Abstract
Objective
Proteinuria occurs commonly among HIV-infected and -uninfected injection drug users (IDUs) and is associated with increased mortality risk. Vitamin D deficiency, highly prevalent among IDUs and potentially modifiable, may contribute to proteinuria. To determine whether vitamin D is associated with proteinuria in this population, we conducted a cross-sectional study in the AIDS Linked to the IntraVenous Experience (ALIVE) Study.
Methods
25(OH)-vitamin D levels were measured in 268 HIV-infected and 614 HIV-uninfected participants. The association between vitamin D deficiency (<10 ng/mL) and urinary protein excretion was evaluated by linear regression. The odds of persistent proteinuria (urine protein-to-creatinine ratio >200 mg/g on two occasions) associated with vitamin D deficiency was examined using logistic regression.
Results
One-third of participants were vitamin D-deficient. Vitamin D deficiency was independently associated with higher urinary protein excretion (P<0.05) among HIV-infected and diabetic IDUs (P-interaction<0.05 for all). Persistent proteinuria occurred in 18% of participants. Vitamin D deficiency was associated with >6-fold odds of persistent proteinuria among diabetic IDUs (odds ratio [OR]=6.29, 95% confidence interval [CI]: 1.54, 25.69) independent of sociodemographic characteristics, co-morbid conditions, body mass index, and impaired kidney function (estimated GFR <60 mL/min|1.73 m2); no association, however, was observed among non-diabetic IDUs (OR=1.06, 95% CI: 0.64, 1.76) (P-interaction<0.05).
Conclusions
Vitamin D deficiency was associated with higher urinary protein excretion among those with HIV infection and diabetes. Vitamin D deficiency was independently associated with persistent proteinuria among diabetic IDUs, although not in non-diabetic persons. Whether vitamin D repletion ameliorates proteinuria in these patients requires further study.
Keywords: Vitamin D deficiency, proteinuria, HIV, injection drug use, diabetes
Introduction
Proteinuria has been consistently associated with higher risk of kidney disease progression, cardiovascular disease, and mortality, independent of kidney function level in the general population and in HIV-infected individuals[1, 2]. HIV-infected persons and at-risk injection drug users (IDUs) are at high risk of developing kidney disease. Nearly one-third of IDUs have proteinuria, and those who are HIV-infected have a 3-fold greater risk for proteinuria[3, 4].
Proteinuria in this patient population likely results from the culmination of several risk factors for kidney disease including diabetes, hepatitis C virus (HCV) infection, and hypertension. In addition to these well-known risk factors, vitamin D deficiency, which is highly prevalent in HIV-infected persons [5, 6], may play a role in the development of proteinuria. Vitamin D deficiency may augment renin-angiotensin system (RAS) activity and exacerbate insulin resistance[7, 8]. Moreover, vitamin D deficiency has been linked to greater inflammation and fibrosis in the kidney, which in turn, may exacerbate proteinuria[9–11].
Previous studies in the general population have demonstrated that higher levels of 25(OH)-vitamin D are associated with lower risk of albuminuria and end-stage renal disease[12, 13]. More importantly, results from small clinical trials of individuals with chronic kidney disease (CKD) suggest that repletion of vitamin D may lower proteinuria[14, 15]. No study, however, has evaluated the association of vitamin D deficiency with proteinuria among IDUs, a patient population at high risk for both vitamin D deficiency and proteinuria and who have risk factors for proteinuria and CKD beyond traditional risk factors. To this end, we conducted a cross-sectional study in the AIDS Linked to the IntraVenous Experience (ALIVE) cohort to evaluate whether vitamin D deficiency was associated with urinary protein excretion and persistent proteinuria among HIV-infected and -uninfected IDUs.
Methods
Study Population
A detailed description of the ALIVE Study has been reported previously[16]. Briefly, the original focus of the ALIVE Study was to investigate the incidence and natural history of HIV infection among IDUs in a community-based observational cohort in Baltimore, Maryland. Participants were initially enrolled in 1988–1989 with subsequent enrollments in 1994–1995, 1998, 2000, and 2005–2008. Of 4376 total IDUs recruited, 1065 were HIV-infected and another 340 seroconverted during follow-up.
The study sample for this analysis was selected from IDUs who were actively followed in 2007 through 2008. For inclusion, individuals had to have stored serum available concurrent with previously obtained serum creatinine and urine protein quantification. In total, 898 participants had serum creatinine and at least one urine protein-to-creatinine ratio available. Stored serum samples for 25(OH)-vitamin D measurement were available in 882 individuals. All participants provided informed consent, and the Johns Hopkins School of Public Health Institutional Review Board approved the study.
Data Collection
During biannual in-person visits, participants completed standardized questionnaires and collection of blood and urine; a subset also completed a physical examination. Questions asked by trained interviewers focused on the participant’s medical and social history and current medications. Questions regarding sensitive risk behaviors were conducted via a computer privately.
The primary outcome of interest was urinary protein excretion, estimated by a spot urine protein-to-creatinine ratio on samples collected concurrently with sera for vitamin D levels. Among individuals with at least two urine samples available (n=737), we also examined persistent proteinuria, defined as a random urine protein-to-creatinine ratio >200 mg/g on two occasions, the clinical definition of proteinuria recommended by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative[17]. The median time elapsed between the two urinary protein quantifications was 1.01 years (interquartile range [IQR]: (0.99–1.08 years).
The primary predictor of interest was 25(OH)-vitamin D which was measured at the Tufts Medical Center Core Laboratory using radioimmunoassay (Diasorin, Stillwater, MN), the method on which clinical cutoffs have been based[18, 19]. One participant whose vitamin D result exceeded the maximum limit of detection for the assay was excluded. Because the distribution of 25(OH)-vitamin D in the study population was skewed, values were log-transformed. Vitamin D levels were examined continuously and categorically, with vitamin D deficiency defined as a level <10 ng/mL [20].
Data on potential confounders were obtained through the questionnaire or through clinical and laboratory testing performed at study visits[21]. Hypertension was defined as a self-reported physician diagnosis of high blood pressure, systolic measurement of 140 mmHg or greater, or a diastolic measurement of 90 mmHg or higher. Diabetes was defined as a self-reported physician diagnosis of this condition. HCV infection was defined based on a positive third generation antibody test result. HIV-uninfected participants underwent HIV antibody testing while HIV-infected individuals underwent HIV-1 RNA and CD4+ cell count measurements after each visit. Estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine using the Modification of Diet in Renal Disease equation[22]. An eGFR<60 mL/min|1.73 m2 defined impaired kidney function.
Statistical Analyses
Initial descriptive analyses utilized Student t-tests or Wilcoxon rank-sum tests for continuous variables and X2 test for categorical variables. Unadjusted and adjusted linear regression models were constructed to evaluate the association between vitamin D deficiency and urinary protein excretion values from visits concurrent with vitamin D samples. We adjusted for sociodemographic and clinical characteristics associated with both vitamin D deficiency and proteinuria (HCV and HIV infection, diabetes, hypertension, BMI, and impaired kidney function). The final model included covariates with P values <0.05 in the unadjusted model. We assessed potential effect modification of the association between vitamin D deficiency and urinary protein excretion by HIV infection, diabetes, and impaired kidney function by inclusion of interaction terms in the linear model and by stratified analyses.
The odds of persistent proteinuria associated with vitamin D deficiency was evaluated with logistic regression models. As in the linear regression models, we adjusted for demographic characteristics, co-morbid conditions, BMI, and impaired kidney function, with the final model including covariates with P values<0.05 in the unadjusted model. Effect modification of the association between vitamin D deficiency and persistent proteinuria by HIV infection, diabetes, and impaired kidney function was evaluated using similar approaches as the linear regression models. Sensitivity analyses in which vitamin D was modeled continuously and categorically with a cutoff of ≥20 and <20 ng/mL were also performed.
Results
Table 1 describes the sociodemographic and clinical characteristics of participants. The majority of participants were African American (92%), cigarette smokers (83%), and HCV seropositive (86%). Nearly one-third had used injection drugs within the prior 6 months. In addition, 27% were taking multivitamins. Half of participants self-reported a prior physician diagnosis of hypertension; while 11% had prior diabetes diagnosis. Fifty-four percent of participants had a BMI of 25 kg/m2 or greater, of whom 48% were obese (BMI ≥30 kg/m2). Of the 268 HIV-infected participants, 55% had recently been on highly active antiretroviral therapy (HAART), 40% had HIV-1 RNA levels <50 copies/ mL, and 66% had CD4+ cell counts >200 cells/mm3.
Table 1.
Characteristics of ALIVE Participants
| n=882 | |
|---|---|
| Mean age, y (SD) | 48.9 (7.9) |
| Black, n (%) | 807 (92) |
| Female, n (%) | 305 (35) |
| Homeless, n (%) | 106 (12) |
| Current cigarette smoker, n (%) | 735 (83) |
| Injection drug use within prior 6 months, n (%) | 320 (36) |
| Diabetic, n (%) | 95 (11) |
| Hypertensive, n (%) | 455 (52) |
| HIV infected, n (%) | 268 (30) |
| Median CD4+ cell count, cells/mm3 (IQR) | 278 (168 – 474) |
| Median HIV-1 RNA, copies/mL (IQR) | 620 (<40 – 21, 600) |
| History of AIDS, n (%) | 46 (5) |
| HAART receipt within 6 months, n (%) | 144 (55) |
| Hepatitis C virus antibody positive, n (%) | 754 (86) |
| Median BMI, kg/m2 (IQR) | 25.5 (22.3 – 30.1) |
| Median 25(OH)-vitamin D level, ng/mL (IQR) | 13.30 (8.99 – 20.31) |
| Median eGFR, mL/min|1.73 m2 (IQR) | 101.6 (84.4 – 114.6) |
| Median first UPCR, mg/g (IQR) | 111 (76 – 199) |
| Median second UPCR, mg/g (IQR) | 104 (70 – 209) |
Abbreviations: SD, standard deviation; IQR, interquartile range; HAART, highly active antiretroviral therapy; eGFR, estimated glomerular filtration rate; UPCR, urine protein-to-creatinine ratio
A total of 218 (25%) and 193 (26%) participants had a urinary protein excretion exceeding 200 mg/g on the first and second samples, respectively. Among the 737 participants who had both first and second urine samples available, the prevalence of persistent proteinuria was 18%. Only 6% of participants had an estimated GFR <60 mL/min|1.73 m2. The median vitamin D level for the overall population was 13.3 ng/mL (IQR: 8.99–20.33 ng/mL), with 30% having vitamin D levels <10 ng/mL. The majority of samples were collected during the fall or winter seasons (77%).
Association between Vitamin D Deficiency and Urinary Protein Excretion
In the unadjusted linear regression models, vitamin D levels <10 ng/mL were significantly associated with 103.26 mg/g greater urinary protein excretion compared to higher vitamin D levels (95% CI: 6.27, 200.25) (Table 2). This association was attenuated after adjustment for sociodemographic factors, co-morbid conditions, BMI, and impaired kidney function (β-coefficient=90.17 mg/g, 95% CI: −0.0004, 180.33). HIV infection and diabetes modified the association between vitamin D deficiency and urinary protein excretion (P-interaction<0.05 for both) (Figure 1). The association between vitamin D deficiency and urinary protein excretion was not statistically significant among HIV-uninfected and non-diabetic participants. In contrast, vitamin D deficiency was associated with a 231.24 mg/g higher urinary protein excretion (95 % CI: 28.15, 434.33) among HIV-infected participants. Similarly among diabetic participants, vitamin D deficiency was associated with significantly higher levels of urinary protein excretion (β-coefficient=722.48 mg/g, 95% CI: 313.40, 1131.56) compared to vitamin D levels >10 ng/mL. While impaired kidney function also modified the association between vitamin D deficiency and urinary protein excretion (P-interaction <0.001), vitamin D deficiency was not significantly associated with urinary protein excretion in the adjusted models stratified by kidney function. Inferences from this analysis, however, were limited by the small number of participants with impaired kidney function.
Table 2.
Linear Association between 25(OH)-Vitamin D Deficiency and Urinary Protein Excretion (N=882)
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Difference in mg/g UPCR (95% CI) |
P-value | Difference in mg/g UPCR (95% CI) |
P-value | |
| Vitamin D <10 ng/mL | 103.26 (6.27, 200.25) | 0.04 | 90.17 (−0.0004, 180.33) | 0.05 |
| Age, per 1 year older | 3.87 (−1.77, 9.52) | 0.18 | --- | |
| Black | 30.74 (−129.05, 190.54) | 0.71 | −64.64 (−214.47, 85.18) | 0.40 |
| Female | 124.51 (31.15, 217.86) | 0.009 | 98.98 (11.12, 186.84) | 0.03 |
| Homeless | −12.56 (−149.95, 124.83) | 0.86 | --- | -- |
| IDU within 6 mos | −17.48 (−110.18, 75.22) | 0.71 | --- | -- |
| HIV-infected | 242.32 (146.74, 337.91) | <0.001 | 156.98 (66.01, 247.94) | 0.001 |
| HCV seropositive | 178.09 (51.59, 304.59) | 0.006 | 72.80 (−46.93, 192.53) | 0.23 |
| Diabetic | 198.19 (54.83, 341.55) | 0.007 | 162.09 (23.95, 300.22) | 0.02 |
| Hypertensive | 206.93 (118.59, 295.28) | <0.001 | 141.97 (54.96, 228.97) | 0.001 |
| BMI, per 1kg/m2 higher | −10.85 (−18.19, −3.51) | 0.004 | −10.86 (−18.05, −3.67) | 0.003 |
| Impaired kidney function | 1067.76 (899.16, 1236.36) | <0.001 | 952.75 (778.10, 1127.40) | <0.001 |
Abbreviations: UPCR, urine protein-to-creatinine ratio; IDU, injection drug use; HCV, hepatitis C virus; BMI, body mass index
Figure 1. Adjusted Difference in Urinary Protein Excretion Associated with Vitamin D Deficiency Stratified by Co-Morbid Conditions.
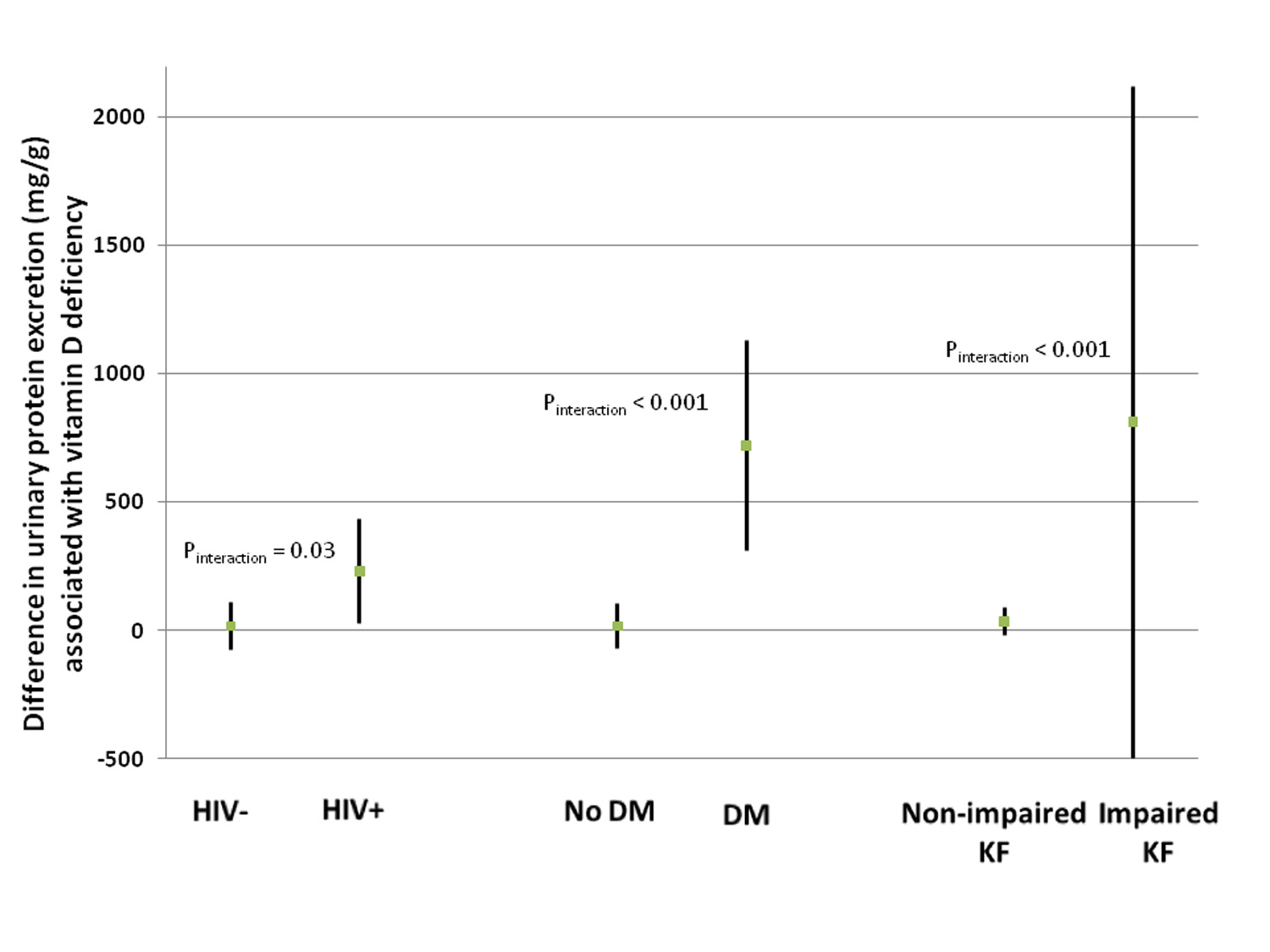
The graph displays the estimated difference in urinary protein excretion associated with vitamin D deficiency stratified by HIV serostatus, diabetes (DM), and impaired kidney function (KF), adjusted for the other co-morbid conditions, race, gender, hepatitis C virus antibody status, and BMI. The □ represents the point estimate, and the associated line shows the 95% confidence interval.
Association of Vitamin D Deficiency and Persistent Proteinuria
Vitamin D deficiency was not associated with persistent proteinuria in the unadjusted models (Table 3). Similar findings were observed after adjustment for sociodemographic characteristics, co-morbid conditions, BMI, and impaired kidney function (OR=1.37, 95% CI: 0.86, 2.18). Diabetes, however, modified the association between vitamin D deficiency and persistent proteinuria (P-interaction = 0.008). Vitamin D deficiency was not associated with persistent proteinuria among non-diabetic participants (OR=1.06, 95% CI: 0.64, 1.76). In contrast, vitamin D levels <10 ng/mL were associated with more than a 6-fold greater odds of persistent proteinuria among diabetic participants (OR=6.29, 95% CI: 1.54, 25.69) compared to those with higher vitamin D levels in fully-adjusted models; ORs for persistent proteinuria among diabetics remained increased in the range of 3–6 irrespective of adjustment factors (Table 3). Neither HIV infection nor impaired kidney function modified the association between vitamin D deficiency and persistent proteinuria. Other independent predictors of persistent proteinuria in the multivariable model included HIV infection (OR=5.02, 95% CI: 3.21, 8.32), HCV seropositivity (OR=7.15, 95% CI: 1.68, 30.41), hypertension (OR=2.00, 95% CI: 1.22, 3.25), and BMI (OR=0.91 per kg/m2, 95% CI: 0.87, 0.96). When modeled continuously, lower vitamin D levels were associated with higher odds of proteinuria among diabetic (OR=5.01 per 10% lower vitamin D, 95% CI: 1.47, 17.00) but not among non-diabetic participants (OR=1.09 per 10% lower vitamin D, 95% CI: 0.75, 1.59). Similar findings were observed in multivariable analyses using a cutoff of <20 ng/mL to define vitamin D deficiency, with no association observed in non-diabetic participants (OR=0.99, 95% CI: 0.59, 1.67) but with the odds of persistent proteinuria associated with vitamin D deficiency attenuated among diabetic participants (OR=3.57, 95% CI: 0.82, 15.42).
Table 3.
Association of Vitamin D Deficiency (25[OH] vitamin D <10 ng/mL) with Persistent Proteinuria, Overall and Stratified by Diabetes Status (n=737)
| Unadjusted | Demographic adjusted† | Demographic adjusted + co- morbid conditions‡ |
Demographic adjusted + co- morbid conditions + BMI¶ |
Demographic adjusted + co- morbid conditions + BMI + impaired kidney function‖ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
P-value | OR (95% CI) |
P-value | OR (95% CI) |
P-value | OR (95% CI) |
P-value | OR (95% CI) |
P-value | |
| Overall | 1.27 (0.85, 1.90) | 0.24 | 1.23 (0.82, 1.84) | 0.32 | 1.39 (0.90, 2.16) | 0.14 | 1.29 (0.82, 2.04) | 0.26 | 1.37 (0.86, 2.18) | 0.18 |
| Diabetic (n=79)* | 3.15 (1.09, 9.04) | 0.03 | 3.47 (1.17, 10.34) | 0.02 | 5.88 (1.67, 20.66) | 0.006 | 4.93 (1.31, 1.08) | 0.02 | 6.29 (1.54, 25.69) | 0.01 |
| Non-diabetic (n=657)* | 1.12 (0.72, 1.74) | 0.62 | 1.04 (0.67, 1.63) | 0.85 | 1.08 (0.67, 1.75) | 0.75 | 1.03 (0.99, 1.06) | 0.90 | 1.06 (0.64, 1.76) | 0.82 |
Adjusted for age and race
Adjusted for demographics and co-morbid conditions (diabetes, hypertension, hepatitis C antibody status, and HIV infection)
Adjusted for demographics, co-morbid conditions, and BMI
Adjusted for demographics, co-morbid conditions, BMI and impaired kidney function (defined as eGFR<60 mL/min/1.73 m2)
Vitamin D and diabetes interaction: P<0.05 in both sets of models
Abbreviations: BMI, body mass index
Discussion
In this study of well-characterized HIV-infected and -uninfected IDUs, vitamin D deficiency was associated with higher levels of urinary protein excretion independent of potential confounders such as sociodemographic factors and BMI. This association was modified by HIV infection, diabetes, and impaired kidney function, with substantially higher urinary protein excretion associated with vitamin D deficiency in participants with any of these conditions. Moreover, among diabetic individuals, severe vitamin D deficiency was associated with a more than 6-fold higher risk of persistent proteinuria independent of the same potential confounders. These observations are important because severe vitamin D deficiency and proteinuria are common among IDUs and have been associated with adverse outcomes. In addition, vitamin D deficiency is a modifiable factor.
Kidney dysfunction and albuminuria are associated with greater risk of cardiovascular complications and mortality in the general and HIV-infected populations[1, 2, 23, 24]. Although the incidence of HIV-associated nephropathy is declining in the context of effective antiretroviral therapy, the prevalence of kidney disease continues to increase among HIV-infected individuals[25, 26]. As this patient population ages, more traditional risk factors for kidney disease such as diabetes and hypertension play a greater role in kidney disease pathogenesis and progression. Understanding the complex interplay among these risk factors, including the contribution of vitamin D, will allow appropriate interventions and management to be determined.
Our results are consistent with recent findings from the general U.S. population and among HIV-uninfected individuals with CKD. Among 15,068 participants in the Third National Health and Nutrition Examination Survey, de Boer et al. demonstrated a linear inverse association between vitamin D levels and albuminuria. Among 1,847 individuals with impaired kidney function, low levels of 25(OH)-vitamin D and 1,25(diOH)-vitamin D were both independently associated with a 2- to 3-fold higher risk of albuminuria[9]. The association between vitamin D and proteinuria observed in our study are further supported by small short-term clinical trials in individuals with CKD who received paricalcitol, an analog of active vitamin D. In 61 HIV-uninfected individuals with CKD and proteinuria >400 mg per day, Fishbane and colleagues showed that participants who received paricalcitol experienced an 18% decline in proteinuria from baseline to 6 months compared to a 3% increase in individuals who received placebo[14].
Our findings, however, suggest that vitamin D deficiency’s role in kidney disease is important primarily among diabetic individuals. Results from prior studies of diabetic mouse models and humans with diabetic nephropathy support the important role of vitamin D deficiency in the pathogenesis and progression of diabetic nephropathy. In a series of experiments by Zhang et al., the combined administration of losartan and paricalcitol prevented albuminuria and restored hyperglycemia-induced glomerular structural changes to a greater degree than either drug alone in murine models of type 1 diabetes[27]. A subsequent study confirmed the favorable effect of losartan and vitamin D on kidney disease in a model of type 2 diabetes[28]. A more recent clinical trial evaluated the effect of paricalcitol on albuminuria among 281 type 2 diabetic individuals with impaired kidney function (median eGFR 39–42 mL/min|1.73 m2) and persistent albuminuria despite RAS blockade[15]. Participants who received 2 mcg of paricalcitol had an average decline in 24-hour urinary albuminuria of 34% from baseline. There was a trend, however, for increasing urinary albumin excretion 60 days after paricalcitol cessation. Individuals who received placebo had no significant change in albuminuria.
Several mechanisms by which vitamin D deficiency may be associated with proteinuria have been proposed. First, vitamin D is a known negative regulator of the RAS which plays a key role in the development of diabetic nephropathy through angiotensin II-induced glomerular injury[29, 30]. In a series of experiments, Li et al. showed that mice lacking the vitamin D receptor had elevated renin and angiotensin II levels and developed hypertension[7]. While blockade of the RAS with ACE-inhibitors or angiotensin-receptor blockers retards diabetic nephropathy progression [31, 32], it also leads to compensatory elevations in renin which may cause renal injury independent of angiotensin II[33]. Li and colleagues also demonstrated, however, that vitamin D repletion of vitamin D-deficient mice decreases renin synthesis[7]. Second, vitamin D deficiency may exacerbate insulin resistance which in turn may cause or worsen albuminuria[34]. Murine studies suggest that insulin activation of the podocyte insulin receptors is important in maintaining the podocyte cytoskeleton and function[35]. Moreover, 1,25(diOH)-vitamin D administration lessens podocyte loss and inhibits hypertrophy after injury[36]. In a meta-analysis of the association between vitamin D and type 2 diabetes, insulin resistance improved with vitamin D repletion among individuals with existing glucose intolerance[37]. Vitamin D may also influence urinary protein excretion through its immunomodulatory effects. Among individuals with kidney disease, vitamin D levels inversely correlated with urinary levels of the pro-inflammatory chemokine, monocyte chemoattractant protein-1 (MCP-1)[38]. In addition, vitamin D may play a role in epithelial mesenchymal transition, the process by which tubular interstitial fibrosis ensues and a mechanism though to contribute to HIV-associated nephropathy[39, 40].
This study has several limitations to consider. First, the cross-sectional design precludes establishing temporality between vitamin D deficiency and proteinuria. Although animal studies and small clinical trials support a causal relationship between vitamin D deficiency and proteinuria especially among diabetics, we could not evaluate whether vitamin D deficiency preceded proteinuria. Due to the observational nature of this study, there may be residual confounding by unknown factors. The majority of study participants were African Americans who are known to have lower vitamin D levels than other ethnic groups. Therefore, our findings may not be generalizable to IDUs of different ethnic background. We evaluated vitamin D continuously, however, so as to not impose conventional cut-offs which may not be physiologically relevant in African Americans. In addition, we utilized a stringent cut-off to define vitamin D deficiency to avoid misclassification of individuals as being vitamin D-deficient. Our findings were significant mainly among diabetic individuals; however, we did not have renal biopsy specimens to confirm the underlying cause of their kidney disease. As the ALIVE Study was initially aimed at understanding the natural history of HIV infection among IDUs, data on other factors which may impact proteinuria, such as use of ACE-inhibitors or angiotensin-receptor blockers, were not collected. Despite these limitations, the ALIVE Study consists of a well-characterized cohort of HIV-infected and uninfected IDUs allowing adjustment for several relevant potential confounders. Our study represents the first study of the association between vitamin D deficiency and persistent proteinuria among IDUs, a population at great risk for both conditions.
In conclusion, vitamin D deficiency was associated with higher urinary protein excretion among IDUs with HIV infection and diabetes. Furthermore, vitamin D deficiency was strongly associated with increased risk of persistent proteinuria among diabetic IDUs. Additional studies are needed to further elucidate the mechanisms by which vitamin D deficiency may promote proteinuria. Vitamin D repletion may improve proteinuria in this patient population. Results from previous small clinical trials, however, are unlikely to be generalizable to IDUs who have additional risk factors for proteinuria apart from traditional risk factors. Therefore, a well-powered clinical trial to validate the observed association between vitamin D deficiency and persistent proteinuria among HIV-infected and uninfected diabetic IDUs is still needed.
Acknowledgements
The study concept and design was conducted by M.M.E., G.D.K., T.T.B., and G.M.L. The statistical analysis and drafting of the manuscript was performed by M.M.E. Critical revision of the manuscript for important intellectual content was performed by all authors.
This study was supported by NIH-NIDA grant P30 DA13868, Tufts University Nutrition Collaborative, a Center for Drug Abuse and AIDS Research. MME is supported by the NIH-NIDDK-sponsored grant K23DK081317. GML is supported by NIH-NIAID grant R01DA026770. The ALIVE Study was supported by NIH-NIDA grants R01DA12568 and R01DA04334 and by NIH-NIAID grant RC1AI086053.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanik EL, Lucas GM, Vlahov D, Kirk GD, Mehta SH. HIV and proteinuria in an injection drug user population. Clin J Am Soc Nephrol. 2010;5:1836–1843. doi: 10.2215/CJN.01030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 5.Adeyemi OM, Agniel D, French AL, Tien P, Weber K, Glesby MJ, et al. Vitamin D deficiency in HIV-infected and un-infected women in the US. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31821ae418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. Am J Clin Nutr. 2010;92:1344–1349. doi: 10.3945/ajcn.110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T, Gutierrez OM, Patel NM, Andress DL, Wolf M, Levin A. Vitamin d deficiency, inflammation, and albuminuria in chronic kidney disease: complex interactions. J Ren Nutr. 2011;21:295–302. doi: 10.1053/j.jrn.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21:966–973. doi: 10.1681/ASN.2009080872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200:207–221. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20:2631–2639. doi: 10.1681/ASN.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 15.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 16.Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Hollis BW, Napoli JL. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem. 1985;31:1815–1819. [PubMed] [Google Scholar]
- 19.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140:2030S–2045S. doi: 10.3945/jn.110.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis. 2011 doi: 10.1093/cid/cir673. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Estrella MM, Parekh RS, Abraham A, Astor BC, Szczech LA, Anastos K, et al. The impact of kidney function at highly active antiretroviral therapy initiation on mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2010;55:217–220. doi: 10.1097/QAI.0b013e3181e674f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George E, Lucas GM, Nadkarni GN, Fine DM, Moore R, Atta MG. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. AIDS. 2010;24:387–394. doi: 10.1097/QAD.0b013e3283359253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 26.Choi AI, Shlipak MG, Hunt PW, Martin JN, Deeks SG. HIV-infected persons continue to lose kidney function despite successful antiretroviral therapy. AIDS. 2009;23:2143–2149. doi: 10.1097/QAD.0b013e3283313c91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105:15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, et al. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int. 2010;77:1000–1009. doi: 10.1038/ki.2010.22. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert RE, Krum H, Wilkinson-Berka J, Kelly DJ. The renin-angiotensin system and the long-term complications of diabetes: pathophysiological and therapeutic considerations. Diabet Med. 2003;20:607–621. doi: 10.1046/j.1464-5491.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- 30.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 31.Chan JC, Ko GT, Leung DH, Cheung RC, Cheung MY, So WY, et al. Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int. 2000;57:590–600. doi: 10.1046/j.1523-1755.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 32.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 33.Veniant M, Menard J, Bruneval P, Morley S, Gonzales MF, Mullins J. Vascular damage without hypertension in transgenic rats expressing prorenin exclusively in the liver. J Clin Invest. 1996;98:1966–1970. doi: 10.1172/JCI119000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fornoni A. Proteinuria, the podocyte, and insulin resistance. N Engl J Med. 2010;363:2068–2069. doi: 10.1056/NEJMcibr1008395. [DOI] [PubMed] [Google Scholar]
- 35.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, et al. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol. 2004;286:F526–F533. doi: 10.1152/ajprenal.00316.2003. [DOI] [PubMed] [Google Scholar]
- 37.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011 doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74:1343–1353. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Spataro BC, Yang J, Dai C, Liu Y. 1,25-dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 2005;68:1500–1510. doi: 10.1111/j.1523-1755.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 40.Yadav A, Vallabu S, Kumar D, Ding G, Charney DN, Chander PN, et al. HIVAN phenotype: consequence of epithelial mesenchymal transdifferentiation. Am J Physiol Renal Physiol. 2010;298:F734–744. doi: 10.1152/ajprenal.00415.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


