briefly, 15 μl of conditioned medium was added to 15 μl of 2× nonreducing sample buffer, resolved under nonreducing conditions on 7.5% SDS–polyacrylamide gels polymerized with 1 mg/ml gelatin (type B from bovine skin; Sigma) as a substrate, and electrophoresed at 125V. Following electrophoresis, the gels were washed in 2.5% Triton X-100 for 30 min with gentle shaking, followed by a 30-min wash in developing buffer (50 mM Tris HCl [pH 8.0], 5 mM CaCl2, and 0.02% NaN3). The gels were incubated overnight in fresh developing buffer at 37 °C, stained in Coomassie brilliant blue R250, and then destained using a solution of 7% acetic acid and 5% methanol. Images of the digested regions representing MMP activity were captured using the digital camera and analyzed using Image-J software.
Statistical analysis
One-way ANOVA followed by Dunnett’s t tests were performed to evaluate the statistical significance of group differences in measured mRNA and protein expression studies in RA synovial fibroblasts. P values less than 0.05 (2-tailed) were considered significant.
RESULTS
Effect of LAR or SAHA on RA synovial fibroblast viability
The results of an MTT-based viability assay using samples obtained from 3 different donors showed that LAR (0.5–5 μM) had no significant effect on the viability of cultured RA synovial fibroblasts (data not shown). However, SAHA (5 μM) increased the RA synovial fibroblast number by ~20%, which was not statistically significant (p>0.05).
Effect of LAR on RA synovial fibroblast HDACs
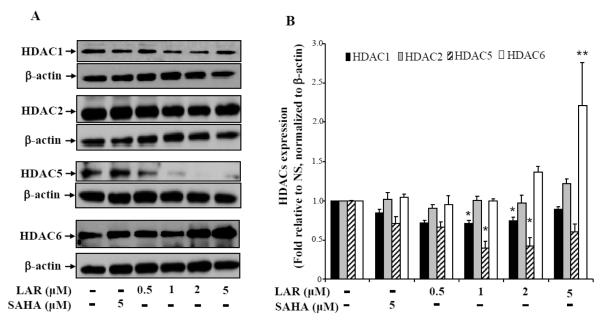
Earlier studies have shown that RA synovial fibroblasts express high level of several HDACs (namely HDAC1, 2, and 8) from class I as compared to the normal or osteoarthritis (OA) synovial fibroblasts (Horiuchi et al., 2009; Grabiec et al., 2011). In the present study, we evaluated the effect of LAR on the constitutive expression of HDACs in RA synovial fibroblasts. Result of the Western blotting analysis showed that HDAC3, 4, and 7 were undetectable in RA synovial fibroblasts (data not shown). However, we observed that HDAC1, 2, 5, and 6 are expressed constitutively by RA synovial fibroblasts (Fig. 2A). Treatment of LAR (0.5-5 μM) for 24 h resulted in a marked ~35% decrease in HDAC1 expression up to 2 μM dose, which was slightly lost at 5 μM dose (Fig. 2A&B). Interestingly, we observed no marked change in the expression of HDAC2 with LAR or SAHA treatment. To our surprise, LAR significantly inhibited the expression of HDAC5, a class II HDAC, in a dose-dependent fashion, ranging from 30 – 58%, with no marked change induced by SAHA (Fig. 2A&B; p<0.05). In another important observation, treatment of RA synovial fibroblasts with LAR significantly induced the expression of HDAC6 in a dose-dependent manner up to ~2.2-fold when compared to the untreated samples (Fig. 2A&B; p<0.01). Overall, we didn’t observe any marked change in these HDACs by the treatment of SAHA (2 μM) at the earlier reported dose (Butler et al., 2002). These results suggest that LAR induced modulation of HDACs may play a role in influencing the outcome of response under stimulation.
Fig. 2.
LAR dose-dependently down-regulates HDAC1 and HDAC5, but upregulates HDAC6 expression in RA synovial fibroblasts. (A) RA synovial fibroblasts (2 × 105/well) were incubated with LAR (0.5–5 μM) or SAHA (5 μM) in serum-free RPMI for 24 h to determine the effect on Class I and II HDACs by Western blotting method. (B) Densitometric analysis of the expression of different HDACs was quantified by determining the intensity of the bands using Image-J software (NIH). These values were normalized with the respective β-actin values and represented as the mean ± SEM of HDAC expression relative to untreated (NS) levels. The results represent values obtained from 5 independent donors’ cells under similar conditions. *p<0.05 vs NS group; **p<0.01 vs NS group.
Effect of LAR on ICAM-1 and VCAM-1 expression in RA synovial fibroblasts in vitro
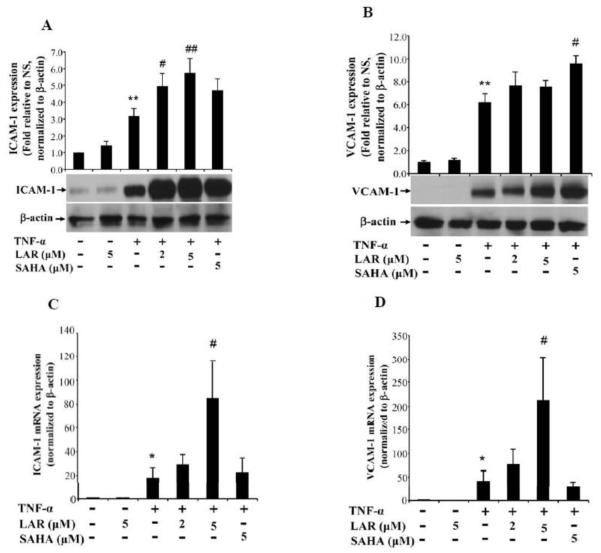
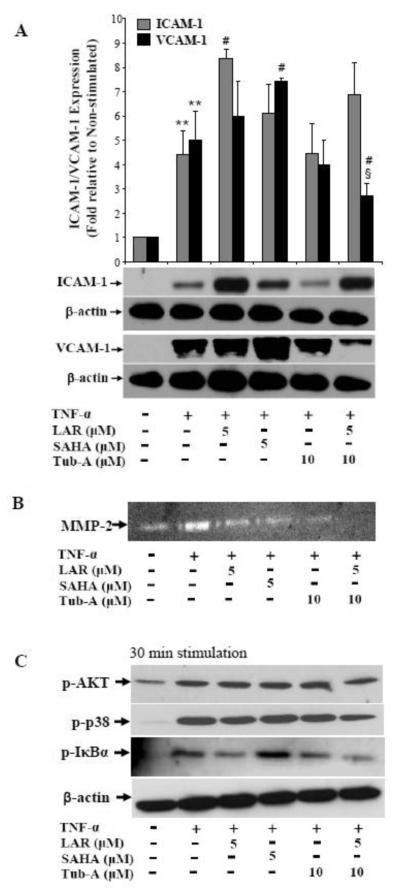
Adhesion molecules ICAM-1 and VCAM-1 produced by RA synovial fibroblasts play an important role in the perpetuation of inflammation by facilitating adhesion of circulating inflammatory cells to the walls of endothelial cells and consequently their extravasation at the site of inflammation or tissue injury (Marrelli et al., 2011). To study the effect of LAR on TNF-α-induced ICAM-1 and VCAM-1 expression, we pretreated RA synovial fibroblasts with or without LAR or SAHA followed by TNF-α stimulation for 24 h. Results of the Western blotting showed that LAR (5 μM) alone had no inducing effect on ICAM-1 or VCAM-1 (Fig. 3). Stimulation of RA synovial fibroblasts with TNF-α (20 ng/ml) resulted in a significant 3.2-fold and 6.2-fold increase in ICAM-1 and VCAM-1 expression, respectively, when compared to the expression in untreated samples (Fig. 3A&B; p<0.05 for both ICAM-1 and VCAM-1). Interestingly, pretreatment of RA synovial fibroblasts with LAR (2 and 5 μM) resulted in a significant 35% and 45% increase in TNF-α-induced ICAM-1 expression, when compared to the expression level with TNF-α alone treatment (Fig. 3A; p<0.05 for 5 μM). Similarly, we found a dose-dependent increase in TNF-α-induced VCAM-1 expression by the addition of LAR (2 and 5 μM) (Fig. 3B; p<0.05).
Fig. 3.
Synergistic induction of TNF-α-induced ICAM-1 and VCAM-1 expression by LAR or SAHA in RA synovial fibroblasts. (A&B) RA synovial fibroblasts were pretreated with LAR (2–5 μM) or SAHA (5 μM) for 2 h followed by TNF-α (20 ng/ml) stimulation for 24 h in serum-free RPMI. Cells were lysed in extraction buffer containing protease inhibitors and utilized for the determination of ICAM-1 and VCAM-1 expression using Western blotting. The intensity of the bands was quantified using Image-J software (NIH). (C&D) For ICAM-1 and VCAM-1 mRNA analysis, RA synovial fibroblasts (2× 105/well) were treated as described above and analyzed for mRNA expression by a qRT-PCR method. Results represent the mean ± SEM of experiments performed using 3-4 different RA synovial fibroblast donors. *p<0.05, **p<0.01 for NS vs TNF-α; #p<0.05, ##p<0.01 for TNF-α vs TNF-α plus LAR.
To determine whether the induction of ICAM-1 and VCAM-1 was not only at translational level, we analyzed ICAM-1 and VCAM-1 mRNA expression in RA synovial fibroblasts by qRT-PCR. After 24 h of TNF-α stimulation, the ICAM-1 and VCAM-1 mRNA expression increased by ~18- and ~42-fold, respectively, when compared to the untreated (NS) group (Fig. 3C&D; p<0.01). Pretreatment of LAR (2 and 5 μM) further induced ICAM-1 and VCAM-1 mRNA expression in RA synovial fibroblasts. Specifically, LAR (5 μM) alone had no inducing effect on ICAM-1 or VCAM-1. However, LAR (2 and 5 μM) further increased TNF-α-induced ICAM-1 mRNA expression by 69% and 373%, respectively (Fig. 3C&D; p<0.05 for 5 μM). Similar concentrations of LAR further induced TNF-α-induced VCAM-1 mRNA expression by 81% and 406%, respectively (Fig. 5B; p<0.05 for 5 μM). Interestingly, SAHA (5 μM) modestly increased ICAM-1 mRNA expression, but inhibited VCAM-1 by ~26% when compared to the TNF-α treated group (Fig. 5A&B). These results were consistent with ICAM-1 and VCAM-1 protein expression analysis and suggest that LAR may enhance the effects of TNF-α via upregulation of upstream signaling events in RA synovial fibroblasts in vitro. Interestingly, we observed a discordant VCAM-1 protein and mRNA correlation in the samples treated with SAHA, which could not be explained from the mechanism point-of-view. While it is open to interpretation, such discordant or delayed correlation of mRNA and protein expression is common in the diseased samples or their stimulation with the treatment, and has been a subject of extensive research in the area of proteomics (Chen et al., 2002; Fournier et al., 2010).
Fig. 5.
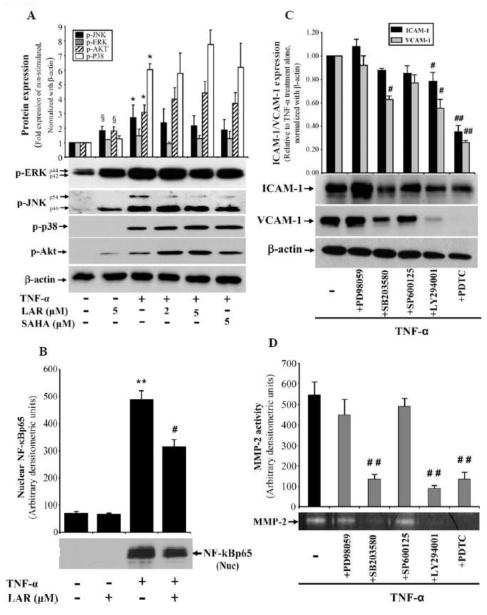
LAR coordinates with TNF-α to induce phosphorylation of AKT and MAPKs, but inhibits NF-κBp65 in RA synovial fibroblasts. (A) RA synovial fibroblasts (2 × 105/well) were pretreated with LAR (2-5 μM) or SAHA (5 μM) for 2 h, followed by stimulation with TNF-α (20 ng/ml) for 30 min in serum-free RPMI. Cells were lysed in extraction buffer containing protease inhibitors and utilized for the determination of p-ERK1/2, p-JNK, p-p38, and p-AKT in RA synovial fibroblasts using Western blotting. (B) Similarly treated RA synovial fibroblasts were utilized for preparation of nuclear fraction as described in Materials and Methods. Nuclear protein (15 μg) was used to analyze nuclear NF-κBp65. The intensity of the bands was quantified using Image-J software (NIH). (C&D) RA synovial fibroblasts were pretreated with the signaling inhibitors for ERK1/2 (PD98059, 10 μM), p38 (SB203580, 10 μM), JNK (SP600125, 10 μM), Akt (LY294001, 20 μM), or NF-κB (PDTC, 200 μM) for 2 h, followed by TNF-α stimulation for 24 h. Cells were lysed to study the expression of ICAM-1 and VCAM-1; conditioned media was collected to analyze MMP-2 activity using gelatin zymography. Values represent the mean ± SEM of 4-6 independent donors’ cells under similar conditions. §p<0.05 for NS vs LAR alone; *p<0.05 or **p<0.01 for NS vs TNF-α; ##p<0.01, TNF-α vs TNF-α plus inhibitor.
Effect of LAR on TNF-α-induced MMP-2 activity in RA synovial fibroblasts
Proinflammatory cytokines such as TNF-α are known inducers of matrix degrading enzymes including MMP-2 (Ahmed et al., 2006; Burrage et al., 2006). We evaluated the effect of LAR on TNF-α-induced MMP-2 activity using gelatin zymography. Results of the densitometric analysis of the gelatin zymograms showed that TNF-α induced MMP-2 activity by almost 92% when compared to the enzymatic activity of MMP-2 in untreated samples (Fig. 4; p<0.01). Interestingly, pretreatment of cells with LAR inhibited TNF-α-induced MMP-2 activity by 28% and 35% in a dose-dependent manner (Fig. 4; p<0.05 for 5 μM). We also observed a marked 28% decrease in TNF-α-induced MMP-2 activity by SAHA (5 μM). These results suggest that LAR may modulate certain signaling pathways activated by TNF-α in RA synovial fibroblasts to suppress in vitro MMP-2 activity.
Fig. 4.
Inhibition of TNF-α-induced MMP-2 activity by LAR in RA synovial fibroblasts. RA synovial fibroblasts were pre-incubated with the LAR (2-5 μM) or SAHA (5 μM) for 2 h, followed by stimulation with TNF-α (20 ng/ml) for 24 h. MMP-2 activity in the cell-free supernatants from different treatment combinations was estimated by gelatin zymography. Values are the mean ± SEM of experiments performed using RA synovial fibroblast obtained from 5 different donors. **p<0.01, NS vs TNF-α; #p<0.05, TNF-α vs TNF-α plus LAR.
LAR enhances TNF-α-induced p38 and AKT pathways, but inhibits JNK and NF-κBp65 activation in RA synovial fibroblasts
To understand the molecular mechanism through which LAR modulates TNF-α response in RA synovial fibroblasts, we studied the effect of LAR on TNF-α-induced pathways that are integral signaling mediators of TNF-α-induced downstream inflammatory proteins in RA pathogenesis (Ahmed et al., 2006; Ahmed et al., 2008). RA synovial fibroblasts were pretreated with LAR or SAHA stimulated with TNF-α for 30 min. Western blot analysis of the phosphorylated MAPKs showed that LAR (5 μM) alone increased the constitutive levels of p-ERK and p-JNK, but not p-p38 MAPK, in RA synovial fibroblasts (Fig. 5A). Specifically, TNF-α stimulation for 30 min resulted in almost 0.5-, 2-, 6-, and 4-fold increase in the expression of p-ERK, p-JNK, p-p38, and p-Akt in RA synovial fibroblasts. Preincubation of LAR differentially modulated the TNF-α-induced MAPK expression in RA synovial fibroblasts (Fig. 5A). In particular, LAR at 2 and 5 μM concentration caused a dose-dependent increase in TNF-α-induced p-p38 and p-Akt by ~20% and ~45%, respectively, but inhibited p-JNK expression by ~30% in TNF-α-stimulated RA synovial fibroblasts (Fig. 5A; p>0.05). Since TNF-α itself is a potent inducer of MAPK pathways, even a moderate increase in its activation by LAR may have profound impact on its downstream signaling events. Therefore, these results suggest that LAR, by selectively activating p38 and Akt pathways, further enhances TNF-α-induced ICAM-1 and VCAM-1 expression in RA synovial fibroblasts.
NF-κBp65 plays a central role in regulating cytokine-induced inflammatory genes including MMP-2 (Ahmed et al., 2006; Ahmed et al., 2008). In the present study, stimulation of RA synovial fibroblasts with TNF-α for 30 mins resulted almost 6-fold induction in the nuclear translocation of NF-κBp65 (Fig. 5B; p<0.01). We further evaluated the effect of LAR on TNF-α-induced nuclear activation of NF-kB in RA synovial fibroblasts. The results from Western blot analysis on the nuclear extracts prepared from TNF-α-stimulation for 30 mins alone or in the presence of LAR (5 μM) showed that LAR inhibits TNF-α-induced NF-κBp65 nuclear translocation in RA synovial fibroblasts by almost 35% (Fig. 5B; p<0.05). These results suggest that the regulation of NF-κB pathway by LAR may be an important mechanism of inhibiting MMP-2 activity in RA synovial fibroblasts.
To further evaluate the role of these signaling pathways in LAR mediated induction of ICAM-1 and VCAM-1, and inhibition of MMP-2 activity, RA synovial fibroblasts were pretreated with signaling inhibitors followed by TNF-α stimulation for 24 h. Western blot analysis showed that p38 and JNK pathways have a modest, whereas Akt and NF-κB pathways have a significant, role in mediating TNF-α-induced ICAM-1 expression (Fig. 5C; p<0.05 and p<0.01). Interestingly, p38, Akt, and NF-κB pathways were found to be the key players in TNF-α-induced VCAM-1 expression and MMP-2 activity in RA synovial fibroblasts (Fig. 5 C&D; p<0.05 and p<0.01). These findings provide evidence for the role of p38, Akt, and NF-κB pathways in mediating LAR induced effects in RA synovial fibroblasts (Fig. 5 C&D).
Involvement of HDAC6 in LAR-induced ICAM-1 and VCAM-1 expression in RA synovial fibroblasts
To understand the influence of LAR on HDAC6 mediated activation of TNF-α-induced ICAM-1 and VCAM-1 expression, we pretreated cells with Tubastatin A (Tub-A; a selective HDAC6 inhibitor, 10 μM) alone or in combination with LAR, followed by TNF-α stimulation. Western blot analysis showed that although Tub-A by itself had a modest effect in inhibiting TNF-α-induced ICAM-1 and VCAM-1 expression, its presence markedly inhibited TNF-α+LAR-induced ICAM-1 and VCAM-1 expression in RA synovial fibroblasts (Fig. 6A; p<0.01 for VCAM-1). Our analysis of zymograms showed that LAR and Tub-A exhibited a combinatorial effect, thereby completely blocking TNF-α-induced MMP-2 activity in these cells (Fig. 6B). These findings validate that LAR might activate class II HDAC (HDAC6) by influencing the upstream signaling cascades to induce ICAM-1 and VCAM-1 in RA synovial fibroblasts.
Fig. 6.
Tub-A suppresses TNF-α+LAR-induced ICAM-1 and VCAM-1 expression and coordinates to inhibit MMP-2 activity in RA synovial fibroblasts. (A) RA synovial fibroblasts were preincubated with LAR (2–5 μM), SAHA (5 μM), or Tub-A (10 μM) in serum-free RPMI for 2 h followed by TNF-α (20 ng/ml) stimulation for 24 h to determine the effect on ICAM-1 and VCAM-1 expression by Western blotting method. Densitometric analysis of the expression was quantified by determining the intensity of the bands using Image-J software (NIH). (B) Conditioned media was utilized for the assessment of MMP-2 activity using gelatin zymography. (C) RA synovial fibroblasts were treated as described in section (A), except that the reaction was terminated within 30 min of TNF-α stimulation to analyze signaling pathways. The results represent values obtained from 3-4 independent donors’ cells under similar conditions. **p<0.01 for NS vs TNF-α; #p<0.05 for TNF-α vs TNF-α plus LAR; §p<0.05 for TNF-α vs TNF-α+LAR+Tub-A.
To further correlate these downstream effects with the modulation of p38, Akt, and NF-κB pathways, we treated cells with LAR and/or Tub-A, followed by TNF-α stimulation for 30 min and probed the cell lysates for the signaling proteins. The result of the analysis showed that although Tub-A alone had no significant inhibitory effect on the phosphorylation of p38 and Akt, it exhibited a marked inhibition of p-p38 and p-Akt activation by TNF-α+LAR in RA synovial fibroblasts (Fig. 6C). In addition, Tub-A further enhanced the effect of LAR in completely inhibiting the phosphorylation of IκBα induced by TNF-α stimulation (Fig. 6C). Overall, these results validate the role of HDAC6, at least in part, in regulating the expression of ICAM-1 and VCAM-1 and MMP-2 activity via these signaling pathways.
DISCUSSION
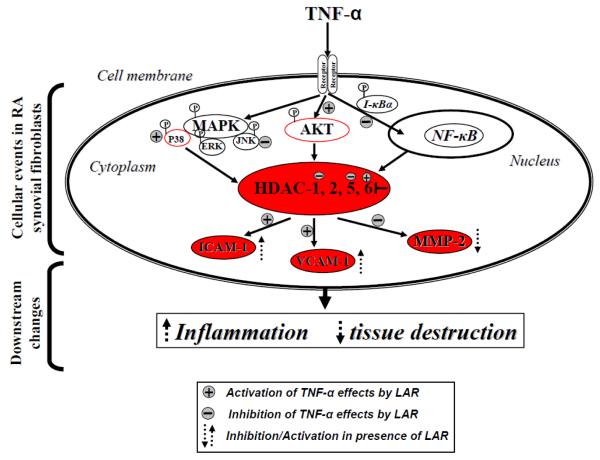
Based on the limited understanding of the role of class I and II HDACs in RA pathogenesis, our two pronged interest was to determine the expression levels of class I and II HDACs in RA synovial fibroblasts and evaluate the effect of newly discovered marine-derived class I HDAC inhibitor, LAR, on TNF-α-induced angiogenic markers in these cells. Some of the interesting findings from this study suggest that LAR, although classified as a class I HDAC inhibitor, may modulate the expression of class II HDACs such as HDAC5 and HDAC6 in RA synovial fibroblasts. Importantly, this study emphasized how LAR modulates the signaling pathways alone or in presence of TNF-α to induce ICAM-1 and VCAM-1 expression. Similar effects of SAHA, a pan-HDAC inhibitor, attest to the notion that different members within each class of HDACs may play a unique, yet important, role in specific disease pathogenesis. In addition, the decrease in LAR-induced ICAM-1 and VCAM-1 expression by Tub-A further provides evidence that the induction of HDAC6 by LAR is an important event in enhancing the effects of TNF-α in RA synovial fibroblasts (Fig. 7 for the proposed mechanism). Thus, developing isoform specific HDAC inhibitors seems more promising therapeutic strategy than designing class specific HDAC inhibitors. It may particularly be important for autoimmune diseases where the presence of multiple proinflammatory cytokines in the process of dysregulated immune function may further exacerbate the detrimental processes such as angiogenesis and tissue destruction.
Fig. 7.
Schematic representation of the mechanism through which LAR upregulates ICAM-1 and VCAM-1 expression and inhibits MMP-2 activity induced by TNF-α. This also underlines the effect of HDAC6 inhibitor (Tub-A) on different signaling steps in down-regulating ICAM-1 and VCAM-1 induced by the combination of TNF-α and LAR. A summary of the present study suggests that LAR, in part, engages HDAC6 to induce ICAM-1 and VCAM-1 expression.
HDACs have been shown to play an important role in epigenetically regulating inflammatory processes at different levels. However, only recent studies have clarified their role in angiogenesis, in particular the epigenetic mechanisms in the regulation of tissue destruction in RA (Buckland, 2011). Studies performed on RA and OA synovial tissues suggest the excessive expression of HDAC1 among other class I HDACs (1, 2, 3, and 8) (Kawabata et al., 2010). A recent study by Gillespie et al, showed higher HDAC activity in the peripheral mononuclear blood cells (PBMCs) obtained from RA patients and the selective inhibition of HDAC3 suppressed selective IL-6 production in these cells (Gillespie et al., 2012). However, in RA synovial fibroblasts we observed a very low expression of HDAC3 suggesting this HDAC has limited role in mediating RA synovial fibroblast aggressiveness. Similarly, the non-specific HDAC inhibitors were shown to inhibit IL-6 production in RA synovial fibroblasts and macrophages by accelerating its mRNA decay (Grabiec et al., 2012). Despite our understanding of the role of HDACs in RA synovial fibroblast biology, the contribution of individual HDACs is not defined. This leaves us with a question of which HDAC can be therapeutically targeted for the treatment of RA. Interestingly, a study by Kawabata et al, pointed to the increased activity and expression of HDAC1, not HDAC2, that correlated with and responded to TNF-α stimulation in RA synovial fibroblasts (Kawabata et al., 2010). Our study partly supports this notion as we observed both HDAC1 and HDAC2 significantly expressed at the constitutive levels and only HDAC1 inhibition was observed with the different concentrations of LAR.
RA is a chronic, systemic autoimmune disease of the synovial joints, which is characterized by synovial hyperplasia mediated via infiltrating leukocytes that release cell adhesion molecules and matrix degrading enzymes to cause bone and cartilage destruction (Ahmed, 2010). Among different cell types present in the diseased synovium, synovial fibroblasts are prominent activators of ICAM-1 and VCAM-1 that facilitate the binding and extravasation of the inflammatory cells in the affected tissue and cause joint damage (Okamoto et al., 2008). VCAM-1 expression is up-regulated in the activated synovial fibroblasts and can further be induced in the presence of inflammatory cytokines (Silverman et al., 2007). ICAM-1, by promoting the mechanism of adhesion in immunologic and inflammatory processes, plays an important role in a variety of inflammatory and neoplastic diseases. In addition, ICAM-1 is also expressed on RA synovial fibroblasts and facilitates the interaction of the matrix protein with the circulating leukocytes (Hanyuda et al., 2003). Studies suggest that blockade of ICAM-1 or VCAM-1 using specific antibodies inhibits the development of collagen-induced arthritis (CIA) and adjuvant-induced arthritis (AIA) in experimental animals (Bullard et al., 1996; Carter et al., 2002). It may be postulated that the increase in TNF-α-induced ICAM-1 and VCAM-1 in the presence of LAR may contribute to the adhesion of recruited leukocytes and other inflammatory cells and eventually amplify the inflammatory milieu in the affected joints. However, further studies are desired to test the effect of LAR on other cell types that are integral part of the joint such as osteoblasts and chondrocytes, which may validate the overall benefit/drawback for such activation in RA joints. Along these lines of discussion, a recent study suggest that LAR induces osteogenic activity (Lee et al., 2011). Although the osteogenic property of LAR in this study was attributed to the induction of RUNX2 and bone morphogenetic proteins (BMP), it may be postulated that induction of ICAM-1 and VCAM-1 may also play an important role in this process. Recent studies suggest that adhesion molecules are essential for an enhanced growth and osteogenic differentiation of human osteoblast-like cells (Defino et al., 2009; Grausova et al., 2011). On a similar note, therapies that upregulate chondrogenic cytokines including adhesion molecules have been proposed as potential strategy to halt the process of cartilage degradation in osteoarthritis (Lo et al., 2013).
The majority of studies so far have addressed the role of class I HDACs and a significant void remains in our understanding of the role of class II HDACs in RA pathogenesis. Results from our study provide novel perspectives on the role and expression of class II HDAC5 and HDAC6 in RA synovial fibroblasts. HDAC5 has been shown to possess a unique anti-angiogenic property and its overexpression resulted in decreased endothelial cell migration, sprouting, and tube formation (Urbich et al., 2009). In another study, the selective inhibition of HDAC5 by siRNA resulted in an increased angiogenesis and enhanced pro-angiogenic gene expression pattern in endothelial cells (Milde et al., 2010). HDAC6 has recently gained significant attention with its emerging role related to the regulation of the acetylation of nonhistone regulatory proteins that participate in cancer-relevant processes (Dallavalle et al., 2012). In contrast to HDAC5, HDAC6 has been shown to promote angiogenesis and play a major role in tumor cell invasion (Yang et al., 2008; Park et al., 2011). However, the role of HDAC6 in RA pathogenesis is not yet studied. In RA, where angiogenesis is a central mechanism of recruitment and retention of leukocyte infiltrates via enhanced blood supply, ICAM-1 and VCAM-1 expression plays a key role in maintaining inflammatory milieu in the affected joints (Okamoto et al., 2008). In the present study, treatment of RA synovial fibroblasts with LAR upregulated HDAC6 expression, which suggests that the induction of ICAM-1/VCAM-1 by LAR alone or in the presence of TNF-α may partly be regulated via HDAC6, in addition to p38 and Akt pathways. This observation is further supported by our results that show downregulation of TNF-α+LAR-induced ICAM-1 and VCAM-1 by HDAC6 inhibitor. Further studies are required to nail down the exact role of HDAC6 inhibition on the expression of angiogenic genes in pre-clinical models of human RA.
MMP-2 is involved in joint destruction observed in RA by facilitating RA synovial fibroblast mediated invasion of microvascular basement membrane and the interstitium (Burrage et al., 2006). We have shown earlier that MMP-2 activation in RA synovial fibroblasts is mediated, in part, by NF-κB pathway (Ahmed et al., 2006; Ahmed et al., 2008). Interestingly, a recent finding suggests HDAC inhibitors SAHA and MS-275 may inhibit MMP-2 and MMP-9 production in synovial fibroblastic cell line E11 (Choo et al., 2010). However, the effect of these HDAC inhibitors and newly discovered LAR on primary human RA synovial fibroblast MMP-2 activity has not been tested. In our results, although LAR cooperated with TNF-α to further induce AKT and MAPKs, it had inhibitory effect on TNF-α-induced NF-κBp65 activation and nuclear translocation. Taken together, our findings support the inhibition of NF-κB signaling pathway as a potential mechanism of MMP-2 regulation by LAR. The addition of Tub-A with LAR completely blocked TNF-α-induced phospho-IκBα and thereby MMP-2 activity in RA synovial fibroblasts, suggesting the involvement of HDAC6 in mediating this process.
In summary, the results of the present study suggest that LAR may also influence class II HDACs, in particular HDAC6, to synergize with some of the detrimental effects of TNF-α. However, further studies are warranted to understand the role of HDAC6 in upregulating de novo protein translation in RA synovial fibroblasts. This study also underlines (a) the role of p38 and Akt pathways in mediating the effects of LAR on TNF-α-induced ICAM-1 and VCAM-1 expression, and (b) the role of NF-κB pathway inhibition by LAR in regulating TNF-α-induced MMP-2 activity in RA synovial fibroblasts in vitro. Overall, validating the exact role of an individual HDAC isoenzyme in RA pathogenesis is extremely important before highly selective HDAC inhibitors could be designed and tested for the treatment of RA.
RESEARCH HIGHLIGHTS.
Largazole enhances TNF-α-induced ICAM-1 and VCAM-1.
Largazole upregulates class II HDAC (HDAC6) in RA synovial fibroblasts.
Largazole increases ICAM-1 and VCAM-1 expression partly via upregulation of p38 and Akt pathways.
A selective HDAC isoform inhibitor may be more effective than a class inhibitor.
Further studies are required to understand the role of class II HDACs in RA.
ACKNOWLEDGMENTS
This work was supported in part by the NIH grants AT-003633 and AR-055741 (SA) and start-up funds from The University of Toledo (SA and LMVT). Authors thank Dr. Nabil Ebraheim (Department of Orthopaedic Surgery at the University of Toledo), National Disease Research Interchange (NDRI), and Cooperative Human Tissue Network (CHTN) for providing RA synovial tissues. Authors also thank Dr. David Fox (Professor and Chair of Rheumatology, University of Michigan, Ann Arbor) for providing RA synovial fibroblasts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP CONTRIBUTION
Participated in research design: Ahmed, Rahman, and Tillekeratne.
Conducted experiments: Ahmed, Beamer, Rahman, Riegsecker, Bellini, Bhansali, and Tillekeratne.
Contributed new reagents or analytic tools: Ahmed, Bhansali, and Tillekeratne.
Performed data analysis: Ahmed, Beamer, Riegsecker, Rahman, Bellini, and Bhansali.
Wrote or contributed to the writing of the manuscript: Ahmed, Rahman, Bhansali, Tillekeratne.
REFERENCES
- Abboud PA, Hake PW, Burroughs TJ, Odoms K, O’Connor M, Mangeshkar P, Wong HR, Zingarelli B. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur. J. Pharmacol. 2008;579:411–417. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Ahmed S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res. Ther. 2010;12:208. doi: 10.1186/ar2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Marotte H, Kwan K, Ruth JH, Campbell PL, Rabquer BJ, Pakozdi A, Koch AE. Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production. Proc. Natl. Acad. Sci. USA. 2008;105:14692–14697. doi: 10.1073/pnas.0802675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006;54:2393–2401. doi: 10.1002/art.22023. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Silverman MD, Marotte H, Kwan K, Matuszczak N, Koch AE. Down-regulation of myeloid cell leukemia 1 by epigallocatechin-3-gallate sensitizes rheumatoid arthritis synovial fibroblasts to tumor necrosis factor alpha-induced apoptosis. Arthritis Rheum. 2009;60:1282–1293. doi: 10.1002/art.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhansali P, Hanigan CL, Casero RA, Tillekeratne LM. Largazole and analogues with modified metal-binding motifs targeting histone deacetylases: synthesis and biological evaluation. J. Med. Chem. 2011;54:7453–7463. doi: 10.1021/jm200432a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers A, West N, Taunton J, Schreiber SL, Bradner JE, Williams RM. Total synthesis and biological mode of action of largazole: a potent class I histone deacetylase inhibitor. J. Am. Chem. Soc. 2008;130:11219–11222. doi: 10.1021/ja8033763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland J. Rheumatoid arthritis: HDAC and HDACi: pathogenetic and mechanistic insights. Nat. Rev. Rheumatol. 2011;7:682. doi: 10.1038/nrrheum.2011.162. [DOI] [PubMed] [Google Scholar]
- Bullard DC, Hurley LA, Lorenzo I, Sly LM, Beaudet AL, Staite ND. Reduced susceptibility to collagen-induced arthritis in mice deficient in intercellular adhesion molecule-1. J. Immunol. 1996;157:3153–3158. [PubMed] [Google Scholar]
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front. Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc. Natl. Acad. Sci. USA. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RA, Campbell IK, O’Donnel KL, Wicks IP. Vascular cell adhesion molecule-1 (VCAM-1) blockade in collagen-induced arthritis reduces joint involvement and alters B cell trafficking. Clin. Exp. Immunol. 2002;128:44–51. doi: 10.1046/j.1365-2249.2002.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabane N, Li X, Fahmi H. HDAC4 contributes to IL-1-induced mPGES-1 expression in human synovial fibroblasts through up-regulation of Egr-1 transcriptional activity. J. Cell. Biochem. 2009;106:453–463. doi: 10.1002/jcb.22027. [DOI] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol. Cell. Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- Choo QY, Ho PC, Tanaka Y, Lin HS. Histone deacetylase inhibitors MS-275 and SAHA induced growth arrest and suppressed lipopolysaccharide-stimulated NF-kappaB p65 nuclear accumulation in human rheumatoid arthritis synovial fibroblastic E11 cells. Rheumatology. 2010;49:1447–1460. doi: 10.1093/rheumatology/keq108. [DOI] [PubMed] [Google Scholar]
- Criswell LA. Gene discovery in rheumatoid arthritis highlights the CD40/NF-kappaB signaling pathway in disease pathogenesis. Immunol. Rev. 2010;233:55–61. doi: 10.1111/j.0105-2896.2009.00862.x. [DOI] [PubMed] [Google Scholar]
- Dallavalle S, Pisano C, Zunino F. Development and therapeutic impact of HDAC6-selective inhibitors. Biochem. Pharmacol. 2012;84:756–765. doi: 10.1016/j.bcp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Defino HL, da Silva Herrero CF, Crippa GE, Bellesini LS, Beloti MM, Rosa AL. In vitro proliferation and osteoblastic phenotype expression of cells derived from human vertebral lamina and iliac crest. Spine. 2009;34:1549–1553. doi: 10.1097/BRS.0b013e3181a9c087. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- Fournier ML, Paulson A, Pavelka N, Mosley AL, Gaudenz K, Bradford WD, Glynn E, Li H, Sardiu ME, Fleharty B, Seidel C, Florens L, Washburn MP. Delayed correlation of mRNA and protein expression in rapamycin-treated cells and a role for Ggc1 in cellular sensitivity to rapamycin. Mol. Cell. Proteomics. 2010;9:271–284. doi: 10.1074/mcp.M900415-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J, Savic S, Wong C, Hempshall A, Inman M, Emery P, Grigg R, McDermott MF. Histone deacetylases are dysregulated in rheumatoid arthritis and a novel histone deacetylase 3-selective inhibitor reduces interleukin-6 production by peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Rheum. 2012;64:418–422. doi: 10.1002/art.33382. [DOI] [PubMed] [Google Scholar]
- Glaser KB. HDAC inhibitors: clinical update and mechanism-based potential. Biochem. Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Grabiec AM, Korchynskyi O, Tak PP, Reedquist KA. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann. Rheum. Dis. 2012;71:424–431. doi: 10.1136/ard.2011.154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabiec AM, Tak PP, Reedquist KA. Function of histone deacetylase inhibitors in inflammation. Crit. Rev. Immunol. 2011;31:233–263. doi: 10.1615/critrevimmunol.v31.i3.40. [DOI] [PubMed] [Google Scholar]
- Grausova L, Kromka A, Burdikova Z, Eckhardt A, Rezek B, Vacik J, Haenen K, Lisa V, Bacakova L. Enhanced growth and osteogenic differentiation of human osteoblast-like cells on boron-doped nanocrystalline diamond thin films. PLoS One. 2011;6:e20943. doi: 10.1371/journal.pone.0020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halili MA, Andrews MR, Sweet MJ, Fairlie DP. Histone deacetylase inhibitors in inflammatory disease. Curr. Top. Med. Chem. 2009;9:309–319. doi: 10.2174/156802609788085250. [DOI] [PubMed] [Google Scholar]
- Hanyuda M, Kasama T, Isozaki T, Matsunawa MM, Yajima N, Miyaoka H, Uchida H, Kameoka Y, Ide H, Adachi M. Activated leucocytes express and secrete macrophage inflammatory protein-1alpha upon interaction with synovial fibroblasts of rheumatoid arthritis via a beta2-integrin/ICAM-1 mechanism. Rheumatology. 2003;42:1390–1397. doi: 10.1093/rheumatology/keg391. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Morinobu A, Chin T, Sakai Y, Kurosaka M, Kumagai S. Expression and function of histone deacetylases in rheumatoid arthritis synovial fibroblasts. J. Rheumatol. 2009;36:1580–1589. doi: 10.3899/jrheum.081115. [DOI] [PubMed] [Google Scholar]
- Huber LC, Stanczyk J, Jungel A, Gay S. Epigenetics in inflammatory rheumatic diseases. Arthritis Rheum. 2007;56:3523–3531. doi: 10.1002/art.22948. [DOI] [PubMed] [Google Scholar]
- Ito T, Ouchida M, Morimoto Y, Yoshida A, Jitsumori Y, Ozaki T, Sonobe H, Inoue H, Shimizu K. Significant growth suppression of synovial sarcomas by the histone deacetylase inhibitor FK228 in vitro and in vivo. Cancer Lett. 2005;224:311–319. doi: 10.1016/j.canlet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Kawabata T, Nishida K, Takasugi K, Ogawa H, Sada K, Kadota Y, Inagaki J, Hirohata S, Ninomiya Y, Makino H. Increased activity and expression of histone deacetylase 1 in relation to tumor necrosis factor-alpha in synovial tissue of rheumatoid arthritis. Arthritis Res. Ther. 2010;12:R133. doi: 10.1186/ar3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, Koch U, De Francesco R, Steinkuhler C, Gallinari P. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl. Acad. Sci. USA. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- Lee SU, Kwak HB, Pi SH, You HK, Byeon SR, Ying Y, Luesch H, Hong J, Kim SH. In Vitro and In Vivo Osteogenic Activity of Largazole. ACS. Med. Chem. Lett. 2011;2:248–251. doi: 10.1021/ml1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WC, Chen WH, Lin TC, Hwang SM, Zeng R, Hsu WC, Chiang YM, Liu MC, Williams DF, Deng WP. Preferential therapy for osteoarthritis by cord blood MSCs through regulation of chondrogenic cytokines. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.03.016. [Epub] [DOI] [PubMed] [Google Scholar]
- Marlor CW, Webb DL, Bombara MP, Greve JM, Blue ML. Expression of vascular cell adhesion molecule-1 in fibroblastlike synoviocytes after stimulation with tumor necrosis factor. Am. J. Pathol. 1992;140:1055–1060. [PMC free article] [PubMed] [Google Scholar]
- Marotte H, Ruth JH, Campbell PL, Koch AE, Ahmed S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology. 2010;49:467–479. doi: 10.1093/rheumatology/kep397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli A, Cipriani P, Liakouli V, Carubbi F, Perricone C, Perricone R, Giacomelli R. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmun. Rev. 2011;10:595–598. doi: 10.1016/j.autrev.2011.04.020. [DOI] [PubMed] [Google Scholar]
- McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in skeletal development and bone mass maintenance. Gene. 2011;474:1–11. doi: 10.1016/j.gene.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde T, Oehme I, Korshunov A, Kopp-Schneider A, Remke M, Northcott P, Deubzer HE, Lodrini M, Taylor MD, von Deimling A, Pfister S, Witt O. HDAC5 and HDAC9 in medulloblastoma: novel markers for risk stratification and role in tumor cell growth. Clin. Cancer Res. 2010;16:3240–3252. doi: 10.1158/1078-0432.CCR-10-0395. [DOI] [PubMed] [Google Scholar]
- Nasu Y, Nishida K, Miyazawa S, Komiyama T, Kadota Y, Abe N, Yoshida A, Hirohata S, Ohtsuka A, Ozaki T. Trichostatin A, a histone deacetylase inhibitor, suppresses synovial inflammation and subsequent cartilage destruction in a collagen antibody-induced arthritis mouse model. Osteoarthritis Cartilage. 2008;16:723–732. doi: 10.1016/j.joca.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Hoshi D, Kiire A, Yamanaka H, Kamatani N. Molecular targets of rheumatoid arthritis. Inflamm. Allergy Drug Targets. 2008;7:53–66. doi: 10.2174/187152808784165199. [DOI] [PubMed] [Google Scholar]
- Park SY, Jun JA, Jeong KJ, Heo HJ, Sohn JS, Lee HY, Park CG, Kang J. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol. Rep. 2011;25:1677–1681. doi: 10.3892/or.2011.1236. [DOI] [PubMed] [Google Scholar]
- Shuttleworth SJ, Bailey SG, Townsend PA. Histone Deacetylase inhibitors: new promise in the treatment of immune and inflammatory diseases. Curr. Drug Targets. 2010;11:1430–1438. doi: 10.2174/1389450111009011430. [DOI] [PubMed] [Google Scholar]
- Silverman MD, Haas CS, Rad AM, Arbab AS, Koch AE. The role of vascular cell adhesion molecule 1/ very late activation antigen 4 in endothelial progenitor cell recruitment to rheumatoid arthritis synovium. Arthritis Rheum. 2007;56:1817–1826. doi: 10.1002/art.22706. [DOI] [PubMed] [Google Scholar]
- Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int. J. Biochem. Cell Biol. 2004;36:372–378. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- Taori K, Paul VJ, Luesch H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008;130:1806–1807. doi: 10.1021/ja7110064. [DOI] [PubMed] [Google Scholar]
- Urbich C, Rossig L, Kaluza D, Potente M, Boeckel JN, Knau A, Diehl F, Geng JG, Hofmann WK, Zeiher AM, Dimmeler S. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009;113:5669–5679. doi: 10.1182/blood-2009-01-196485. [DOI] [PubMed] [Google Scholar]
- Yang Y, Rao R, Shen J, Tang Y, Fiskus W, Nechtman J, Atadja P, Bhalla K. Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res. 2008;68:4833–4842. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Taori K, Kim H, Hong J, Luesch H. Total synthesis and molecular target of largazole, a histone deacetylase inhibitor. J. Am. Chem. Soc. 2008;130:8455–8459. doi: 10.1021/ja8013727. [DOI] [PubMed] [Google Scholar]
- Yurek-George A, Habens F, Brimmell M, Packham G, Ganesan A. Total synthesis of spiruchostatin A, a potent histone deacetylase inhibitor. J. Am. Chem. Soc. 2004;126:1030–1031. doi: 10.1021/ja039258q. [DOI] [PubMed] [Google Scholar]